Abstract
Speckle-tracking echocardiography is increasingly utilized to assess strain in a variety of pathologies. Strain measurements have demonstrated utility following heart-transplant (HTx) and may aid in the detection of rejection and cardiac allograft vasculopathy (CAV). Strain parameters have not been well defined in the pediatric HTx population. This study aimed to describe strain in pediatric HTx recipients compared to controls and assess changes over time. All pediatric HTx recipients with available echocardiograms (2004–2015) without rejection or CAV were identified. Longitudinal and circumferential strain were measured at <1 month, 1-year, 3-years, and 5-years post-transplant and compared to controls. A total of 218 echocardiograms were analyzed in 79 HTx recipients. At <1 month post-transplant, there was significant decrement in longitudinal strain (GLS −14.6 vs. −19.2,p<0.001) with concurrent augmentation of circumferential strain (GCS −27.3 vs. −24.3,p=0.005). By 1-year post-HTx, all strain parameters normalized and were not significantly different from the control population. In the absence of graft complications, strain parameters did not change up to 5-years post-transplant. Abnormal longitudinal strain parameters are present in the early post-HTx period with a compensatory increase in circumferential strain. These changes normalize by 1-year post-transplant and do not change over time in the absence of graft complications.
Introduction
Two-dimensional speckle tracking echocardiography (STE) is an emerging technology that provides an angle-independent measure of myocardial deformation1,2. Through the calculation of myocardial strain and strain rate, deformation imaging allows for a more direct quantification of regional and global myocardial contraction and may allow detection of subclinical cardiac disease. This technology is becoming increasingly utilized across a wide spectrum of cardiovascular pathology, including patients following heart transplantation (HTx). Alterations in strain measured by STE have been documented in the setting of allograft rejection3–5 as well as cardiac allograft vasculopathy (CAV)6,7, and may aid in the non-invasive assessment of left ventricular diastolic dysfunction8,9.
Numerous studies have reported normal reference values for STE-measured strain in both the pediatric and adult populations10–20. Despite this, there are limited published data in ‘healthy’ HTx recipients. The few studies that are available suggest that longitudinal strain parameters as measured by STE are lower in HTx recipients at 1-year post-transplant compared to controls21,22. Additionally, while at least one study demonstrated no deterioration in strain over time in adult HTx recipients23, the changes in strain parameters over time following HTx have not been well-described in the pediatric population.
To fully recognize the potential of STE to detect graft complications, it is critical to define reference values and the changes in these parameters over time. Moreover, longitudinal assessment of strain in pediatric HTx recipients without rejection or CAV allows for a better understanding of myocardial remodeling after HTx. The aim of this study was to define reference values of strain, strain rate, and diastolic strain parameters in pediatric HTx recipients and to assess changes in these measurements over time in the absence of rejection or CAV. We hypothesized that strain parameters would be lower in HTx recipients compared to healthy controls and that these values would remain stable over time in the absence of graft complications.
Methods
Patient Selection
This was a retrospective study and was approved by the Vanderbilt University Institutional Review Board. All pediatric patients who underwent HTx at Vanderbilt University Medical Center from 1999–2015 were included. Our center utilizes routine induction therapy with anti-thymocyte globulin before transitioning to maintenance immunosuppression with tacrolimus and mycophenolate mofetil. Steroids are used sparingly and discontinued prior to discharge in the majority of patients. Echocardiograms were identified based on time post-transplant and included echocardiograms obtained: (1) At the time of initial discharge or 1-month post-transplant (whichever came first), (2) 1-year post-transplant, (3) 3-years post-transplant, and (4) 5-years post-transplant. Only one echocardiogram per patient was analyzed at each time point. Echocardiogram exclusion criteria were: (1) echocardiogram not in digital imaging and communications in medicine (DICOM) format (echocardiograms performed prior to 2004); (2) inadequate image quality; (3) prior episode of rejection or diagnosis of CAV (all subsequent echocardiograms were excluded after a diagnosis of either rejection or CAV). Rejection was defined as an event that prompted augmentation of the immunosuppression regimen. This includes patients with clinical evidence of rejection with or without endomyocardial biopsy and those with endomyocardial biopsies with ISHLT grade ≥2R (2004 grading system24), grade 1B (1990 grading system25), or ≥pAMR 226. Our center routinely performs C4d immunofluorescence on all biopsy specimens. CAV was defined as detectable lumen narrowing on routine coronary angiography. Coronary angiograms were interpreted by 1 of 3 experienced interventional cardiologists, but not reviewed by study personnel. Our center utilizes primarily non-invasive echocardiographic surveillance for rejection with catheterizations and coronary angiography performed annually following the first year post-HTx. During the first post-HTx year, 1 to 4 biopsies are performed dependent on patient age at HTx. Biopsies are also performed when clinically indicated.
Healthy control population
STE measurements were also performed on a group of 142 healthy pediatric patients aged 0–18 years. Healthy control patients were retrospectively identified and had echocardiograms obtained between December 2012 and April 2015 for other indications (e.g. murmurs) and were without detectable cardiovascular pathology. A complete chart review was conducted for each control patient and all patients with documented cardiovascular disease were removed from the study. All echocardiograms were interpreted as normal and all studies were reviewed by study personnel to insure that no pathology was missed. Exclusion criteria were the following: inadequate image quality, congenital or acquired heart disease including arrhythmia, first degree relative with cardiomyopathy, history of prematurity, obesity, and a diagnosis of a genetic abnormality. No significant variation in strain parameters was noted in our healthy control population based on age or gender and therefore no matching was performed and transplant recipients were compared to the entire control population.
Echocardiography
Images were obtained on Siemens Acuson Sequoia (Siemens Medical Solutions, Erlangen, Germany) and Philips IE33 and EPIQ7 ultrasound machines (Philips Medical Systems, Best, The Netherlands). The same machines were utilized for both the control group and the HTx group. Echocardiograms were obtained using a standard HTx protocol. To ensure image quality and consistency, HTx patients at our institution are imaged by a core group of 6 sonographers with additional training in the HTx protocol. This protocol includes multiple acquisitions of the short axis at the level of the papillary muscles and the apical 4-chamber view to ensure adequate visualization of the endocardial border. Although the protocol includes functional assessment at the base and apex in the short axis and the 2-chamber and 3-chamber apical views, these images were not analyzed in this study due to variable image quality.
Post-processing
STE measurements were performed using vendor independent software (Cardiac Performance Analysis, TomTec Imaging Systems, Unterschleissheim, Germany). Images were imported in the standard DICOM format with a standard frame rate of 30 Hz. Measurements included global longitudinal strain (GLS), global circumferential strain (GCS), longitudinal strain rate (LSR), circumferential strain rate (CSR), peak longitudinal early diastolic strain rate (LDSR), and peak circumferential early diastolic strain rate (CDSR). Circumferential strain and strain rate measurements were obtained from a parasternal short axis image at the level of the papillary muscles and longitudinal strain and strain rate measurements were obtained from an apical 4-chamber view. Measurements were completed for one cardiac cycle, from end-diastole to end-diastole. Measurements were performed by two readers with expertise in STE (MS and CH), blinded to clinical history. One set of measurements was performed for each echocardiogram. To insure consistency in tracking and measurement technique, all measurements were reviewed and validated by an additional reader with expertise in STE who was also blinded to clinical history (JG). Standard echocardiographic measurements were also collected for each study including ejection fraction (measured using either Simpson’s biplane technique or 5/6 area length method), medial and lateral tissue Doppler velocities as well as medial and lateral E/E’.
Statistical analysis
Patient demographics were assessed with standard summary statistics. Longitudinal changes in strain measurements were described and at each time point post-transplant, strain measurements were compared to the healthy control population using the Wilcoxon rank sum test. A Bonferroni correction was used given multiple comparisons. Given the variable patient populations at each time point, data were subsequently analyzed only for patients with measurements at each time interval using a repeated measures ANOVA. Standard deviations were calculated for this group to assess intra-patient variability. Changes in standard echocardiographic measures over time including ejection fraction and tissue Doppler velocities were described. Linear regression was performed to assess the impact of donor ischemic time and donor-to-recipient weight ratio on baseline strain measurements post-transplant. Approximately 10% of the transplant recipients were randomly selected and strain measurements were repeated to assess reproducibility and intraclass correlation coefficients were calculated. All statistical analyses were performed in R version 3.3.3 (R core team (2017), R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria) or STATA version 13 (StataCorp LLC; College Station, TX) with α<0.05 considered statistically significant. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Vanderbilt27.
Results
A total of 247 echocardiograms were obtained during the study period and eligible for inclusion. Echocardiographic images were suboptimal for accurate STE assessment in 29 studies (11.7%) and these were excluded. In total, 218 echocardiograms were analyzed from 79 unique HTx recipients. A total of 63 echocardiograms were analyzed <1 month, 68 at 1-year, 51 at 3-years, and 36 at 5-years post-transplant. The decline in echocardiogram numbers was secondary to varying follow-up intervals and exclusion secondary to the development of rejection or CAV. A total of 26 patients were excluded due to rejection or CAV. Demographics of the included patients are shown in Table 1. This group was predominantly male (58.2%), Caucasian (68.4%), and UNOS status 1A at transplant (65.8%). The control population was comprised of 142 pediatric patients with normal echocardiograms. In this group, 79 patients were male (55%) and the median age was 5.5 years (range 0 – 18 years).
Table 1.
Demographics of included patients at each time point
| Total (N=79) | Baseline (N=63) | 1 year (N=68) | 3 years (N=51) | 5 years (N=36) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Male Gender | 46 | (58.2%) | 36 | (60.%) | 37 | (56.9%) | 28 | (58.3%) | 21 | (63.6%) |
| Race | ||||||||||
| Caucasian | 54 | (68.4%) | 42 | (70.%) | 44 | (67.7%) | 32 | (66.7%) | 23 | (69.7%) |
| African-American | 18 | (22.8%) | 13 | (21.7%) | 14 | (21.5%) | 12 | (25.%) | 8 | (24.2%) |
| Other | 7 | (8.8%) | 5 | (8.3%) | 7 | (10.8%) | 4 | (8.3%) | 2 | (6.1%) |
| Diagnosis | ||||||||||
| Cardiomyopathy | 37 | (46.8%) | 25 | (41.7%) | 32 | (49.2%) | 25 | (52.1%) | 13 | (39.4%) |
| Congenital heart disease | 37 | (46.8%) | 34 | (56.7%) | 31 | (47.7%) | 22 | (45.8%) | 19 | (57.6%) |
| Retransplant | 5 | (6.3%) | 1 | (1.7%) | 2 | (3.1%) | 1 | (2.1%) | 1 | (3.%) |
| Age at transplant | 2.8 | (0.46 – 15) | 3 | (0.46 – 15.2) | 2.9 | (0.46 – 4.9) | 1.5 | (0.42 – 12.2) | 1.6 | (0.42 – 8.4) |
| Blood type | ||||||||||
| O | 45 | (57.%) | 35 | (58.3%) | 37 | (56.9%) | 29 | (60.4%) | 25 | (75.8%) |
| A | 22 | (27.9%) | 16 | (26.7%) | 17 | (26.2%) | 13 | (27.1%) | 5 | (15.2%) |
| B | 10 | (12.7%) | 7 | (11.7%) | 9 | (13.8%) | 4 | (8.3%) | 3 | (9.1%) |
| AB | 2 | (2.5%) | 2 | (3.3%) | 2 | (3.1%) | 2 | (4.2%) | 0 | - |
| Listing status at transplant | ||||||||||
| 1A | 52 | (65.8%) | 50 | (83.3%) | 54 | (83.1%) | 38 | (79.2%) | 26 | (78.8%) |
| 1B | 12 | (15.2%) | 4 | (6.7%) | 4 | (6.2%) | 3 | (6.2%) | 2 | (6.1%) |
| 2 | 15 | (19.%) | 6 | (10.%) | 7 | (10.8%) | 7 | (14.6%) | 5 | (15.2%) |
| Pre-transplant mechanical support | 12 | (15.4%) | 9 | (15.%) | 9 | (13.8%) | 6 | (12.5%) | 3 | (9.1%) |
| Ventilator at transplant | 13 | (16.9%) | 9 | (15.3%) | 11 | (16.9%) | 9 | (18.8%) | 5 | (15.2%) |
| Ischemic time (minutes) | 204 | (175 – 242) | 202 | (172 – 237) | 202 | (174 – 243) | 200 | (150 – 234) | 192 | (139 – 231) |
| Donor-recipient weight ratio | 1.2 | (1 – 1.8) | 1.2 | (1 – 1.8) | 1.2 | (1 – 1.8) | 1.3 | (1 – 1.9) | 1.3 | (1 – 1.9) |
Data expressed as median (IQR) for continuous variables and N(%) for categorical variables
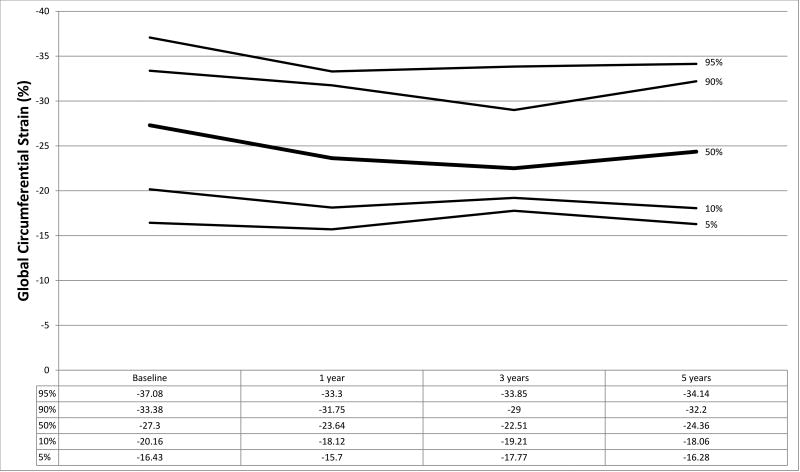
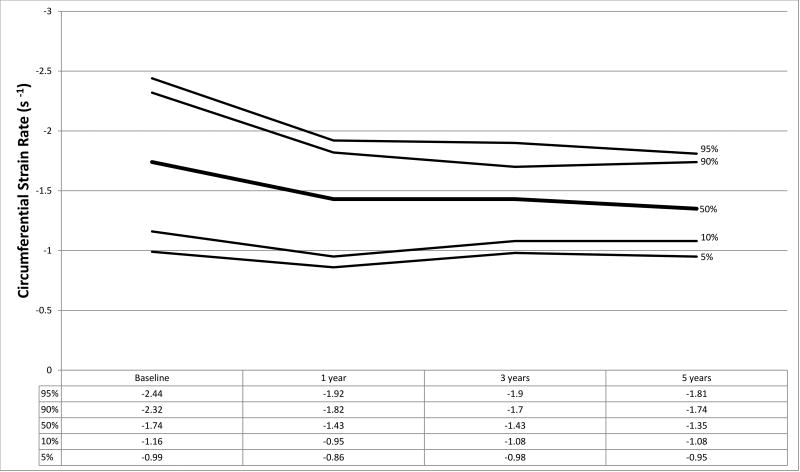
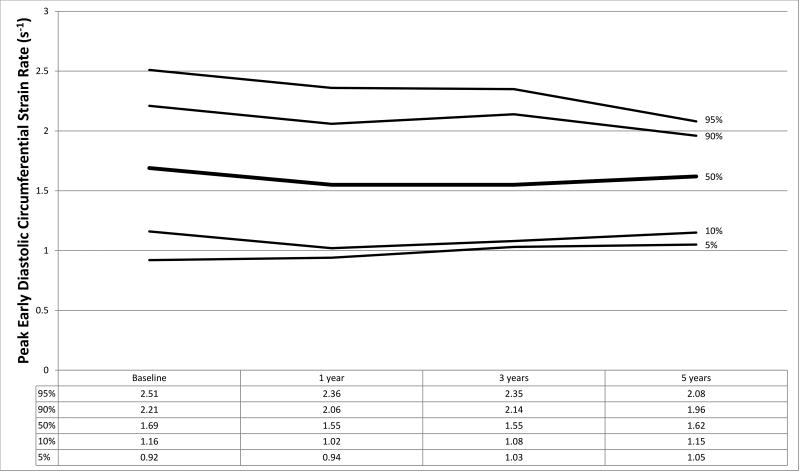
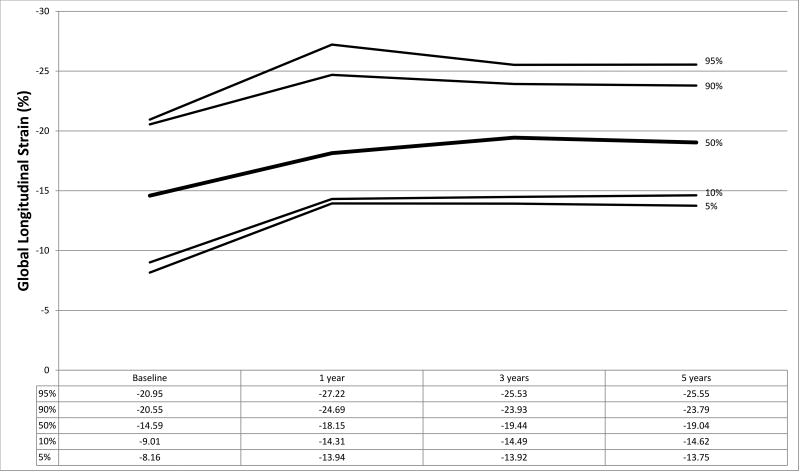
Strain measurements from HTx recipients and the normal control population are shown in Table 2 with graphical representation of strain parameters in HTx recipients shown in Figures 1a–1f. In the control population, median GLS and GCS were −19.18 (IQR −17.31, −21.61) and −24.27 (IQR −21.91, −28.03) respectively. At baseline, HTx recipients demonstrated significantly worse GLS (−14.59, p<0.001) and augmented GCS (−27.30, p=0.005) compared to the control group. LSR (−0.84 vs. −1.03, p<0.001) and LDSR (0.98 vs. 1.25, p<0.001) were also noted to be worse in HTx recipients with concurrent augmentation of CSR (−1.74 vs. −1.45, p<0.001) and CDSR (1.69 vs. 1.51, p=0.002) (Table 2). These results remained significant when accounting for multiple comparisons using a Bonferroni correction (p<0.0125 considered statistically significant). No significant association was found between baseline GLS or GCS and donor ischemic time or donor-to-recipient weight ratio (R2<0.05 for all analyses).
Table 2.
Strain measurements of transplant recipients compared to normal controls
| Normal Controls (N=142) |
Baseline (N=63) | 1 year (N=68) | 3 years (N=51) | 5 years (N=36) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Circumferential Strain | p-value* | p-value* | p-value* | p-value* | |||||
|
|
|
|
|
||||||
| Global circumferential strain | −24.27 (−21.91, −28.03) | −27.3 (−23.87, −30.01) | 0.005 | −23.64 (−19.96, −26.79) | 0.241 | −22.51 (−20.86, −26.87) | 0.252 | −24.36 (−21.41, −28.43) | 0.949 |
| Average circumferential strain rate | −1.45 (−1.25, −1.65) | −1.74 (−1.47, −1.98) | <0.001 | −1.43 (−1.16, −1.67) | 0.166 | −1.43 (−1.19, −1.56) | 0.160 | −1.35 (−1.2, −1.61) | 0.136 |
| Peak diastolic circumferential strain rate | 1.51 (1.29, 1.71) | 1.69 (1.38, 2.06) | 0.002 | 1.55 (1.38, 1.85) | 0.145 | 1.55 (1.29, 1.85) | 0.277 | 1.62 (1.31, 1.82) | 0.271 |
| Longitudinal Strain | |||||||||
| Global longitudinal strain | −19.18 (−17.31, −21.61) | −14.59 (−11.11, −17.59) | <0.001 | −18.15 (−16.12, −21.67) | 0.272 | −19.44 (−16.19, −21.63) | 0.825 | −19.04 (−16.06, −21.57) | 0.900 |
| Average longitudinal strain rate | −1.03 (−0.9, −1.26) | −0.84 (−0.7, −1.09) | <0.001 | −1.06 (−0.93, −1.24) | 0.679 | −0.95 (−0.86, −1.17) | 0.062 | −1.07 (−0.86, −1.29) | 0.965 |
| Peak diastolic longitudinal strain rate | 1.25 (1.05, 1.47) | 0.98 (0.69, 1.24) | <0.001 | 1.32 (1.09, 1.58) | 0.209 | 1.39 (1.04, 1.62) | 0.272 | 1.33 (1.12, 1.6) | 0.184 |
p-values from the Wilcoxon Rank Sum test for pairwise comparison of each time point with the normal control group
Data expressed as median (25%, 75%)
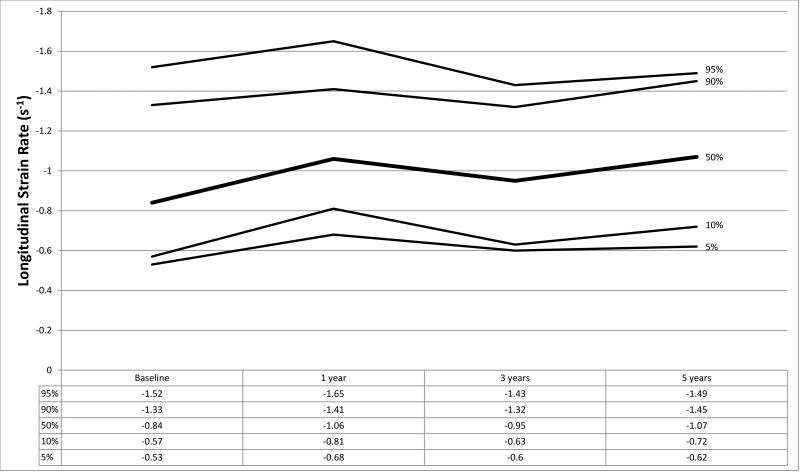
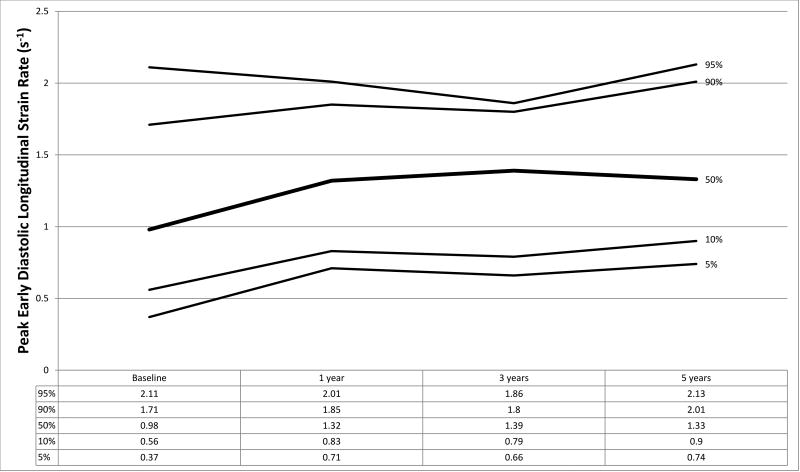
Figure 1.
a–f. Percentiles at each time point for a) global circumferential strain, b) circumferential strain rate, c) peak circumferential early diastolic strain rate, d) global longitudinal strain, e) longitudinal strain rate, and f) peak longitudinal early diastolic strain rate.
Differences in strain parameters resolved by 1-year post-HTx across all measurements. In the absence of rejection or CAV, no changes in strain parameters were observed over time from 1 year post-HTx up to 5 years post-HTx (Table 2). Ejection fraction in HTx recipients remained stable across all time points with a mean of 62%, 63.2%, 62.3%, and 63.2% at baseline, 1-year, 3-years, and 5-years respectively. Medial and lateral tissue Doppler velocities were lower at baseline compared to all other time points (medial E’: 9.7 cm/s at baseline vs. 13.2, 13.9, and 13 cm/s at 1-, 3-, and 5-years respectively; lateral E’: 13.4 cm/s at baseline vs. 16.3, 16.9, and 16 cm/s at 1-, 3-, and 5-years respectively) (p<0.001 for all pairwise comparisons of baseline with other time points using a paired t-test). There were no differences in medial or lateral E/E’ values across any time points. In total, 25 (32%) patients had data collected at all time intervals. Strain measurements for this group are shown in Table 3. Repeated measures ANOVA demonstrated significant changes from baseline values for all longitudinal and circumferential strain parameters. Excluding baseline measurements, standard deviations were calculated at the patient level for each measurement and reported in Table 4.
Table 3.
Strain measurements of transplant recipients with measurements at all time points
| Baseline (N=25) | 1 year (N=25) | 3 years (N=25) | 5 years (N=25) | ||
|---|---|---|---|---|---|
| Circumferential Strain | p-value* | ||||
|
|
|||||
| Global circumferential strain | −28.4 (−24.14, −30.33) | −24.99 (−19.74, −27.84) | −25.72 (−21.68, −28.28) | −25.14 (−20.95, −28.82) | 0.01 |
| Average circumferential strain rate | −1.77 (−1.54, −2.14) | −1.47 (−1.23, −1.69) | −1.44 (−1.2, −1.64) | −1.36 (−1.19, −1.61) | <0.001 |
| Peak diastolic circumferential strain rate | 1.99 (1.51, 2.18) | 1.62 (1.43, 1.73) | 1.55 (1.35, 1.85) | 1.51 (1.26, 1.78) | <0.001 |
| Longitudinal Strain | |||||
| Global longitudinal strain | −15.78 (−11.89, −19.99) | −19.83 (−17.48, −23.67) | −20.08 (−15.95, −22.87) | −19.53 (−18.06, −22.08) | <0.001 |
| Average longitudinal strain rate | −1.01 (−0.68, −1.15) | −1.21 (−0.93, −1.36) | −1.01 (−0.87, −1.24) | −1 (−0.86, −1.29) | 0.017 |
| Peak diastolic longitudinal strain rate | 1.07 (0.74, 1.3) | 1.57 (1.2, 1.74) | 1.45 (1.02, 1.69) | 1.33 (1.13, 1.61) | 0.003 |
p-value from repeated measures ANOVA across all time points
Data expressed as median (25%, 75%)
Table 4.
Range of standard deviations at the patient level for all measurements
| Standard Deviation | |||
|---|---|---|---|
|
|
|||
| Minimum | Maximum | Average | |
| Circumferential Strain | |||
| Global circumferential strain | 0.28 | 8.67 | 3.15 |
| Average circumferential strain rate | 0.02 | 0.44 | 0.2 |
| Peak diastolic circumferential strain rate | 0.07 | 0.56 | 0.26 |
| Longitudinal Strain | |||
| Global longitudinal strain | 0.36 | 4.12 | 2.12 |
| Average longitudinal strain rate | 0.05 | 0.35 | 0.2 |
| Peak diastolic longitudinal strain rate | 0.05 | 0.46 | 0.23 |
For patients with measurements at all time points (N=25)
Excluding baseline measurements
Strain measurements were repeated on a total of 22 transplant patients by a second independent reviewer who was blinded to the initial results. GCS and CSR both demonstrated good interrater reliability with intraclass correlation coefficients of 0.873 (95%CI: 0.723–0.945) and 0.847 (95%CI: 0.671–0.933) respectively. Peak diastolic circumferential strain rate demonstrated less optimal but still acceptable interrater reliability with an intraclass correlation coefficient of 0.643 (95%CI: 0.320–0.834). Interrater reliability for longitudinal strain measurements was good for GLS with an intraclass correlation coefficient of 0.793 (95%CI: 0.570–0.908), but not as good for LSR and LDSR with intraclass correlation coefficients of 0.525 (95%CI: 0.150–0.770) and 0.599 (0.254–0.810) respectively.
Discussion
Our analysis demonstrates that pediatric HTx recipients have decreased longitudinal strain parameters with concurrent augmentation of circumferential strain in the early post-HTx period. These changes normalize by 1-year and do not change significantly up to 5-years post-HTx in the absence of clinically evident rejection or CAV. These results further elucidate the myocardial remodeling that occurs after HTx and suggest that minimal changes occur after 1-year post-HTx. These data will aid in graft assessment using STE by providing reference values in this unique population.
The relative decrement in longitudinal strain and augmentation in circumferential strain following a period of ischemia is not surprising and may be explained by myocardial fiber structure and myocardial blood supply. Longitudinal fibers are present in a subendocardial location and therefore may be more susceptible to ischemic challenges22,28–30. Additionally, it has been suggested that circumferential fibers have a greater functional reserve compared to longitudinal fibers and this may help to explain the relative augmentation of circumferential strain early post-HTx30. Prior studies have demonstrated augmentation of circumferential strain in the presence of a systemic right ventricle, suggesting that these fibers may adapt to ventricular stress31. Lastly, it is unclear how denervation and subsequent catecholamine dependence impacts myocardial fiber function and this may also contribute to the changes seen in the early post-HTx period.
Similar to our study, two prior reports have documented abnormal longitudinal strain in HTx recipients21,22. However, in contrast to these studies, we demonstrate that differences in longitudinal strain occur early post-HTx and resolve by 1-year. The reason for these differences is unclear, but may result from center variation in myocardial preservation, ischemic time, and post-transplant management. Each of these studies has a relatively small sample size and our analysis is the largest reported cohort of ‘healthy’ HTx recipients to date. Importantly, we also demonstrate that longitudinal and circumferential strain measurements remain stable up to 5-years post-HTx following initial normalization. This finding is similar to the study by Pichler et al. that demonstrated no deterioration in longitudinal strain 3 years following HTx in adults23. While left ventricular strain measurements normalized by 1-year post-HTx in our analysis, prior studies have demonstrated the persistence of right ventricular systolic dysfunction up to 1-year post-HTx32. Therefore, measurable differences may be present beyond 1-year post-HTx that are not evident based on the assessment of left ventricular strain in isolation.
Given the apparent stability of longitudinal and circumferential strain measurements over time, a significant change from baseline may prove to be a valuable tool for non-invasive graft assessment. In fact, decreases in strain have been reported with allograft rejection as well as CAV7,33–35. More recently, diastolic strain parameters have been shown to correlate with pulmonary capillary wedge pressure, indicating that diastolic strain measurements may be a useful tool for non-invasive graft assessment9. Our analysis provides baseline measurements of peak diastolic strain rate and lays the groundwork for further research on this topic.
Patients in our analysis demonstrated stable ejection fraction over time despite measurable differences in both longitudinal and circumferential strain parameters. Similar changes were also noted in medial and lateral tissue Doppler velocities. Strain measured by STE may allow detection of subclinical myocardial dysfunction and is likely more sensitive than ejection fraction alone in detecting alterations in graft function.
Limitations
Our study has inherent limitations. There is significant center variation in the management of pediatric HTx recipients, and differences in management strategies may impact the generalizability of these results. Additionally, given the unavailability of certain echocardiographic views, segmental analysis was not possible and therefore our study focused on global strain assessment. While the 12% of echocardiograms with inadequate image quality may have skewed the study results, we consider this a low rate of unacceptable images, reinforcing the potential utility of STE in this patient population. Given differences in echocardiogram quality, follow-up intervals, and dropout due to the development of rejection or CAV, our study cohort varied over time, representing a potential limitation. The use of third party strain analysis software improves the generalizability of this study as images from multiple vendors can be analyzed with Cardiac Performance Analysis Software. However, the lower temporal resolution of images at 30 Hz may lead to underestimation of values. While longitudinal and circumferential strain values are less likely to be affected by frame rate36,37, strain rates may be more susceptible. In addition, there may be variation in strain measurements based on the software package used, as robust standardization has not yet been pursued36,38. In addition, our analysis demonstrates the potential for significant intra-patient variability, which may limit the clinical utility of serial strain measurements. While ischemic time was not found to be associated with baseline strain measurements in our analysis, ischemic time is fairly normally distributed at our center and therefore it is unclear what impact significantly shorter or longer ischemic times may have on these parameters. Lastly, it is possible that subclinical rejection episodes occurred during the study period, as our center utilizes surveillance biopsy sparingly.
Conclusions
Pediatric HTx recipients demonstrate worse longitudinal systolic and diastolic strain in the early post-HTx period with concurrent augmentation in circumferential strain parameters. These changes normalize by 1-year post-HTx and do not change over time in the absence of clinically evident rejection or CAV. For this reason, a significant change from baseline in strain parameters may warrant further investigation.
Acknowledgments
Acknowledgements / Funding sources:
This project was generously supported by the Enduring Hearts Foundation and the Thrasher Research Fund (JG). Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL123938 (Bethesda, MD) (JHS). The project was also supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06 (Bethesda, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- HTx
Heart transplant
- CAV
cardiac allograft vasculopathy
- STE
speckle tracking echocardiography
- GLS
global longitudinal strain
- GCS
global circumferential strain
- LSR
longitudinal strain rate
- CSR
circumferential strain rate
- LDSR
peak longitudinal early diastolic strain rate
- CDSR
peak circumferential early diastolic strain rate
Footnotes
Disclosures:
None
References
- 1.Dandel M, Hetzer R. Echocardiographic strain and strain rate imaging--clinical applications. International journal of cardiology. 2009;132(1):11–24. doi: 10.1016/j.ijcard.2008.06.091. [DOI] [PubMed] [Google Scholar]
- 2.Clemmensen TS, Eiskjaer H, Kofoed-Nielsen PB, Hoyer S, Poulsen SH. Case of Acute Graft Failure during Suspected Humoral Rejection with Preserved Ejection Fraction, but Severely Reduced Longitudinal Deformation Detected by 2D-Speckle Tracking. Case reports in transplantation. 2014;2014:173589. doi: 10.1155/2014/173589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Podrouzkova H, Bedanova H, Tretina M, et al. Decrease in longitudinal strain in heart transplant recipients is associated with rejection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(4):601–606. doi: 10.5507/bp.2015.020. [DOI] [PubMed] [Google Scholar]
- 4.Mingo-Santos S, Monivas-Palomero V, Garcia-Lunar I, et al. Usefulness of Two-Dimensional Strain Parameters to Diagnose Acute Rejection after Heart Transplantation. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015;28(10):1149–1156. doi: 10.1016/j.echo.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Lisi M, Cameli M, Tacchini D, Ballo P, Maccherini M, Mondillo S. Two-dimensional speckle tracking echocardiography of acute cardiac transplant rejection following pregnancy. Journal of clinical ultrasound : JCU. 2012;40(7):451–454. doi: 10.1002/jcu.21886. [DOI] [PubMed] [Google Scholar]
- 6.Zoeller BB, Miyamoto SD, Younoszai AK, Landeck BF., 2nd Longitudinal Strain and Strain Rate Abnormalities Precede Invasive Diagnosis of Transplant Coronary Artery Vasculopathy in Pediatric Cardiac Transplant Patients. Pediatric cardiology. 2016;37(4):656–662. doi: 10.1007/s00246-015-1328-9. [DOI] [PubMed] [Google Scholar]
- 7.Clemmensen TS, Logstrup BB, Eiskjaer H, Poulsen SH. Evaluation of longitudinal myocardial deformation by 2-dimensional speckle-tracking echocardiography in heart transplant recipients: Relation to coronary allograft vasculopathy. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014 doi: 10.1016/j.healun.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Buddhe S, Richmond ME, Gilbreth J, Lai WW. Longitudinal Strain by Speckle Tracking Echocardiography in Pediatric Heart Transplant Recipients. Congenital heart disease. 2015;10(4):362–370. doi: 10.1111/chd.12263. [DOI] [PubMed] [Google Scholar]
- 9.Lu JC, Magdo HS, Yu S, et al. Usefulness of Diastolic Strain Measurements in Predicting Elevated Left Ventricular Filling Pressure and Risk of Rejection or Coronary Artery Vasculopathy in Pediatric Heart Transplant Recipients. The American journal of cardiology. 2016;117(9):1533–1538. doi: 10.1016/j.amjcard.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Bussadori C, Moreo A, Di Donato M, et al. A new 2D-based method for myocardial velocity strain and strain rate quantification in a normal adult and paediatric population: assessment of reference values. Cardiovasc Ultrasound. 2009;7:8. doi: 10.1186/1476-7120-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallaire F, Slorach C, Bradley T, et al. Pediatric Reference Values and Z Score Equations for Left Ventricular Systolic Strain Measured by Two-Dimensional Speckle-Tracking Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2016;29(8):786–793. e788. doi: 10.1016/j.echo.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Kocabay G, Muraru D, Peluso D, et al. Normal left ventricular mechanics by two-dimensional speckle-tracking echocardiography. Reference values in healthy adults. Rev Esp Cardiol (Engl Ed) 2014;67(8):651–658. doi: 10.1016/j.rec.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Levy PT, Machefsky A, Sanchez AA, et al. Reference Ranges of Left Ventricular Strain Measures by Two-Dimensional Speckle-Tracking Echocardiography in Children: A Systematic Review and Meta-Analysis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2016;29(3):209–225. e206. doi: 10.1016/j.echo.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus KA, Mavinkurve-Groothuis AM, Barends M, et al. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2011;24(6):625–636. doi: 10.1016/j.echo.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Jashari H, Rydberg A, Ibrahimi P, et al. Normal ranges of left ventricular strain in children: a meta-analysis. Cardiovasc Ultrasound. 2015;13:37. doi: 10.1186/s12947-015-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marwick TH, Leano RL, Brown J, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2(1):80–84. doi: 10.1016/j.jcmg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Menting ME, McGhie JS, Koopman LP, et al. Normal myocardial strain values using 2D speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography. 2016;33(11):1665–1675. doi: 10.1111/echo.13323. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Maruyama A, Ichihashi K. Myocardial strain of the left ventricle in normal children. J Cardiol. 2012;60(2):145–149. doi: 10.1016/j.jjcc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Takigiku K, Takeuchi M, Izumi C, et al. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circulation journal : official journal of the Japanese Circulation Society. 2012;76(11):2623–2632. doi: 10.1253/circj.cj-12-0264. [DOI] [PubMed] [Google Scholar]
- 20.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2013;26(2):185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Saleh HK, Villarraga HR, Kane GC, et al. Normal left ventricular mechanical function and synchrony values by speckle-tracking echocardiography in the transplanted heart with normal ejection fraction. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30(6):652–658. doi: 10.1016/j.healun.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Kailin JA, Miyamoto SD, Younoszai AK, Landeck BF. Longitudinal myocardial deformation is selectively decreased after pediatric cardiac transplantation: a comparison of children 1 year after transplantation with normal subjects using velocity vector imaging. Pediatric cardiology. 2012;33(5):749–756. doi: 10.1007/s00246-012-0205-z. [DOI] [PubMed] [Google Scholar]
- 23.Pichler P, Binder T, Hofer P, et al. Two-dimensional speckle tracking echocardiography in heart transplant patients: three-year follow-up of deformation parameters and ejection fraction derived from transthoracic echocardiography. European heart journal cardiovascular Imaging. 2012;13(2):181–186. doi: 10.1093/ejechocard/jer239. [DOI] [PubMed] [Google Scholar]
- 24.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. The Journal of heart transplantation. 1990;9(6):587–593. [PubMed] [Google Scholar]
- 26.Berry GJ, Angelini A, Burke MM, et al. The ISHLT working formulation for pathologic diagnosis of antibody-mediated rejection in heart transplantation: evolution and current status (2005–2011) The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30(6):601–611. doi: 10.1016/j.healun.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH. Left ventricular fibre architecture in man. Br Heart J. 1981;45(3):248–263. doi: 10.1136/hrt.45.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spotnitz HM. Macro design, structure, and mechanics of the left ventricle. J Thorac Cardiovasc Surg. 2000;119(5):1053–1077. doi: 10.1016/S0022-5223(00)70106-1. [DOI] [PubMed] [Google Scholar]
- 30.Pauliks LB, Pietra BA, Kirby S, et al. Altered ventricular mechanics in cardiac allografts: a tissue Doppler study in 30 children without prior rejection events. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24(11):1804–1813. doi: 10.1016/j.healun.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 31.Pettersen E, Helle-Valle T, Edvardsen T, et al. Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. Journal of the American College of Cardiology. 2007;49(25):2450–2456. doi: 10.1016/j.jacc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 32.Lunze FI, Colan SD, Gauvreau K, et al. Cardiac allograft function during the first year after transplantation in rejection-free children and young adults. Circ Cardiovasc Imaging. 2012;5(6):756–764. doi: 10.1161/CIRCIMAGING.112.976613. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz Ortiz M, Pena ML, Mesa D, et al. Impact of Asymptomatic Acute Cellular Rejection on Left Ventricle Myocardial Function Evaluated by Means of Two-Dimensional Speckle Tracking Echocardiography in Heart Transplant Recipients. Echocardiography. 2014 doi: 10.1111/echo.12623. [DOI] [PubMed] [Google Scholar]
- 34.Sera F, Kato TS, Farr M, et al. Left ventricular longitudinal strain by speckle-tracking echocardiography is associated with treatment-requiring cardiac allograft rejection. Journal of cardiac failure. 2014;20(5):359–364. doi: 10.1016/j.cardfail.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Sehgal S, Blake JM, Sommerfield J, Aggarwal S. Strain and strain rate imaging using speckle tracking in acute allograft rejection in children with heart transplantation. Pediatric transplantation. 2015;19(2):188–195. doi: 10.1111/petr.12415. [DOI] [PubMed] [Google Scholar]
- 36.Koopman LP, Slorach C, Manlhiot C, et al. Assessment of myocardial deformation in children using Digital Imaging and Communications in Medicine (DICOM) data and vendor independent speckle tracking software. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2011;24(1):37–44. doi: 10.1016/j.echo.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Risum N, Ali S, Olsen NT, et al. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2012;25(11):1195–1203. doi: 10.1016/j.echo.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Koopman LP, Slorach C, Hui W, et al. Comparison between different speckle tracking and color tissue Doppler techniques to measure global and regional myocardial deformation in children. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2010;23(9):919–928. doi: 10.1016/j.echo.2010.06.014. [DOI] [PubMed] [Google Scholar]