Abstract
Chronic pain is an important and understudied comorbidity in people living with HIV (PLWH). We conducted a pilot trial of Skills TO Manage Pain (STOMP), an innovative social cognitive theory-based pain self-management intervention tailored to PLWH, to assess feasibility, acceptability, and preliminary efficacy. Eligibility criteria included being HIV+, ≥ moderate pain for ≥ 3 months and a score of ≥ 4 on the three-item PEG pain severity and interference scale. Participants were randomized in a 1:1 fashion to STOMP or a usual care comparison. Among 22 participants randomized to STOMP, median session attendance was 9/12 (75%). Of 19 STOMP participants surveyed, 13 reported being “much better” overall since beginning treatment. Brief pain inventory-total scores decreased by 2 points in the intervention group and 0.9 in the control group (p = 0.11). STOMP is feasible, acceptable, and shows preliminary evidence of efficacy and promise for a full-scale trial.
Keywords: HIV, Pain, Social cognitive theory, Self-management
Introduction
Chronic pain, defined as pain lasting for at least 3 months, is an important comorbidity in people living with HIV (PLWH). Although prevalence estimates vary depending on sampling and measurement methods, as many as 30–85% of PLWH experience chronic pain [1–3]. Chronic pain in the modern antiretroviral era includes a predominance of musculoskeletal pain [4, 5], is associated with significant functional disability [6], and in some individuals, suboptimal retention in HIV primary care [7].
Given the unique biopsychosocial milieu experienced by PLWH [8], interventions for chronic pain should be developed for and tested in this population. However, a recent systematic review found only 11 studies of interventions for chronic pain that have been tested in PLWH [9]. Seven of the interventions were pharmacologic, four were non-pharmacologic (two behavioral interventions), and most studies were limited by lack of randomization or short-term follow-up. The two behavioral interventions tested both included cognitive behavioral therapy delivered by clinical psychologists, a resource often not available in HIV treatment settings. Both studies were limited by poor session adherence.
Due to the serious risks and modest benefits of medications such as opioids for individuals with chronic pain, the 2016 Department of Health and Human Services National Pain Strategy underscored the urgent importance of developing cost-effective, scalable behavioral interventions, called pain self-management (PSM) interventions, to treat chronic pain [10]. These interventions promote building PSM skills, such as cognitive reframing and increasing physical activity to manage pain, and can be administered as a complement or alternative to pharmacologic approaches. To optimize treatment effects, it is critical to tailor interventions to the specific needs of the target population, in this case PLWH, and incorporate behavior change theory [11].
We developed a social cognitive theory (SCT)-based PSM intervention tailored to PLWH called Skills TO Manage Pain (STOMP). Using other PSM intervention manuals as a starting point [12, 13], STOMP’s development was informed by extensive qualitative inquiry of patients and providers [14], and an intervention mapping technique that integrates qualitative findings and theory into every intervention component [15]. The result is an HIV primary care clinic-based 12-session intervention that includes group, peer, and one-on-one skill building components and incorporates the SCT constructs of self-regulation, self-efficacy, observational learning, and outcome expectations.
STOMP is an innovative approach to pain management in PLWH for several reasons. STOMP is the first behavioral intervention to apply the PSM approach to PLWH. Additionally, STOMP’s approach to addressing pain is novel. We are aware of only one other chronic pain intervention that uses peers in a very different way—to deliver one-on-one PSM skill-building content [16]. To our knowledge, STOMP is the first PSM intervention to include peers in order to share personal experiences and model adaptive PSM behaviors, which we hypothesize will lead to improved self-efficacy. Further, while psychologist-led pain CBT groups are common in clinical practice, they are typically used as an efficient way to deliver content rather than for social support, and have not incorporated peer leaders.
The primary objective of this study was to assess STOMP’s feasibility and acceptability, including session adherence and participant experience with the intervention. We also conducted exploratory analyses of the preliminary impact of STOMP on pain-related outcomes.
Methods
We conducted a pilot randomized controlled trial of STOMP compared to usual care (http://Clinicaltrials.gov: NCT02824562). Our approach to the design and reporting of this pilot trial was informed by Thabane et al.’s adaptation of the CONSORT statement [17]. The study protocol was approved by the university’s Institutional Review Board.
Participants and Setting
PLWH and chronic pain were recruited from an HIV clinic in the southeastern US [18]. This clinic provides comprehensive care, including primary and specialty care, mental health services, case management, and a pharmacy.
Study participants were recruited using fliers, provider referrals, and by querying the clinic’s pain patient reported outcome (PROs). Pain PROs included the two-item brief chronic pain questionnaire (BCPQ), which asks participants about pain duration and severity [19, 20], and the three-question PEG, which asks about pain severity and pain-related functional impairment (pain-related interference with general activities and enjoyment of life) on a scale of 0–10 [21].
Potential participants were initially screened by phone using the BCPQ and PEG. Those who met the study’s inclusion criteria were invited for an in-person pre-screening visit. At the pre-screening visit, potential participants were again screened using the BCPQ and PEG. We excluded individuals who reported planning surgery or other major treatment during the subsequent few months, extended travel plans, or being unavailable to participate in group sessions on the days/times they were offered. During an initial assessment, participants were asked about transportation barriers, and transportation vouchers (bus and gas) were provided throughout the intervention as needed.
Enrollment visits for potentially eligible participants were scheduled within approximately 2 months of the intervention’s anticipated start date to ensure that the participant continued to meet inclusion criteria and participant commitment to the study (i.e., a brief run-in period). Participants were consented, enrolled, and completed a battery of questionnaires described below. Then participants were randomized to STOMP versus usual care. Note that individuals randomized to STOMP also continued to receive usual care, which we assessed systematically in both groups (see “Control: Usual Care” section, below). Randomization was conducted during the enrollment visit in a 1:1 fashion. The study statistician generated a block randomization scheme with block sizes of two, four, or six.
Interventionists
This study used four paid interventionists: two peers with HIV and chronic pain (“pain pals”) and two research staff (“pain coaches”) to work in pain coach–pain pal pairs. Each pair was responsible for the same 10 participants during the study period and co-led group sessions. Pain coaches also delivered the one-on-one sessions.
The pain pal role was created to be responsive to participants’ desire to learn from someone with shared experiences relating to HIV and chronic pain [14]. We also hypothesized that learning by watching a peer model healthy PSM behaviors (a SCT construct) would improve participants’ self-efficacy, or confidence in their own abilities. Pain pals were patients identified by clinic leadership as having excellent PSM skills and were hired as paid study staff. Participants also saw a role for learning PSM skills from a knowledgeable expert—the pain coach. The pain coaches had master’s degrees in health education or social work, and had served as interventionists on prior HIV behavioral trials.
Pain pals and pain coaches received training on chronic pain in HIV and on the study protocol. All attended two trainings: (1) a half-day, pain psychology group session delivered by a pain psychologist, and (2) a day-long training workshop with the investigators and staff who developed and tested the PSM intervention on which STOMP was structured [12]. Pain coaches initially delivered all one-on-one sessions to their pain pal partner, which served as training for both parties. These sessions were audio-recorded so that the pain coaches could receive individualized feedback. Debriefing sessions with WD and JSM were held weekly throughout the intervention.
STOMP Intervention
STOMP consisted of 12 sessions: 6 individual and 6 group sessions alternating weekly for 12 weeks:
One-on-one sessions the purpose of the one-on-one sessions was to build PSM skills. These sessions were led by the pain coaches. Based on input from our qualitative work, we developed 10 one-on-one sessions; all participants received a pain education session, and were allowed to select 5 of the remaining 9 sessions (physical activity and your pain, losing weight to improve your pain, relaxation skills to prevent your pain, sleeping better to help your pain, thinking differently about your pain, building self-worth, talking with our family and friends about pain, taking opioid pain medications).
Group sessions the purpose of the group sessions was to enhance peer support related to chronic pain, an important theme that emerged from our qualitative work [14]. The group sessions were co-led by the pain coach–pain pal pair. Each session included sharing reflections on lessons learned and goals set during one-on-one sessions, and challenges encountered.
STOMP incorporates several SCT constructs. These include self-regulation (e.g., self-monitoring by completing homework between one-on-one sessions, goal-setting at every session), self-efficacy (e.g., social modeling through the use of pain pals who are successful pain self-managers), observational learning (e.g., observing others’ successes at group sessions), and outcome expectations (e.g., encouragement at group and one-on-one sessions that participation will lead to improvement).
Control: Usual Care
The control group received usual care, meaning any other pharmacologic and non-pharmacologic treatments for chronic pain provided by their clinicians and not related to the study. We systematically documented participants’ receipt of usual care in both arms, including medications, physical therapy, and clinic visits that could help pain (e.g., pain specialist, psychologist). A usual care control allowed us to estimate retention rates of controls not receiving any active treatment in pain trials, informing the development of an enhanced usual care control in the planned full-scale trial of STOMP.
Sample Size
The goal total sample size was 40, with 20 participants per arm, a sample size generally sufficient to investigate feasibility/acceptability [22].
Feasibility and Acceptability Outcomes
Feasibility outcomes included recruitment, randomization, retention, timely completion of the intervention, and completion of an outcome assessment battery. Acceptability was assessed by semi-structured qualitative interviews at the midpoint and end of the study, and treatment satisfaction questionnaires. These outcomes are described in more detail in Table 1.
Table 1.
Intervention feasibility and acceptability outcomes
| Feasibility outcomes | |
| Recruitment | Our goal was to be able to recruit all 40 participants using study fliers, referrals from primary care providers, and if needed, a database of individuals from the clinic with chronic pain based on a recent patient reported outcome questionnaire. We determined the approach-to-enroll ratio, which we will use to estimate the number of participants needed to approach for the full-scale trial to achieve our desired sample size |
| Randomization | Not all pilot trials involve randomization, as the purpose of a pilot trial is to assess feasibility and acceptability rather than to assess the differences in outcomes between the intervention and a control group. However, there is a paucity of chronic pain intervention studies among individuals with HIV, who have an especially high burden of chronic pain. Therefore, we investigated the feasibility and acceptability of randomization to a usual care control among PLWH and chronic pain |
| Retention | Given the Center for Disease Control and Prevention’s benchmark for behavioral interventions for adherence and retention to HIV treatment and care, our goal was for participants to complete an average of 80% of all study visits. Individuals who missed sessions were called to ask about barriers to attendance |
| Completion in allotted time | Group sessions occurred every other week on a fixed schedule. However, one-on-one sessions were scheduled at the participant’s convenience. We determined what percentage of participants would be able to complete all one-on-one sessions within the study period (16 weeks) |
| Outcome assessment completion | The full-scale trial will assess distal outcomes including pain and pain-related functional impairment, as well as more proximal outcomes such as SCT constructs (e.g., self-efficacy), and potential confounders of effect (e.g., mood) identified in our previously published conceptual framework. A goal of the present study was to evaluate the feasibility of administering a battery of outcome assessments. Additionally, outcome assessments were conducted immediately following study completion. Our goal was to complete outcome assessments on 80% of individuals randomized to the study within 1 month of the participant’s last session |
| Acceptability outcomes | |
| Participant experience with the study | We conducted qualitative interviews with patient participants at the mid-point and end of the trial. The purpose of these interviews was to assess participants’ experience with the study and assess the need for modifications. Participants were asked what they liked and did not like about the intervention, what if anything they noticed changed during the intervention, and what they would change about the intervention in the future |
| Participant satisfaction | Participants completed a treatment satisfaction questionnaire after the intervention was completed |
Exploratory Assessment of STOMP’s Efficacy
Outcome assessors were blinded to the intervention condition. Pre and post-intervention study questionnaires were informed by the IMMPACT guidelines on outcomes relevant to pain clinical trials [23], and included SCT constructs hypothesized to be impacted by the intervention:
Brief pain inventory (BPI)-total score a composite measure of pain and function [24].
PEG as described above [21].
The pain self-efficacy questionnaire (higher scores indicates better pain self-efficacy, and scores of 40 or more have been associated with better outcomes [25]). Collection of data on other SCT constructs such as self-regulation and outcome expectations was piloted during the study but was not of sufficient quality to merit reporting.
Tampa kinesiophobia scale (higher scores indicate greater fear of pain with movement, a maladaptive coping mechanism; mild = 23, moderate = 33, severe = 43 [26]), and the pain catastrophizing scale (higher scores indicate catastrophizing, clinically relevant catastrophizing = 30 or more [27]).
Other questionnaires included the PHQ-8 for depressive symptoms (higher scores indicate worse depressive symptoms, a score of 10 or greater is considered moderate depressive symptoms) [28]; the AUDIT-C for alcohol use (used gender-specific version, ≥ 2 is considered to be at-risk drinking) [29], and the ASSIST for substance use [30].
Analyses
Outcomes were reported as means and standard deviations for continuous variables, and frequencies and percentages for categorical variables. For continuous variables t-tests were used to compare change scores between the two groups, and Fisher’s exact tests were used to compare percentages. Interviews were analyzed thematically by two independent coders (SRY, JSM). Discrepancies were reconciled, a code book was created, and the lead analyst (SRY) coded the remaining transcripts with continued input from the other coder. Representative quotes are presented for themes that helped us understand why and how the intervention could work.
Results
Participant Flow
Recruitment and enrollment lasted 13 weeks (July–October 2016). Figure 1 details participant flow through the study. Ninety-eight potential participants initiated contact with study staff. Of these 45 participants who completed the enrollment visit, 43 were randomized. One participant was withdrawn because the recruitment goal had been achieved, and one participant was unintentionally not randomized and was placed in the control arm.
Fig. 1.
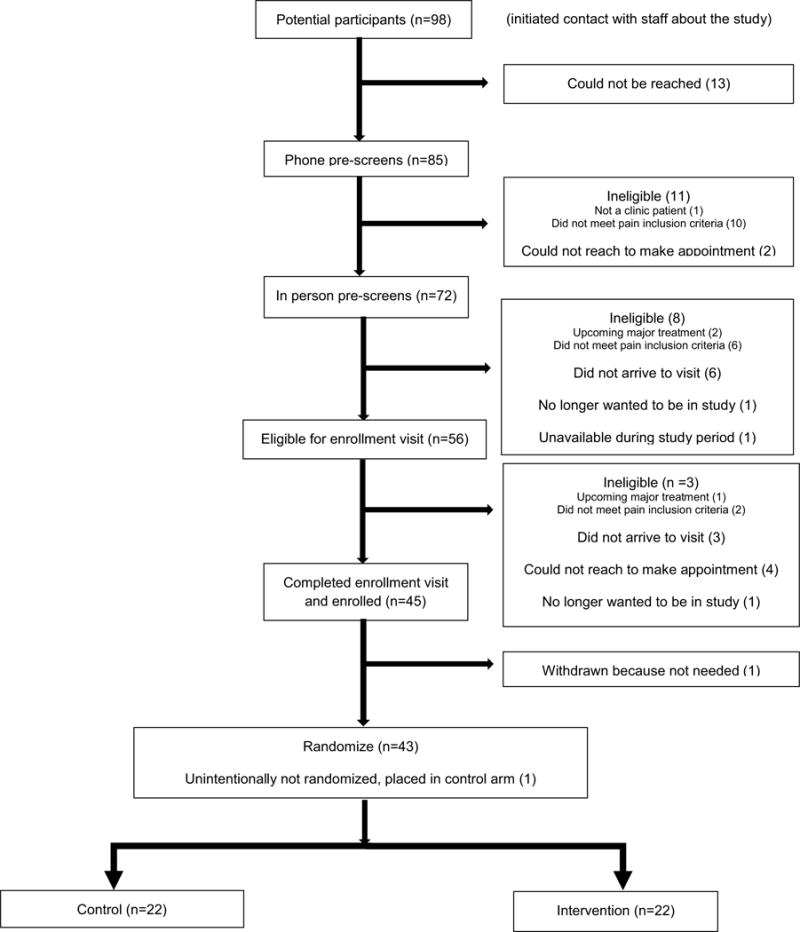
CONSORT diagram
Baseline Characteristics
Baseline demographic and clinical characteristics are presented in Table 2. Overall, participants (N = 44) had a median age of 51 years, 25 were female, 38 were Black, and 42 were virologically suppressed. Nearly all (42) participants reported that transportation vouchers would help them attend study visits. The most common pain locations were low back, knee, and numbness/tingling in the hands and feet. The mean BPI-total score (0–10) was 7.8 (SD 2.1) in the intervention group and 7.4 (SD 1.6) in the control group. Baseline pain self-efficacy scores were similarly low in both groups, and catastrophizing was similarly high. The majority of participants reported current or prior substance use.
Table 2.
STOMP pilot trial baseline data
| Intervention N = 22 | Control N = 22 | |
|---|---|---|
| Age (median, IQR) (EMR) | 51 (48–55) | 51 (46–57) |
| Female gender, n (%) (EMR) | 11 (50) | 14 (64) |
| Race, n (%) (EMR) | ||
| White | 3 (14) | 2 (9) |
| Black | 19 (86) | 19 (86) |
| Other | 0 (0) | 1 (5) |
| VL < 200 copies/mL, n (%) (EMR) | 21 (95) | 21 (95) |
| CD4+ T cell count (median, IQR) (EMR) | 910 (384–1023) | 581 (400–714) |
| Pain location, n (%) (RedCap) | ||
| Numbness/tingling hands/feet | 12 (55) | 7 (32) |
| Headache | 5 (23) | 5 (23) |
| Abdominal pain | 4 (18) | 2 (9) |
| Low back | 16 (73) | 18 (82) |
| Hip | 7 (32) | 10 (45) |
| Shoulder | 10 (45) | 6 (27) |
| Knee | 12 (55) | 13 (59) |
| Pain everywhere in your body | 6 (27) | 6 (27) |
| Transportation: (RedCap) | ||
| Importance on scale 1–10 (median, IQR) | 9 (5–10) | 6 (3–9) |
| Assistance would help participant attend sessions | 22 (100) | 20 (91) |
| Locations of chronic pain care, n (%) (RedCap) | ||
| Primary care at HIV clinic | 21 (95) | 16 (73) |
| Primary care outside of HIV clinic | 0 (0) | 2 (9) |
| Urgent care/sick call | 1 (5) | 0 (0) |
| Emergency room | 4 (18) | 3 (14) |
| Pain specialist | 1 (5) | 7 (32) |
| Other (specify) | UAB ambulatory clinics 1 (5), knee doctor 1 (5) | Orthopedist 1 (5), self-medication 1 (5), spine/arthritis doctor 1 (5) |
| Current pain co-interventions, n (%) (RedCap) | ||
| Pain clinic | 4 (18) | 4 (18) |
| Seen by a counselor, psychiatrist, or psychologist | 12 (55) | 13 (59) |
| Physical therapy | 0 (0) | 1 (5) |
| Acupuncture | 0 (0) | 0 (0) |
| Massage | 0 (0) | 1 (5) |
| Providers seen in health system (ever) (EMR) | ||
| Addiction individual and/or group session | 11 (50) | 12 (55) |
| Neurology | 3 (14) | 3 (14) |
| Palliative care | 6 (27) | 12 (55) |
| Psychiatry | 11 (50) | 12 (55) |
| Psychology | 10 (45) | 11 (50) |
| Pain medications (EMR)—opioids, acetaminophen, NSAIDS, muscle relaxants at study start | 7 (32) | 11 (50) |
| Opioid pain medications at study start (EMR) | 3 (14) | 5 (23) |
| Primary care visit in last 12 weeks (EMR) | 15 (68) | 17 (77) |
| Urgent care visit in last 12 weeks (EMR-sick call) | 0 (0) | 4 (18) |
| ER visits in the last 12 weeks (EMR) | 5 (23) | 5 (23) |
| Medical hospitalizations in the last 12 weeks (EMR) | 4 (18) | 5 (23) |
| PEG (0–10), mean SD (RedCap) | 8.2 (1.3) | 8.0 (1.4) |
| BPI-total score (0–10), mean SD (RedCap) | 7.1 (2.1) | 7.4 (1.6) |
| Pain self-efficacy questionnaire (0–60), mean SD (RedCap) | 32.9 (16.5) | 31.6 (19.8) |
| Tampa kinesiophobia scale (10–40), mean SD (RedCap) | 24.2 (5.4) | 22.7 (6.5) |
| Pain catastrophizing scale (0–52), mean SD (RedCap) | 30.7 (15.0) | 29.2 (16.6) |
| Currently taking ART | 22 (100) | 21 (95) |
| Of those, any ART missed over last 2 weeks Y/N | 4 (18) | 2 (10) |
| PHQ-8 (0–24, median, IQR) (RedCap) | 8.5 (5.4) | 9.3 (6.2) |
| AUDIT-C ≥ 2 (RedCap) | 3 (14) | 3 (14) |
| ASSIST (RedCap): substance use other than marijuana, non-prescribed opioids | ||
| Current | 2 (9) | 1 (5) |
| Prior | 14 (64) | 18 (82) |
| Never | 6 (27) | 3 (14) |
Missing values: Tampa kinesiophobia 5, pain catastrophizing 1, PHQ-8 1, AUDIT-C 2
EMR data pulled from the Electronic Medical Record, RedCap patient self-report collected by study staff using RedCap, an online data collection tool programmed for the purposes of this study
Feasibility
We recruited and randomized 44 participants. The ratio of participants who approached staff to those who were randomized was 98/44 = 2.1. Of the 22 total participants in the intervention group, the median number of group sessions attended was 3.5 out of 6 (IQR 3–5), the median number of individual sessions attended was 6 out of 6, and the median number of total sessions attended was 9 out of 12 or 75% (IQR 8–11). Seventeen participants (77.3%) completed all six one-on-one sessions within the 16-week study period. Reasons for missed sessions included personal or family illness, a conflicting medical appointment, work conflict, voting, and major holidays.
Among the 22 intervention participants, the most commonly selected topics to be discussed during the one-on-one sessions were physical activity (17), relaxation (17), stress (15), sleep (12), weight loss (11), and thinking differently about your pain (10).
Of 44 participants, 36 (82%) completed outcome assessments within 1 month of completing the intervention (or within 1 month after the last group session for the control participants). Of the remaining 8, 5 were from the control group and 3 were from the intervention group. Of the five control participants who did not complete outcome assessments one died, three had incorrect phone numbers, and one was in jail. Of the three intervention participants who did not complete outcome assessments, two had incorrect phone numbers and one was in jail.
Acceptability
Three major themes emerged from the qualitative interviews: helpfulness of the intervention in reducing pain, behavioral changes as a result of the intervention, and the benefits of the multi-component intervention.
Participants indicated that the intervention helped relieve pain. One participant stated: “Sometimes you get where you say, what can they tell me? I’ve been through it all. None of it helped. I really didn’t look for it to help me as much as it has. So, I’m gung ho about trying some more” (65-year-old African-American female).
Participants described a variety of behavioral changes made as a result of the intervention, including increased physical activity, focusing less on the pain, and thinking differently about pain. One participant noted that the intervention reduced reliance on pain medications: “It’s benefited me a lot because I don’t have to take pain pills. I’m learning to not take like Tylenols or the Aspirin anymore. I’m learning just to exercise and if I exercise or stretch or cut back on what I eat, I feel better about myself and I love that part” (32-year-old African-American male).
Participants talked about how intervention components—group, peer, and one-on-one skill building sessions—came together to make the intervention work. One participant reflected: “Just in having the ability to meet with people, have a support group, meet one on one, gain the tools, different avenues in order to deal with your pain, and have somebody who’s willing to listen about your pain. That’s the job” (46-year-old African-American female).
The support of a peer leader was also important. One participant shared: “They know where you’re coming from and if, at any reason, at any time, you feel like, I’m the worst one, he could tell us things that was like, wow…He could understand where you were coming from…if you’ve never had pain, I ain’t going to say you can’t talk about or teach it or infiltrate it, but it’s nothing like me actually being there” (65-year-old African-American female).
Seventeen of 19 respondents (89%) to the treatment satisfaction questionnaire reported being very satisfied, 1 moderately satisfied, and 1 neither satisfied nor dissatisfied with the intervention. Thirteen intervention participants reported being “much better” overall since they began treatment, 5 “a little better”, and 1 no change. Almost all [18] participants reported that they would return to the intervention in the future.
Preliminary Efficacy
Table 3 summarizes the changes in outcome measures between the intervention and control group. BPI-total scores decreased on average by 2 points in the intervention group and 0.9 points in the control group (on a scale of 0–10). This is a difference of 1.1, with an effect size of 0.6. BPI-total scores decreased by 1 or more in 13 (68%) intervention group participants and 9 (53%) control group participants (p = 0.49). Pain catastrophizing decreased on average by 8.6 points in the intervention group and 4 points in the control group (p = 0.25). Pain self-efficacy decreased slightly and kinesiophobia increased slightly, but neither were statistically significant.
Table 3.
Changes in measures before and after the intervention
| Intervention N = 19 | Control N = 17 | p value (between group)* | |
|---|---|---|---|
| Mean (SD) (positive is increase, negative is decrease) | |||
| PEG | − 1.5 (1.9) | − 1.4 (2.3) | 0.93 |
| BPI-total | − 2.0 (2.1) | − 0.9 (1.6) | 0.11 |
| Pain self-efficacy questionnaire | 4.2 (17.8) | 7.4 (15.4) | 0.58 |
| Tampa kinesiophobia scale | 1.1 (5.4) | − 0.2 (2.7) | 0.43 |
| Pain catastrophizing scale | − 8.6 (11.4) | − 4.0 (11.5) | 0.25 |
Missing values: Tampa kinesiophobia scale 3, control 3 intervention, pain catastrophizing 1 intervention
t-test
Discussion
For the reasons described above, STOMP represents an important innovation in pain treatment for PLWH. This pilot study was a critical first step in its evaluation. Given the poor session adherence seen in previously published behavioral interventions tested in PLWH, STOMP’s feasibility and acceptability was noteworthy. In particular, participants attended 75% of sessions, indicated that it helped improve their pain and function, reported high levels of satisfaction with the intervention. Preliminary findings suggest the intervention’s potential impact on pain and pain-related functional impairment.
We believe that the feasibility of this study is generalizable to other study settings. We purposely used staff interventionists with backgrounds often found in HIV clinical care and research settings. Peer interventionists were easily identified and retained for the entire study. The training required was sufficient to ensure fidelity to the study protocol and carry it out, but not overly onerous. Additionally, the qualitative interviews indicated that the group component is an essential ingredient to the intervention.
We were encouraged by the preliminary impact of our intervention. Although this study was not powered to test efficacy, individuals in the intervention group experienced a two-point decrease in their BPI-total score, which is considered moderately clinically meaningful [23]. This difference exceeds what has been found in other studies of chronic pain interventions in PLWH [9]. Additionally, this was a 1.1-point greater decrease than the control group, which exceeds the cutoff for the minimum clinically important difference [31]. However, due to the small sample size, these findings are preliminary and a fully powered study is needed before conclusions about efficacy can be confidently drawn. Unlike high dropout rates (> 20%) due to lack of effect or adverse seen in studies of opioids [32], dropout was modest and typically due to extenuating personal circumstances. We were also encouraged by our participants’ positive response to the intervention in interviews and on the treatment satisfaction survey. We believe this reflects our systematic intervention development process which included extensive tailoring based on the expressed needs of both participants and clinicians.
This study has limitations. It was conducted at one site, and among participants who were mostly virologically suppressed (and therefore likely retained in care and adherent to antiretroviral therapy). While we believe its feasibility is likely generalizable for the reasons described above, this was not specifically investigated. Also, this study was conducted among individuals who self-selected to participate. This may create bias towards a more feasible, acceptable, and efficacious intervention. A different intervention would likely be needed for individuals reluctant to engage in this type of chronic pain management approach.
In sum, STOMP is feasible and acceptable, and is therefore ready to be tested in a full-scale trial. Positive findings would lead to subsequent dissemination/implementation research on STOMP in HIV treatment settings.
Acknowledgments
All authors contributed substantially to study design and analysis, as well as manuscript preparation. AW and DL conducted the statistical analyses.
Funding This work was supported by the National Institutes of Health (K23MH104073[JSM]), K24DA037034 [MOJ], R01DA039046 [JS], CNICS (R24 AI067039), and the University of Alabama at Birmingham (UAB) Center for AIDS Research, an NIH Funded Program (P30 A1027767) that was made possible by the following institutes: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, and OAR; and the VA PRIME Health Services Research and Development Center (CIN 13-047).
Footnotes
Compliance with Ethical Standards
Conflict of interest Dr. Kertesz reports ownership of stock in Merck and Abbot, amount to less than 3% of assets but no income, honoraria, grants, or other associations with any drug company. Dr. Starrels receives research support from the Opioid Post-marketing Requirement Consortium to conduct FDA-mandated observational research. The following authors declare no conflicts of interest: Dr. Merlin, Mr. Westfall, Drs. Long, Davies, Saag, Demonte, Young, Kerns, Bair, Turan, Kilgore, Clay, Pekmezi, and Johnson.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained. This article does not contain any studies with animals performed by any of the authors.
References
- 1.Merlin JS, Westfall AO, Raper JL, Zinski A, Norton WE, Willig JH, et al. Chronic pain, psychiatric, and substance abuse comorbidities: implications for adherence to HIV care, ART, and virologic suppression; International workshop on HIV observational databases; Athens, Greece. March 2012. [Google Scholar]
- 2.Merlin JS, Cen L, Praestgaard A, Turner M, Obando A, Alpert C, et al. Pain and physical and psychological symptoms in ambulatory HIV patients in the current treatment era. J Pain Symptom Manag. 2012;43(3):638–45. doi: 10.1016/j.jpainsymman.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain. 2011;12(9):1004–16. doi: 10.1016/j.jpain.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiao JM, So E, Jebakumar J, George MC, Simpson DM, Robinson-Papp J. Chronic pain disorders in HIV primary care: clinical characteristics and association with healthcare utilization. Pain. 2015 doi: 10.1097/j.pain.0000000000000462. [DOI] [PubMed]
- 5.Perry BA, Westfall AO, Molony E, Tucker R, Ritchie C, Saag MS, et al. Characteristics of an ambulatory palliative care clinic for HIV-infected patients. J Palliat Med. 2013;16(8):934–7. doi: 10.1089/jpm.2012.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merlin JS, Westfall AO, Chamot E, Overton ET, Willig JH, Ritchie C, et al. Pain is independently associated with impaired physical function in HIV-infected patients. Pain Med. 2013 doi: 10.1111/pme.12255. [DOI] [PMC free article] [PubMed]
- 7.Merlin JS, Westfall AO, Raper JL, Zinski A, Norton WE, Willig JH, et al. Pain, mood, and substance abuse in HIV: implications for clinic visit utilization, ART adherence, and virologic failure. J Acquir Immune Defic Syndr. 2012;61(2):164–70. doi: 10.1097/QAI.0b013e3182662215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merlin JS, Zinski A, Norton WE, Ritchie CS, Saag MS, Mugavero MJ, et al. A conceptual framework for understanding chronic pain in patients with HIV. Pain Pract. 2013 doi: 10.1111/papr.12052. [DOI] [PubMed]
- 9.Merlin JS, Bulls HW, Vucovich LA, Edelman EJ, Starrels JL. Pharmacologic and non-pharmacologic treatments for chronic pain in individuals with HIV: a systematic review. AIDS Care. 2016 doi: 10.1080/09540121.2016.1191612. [DOI] [PMC free article] [PubMed]
- 10.Department of Health and Human Services UG. National Pain Strategy: a comprehensive population health strategy for pain. https://iprcc.nih.gov/docs/HHSNational_Pain_Strategy.pdf. Accessed 6 July 2016.
- 11.Green LW, Kreuter MW. Health program planning: an educational and ecological approach. 4th. Boston: McGraw-Hill; 2005. [Google Scholar]
- 12.Kroenke K, Bair M, Damush T, Hoke S, Nicholas G, Kempf C, et al. Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) study: design and practical implications of an intervention for comorbid pain and depression. Gen Hosp Psychiatry. 2007;29(6):506–17. doi: 10.1016/j.genhosppsych.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Eyer JC, Thorn BE. The Learning About My Pain study protocol: reducing disparities with literacy-adapted psychosocial treatments for chronic pain, a comparative behavioral trial. J Health Psychol. 2015 doi: 10.1177/1359105315570985. [DOI] [PubMed]
- 14.Merlin JS, Young SR, Johnson MO, Saag M, Demonte W, Modi R, et al. Using patient perspectives to inform the development of a behavioral intervention for chronic pain in patients with HIV: a qualitative study. Pain Med. 2016 doi: 10.1093/pm/pnw150. [DOI] [PMC free article] [PubMed]
- 15.Bartholomew LK, Parcel GS, Kok G, Gottleib NH, Fernandez ME. Planning health promotion programs: an intervention mapping approach. San Francisco: Jossey-Bass; 2011. [Google Scholar]
- 16.Matthias MS, Kukla M, McGuire AB, Bair MJ. How do patients with chronic pain benefit from a peer-supported pain self-management intervention? A qualitative investigation. Pain Med. 2016 doi: 10.1093/pm/pnw138. [DOI] [PubMed]
- 17.Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallant JE, Adimora AA, Carmichael JK, Horberg M, Kitahata M, Quinlivan EB, et al. Essential components of effective HIV care: a policy paper of the HIV Medicine Association of the Infectious Diseases Society of America and the Ryan White Medical Providers Coalition. Clin Infect Dis. 2011;53(11):1043–50. doi: 10.1093/cid/cir689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merlin JS, Walcott MM, Herbey I, Chamot E, Ritchie C, Saag MS, et al. Qualitative investigation of a brief chronic pain screening tool in HIV-infected patients. AIDS Patient Care STDS. 2014 doi: 10.1089/apc.2014.0006. [DOI] [PMC free article] [PubMed]
- 20.Merlin JS, Westfall AO, Chamot E, Saag M, Walcott M, Ritchie C, et al. Quantitative evaluation of an instrument to identify chronic Pain in HIV-infected individuals. AIDS Res Hum Retrovir. 2015;31(6):623–7. doi: 10.1089/aid.2014.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733–8. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626–9. doi: 10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11(2):153–63. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Neblett R, Hartzell MM, Mayer TG, Bradford EM, Gatchel RJ. Establishing clinically meaningful severity levels for the Tampa Scale for Kinesiophobia (TSK-13) Eur J Pain. 2016;20(5):701–10. doi: 10.1002/ejp.795. [DOI] [PubMed] [Google Scholar]
- 27.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merri-field T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–65. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 30.Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24(3):217–26. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- 31.Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3–14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed]


