Abstract
Purpose
To determine the treatment effect of oral acetazolamide on refractory inflammatory macular edema.
Methods
A retrospective review identified patients with uveitic or pseudophakic macular edema treated with acetazolamide between 2007 and 2014. Visual acuity and central macular subfield thickness (CST) was determined at baseline and at first follow up. Baseline optical coherence tomography (OCT) features were analyzed as predictors of acetazolamide response.
Results
Sixteen patients (19 eyes) of 61 screened met all criteria. Mean age was 57.9 years (19.7-81.1). The most common diagnosis was idiopathic uveitis (n=6, 31.6%). Mean uveitis duration was 4.4 years (0.2-27.5). Average CST decreased significantly (from 471.8 ± 110.6 to 358.3 μm ± 50.4) (p<0.0001). Average visual acuity (LogMAR) improved significantly from 20/54 (0.43 ± 0.25) to 20/37 (0.27 ± 0.16)(p=0.003). Pretreatment OCTs demonstrated intraretinal fluid (n=19, 100%), subretinal fluid (n=8, 42.1%), epiretinal membrane (n=13, 68.3%), and vitreomacular traction (n=1, 5.2%). No OCT characteristic was predictive of a response to therapy.
Conclusion
There is a significant benefit to vision and CST following acetazolamide treatment in patients with inflammatory macular edema. In patients with refractory inflammatory macular edema, treatment with acetazolamide can provide anatomic and visual benefit without corticosteroid-related adverse effects.
Keywords: Acetazolamide, Carbonic anhydrase inhibitor, Central subfield thickness, Inflammation, Macular edema, Uveitis
INTRODUCTION
Inflammatory cystoid macular edema (CME) is a common, vision-threatening complication in uveitis patients1–3 and is thought to be the mechanism underlying chronic CME following cataract surgery (Irvine-Gass syndrome).4–6 Standard treatments for CME include topical, periocular, and intravitreal corticosteroids.7 Despite availability of these modalities, treatment of inflammatory CME remains challenging as patients can develop steroid resistance, or develop corticosteroid complications such as ocular hypertension, glaucoma, or cataract.8 Alternative therapies include systemic immune suppression, biologics (interferon α and β, anti-tumor necrosis alpha, and anti-interleukin-6 agents), local options (intravitreal methotrexate, sirolimus, and anti-vascular endothelial growth factor agents), and surgical options (pars plana vitrectomy with or without internal limiting membrane peel) (reviewed elsewhere9, 10). Oral acetazolamide therapy was first reported as a therapy for chronic uveitic macular edema in 1988.11 While the specific mechanism of action is unknown, it has been shown to increase the rate of subretinal fluid resorption in experimental retinal detachment,12 and to increase the rate of vitreous fluorescein clearance.13, 14 However, two placebo-controlled cross-over studies conducted in the 1990’s, using oral acetazolamide for uveitic CME, identified reduced fluorescein angiographic edema, but failed to show significant improvements in vision.13, 15 Despite these results, acetazolamide continues to be used for patients with uveitic CME with reported benefits.16–18
Previous studies of acetazolamide’s effects on uveitic CME were performed prior to the adoption of optical coherence tomography (OCT) as the preferred method for detecting and monitoring macular edema.19 We therefore set out to analyze the effect of acetazolamide therapy on inflammatory CME as defined by change in central macular subfield thickness (CST), as well as reassess its effect on visual acuity. Additionally, we hypothesized that there are OCT characteristics on baseline scans that would predict an anatomic response to oral carbonic anhydrase inhibitor (CAI) therapy. Identifying the presence of a quantifiable impact on inflammatory CME and the types of eyes most likely to improve with therapy would help tailor treatment plans for patients with chronic CME.
MATERIALS AND METHODS
This study was approved by the University of Washington Institutional Review Board and was performed in accordance with the tenets of the Declaration of Helsinki. A retrospective chart review was performed of patients treated with acetazolamide or methazolamide by two providers (TL and RVG) off-label for uveitic and Irvine-Gass-related macular edema between 01/01/2007 and 07/31/2014. Inflammatory CME was defined by the presence of macular edema (CST > 320 μm)20 and cystoid intraretinal spaces on OCT images21, in a patient with active or inactive anterior, intermediate, posterior, or panuveitis, or with angiographic edema and late disc leakage by fluorescein angiography > 60 days after cataract surgery. Patients were included if they were 18 years of age or older, and had OCT imaging at baseline and at first follow-up within 3 months of starting acetazolamide. Spectral-domain OCT images were obtained using a single device (Spectralis HRA+OCT, Heidelberg Engineering, Heidelberg Germany). CST measurement was determined using the inbuilt Spectralis mapping software. A 20% decrease in CST was used as a clinically significant change, as recommended by previous studies.22 Visual acuity was recorded using Snellen notation and converted to Logarithm of the Minimal Angle of Resolution (LogMAR) for analysis. Exclusion criteria included any changes in oral prednisone dose or other systemic immunomodulator within the 4 weeks preceding initiation of acetazolamide therapy, or addition of local steroid therapy (including topical 0.05% difluprednate, periocular or intravitreal steroid injection) during the interval between baseline and follow-up imaging, or treatment with oral CAIs for reasons other than CME. Baseline OCT imaging was performed on the CAI prescription date, or within the previous 2 weeks. Images were scored by two masked specialists trained in both uveitis and medical retina (KLP, CSL) for the presence of epiretinal membranes (ERM), cystic intraretinal fluid, subretinal fluid, and vitreomacular traction. Chart information included demographics (age, gender), diagnosis, oral and topical ophthalmic medications, duration of uveitis diagnosis, date of cataract surgery and any associated complications, date of baseline and follow-up OCT, CST, best corrected visual acuity, date of CAI discontinuation and reason (resolved macular edema, side effect, other), and any side effects of therapy. A medication score was determined for each eye. The score is a combination of all topical and systemic anti-inflammatory medications prescribed for each patient/eye. Compliance was not ascertained. For topical medications, each drop/day of prednisolone acetate and topical NSAIDs was assigned one point (four times a day dosing = 4 points). Each drop/day of Durezol was assigned 2 points (four times a day dosing = 8 points). For systemic medications, each 10 mg of prednisone was assigned a score of 1 (20 mg = 2 points), and all other systemic medications were given a score of 1 for their presence or absence.
The Wilcoxon signed-rank test was used to evaluate the macular thickness and visual acuity changes attributable to CAI therapy. Uni- and multivariate logistic regressions were used to test for OCT characteristics as predictors of CST response to CAI treatment. Spearman’s rank correlation coefficient was used to evaluate medication score and change in CST. Correlation was defined as very weak/none (r < 0.1), weak (0.1< r < 0.3), moderate (0.3 < r < 0.6), strong (0.6 < r < 0.8), or very strong (0.8 < r < 1). Analyses were performed with R version 3.2.5 (https://www.r-project.org/), and graphed using Prism GraphPad version 7.0.
RESULTS
Sixty-one patients with a history of uveitis and treatment with acetazolamide were identified. Twenty-seven patients were excluded from analysis due to recent initiation or increased dose of topical 0.05% difluprednate, oral prednisone, or systemic immunomodulation. Eighteen patients were excluded due to OCT scans outside the required time-period. The final group consisted of 16 patients (19 eyes) (Table). Both eyes of three patients were eligible for the study (total 6 eyes). Nine patients (56%) were female, with average age of 57.9 years (range 19.7-81.1 yrs.). The most common anatomic location of uveitis was anterior n=8 (42.1%), and the average duration of uveitis 4.4 years (range 0.2-27.5 yrs.). All patients had received prior treatment for macular edema. Four eyes with pseudophakic CME and inflammatory signs were included, all greater than 60 days from the date of surgery.
Table.
Demographics and characteristics of subjects with inflammatory macular edema treated with acetazolamide.
| Eye # | Age | M/F | Uveitis dx (years) | Uveitis Diagnosis | Prior edema treat. | Systemic meds | Topical meds | Study interval (days) | VA 1 | VA 2 | CST 1 | CST 2 | Change CST (um) | (%) | Side Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BILATERAL
| |||||||||||||||
| 1R | 52 | F | 7.1 | Idiopathic intermediate | none | none | none | 35.0 | 20/25 | 20/30 | 400 | 370 | 30 | 8 | C, DI, F, N |
| 1L | PA, K, STTA | K QID, PA QID | 20/80 | 20/60 | 498 | 353 | 145 | 29 | |||||||
| 2R | 63 | M | 0.3 | Idiopathic intermediate | D, Pred | Pred 10 mg | D BID | 20.0 | 20/40 | 20/30 | 455 | 440 | 15 | 3* | DI, DY, HL |
| 2L | D, Pred | D BID | 20/40 | 20/30 | 488 | 431 | 57 | 12* | |||||||
| 3R | 70 | F | 0.2 | Pseudophakic macular edema | PA, D, K | none | none | 55.0 | 20/32 | 20/30 | 393 | 386 | 7 | 2 | DE, ED, HY, MA |
| 3L | PA, D, K | none | 20/70 | 20/30 | 499 | 408 | 91 | 18 | |||||||
|
UNILATERAL
| |||||||||||||||
| 7R | 24 | M | 0.9 | HLA-B27 AU | PA | Pred 20 mg | D QID, N TID | 14.0 | 20/50 | 20/20 | 767 | 362 | 405 | 53 | none |
| 8L | 67 | M | 10.5 | Idiopathic AU | D, N | none | D QID, N TID | 30.0 | 20/50 | 20/30 | 585 | 341 | 244 | 42 | none |
| 9R | 61 | F | 3.0 | Anterior and posterior scleritis | D, N, Pred | MTX 15 mg, Pred 20 mg | D 6× a day, N TID | 39.0 | 20/125 | 20/60 | 595 | 376 | 219 | 37 | IA, PR |
| 10L | 20 | M | 1.4 | DUSN | STTA | none | none | 21.0 | 20/32 | 20/25 | 583 | 374 | 209 | 36 | DY, PR |
| 11R | 68 | F | 3.1 | Prior ARN | N, PA, IB, PPV/MP | MTX 25 mg | D QID | 30.0 | 20/50 | 20/50 | 471 | 310 | 161 | 34 | DI, G, PR |
| 12R | 72 | F | 1.8 | Chronic AU | D, K | none | PA TID | 28.0 | 20/50 | 20/30 | 399 | 251 | 148 | 37 | PR |
| 13L | 38 | F | 27.5 | JIA associated AU | PA | none | PA BID | 28.0 | 20/125 | 20/40 | 449 | 318 | 131 | 29 | DY, ML, PR |
| 14L | 79 | M | 1.4 | Pseudophakic macular edema | PA, IVTA, IB | none | none | 43.0 | 20/100 | 20/100 | 573 | 444 | 129 | 23 | C, F, DY, PR |
| 15R | 65 | F | 2.4 | Birdshot choroiditis | PA, D, K, N, STTA, Pred | MTX 15mg, Pred 20mg, | D QID, N TID | 28.0 | 20/40 | 20/40 | 390 | 337 | 53 | 14 | F, DI, DY, PR |
| 16L | 58 | F | 4.1 | HLA-B27 AU | D, N, STTA, IVTA, | Humira 40mg Q 2 wk | D QID, N QID | 35.0 | 20/40 | 20/30 | 341 | 305 | 36 | 11* | F, DY, PO, PR |
| 17L | 52 | M | 5.8 | Sarcoidosis | PA, D, STTA, Pred, PPV/MP | MTX 25mg, AZA 100 mg, Pred 10 mg | D QID, | 38.0 | 20/40 | 20/30 | 385 | 357 | 28 | 7 | none |
| 18L | 81 | M | 2.5 | Pseudophakic macular edema | PA | none | PA BID, | 42.0 | 20/40 | 20/40 | 327 | 302 | 25 | 8* | PR |
| 19L | 46 | F | 3.7 | Demyelination associated | D, N | none | D TID, N BID | 21.0 | 20/50 | 20/50 | 366 | 342 | 24 | 7 | F, PR |
Complete resolution of macular edema
|
| |||
| Treatment/Medications | Diagnosis | Side effects | Other |
|
| |||
| PA= 1% Prednisolone acetate D= 0.5% Difluprednate Pred= Oral prednisone N= 0.1 % Nepafenac K= 0.5% Ketorolac STTA= Subtenons triamcinolone IB= Intravitreal bevacizumab IVTA= Intravitreal triamcinolone AZA= Azathioprine MTX= Methotrexate PPV/MP= Pars plana vitrectomy with internal limiting membrane peel BID= Twice a day TID= Three times a day QID= Four times a day |
ARN= Acute retinal necrosis AU= Anterior Uveitis DUSN= Diffuse unilateral subacute neuroretinitis HLAB27= human leukocyte antigen B27 JIA= Juvenile Idiopathic Arthritis associated |
C= Confusion/Disorientation DE= Dehydration DI= Diarrhea DY= Dysguesia ED= Emergency visit F= Fatigue G= Gastroesophageal reflux HL= Hearing Loss HY= Hypotension IA= Increased Appetite MA= Malaise ML= Memory Loss N= Nausea PO= Polydipsia PR= Paresthesia |
CST= Central subfield thickness VA= Visual acuity CAI= Carbonic anhydrase inhibitor SE= Side effect D/C= Discontinuation IC= Insurance Coverage |
|
| |||
All patients were treated with twice daily 500mg acetazolamide extended-release capsules (Diamox Sequels ®). This medication was continued until first follow-up except patient #1 who returned taking 250 mg acetazolamide tablets three times daily due to insurance coverage, patient #3 who stopped treatment due to dehydration requiring treatment, and patient #13 taking methazolamide 50 mg three times daily due to prior intolerance of acetazolamide side effects. At the time of CAI initiation, 7/16 (44%) patients were taking systemic prednisone and/or other immunomodulators, and 14/19 (74%) eyes were being treated with topical steroid drops (n=4 with 1% prednisolone acetate and n=10 with 0.05% difluprednate). Average time from treatment initiation to initial follow-up (study end point) was 32 days (range 14-60 days). 13/16 (81%) of patients reported adverse effects from CAI therapy. These included paresthesias (n=10), dysgeusia (n=6), fatigue (n=5), and diarrhea (n=3). One patient presented to hospital for management of dehydration attributed to acetazolamide. This was the only patient to discontinue all CAI treatment prior to first follow-up.
In addition to oral CAI therapy, 4 patients were treated chronically with topical dorzolamide for intraocular pressure. There were no changes in therapy over the study period. Ten eyes were pseudophakic at baseline. In 2 of these eyes (3R, 3L), CME developed within 1 month of cataract surgery, and both eyes entered the study less than 3 months postoperatively. One of these eyes (19L) developed macular edema 3 months after uncomplicated cataract surgery in the setting of prior chronic anterior and intermediate uveitis. The remaining 7/10 eyes had a remote history of cataract surgery (> 2 years) prior to study entry. Two of these eyes (14L, 18L) developed CME immediately after cataract surgery, but entered the study > 2 years after failure of prior treatment. In the remaining 5 pseudophakic eyes (8L, 12R, 13L, 16L, 17L), the development of CME was not attributed to surgery. Eye 9R entered the study 3 months after combined retinal surgery and intravitreal triamcinolone for an epiretinal membrane in the setting of prior scleritis.
All eyes experienced a decrease in CST with treatment (range 7 μm - 405 μm) (Figure 1). Mean CST decreased significantly from 471.8 μm ± 110.6 to 358.3 μm ±50.4 (p<0.0001, Wilcoxon-rank sum). In this study, 9/19 (47%) eyes had a 20% or greater decrease in CST. Four additional eyes (2R, 2L, 16L, and 18L) had complete resolution of their macular edema but did not meet this threshold. Thus a total of 13/19 (68%) eyes demonstrated a clinically significant decrease in CST or total resolution of their CME following the addition of acetazolamide. Mean visual acuity (LogMAR) improved significantly from 20/54 (0.43 ± 0.25) to 20/37 (0.27 ± 0.16) (p=0.003) at first follow-up. Twelve of nineteen eyes (63%) gained at least one line of visual acuity between baseline and their first follow up. The visual acuity in six eyes (32%) was unchanged, while one eye had a decrease from 20/25 to 20/30 (Eye #1R).
Figure 1.
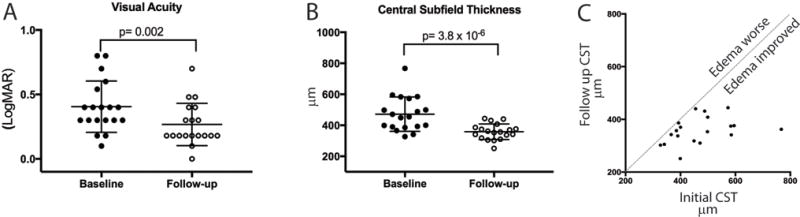
Significant improvement in visual acuity and central subfield thickness (CST) in patients with inflammatory macular edema treated with acetazolamide. A. Visual acuity before (filled circles) and at first follow up (open circles). B. Central subfield thickness obtained from macular optical coherence tomography obtained prior to treatment (filled circles) and at first follow-up (open circles). C. Comparison of initial CST (X axis) and after treatment with acetazolamide (Y axis). Each point represents one patient. The dotted line indicates the case where there was no change in CST.
The change in CST was then evaluated for an association with the dose and number of systemic and topical anti-inflammatory medications each eye was receiving at the time of treatment with acetazolamide (Figure 2). A weak, and non-significant correlation r=0.296 (95% CI, −0.1966 to 0.6694, p 0.22) was found between the degree of CST improvement and treatment with more anti-inflammatory medications, and this was primarily impacted by topical therapy r=0.382 (−0.1624 to 0.6885, p=0.17). There was no correlation to the systemic anti-inflammatory score r= 0.0738 (−0.4058 to 0.5215, p=0.76).
Figure 2.
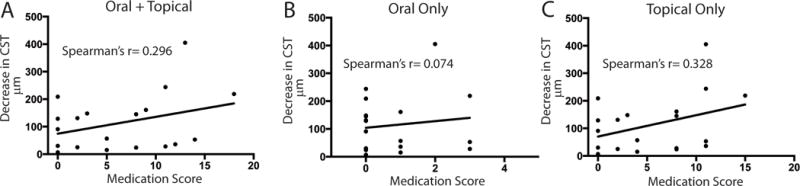
There is not a significant correlation between change in central subfield thickness (CST) and oral or topical anti-inflammatory treatment. The absolute value of the difference in CST from baseline to follow up (Y axis) is plotted against the medication score for A. All oral and topical anti-inflammatory medications B. Oral anti-inflammatory medications only, and C. Topical anti-inflammatory medications only. P values for all correlations are > 0.1.
Baseline and follow-up images for patients are shown in Figures 3 and 4. Pretreatment OCTs were scored for anatomic complications of chronic inflammation. All eyes had cystic intraretinal fluid, 9/19 (47%) eyes had subretinal fluid, 11/19 (58%) eyes had epiretinal membranes, and 1/19 eyes had vitreomacular traction. By uni- and multivariate logistical regression, no OCT feature listed above was identified that predicted improvement in CST. To determine if there was a difference in response to CAI treatment due to lens status (phakia versus pseudophakia), the pretreatment and post treatment CST and visual acuity was compared between groups. There was no statistically significant difference (Wilcoxon-rank sum) between the phakic and pseudophakic groups before or after treatment with respect to average CST (p = 0.243) or visual acuity (p = 1.0). However, one case of recalcitrant pseudophakic CME did demonstrate a close temporal relationship between CME relapse and recovery in response to CAI therapy (Figure 5). These results extend beyond the primary endpoint of this study, but are in line with previous reports in patients with pseudophakic CME treated with oral acetazolamide.11
Figure 3.
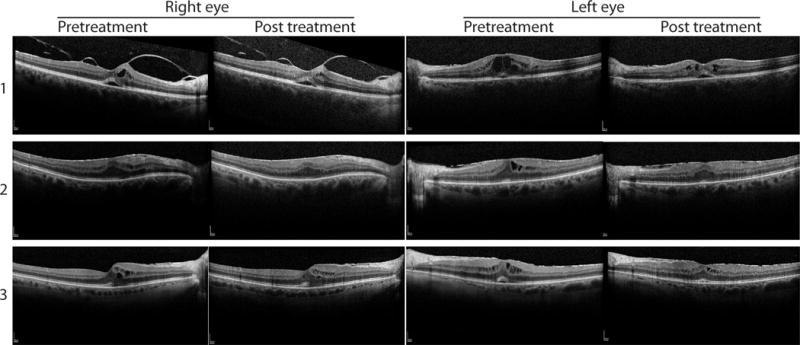
Central macular ocular coherence tomography (OCT) of patients with bilateral inflammatory macular edema treated with acetazolamide. Pre- and post-treatment foveal OCT images for the right and left eyes for three patients. Top row: patient #1. Middle row: patient #2. Bottom row: patient #3.
Figure 4.
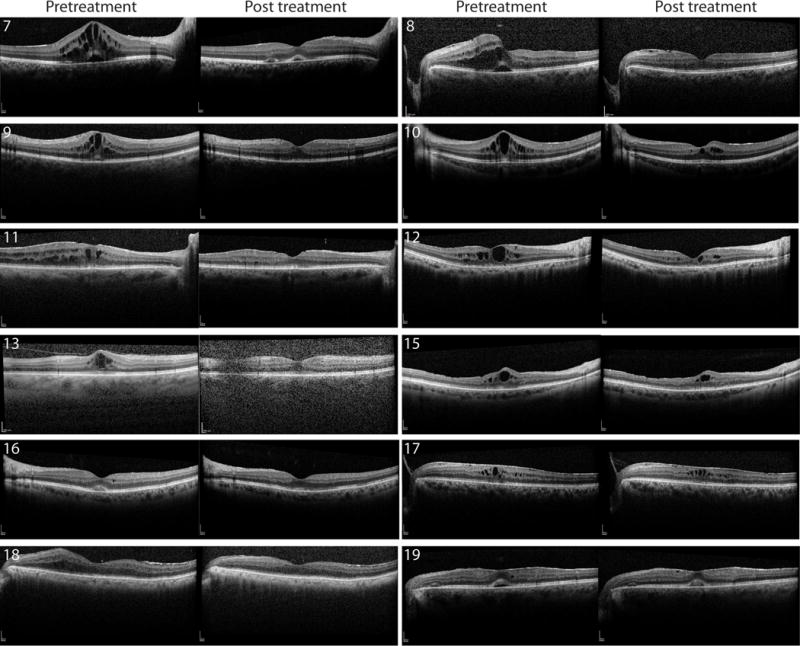
Macular ocular coherence tomography (OCT) of patients with unilateral inflammatory macular edema treated with acetazolamide. Pre- and post-treatment foveal OCT images for twelve eyes (patients 7-13, 15-19). Patient 14 shown in Figure 4.
Figure 5.
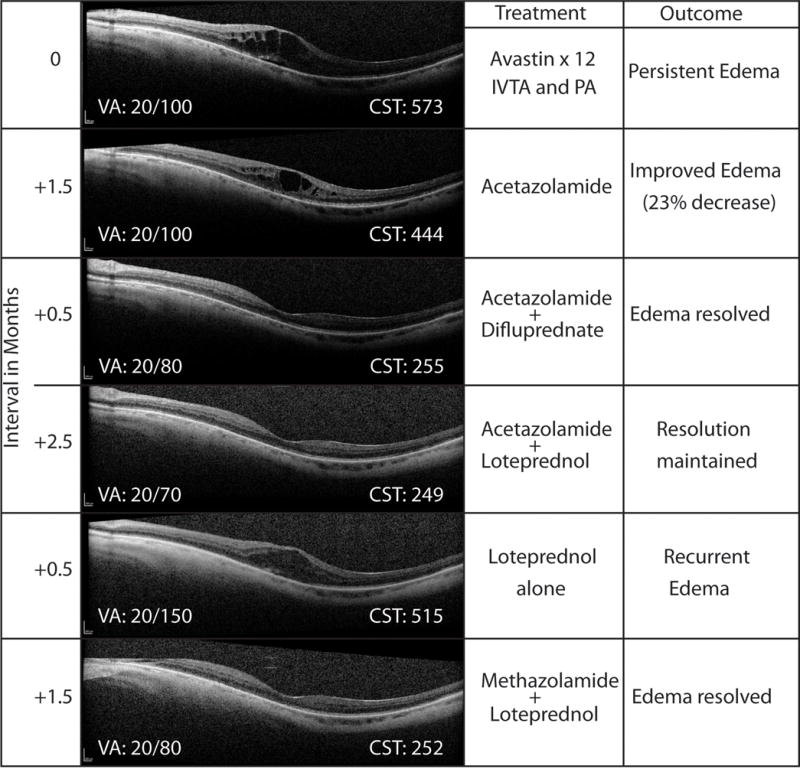
A case of pseudophakic cystoid macular edema (#14L) unresponsive to topical 1% prednisolone acetate (PA), intravitreal triamcinolone (IVTA), and twelve prior intravitreal bevacizumab injections, that responded to acetazolamide 500 mg twice daily, and resolved completely with the addition of 0.05% difluprednate 4×/day. Resolution was maintained with acetazolamide plus 0.5% loteprednol etabonate. Recurrence occurred rapidly after discontinuation of acetazolamide (side effects) despite continued topical loteprednol. Edema resolved again with the addition of methazolamide 50 mg twice daily. This regimen maintained resolution for over three years. VA: visual acuity. CST: central macular subfield thickness.
DISCUSSION
This is the first study to use OCT to investigate the effects of treatment with acetazolamide on inflammatory macular edema. The majority of patients demonstrated benefit by OCT parameters, and experienced an improvement in visual acuity. This study found an average increase in vision of 0.16 LogMAR (8 ETDRS letters) with acetazolamide treatment. This is in line with previous studies showing statistically significant visual improvement from approximately 20/80 at baseline to 20/63 after 4 weeks of therapy. However, upon completion of these previous studies, there was no significant difference in vision when compared to placebo.13, 15 The current study did not include a control group which limits our conclusions regarding the role of natural history in the results. However, many of these patients had a history of chronic disease that had failed multiple prior therapies.
Two uncontrolled retrospective studies reported that patients with uveitic CME treated with long-term acetazolamide therapy did show significant improvements in vision over time.16, 18 This led some to hypothesize that the benefits of acetazolamide therapy over placebo may not be immediately apparent, but could translate into visual benefit over more extended periods.10 The current study would be consistent with this hypothesis, by demonstrating that most patients had a quantifiable short-term benefit of therapy on CST, a factor that has been linked to long-term visual outcome in uveitis patients.22 We did not evaluate long-term outcomes in this study. Long-term use of CAIs is often complicated by unpleasant side effects. Many of our patients reported common CAI side effects and one patient stopped therapy due to significant dehydration. Discontinuation of treatment may prevent patients from achieving the long-term benefit identified in previous studies, but for those patients who can tolerate therapy, acetazolamide may be a therapeutic option.
The above studies examining the use of oral CAIs for uveitic CME were conducted before the widespread use of OCT. Previously utilized outcome parameters include visual acuity, biomicroscopy, fluorescein angiographic leakage, and Amsler grid testing.14, 16–18 We know, however, that in the context uveitic CME, OCT provides clinically useful information and is now recommended as the first-line diagnostic modality.19 Untreated macular edema can lead to macular atrophy and permanent visual loss, underscoring the importance of both detecting and treating subtle macular edema to preserve long-term visual acuity.23
In the current case series, no clear predictors of response to therapy as judged by improved CST were identified by the OCT analysis. Other studies have identified that patients with uveitic CME complicated by epiretinal membranes with retinal striae are less responsive to standard therapy,24 while those with subretinal fluid are more responsive.25 However, neither quantitative nor qualitative analysis in this study suggested such a trend for acetazolamide therapy.
Pseudophakic CME was included in our study given the preponderance of evidence supporting its inflammatory-mediated pathogenesis.4–6 We found comparable improvements in both vision and CST in pseudophakic patients compared with phakic patients, and further saw efficacy in those patients with inflammation more typical of Irvine-Gass syndrome (i.e. minimal inflammatory signs). Elevated levels of prostaglandins and other cytokines have been implicated in this condition, and may lead to long-term disruption of the blood-retina barrier and RPE function in chronic cases. This is in contrast to other causes of CME, such as ischemic, degenerative, or drug-related etiologies. While mechanical elements likely contribute in a subset of patients, the inflammatory etiology in pseudophakic macular edema is supported by the primarily anti-inflammatory treatment algorithm employed for the majority of patients.6 In addition, clinical signs of inflammation on examination such as anterior chamber and vitreous cells are common.4 Studies comparing intraocular cytokine levels between typical uveitides by Standardization of Uveitis Nomenclature criteria versus pseudophakic CME are lacking, but could be pursued to further explore the common inflammatory mechanisms. Ultimately, in both uveitic and pseudophakic patients, similar signs of RPE dysfunction develop, and may account for the response to oral CAIs.
The current study has several limitations, including small sample size, retrospective design, variable time to primary endpoint, and lack of a comparison group. The best control group would be patients with the same inclusion/exclusion criteria but that were not treated with a CAI. However, it is not in the practice pattern of the treating physicians in this study to withhold acetazolamide except in cases where the medication is contraindicated. The number of patients meeting these criteria was not predicted to be of sufficient numbers to provide an adequate control population, thus introducing potential selection bias for treatment. Further studies with larger patient numbers or case-control design may identify potential predictors of response. The number of patients in this analysis was limited by our strict exclusion criteria for any changes in other medications that could impact CME. While we cannot determine if there was an additional benefit of acetazolamide therapy in the 27 patients excluded for this reason, we did see an overall decrease in CST in this population as well (data not shown).
In summary, addition of acetazolamide therapy to patients with recalcitrant inflammatory CME can provide a significant anatomical and functional benefit. Acetazolamide addresses the dysfunction of the RPE that may account for the poor responsiveness of CME to anti-inflammatory treatment in long-standing cases of uveitic and pseudophakic macular edema. Treatment will often be limited by side effects, but in patients with chronic CME who have failed or are intolerant of other therapies, use of oral CAI therapy should be considered.
BRIEF SUMMARY.
Addition of acetazolamide therapy to the treatment of uveitic macular edema can provide a significant improvement in macular thickness and visual acuity at first follow up when compared to baseline.
Acknowledgments
This study was supported by an unrestricted departmental grant and a career development award (KLP) from Research to Prevent Blindness (New York, NY) NEI K08EY023998 (KLP), NEI K23EY024921 (CL), P30-EY001730 (RVG) (Bethesda, MD), and by the generous support of Joe and Cynthia Gensheimer, and a gift from the Mark J. Daily, MD Research Fund.
Footnotes
No authors have any proprietary interest related to this manuscript.
This work was presented as a paper presentation at the Association for Research in Vision and Ophthalmology annual meeting in Baltimore, MD, on 5/8/17
References
- 1.Altaweel MM, Gangaputra SS, Thorne JE, et al. Morphological assessment of the retina in uveitis. J Ophthalmic Inflamm Infect. 2016;6:33. doi: 10.1186/s12348-016-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durrani OM, Tehrani NN, Marr JE, et al. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88:1159–1162. doi: 10.1136/bjo.2003.037226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grajewski RS, Boelke AC, Adler W, et al. Spectral-domain optical coherence tomography findings of the macula in 500 consecutive patients with uveitis. Eye (Lond) 2016;30:1415–1423. doi: 10.1038/eye.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557–634. [PMC free article] [PubMed] [Google Scholar]
- 5.Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Surv Ophthalmol. 2002;47(Suppl 1):S203–218. doi: 10.1016/s0039-6257(02)00294-1. [DOI] [PubMed] [Google Scholar]
- 6.Guo S, Patel S, Baumrind B, et al. Management of pseudophakic cystoid macular edema. Surv Ophthalmol. 2015;60:123–137. doi: 10.1016/j.survophthal.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Tomkins-Netzer O, Lightman S, Drye L, et al. Outcome of Treatment of Uveitic Macular Edema: The Multicenter Uveitis Steroid Treatment Trial 2-Year Results. Ophthalmology. 2015 doi: 10.1016/j.ophtha.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118:1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fardeau C, Champion E, Massamba N, LeHoang P. Uveitic macular edema. Eye (Lond) 2016;30:1277–1292. doi: 10.1038/eye.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis J. Current concepts in the management of uveitic macular edema. Johns Hopkins Advanced studies in Ophthalmology. 2010;7:60–66. [Google Scholar]
- 11.Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988;106:1190–1195. doi: 10.1001/archopht.1988.01060140350030. [DOI] [PubMed] [Google Scholar]
- 12.Marmor MF, Negi A. Pharmacologic modification of subretinal fluid absorption in the rabbit eye. Arch Ophthalmol. 1986;104:1674–1677. doi: 10.1001/archopht.1986.01050230112043. [DOI] [PubMed] [Google Scholar]
- 13.Farber MD, Lam S, Tessler HH, et al. Reduction of macular oedema by acetazolamide in patients with chronic iridocyclitis: a randomised prospective crossover study. Br J Ophthalmol. 1994;78:4–7. doi: 10.1136/bjo.78.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuboi S, Pederson JE. Experimental retinal detachment. X. Effect of acetazolamide on vitreous fluorescein disappearance. Arch Ophthalmol. 1985;103:1557–1558. doi: 10.1001/archopht.1985.01050100133034. [DOI] [PubMed] [Google Scholar]
- 15.Whitcup SM, Csaky KG, Podgor MJ, et al. A randomized, masked, cross-over trial of acetazolamide for cystoid macular edema in patients with uveitis. Ophthalmology. 1996;103:1054–1062. doi: 10.1016/s0161-6420(96)30567-8. discussion 1062-1053. [DOI] [PubMed] [Google Scholar]
- 16.Schilling H, Heiligenhaus A, Laube T, et al. Long-term effect of acetazolamide treatment of patients with uveitic chronic cystoid macular edema is limited by persisting inflammation. Retina. 2005;25:182–188. doi: 10.1097/00006982-200502000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Steinmetz RL, Fitzke FW, Bird AC. Treatment of cystoid macular edema with acetazolamide in a patient with serpiginous choroidopathy. Retina. 1991;11:412–415. [PubMed] [Google Scholar]
- 18.Zierhut M, Thiel HJ, Schlote T. Treatment of uveitic macular edema with acetazolamide. Doc Ophthalmol. 1999;97:409–413. doi: 10.1023/a:1002469503835. [DOI] [PubMed] [Google Scholar]
- 19.Kempen JH, Sugar EA, Jaffe GJ, et al. Fluorescein angiography versus optical coherence tomography for diagnosis of uveitic macular edema. Ophthalmology. 2013;120:1852–1859. doi: 10.1016/j.ophtha.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grover S, Murthy RK, Brar VS, Chalam KV. Comparison of retinal thickness in normal eyes using Stratus and Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:2644–2647. doi: 10.1167/iovs.09-4774. [DOI] [PubMed] [Google Scholar]
- 21.Iannetti L, Spinucci G, Abbouda A, et al. Spectral-domain optical coherence tomography in uveitic macular edema: morphological features and prognostic factors. Ophthalmologica. 2012;228:13–18. doi: 10.1159/000337234. [DOI] [PubMed] [Google Scholar]
- 22.Sugar EA, Jabs DA, Altaweel MM, et al. Identifying a clinically meaningful threshold for change in uveitic macular edema evaluated by optical coherence tomography. Am J Ophthalmol. 2011;152:1044–1052.e1045. doi: 10.1016/j.ajo.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forooghian F, Yeh S, Faia LJ, Nussenblatt RB. Uveitic foveal atrophy: clinical features and associations. Arch Ophthalmol. 2009;127:179–186. doi: 10.1001/archophthalmol.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehpamer B, Moshier E, Pahk P, et al. Epiretinal membranes in uveitic macular edema: effect on vision and response to therapy. Am J Ophthalmol. 2014;157:1048–1055. doi: 10.1016/j.ajo.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehpamer B, Moshier E, Goldberg N, et al. Subretinal fluid in uveitic macular edema: effect on vision and response to therapy. Am J Ophthalmol. 2013;155:143–149. doi: 10.1016/j.ajo.2012.06.028. [DOI] [PubMed] [Google Scholar]


