Abstract
Background
The effects of multiple sclerosis (MS) on cognition have gained increasing recognition as one of the major disabling symptoms of the disease. Despite the prevalence of these symptoms and their impact on quality of life, limited attention has been given to strategies that might help manage the cognitive changes commonly experienced by persons with MS.
Objective
The primary purpose of this study was to determine the effectiveness of a novel computer-assisted cognitive rehabilitation intervention MAPSS-MS (Memory, Attention, Problem Solving Skills in MS) in a multi-site trial with persons with MS.
Methods
Persons with MS (N = 183) with cognitive concerns were randomly assigned to either the 8-week MAPSS-MS intervention or usual care plus freely available computer games. Participants completed self-report and performance measures of cognitive functioning, compensatory strategies and depression at baseline, immediately after the MAPSS-MS intervention, and three and six months post-intervention. Changes in study outcomes were analyzed using intention to treat methodology, ANOVA with repeated measures, and ANCOVA.
Results
Both groups improved significantly on all outcome measures. The intervention group outperformed the comparison group on all measures, and there were statistically significant differences on selected measures.
Conclusion
Findings suggest that MAPSS-MS is a feasible intervention that could be broadly implemented in community settings. It has been shown to be modestly successful in improving cognitive functioning.
Keywords: Multiple sclerosis, Cognitive rehabilitation, Randomized controlled trial
The debilitating and widespread effects of Multiple Sclerosis (MS) on cognition are thought to occur in 50–75% of persons with the disease. Cognitive impairment, “potentially the most disabling symptom of the disease”.1, p53 has been systematically studied only in the last 20 years, and even now limited attention is given to strategies to help manage cognitive changes and related disability in everyday life.2 Yet, numerous studies of persons with MS have found deficits in tasks involving recent memory, attention, information processing (including processing speed), executive functions (including verbal learning) and visuospatial abilities.1–4 Subcortical brain injury caused by plaque and inflammation and the irreversible axonal loss are thought to be responsible for cognitive deficits.1,5 These deficits may occur at any point in the disease and are only weakly correlated with physical impairment.3
Cognitive impairment can have major effects on both the work and social lives of persons with MS.6 Memory problems can be devastating to individuals with MS because they directly determine activities a person can reliably engage in as part of everyday life. The fact that persons with MS are generally diagnosed as young adults and live with this condition over a lifetime magnifies the impact of disabling cognitive symptoms on work, family and social activities. Persons with MS with cognitive impairment have difficulty meeting the demands of their jobs, are more likely to be unemployed, require greater assistance and are less likely to engage in social activities than cognitively intact persons with MS and healthy controls.7,8 Indeed, cognitive impairment is the leading predictor of the high rates of occupational disability seen in persons with MS.9
Although neurological damage in the brain occurs with MS, recent studies suggest the possibility of neuroplasticity for cognitive tasks in persons with MS in response to training. Findings suggest enhanced recruitment of brain networks serving trained functions and the possibility of behavioral compensation for damaged neurons in persons with MS.10 A search of PubMed (1994–2017) identified twenty-two published studies of computer-assisted cognitive rehabilitation programs for persons with MS including fifteen that used an RCT design. Most studies utilized small samples (less than 100 participants), involved a wide variety of individually supervised computer training and yielded mixed results. Differential gains for the intervention groups were most likely to be found in the areas of attention and processing speed,10–14 although positive change in verbal memory and executive function have also been reported.2,9,11,14–16 No interventions, other than the investigators’ work described below,2 appear to have combined computer training with a group intervention to build compensatory cognitive skills within a general health promotion framework.
We designed and refined the MAPSS-MS intervention in an earlier randomized single-blind study.2 The intervention included eight weekly 2-h group sessions focused on building efficacy for use of cognitive compensatory strategies and lifestyle activities that support cognitive functioning (e.g., sleep and rest, exercise) paired with a home-based computer-assisted cognitive training program. The computer component enabled participants (N = 63) to engage in practice sessions without leaving their homes. Overall, the study supported the feasibility of the MAPSS-MS intervention (compliance with the intervention was high and computer training delivered in the home was acceptable)17 and the efficacy of this innovative intervention in producing greater gains in use of compensatory strategies and performance on neuropsychological tests of verbal memory for those in the intervention group.2
The purpose of this study was to test the refined MAPSS-MS intervention in a larger multi-site trial with a six-month follow-up. We hypothesized that, compared to persons in the usual care plus computer games group, persons with MS who participate in the MAPSS-MS intervention would:
➢ Demonstrate significantly greater improvements in verbal memory, compensatory cognitive strategies and cognitive-related IADL performance immediately, and 3 and 6 months post-intervention; and
➢ Demonstrate significantly greater improvements in self-efficacy, perceived cognitive abilities, non-verbal memory, attention/processing speed, verbal fluency and complex scanning and tracking immediately, and 3 and 6 months post-intervention.
Methods
A randomized controlled trial (NCT 03200899) was conducted with 183 persons with MS to evaluate the efficacy of the MAPSS-MS intervention in improving the primary outcome of overall neurocognitive competence in activities of daily living (verbal memory performance, use of cognitive strategies and performance on cognitive-related IADLs). The comparison group received usual-care plus information about publicly available computer games. The effects of the intervention on outcomes were assessed over an 8-month period, with measurements at baseline, immediately after the 8-week MAPPS intervention, and at 3 and 6 months post-intervention. Statistical significance was set at p < .05.
Data collection procedures
Using effect size estimates from prior research2 a total sample size of 180 was determined to be sufficient to detect an effect size (f) of .28 as statistically significant (assuming an alpha level of .05).18 Following approval by the Institutional Review Board for the University of Texas at Austin, participants were recruited from three large metropolitan communities in Texas: Houston, San Antonio and Dallas. Participants were recruited via physician referral, targeted mailings to persons with MS on the mailing list of the National MS Society, contact with support groups, and notices in MS newsletters and web sites. The project staff used a script to screen potential participants and explain the study requirements to those who called the toll-free number and met eligibility criteria (18–60 years of age, able to understand and comply with the study protocol, visual acuity with correction sufficient to work on a computer screen, clinically definite MS for at least 6 months and exacerbation free for 90 days). The Perceived Deficits Questionnaire19 was administered by phone, and those scoring at least 10 (indicating some problems in at least 5 areas) were eligible to participate.
The 347 persons who were initially screened as “eligible” were later contacted by phone to invite their participation during specific dates. Those who agreed to the schedule (repeated neuropsychological testing, classes and computer practice) were mailed a packet containing a written consent form and baseline data collection packet to complete. When the completed packet was returned, participants were scheduled for neuropsychological testing. Neuropsychological assistants, trained in the study protocol and supervised by a neuropsychologist consultant to the study, administered the tests at each site.
Prior to the initiation of data collection, we used a 1:1 ratio to randomly assign participants to groups. Randomization assignment was recorded on a letter sealed in an opaque envelope. Following the completion of baseline testing, the project manager opened the next envelope in the sequence and assigned the participant to either the active intervention or the usual care comparison group. The testers conducting neuropsychological assessments were blinded to participants’ group assignment and participants were not informed of their specific group assignment (intervention or comparison). Neuropsychological testing and questionnaire data were collected from all subjects at baseline, following completion of the intervention, and 3 and 6 months post-intervention.
Intervention program
The MAPSS-MS intervention aims to help persons with MS acquire the highest level of cognitive functioning and functional independence. The intervention includes group sessions (2 h per week for 8 weeks) focused on building efficacy for use of cognitive strategies and a home-based computer training program (45 min three times per week). Based on the integration of Bandura’s self-efficacy theory20 with the investigators’ prior intervention studies,21,22 the program’s conceptual model proposes accurate knowledge of cognitive problems, lifestyle adjustments (sleep, stress management, physical activity) that support cognitive functioning, and self-efficacy to manage cognitive challenges will support persons with MS in the use of compensatory cognitive strategies and cognitive skills.
Group component
The first four sessions focused on common cognitive problems experienced with MS (attention and processing speed, memory and language, visuospatial and executive functioning) and development of relevant compensatory strategies. The final four sessions focused on lifestyle behaviors to support cognitive functioning, including managing fatigue and stress and increasing physical activity. Process aspects of the intervention focused on building self-efficacy for maximizing cognitive functioning through verbal persuasion, performance accomplishment of new behaviors and role modeling.35 Detailed intervention materials are available from the first author.
Computer protocol
Following a successful pilot, the PIs contracted with Lumos Labs for use of components of the Lumosity program to reduce MS-specific deficits. The Lumosity program delivers interactive programs that run directly in standard web browsers and is designed to adapt to the individual user and offers novel, engaging, and challenging tasks within an integrated, hierarchical structure.23 The facilitator prescribed exercises from a study-specific protocol addressing the most common deficits experienced by persons with MS (attention, memory, flexibility, and problem solving). The games/tasks (see Table 1) were arranged so that the most basic cognitive skills (attention) were addressed first. Each participant was asked to complete 3 sessions (45–60 min of training) a day three times a week, (approximately 45 games) and to keep a written log of practice time. The researchers monitored exercise practice and completion.
Table 1.
Selected examples of cognitive tasks/games in Lumosity program.
| Game | Cognitive Domain | Construct |
|---|---|---|
| Birdwatching | Attention | Visual field |
| WordBubbles | Flexibility/Executive Functioning | Verbal fluency |
| MonsterGarden | Memory | Working memory |
| BytheRules | Problem Solving | Logical reasoning |
| PenguinPursuit | Processing Speed | Spatial orientation |
Comparison group
Persons randomly assigned to the comparison group received their usual care and a referral to “MyBrainGames”, available for free at MultipleScerosis.com. The games challenge processing speed, working memory attention and task switching ability. Participants were asked to keep a log of any practice time. These participants also received weekly “check-in” calls from research staff during the 8 weeks of the intervention period.
The small group-based intervention and the 6 months of follow-up testing were delivered to 10 cohorts of participants across the three sites over a 34-month period. To promote consistency in testing and intervention delivery, the researchers trained neuropsychological testers and facilitators at each site in the study procedures. A “booster” session was held with facilitators approximately one year after they had begun conducting groups to reinforce key components of the intervention. In addition, the facilitators audio-recorded their group sessions, and the second author listened to a sample of these tapes to determine that the intervention was being delivered as intended.
Measures
A Background Information Sheet (BIS) was used to collect information on demographic and disease characteristics to describe the sample. Participants completed the Self-Administered Expanded Disability Status Scale (EDSS-S)24 as a measure of impairment due to MS. Scores on the EDSS-S can range from 0 to 9.5, and have a strong (r = 0.89) intraclass correlation with physician ratings.24
Cognitive Performance Outcomes
Five neuropsychological tests from the Minimal Assessment of Cognitive Function in MS (MACFIMS), were used to measure cognitive performance. Benedict and colleagues25 presented evidence for the reliability, construct and concurrent validity of the MACFIMS battery in a sample of 291 persons with MS. Based on the findings from our earlier pilot study,2 we selected 5 of the 7 tests to limit redundancy and participant burden. The 70-min battery included the following widely used tests: the Controlled Oral Word Association Test (COWAT)26 - verbal fluency and word finding; The California Verbal Learning Test, 2nd ed, (CVLT-II)27 - verbal learning and remembering; The Brief Visuospatial Memory Test – Revised (BVMT-R)28 -nonverbal learning and memory; The Paced Auditory Serial Addition Test (PASAT)29 - auditory information processing speed and flexibility, as well as calculation abilities; and The Symbol Digit Modalities Test (SDMT)30 - complex scanning and visual tracking. All of these show strong reliability and were administered by a trained rater following standardized testing protocols. The use of alternate forms when available and the time intervals between repeated testing helped limit the learning of specific test stimuli and mitigated practice effects.
IADL Performance Outcome
Performance on cognitive-related instrumental activities of daily living (IADL) was measured using the Everyday Problems Test-Revised(EPT-R)31 This test, originally developed as a key outcome in the ACTIVE trials of older adults,32 assesses cognitive ability to reason and solve problems encountered in daily living.33 The instrument developer (Willis, personal communication) granted permission for creation of a shortened form of the EPT that eliminated 12 items that were consistently answered correctly by most participants in a pilot study. The reliability coefficient for the revised 30-item version, the EPT-R, was 0.83, and 2-month test/retest reliability was 0.74. The EPT-R had significant, moderately strong positive correlations (.40–.60) with the neuropsychological performance tests in pilot work by the investigators.
Self-Report Outcomes
The 17-item General Self-Efficacy Scale34 was used as a measure of confidence in the ability to affect outcomes in various contexts and situations. Given the well known association of depression with working memory in those with MS, the 10-item Center for Epidemiologic Studies Depression Scale (CES-D)35 was used. Participants rated how often they have experienced specific symptoms during the past week; higher summated scores indicate more depressive symptoms. The Strategy Subscale of the Multi-Factorial Memory Questionnaire36 was used as a measure of use of memory strategies. The 19 items describe various memory aids and strategies (e.g. making to do lists). Respondents indicate how frequently they used each strategy during the past 2 weeks using a 5-point scale (never to all the time). The PROMIS v1.0-Applied Cognition-Abilities-Short Form 8a was used to assess self-reported cognitive function. It has demonstrated acceptable psychometric properties in a sample of community-dwelling persons with MS.31 Participants rate how positively (1 = Not at all, 5 = Very much) they assess 8 items (e.g., attention, thinking, memory) over the preceding 7 days. Scores range from 8 to 40, and higher scores indicate greater perceived cognitive ability.
Data analysis
Data analyses were conducted using SPSS, version 24.0 for Windows (SPSS Inc., Chicago, IL, USA). Neuropsychological data were scored by the testers and verified by a second staff member. Data were entered and checked for accuracy. Internal consistency reliability coefficients for all self-report measures were .80 or above. Descriptive analyses were performed to obtain a profile of the sample. The groups were compared at baseline on demographic and disease variables. Preliminary analysis for tests of assumptions such as normality, independence of errors, homogeneity of variance, and outliers suggested that assumptions had generally been met and therefore data transformations were deemed unnecessary.
Missing data are often problematic for randomized controlled trials and various strategies are used to manage missing data.37 For the current study, we employed intention-to-treat strategies to handle missing data using a “last observation carried forward” (LOCF) strategy in which missing data points are imputed based on the participant’s existing data. When data were found to be missing, the value was carried forward for all remaining points.38 Therefore, the results presented include data for all participants at each of the four time points.
To account for cohort and city effects, multi-level analyses using SAS Proc Mixed were conducted. These analyses revealed very minimal city and cohort effects, thus these factors were not included in subsequent analyses. A repeated measures ANOVA (RM-ANOVA) design was utilized to determine time and time by group effects on performance and self-report outcomes. Because of the large variability among individuals with MS, we determined that it would be appropriate to use Analysis of Covariance (ANCOVA) to control for the differences among participants that are not germane to assessing the magnitude of the difference attributable to the treatment.39 Therefore, ANCOVAs were also performed to determine the differences between the intervention group and comparison groups at T2, T3, and T4, after controlling for baseline scores. Separate analyses were conducted for each outcome measure.
Results
Fig. 1 depicts the flow of participants from recruitment through randomization and data collection. Of the 441 persons who responded to recruitment materials and assessed for eligibility, 94 (21%) did not meet inclusion criteria, 154 (35%) ultimately declined to participate due to scheduling conflicts or failure to respond and 10 (2%) did not complete the paperwork for the study or declined due to health reasons.
Fig. 1.
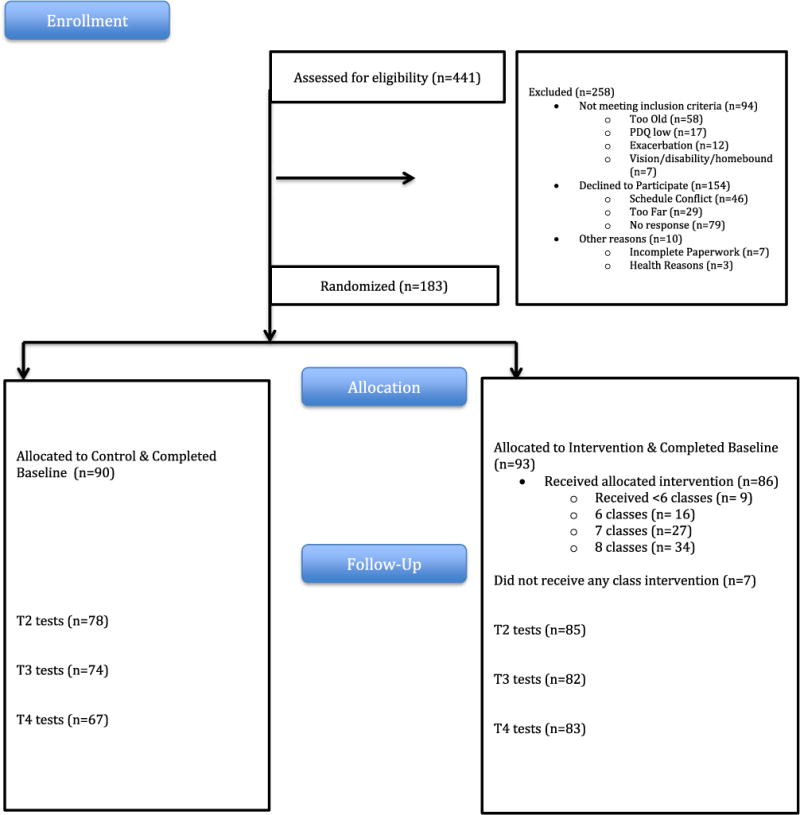
Flow diagram.
As seen in Fig. 1, 183 persons with MS and perceived cognitive difficulties (93 intervention, 90 control) completed baseline measures, were randomized to a group and included in the analysis. Consistent with most studies of persons with MS, the majority of participants were female (87%), white (75%), and married (64%). Participants ranged in age from 26 to 60 (mean = 49.6 ± 8.0 years). In general, the study participants were well-educated as almost all (96%) had completed high school and 60% had completed a bachelor’s degree or higher (See Table 2).
Table 2.
Sample demographics (N = 183).
| Characteristic | Categories | Intervention Group (n = 93)
|
Comparison Group (n = 90)
|
Total (n = 183)
|
|---|---|---|---|---|
| n(%)a | n(%)a | n(%)a | ||
| Gender | Male | 13 (14%) | 10 (11%) | 23 (13%) |
| Female | 80 (86%) | 80 (89%) | 160 (87%) | |
| Age | 20-35 years | 4 (4%) | 8 (9%) | 12 (7%) |
| 36-50 years | 39 (41%) | 34 (38%) | 73 (40%) | |
| 51-60 years | 50 (54%) | 47 (53%) | 97 (54%) | |
| Mean (SD) | 49.8 (7.5) | 49.4 (8.5) | 49.6 (8.0) | |
| Range | 29–60 | 26–60 | 26–60 | |
| Education | < High School | 4 (4%) | 4 (5%) | 8 (4%) |
| High School Grad | 17 (19%) | 23 (26%) | 40 (22%) | |
| Associate Degree | 11 (12%) | 15 (17%) | 26 (14%) | |
| Bachelor’s Degree | 44 (48%) | 33 (37%) | 77 (43%) | |
| Graduate Degree | 16 (17%) | 14 (16%) | 30 (17%) | |
| Race/Ethnicity | Non-Hispanic | 84 (90%) | 81 (90%) | 165 (90%) |
| Spanish/Hispanic | 9 (10%) | 9 (10%) | 18 (10%) | |
| White | 73 (79%) | 64 (71%) | 137 (75%) | |
| African American | 17 (18%) | 17 (19%) | 34 (19%) | |
| Multiple categories | – | 1 (1%) | 1 (1%) | |
| Other | 3 (3%) | 8 (9%) | 11 (6%) | |
| Marital Status | Married | 56 (60%) | 61 (65%) | 117 (64%) |
| Un-married | 37 (40%) | 29 (35%) | 66 (36%) | |
| Employment Status | Full-time | 25 (27%) | 28 (31%) | 53 (29%) |
| Part-time | 5 (5%) | 3 (3%) | 8 (4%) | |
| Unemployed-Disability | 32 (36%) | 39 (44%) | 71 (39%) | |
| Retired | 12 (13%) | 5 (6%) | 17 (9%) | |
| Other | 8 (8%) | 2 (2%) | 10 (6%) | |
| Years Since Diagnosis | Mean (SD) | 13.9 (8.05) | 12.1 (8.07) | 13.0 (8.08) |
| Range | 1–34 | 1–32 | 1–34 | |
| MS Type | Benign Sensory | 3 (3%) | 3 (3%) | 6 (3%) |
| Relapsing-Remitting | 64 (69%) | 61 (69%) | 125 (69%) | |
| Primary Progressive | 3 (3%) | 5 (6%) | 8 (4%) | |
| Secondary Progressive | 14 (90%) | 10 (11%) | 24 (13%) | |
| Progressive-Relapsing | 1 (1%) | 1 (1%) | 2 (1%) | |
| Unable to choose one/don’t know | 8 (9%) | 9 (10%) | 17 (9%) | |
| EDSS Total | Mean (SD) | 5.1 (1.63) | 5.3 (1.5) | 5.2 (1.56) |
| Range | 1.0–9.0 | 2.0–8.0 | 1.0–9.0 |
Percentage totals may not add to 100% due to rounding.
On average, this sample had mild to moderate impairment from MS with mean EDSS score of 5.2 ± 1.56. Participants had been diagnosed for an average of 13.4 years (SD 7.99) and most (68%) reported that they had relapsing-remitting MS. There were no statistically significant baseline differences between groups on the self-report measures or in age, years of education, or time since diagnosis.
Overall, participation in the intervention was high. The mean number of classes attended was 6.4 (SD = 2.3, range 0–8) for those randomized to the MAPSS-MS group. Although individuals varied their computer training time from week to week, 68% met or exceeded the total time prescribed over the 8-week intervention. About half met or exceeded the goal each week. By contrast, 41 of the 90 comparison group participants reported time spent on MyBrain game, ranging from an average of 10 min per week to 370 min; half listed 45 min or less per week.
Change over time analyses
Means, standard deviations, and F-values for each outcome measure at each time point are shown in Table 3. The findings indicated that both the intervention and control groups reported significant change over time on all measures (F for time effect). A marginal time × group effect was found for the CVLT Delayed score (F = 2.28, p = .051), the 3-sec PASAT (F = 2.86, p = .053) and PROMIS Cognitive Abilities (F = 2.59, p = .059) suggesting that the intervention group was making greater gains than the comparison group. On the CESD, the time by group effect was significant (F = 2.01, P < .05); the intervention group’s reported depressive symptoms decreased, while the control group remained about the same.
Table 3.
Means/SDs by time and group (intention-to-treat).
| Scale | Group | Time 1 (N = 183) | Time 2 (N = 183) | Time 3 (N = 183) | Time 4 (N = 183) | F Time Effect | F Group by Time Effect |
|---|---|---|---|---|---|---|---|
| + CVLT Total | Comparison | 49.6 ± 10.3 | 49.9 ± 11.5 | 54.7 ± 12.3 | 53.6 ± 12.9 | 41.166*** | 0.133 |
| Intervention | 52.4 ± 12.0 | 53.0 ± 12.6 | 57.2 ± 12.3 | 56.1 ± 12.9 | |||
| + CVLT Delay | Comparison | 11.0 ± 3.6 | 10.6 ± 3.7 | 11.9 ± 3.7 | 11.6 ± 3.7 | 16.811*** | 2.676 |
| Intervention | 11.6 ± 3.4 | 11.9 ± 3.5 | 12.4 ± 3.5 | 12.4 ± 3.6 | |||
| BVMT Total | Comparison | 20.2 ± 6.9 | 22.6 ± 6.6 | 20.7 ± 6.1 | 20.1 ± 6.7 | 25.950*** | 0.331 |
| Intervention | 21.7 ± 6.4 | 24.3 ± 6.1 | 21.9 ± 6.8 | 21.9 ± 7.0 | |||
| BVMT Delay | Comparison | 8.0 ± 2.7 | 8.5 ± 2.8 | 7.7 ± 2.5 | 7.5 ± 2.7 | 18.996*** | 0.511 |
| Intervention | 8.4 ± 2.7 | 9.3 ± 2.4 | 8.2 ± 2.6 | 8.2 ± 2.7 | |||
| + PASAT 3 Seconds | Comparison | 40.1 ± 13.2 | 42.5 ± 12.6 | 44.3 ± 12.4 | 45.9 ± 11.8 | 59.330*** | 2.860 |
| Intervention | 41.0 ± 14.4 | 46.1 ± 12.1 | 46.9 ± 11.6 | 47.6 ± 11.9 | |||
| + PASAT 2 Seconds | Comparison | 28.5 ± 11.4 | 30.7 ± 12.1 | 31.6 ± 12.5 | 33.4 ± 12.2 | 34.580*** | 2.145 |
| Intervention | 29.3 ± 13.2 | 33.0 ± 12.0 | 35.1 ± 12.4 | 34.8 ± 13.2 | |||
| SDMT | Comparison | 49.0 ± 12.4 | 50.6 ± 11.5) | 50.7 ± 12.2 | 52.0 ± 12.4 | 23.203*** | 1.210 |
| Intervention | 49.8 ± 11.8 | 52.4 ± 12.6 | 52.8 ± 13.0 | 54.6 ± 12.2 | |||
| + COWAT | Comparison | 36.2 ± 11.8 | 35.9 ± 11.3 | 38.1 ± 11.7 | 36.9 ± 11.9 | 10.808*** | 1.831 |
| Intervention | 36.4 ± 11.1 | 37.9 ± 11.3 | 40.2 ± 12.7 | 39.5 ± 12.1 | |||
| + EPT-R | Comparison | 22.8 ± 4.9 | 23.0 ± 4.5 | 23.1 ± 4.3 | 23.5 ± 4.4 | 10.359*** | 1.186 |
| Intervention | 22.9 ± 4.6 | 23.4 ± 5.0 | 23.8 ± 4.8 | 24.2 ± 4.8 | |||
| + PROMIS Cog Ability | Comparison | 22.5 ± 7.8 | 23.8 ± 7.0 | 23.4 ± 7.3 | 23.0 ± 7.8 | 7.559*** | 2.590 |
| Intervention | 22.5 ± 7.2 | 25.1 ± 7.2 | 25.6 ± 7.7 | 25.6 ± 7.4 | |||
| + Memory Strategy | Comparison | 36.7 ± 11.8 | 40.4 ± 11.1 | 39.6 ± 11.3 | 39.5 ± 11.2 | 12.736*** | 0.296 |
| Intervention | 38.4 ± 13.4 | 41.7 ± 11.0 | 40.5 ± 11.4 | 40.2 ± 11.0 | |||
| + Self-efficacy | Comparison | 60.3 ± 11.0 | 61.4 ± 11.8 | 62.5 ± 11.4 | 61.1 ± 12.0 | 4.990** | 0.657 |
| Intervention | 61.5 ± 12.2 | 62.4 ± 11.6 | 64.0 ± 10.6 | 63.7 ± 11.1 | |||
| + CESD | Comparison | 11.4 ± 5.8 | 11.4 ± 6.3 | 11.5 ± 6.6 | 10.5 ± 5.9 | 3.453*** | 2.914* |
| Intervention | 11.4 ± 6.2 | 9.7 ± 5.6 | 9.9 ± 6.2 | 10.1 ± 6.2 |
Note.
F tests use the Greenhouse-Geisser statistic. All other F tests use the Wilks Lambda statistic.
p < .05,
p < .01,
p < .001.
Table 4 shows results of the ANCOVA analysis for each outcome measure adjusting for baseline scores on that measure. These analyses revealed that immediately following the intervention, the intervention group scored significantly higher than the comparison group on the CVLT Delayed score (F = 6.47, p = .012), the PASAT3, (F = 7.72, p = .006) and lower on the CESD, (F = 7.63, p = .006). At 3 months post-intervention, the intervention group scored significantly higher than the comparison group on the PROMIS Cognitive Abilities (F = 5.33, P < .05), PASAT3 (3.92, p < .05), PASAT2 (F = 5.78, P < .05), and lower on the CESD (F = 5.04, p < .05). At 6 months post-intervention the intervention group scored significantly higher on PROMIS Cognitive Abilities (F = 6.62, p < .05), SDMT (F = 4.09, p < .05), and the COWAT (F = 4.42, p < .05). With the exception of the CESD where the Intervention group reported fewer depressive symptoms, the intervention group scored higher than the comparison group in all post-treatment analyses.
Table 4.
ANCOVA Results at Time 2, 3 and 4 by Group (Intention-to-treat data, N = 183, Intervention = 93, Control = 90).
| Time
|
T2
|
T3
|
T4
|
|||
|---|---|---|---|---|---|---|
| Scales | Group F | p-value | Group F | p-value | Group F | p-value |
| Memory Strategy | 0.015 | 0.904 | 0.051 | 0.821 | 0.102# | 0.750 |
| CESD | 7.626 | 0.006** | 5.039 | 0.026* | 0.383 | 0.537 |
| Self-efficacy | 0.010 | 0.919 | 0.395# | 0.531 | 2.072# | 0.152 |
| PROMIS Cognitive Ability | 2.116þ | 0.147 | 5.334 | 0.022* | 6.617 | 0.011* |
| CVLT Total | 0.270 | 0.604 | 0.002 | 0.961 | 0.009 | 0.926 |
| CVLT Delay | 6.470 | 0.012* | 0.002 | 0.968 | 0.812 | 0.369 |
| BVMT Total | 1.122 | 0.291 | 0.047 | 0.828 | 1.093 | 0.297 |
| BVMT Delay | 3.159 | 0.077 | 0.506 | 0.478 | 1.060 | 0.305 |
| PASAT 3 Seconds | 7.719 | 0.006** | 3.917# | 0.049* | 1.112 | 0.293 |
| PASAT 2 Seconds | 2.460 | 0.119 | 5.783 | 0.017* | 0.362 | 0.548 |
| SDMT | 1.978 | 0.161 | 1.972 | 0.162 | 4.086 | 0.045* |
| COWAT | 2.963 | 0.087 | 2.658# | 0.105 | 4.418 | 0.037* |
| EPT-R Total | 0.290# | 0.591 | 2.835# | 0.094 | 2.320# | 0.129 |
Note:
p < .05,
p < .01,
p < .001,
Violated the Levene’s Test of Equality of Error Variances.
Violated the assumption of homogeneity of regression with significant Time 1 by Group interactions.
Discussion
This multi-site trial of the MAPSS-MS intervention provided additional evidence that persons with MS can enhance their cognitive functioning and confirmed the feasibility of the intervention in diverse community settings. Participation in the group sessions and computer training was high at all sites. It is challenging to keep people motivated to continue practicing the brain training programs, particularly when they find the exercises to be difficult. In addition, technical difficulties with the programs can temporarily disrupt participants’ ability to utilize the games; a problem for a study that is attempting to tightly control the intervention delivery.
Changes in scores over time were statistically significant and in the desired direction for both groups–including greater self-efficacy, more frequent use of compensatory strategies, and improved performance on neuropsychological tests. Because of the great variability among people with MS in the nature of their impairments and course of the disease, as well as many other factors not measured in this study, we decided to control for individual differences by using baseline measures as covariates. When controlling for baseline scores, the intervention group outperformed the comparison group on all measures, including significant differences on the CVLT Delayed score, the PASAT3, and CESD (immediately post-intervention); on the PROMIS Cognitive Abilities, PASAT3, PASAT2, and CESD (at 3 months post-intervention); and PROMIS Cognitive Abilities, SDMT, and the COWAT (at 6 months post-intervention). So, while the observed differences between groups were small, the overall pattern suggests somewhat greater positive change for the intervention group. However, it is unknown if the positive effects observed will persist if individuals do not continue to engage in training activities.
The fact that both groups demonstrated improvements over time underscores the importance that many people with MS place on dealing with their cognitive limitations. In contrast to a true control group who gets no treatment, the comparison group in this study was encouraged to try the MyBrain games made available by the MS Society and approximately half of the comparison group turned in logs reporting they had attempted this program. So, our findings must be interpreted in the context of increasing attention to this topic in the MS community, which may have encouraged participants in both groups to take steps to improve their cognitive abilities. Of note, both groups reported using more memory strategies at Time 2 compared to what they reported at baseline. It is certainly possible that they “learned” about additional strategies they might utilize simply from completing the initial baseline measure.
The statistically significant time by group effect on the CESD speaks to the positive effects of the group intervention in helping participants reframe the challenges they face, particularly in the area of cognitive abilities. The fact that the difference between the groups was strongest immediately following the intervention tends to support the assertion that on-going interpersonal support can be important for people with MS, who in previous research have frequently reported depression.
Many other cognitive RCTs reported in the literature have recruited participants through clinical settings where the intervention has been described as a “rehabilitation intervention” and participants in these studies were often closely monitored on an individual basis as they practiced exercises designed to build skills in specific cognitive domains. By contrast, the MAPSS-MS recruitment brochure described it as study of “strategies to help you improve cognitive functioning in everyday life”. Our study employed a more pragmatic approach addressing strategies that would help participants in their activities of daily living while practicing computer exercises targeting a variety of cognitive areas. Consequently, it may not be surprising that participants in our study did not demonstrate as consistent a pattern of gain (relative to the comparison group) in specific neurocognitive domains as was observed in some previous studies. Unfortunately, our efforts to capture the study’s impact on activities of daily living were limited by the measures of daily functioning ability available. Most measures target a relatively limited range of tasks and have yet to demonstrate strong ecological validity. The development of reliable and valid measures of the cognitive abilities needed to function effectively in every-day life should be a priority for future research in this area.
Our results must be interpreted with caution because of the convenience sample, the limitation to English-speakers, and the potential for selection bias in this generally well educated and moderately impaired group. This sample may have been highly motivated to learn and use compensatory strategies, which may be reflected in the improvement observed in participants in both arms of the study. Treatment contamination is difficult if not impossible to control in more pragmatic community-based studies. In each community, there are various activities and resources available to people with MS, so participants may have known each other and shared information about the study. There are also numerous online cognitive training programs available to the public that may have enabled comparison group participants to build their cognitive abilities.
Conclusion
Persons with MS are in urgent need of interventions to improve cognitive skills to support their functioning in everyday life. The MAPSS-MS program that integrates the positive effects of group interventions to build self-efficacy for compensatory and lifestyle behaviors with individual home-based computer training could be broadly implemented in community settings and has been shown to be modestly successful in increasing cognitive functioning. Future research should explore if one or both components of the interventions were responsible for changes in outcomes by examining the relative impact of each component and explore the use of alternate delivery formats for those with mobility and transportation barriers.
Acknowledgments
The authors wish to acknowledge Ana Todd, PhD, RN, Ashley Henneghan PhD RN, Nicole Gloris Hargrove, BSN, RN and Richard Fulbright, PhD for assistance with recruitment and data collection. We also thank the National MS Society and the neurologists, neuropsychologists, testers and intervention facilitators in Houston, Dallas and San Antonio who assisted with the study.
Funding
This work was supported by the National Institutes of Health, National Institute of Nursing Research 1R01NR014362. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
This work was funded by an NIH grant and was reviewed and approved by the University of Texas at Austin IRB. All authors meet the criteria for authorship credit
Footnotes
Conflicts of interest
The author(s) declare(s) that there is no conflict of interest regarding the publication of this article.
References
- 1.Pierson SH, Griffith N. Treatment of cognitive impairment in multiple sclerosis. Behav Neurol. 2006;17(1):53–67. doi: 10.1155/2006/545860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuifbergen AK, Becker H, Perez F, Morrison J, Kullberg V, Todd A. A randomized controlled trial of a cognitive rehabilitation intervention for persons with multiple sclerosis. Clin Rehabil. 2012;26(10):882–893. doi: 10.1177/0269215511434997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien AR, Chiaravalloti N, Goverover Y, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil. 2008;89(4):761–769. doi: 10.1016/j.apmr.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Goretti B, Portaccio E, Zipoli V, et al. Impact of cognitive impairment on coping strategies in multiple sclerosis. Clin Neurol Neurosurg. 2010;112(2):127–130. doi: 10.1016/j.clineuro.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 6.Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L. Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler. 2001;7(5):340–344. doi: 10.1177/135245850100700511. [DOI] [PubMed] [Google Scholar]
- 7.Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis II. Impact on employment and social functioning. Neurology. 1991;41:692–696. doi: 10.1212/wnl.41.5.692. [DOI] [PubMed] [Google Scholar]
- 8.Shevil E, Finlayson M. Perceptions of persons with multiple sclerosis on cognitive changes and their impact on daily life. Disabil Rehabil. 2006;28:779–788. doi: 10.1080/09638280500387013. [DOI] [PubMed] [Google Scholar]
- 9.Rahn K, Slusher B, Kaplin A. Cognitive impairment in multiple sclerosis: a forgotten disability remembered. Cerebrum: the Dana Forum on Brain Science. 2012;2012:14. Retrieved from http://dana.org/news/cerebrum/detail.aspx?id=39986. [PMC free article] [PubMed] [Google Scholar]
- 10.Filippi M, Riccitelli G, Mattioli F, et al. Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures–an explorative study. Radiology. 2012;262(3):932–940. doi: 10.1148/radiol.11111299. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrandt H, Lanz M, Hahn HK, et al. Cognitive training in MS: effects and relation to brain atrophy. Restor Neurol Neurosci. 2007;25:33–43. [PubMed] [Google Scholar]
- 12.Amato MP, Goretti B, Viterbo RG, et al. Computer-assisted rehabilitation of attention in patients with multiple sclerosis: results of a randomized, double-blind trial. Mult Scler. 2014;20:91–98. doi: 10.1177/1352458513501571. [DOI] [PubMed] [Google Scholar]
- 13.Mattioli F, Bellomi F, Stampatori C, et al. Two years follow up of domain specific cognitive training in relapsing remitting multiple sclerosis: a randomized clinical trial. Front Behav Neurosci. 2016;10:28. doi: 10.3389/fnbeh.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Martin MY, Gonzalez-Platas M, Eguia-Del Rio P, Croissier-Elias C, Jimenez Sosa A. Efficacy of a short cognitive training program in patients with multiple sclerosis. Neuropsychiatr Dis Treat. 2017;13:245–252. doi: 10.2147/NDT.S124448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tesar N, Bandion K, Baumhackl U. Efficacy of a neuropsychological training programme for patients with multiple sclerosis – a randomized controlled trial. Wien Klin Wochenschr. 2005;117(21–22):747–754. doi: 10.1007/s00508-005-0470-4. [DOI] [PubMed] [Google Scholar]
- 16.De Giglio L, De Luca F, Prosperini L, et al. A low-cost cognitive rehabilitation with a commercial video game improves sustained attention and executive functions in multiple sclerosis: a pilot study. Neurorehabil Neural Repair. 2015;29:453–461. doi: 10.1177/1545968314554623. [DOI] [PubMed] [Google Scholar]
- 17.Stuifbergen AK, Becker H, Morgan S, Morrison JD, Perez F. Home-based computer-assisted cognitive training: feasibility and perceptions of persons with MS. Int J MS Care. 2011;13:189–198. doi: 10.7224/1537-2073-13.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faul F, Erdfelder E, Buchner A, Lang AG. G*Power Version 3.1.3. Computer Software. Germany: Universit€at Kiel; 2011. Available from: http://www.psycho.uniduesseldorf.de/abteilungen/aap/gpower3/download-andregister. [Google Scholar]
- 19.Sullivan JIL, Edgley K, DeHoux E. A survey of multiple sclerosis, part 1: perceived cognitive problems and compensatory strategy use. Can J Rehabil. 1990;4(2):99–105. [Google Scholar]
- 20.Bandura A. Self-efficacy: The Exercise of Control. New York: WH Freeman and Company; 1997. [Google Scholar]
- 21.Stuifbergen AK, Becker H, Blozis S, Timmerman G, Kullberg V. A randomized clinical trial of a wellness intervention for women with multiple sclerosis. Arch Phys Med Rehabil. 2003;84(4):467–476. doi: 10.1053/apmr.2003.50028. [DOI] [PubMed] [Google Scholar]
- 22.Stuifbergen AK, Blozis SA, Becker H, Phillips L, Timmerman G, Kullberg V, Morrison J. A randomized controlled trial of a wellness intervention for women with fibromyalgia a syndrome. Clin Rehabil. 2010;24:305–318. doi: 10.1177/0269215509343247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy J, Scanlon M. The science behind Lumosity. 2012 Retrieved at: http://www.lumosity.com/documents;the_science_behind_lumosity.pdf.
- 24.Bowen J, Gibbons L, Gianas A, Kraft GH. Self-administered Expanded Disability Status Scale with functional system scores correlates well with a physician-administered test. Mult Scler. 2001;7:201–206. doi: 10.1177/135245850100700311. [DOI] [PubMed] [Google Scholar]
- 25.Benedict RH, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) J Int Neuropsychol Soc. 2006;12(4):549–558. doi: 10.1017/s1355617706060723. [DOI] [PubMed] [Google Scholar]
- 26.Benton AL, Sivan AB, Hamsher K, Varnery NR, Spreen O. Contributions to Neuropsychological Assessment. second. New York: Oxford University Press; 1994. [Google Scholar]
- 27.Delis DC, Kaplan E, Kramer JH. California verbal Learning Test Manual adult version. second. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 28.Benedict RH. Brief Visuospatial Memory Test – Revised: Professional Manual. Odessa, FL: Psychosocial Assessment Resources, Inc; 1997. [Google Scholar]
- 29.Gronwall DM. Paced auditory serial addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- 30.Smith A. Symbol Digit Modalities Test Manual. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- 31.Becker H, Stuifbergen A, Morrison J. Promising new approaches to assess cognitive functioning in people with multiple sclerosis. Int J MS Care. 2012;14:71–76. doi: 10.7224/1537-2073-14.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross AL, Rebok GW, Unverzagt FW, Willis SL, Brandt J. Cognitive predictors of everyday functioning in older adults: results from the ACTIVE Cognitive Intervention Trial. J Gerontol B Psychol Sci Soc Sci. 2011;66(5):557–566. doi: 10.1093/geronb/gbr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willis SL, Marsiske M. Manual for Everyday Problems Test. University Park, PA: Pennsylvania State University; 1993. [Google Scholar]
- 34.Sherer M, Maddux J, Mercandante B, Prentice-Dunn S, Jacobs B, Rogers R. The self-efficacy scale: construction and validation. Psychol Rep. 1982;51:663–671. [Google Scholar]
- 35.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 36.Troyer AK, Rich JB. Psychometric properties of a new metamemory questionnaire for older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57:19–27. doi: 10.1093/geronb/57.1.p19. [DOI] [PubMed] [Google Scholar]
- 37.Enders CK. Applied Missing Data Analysis. New York, NY: Guilford Press; 2010. [Google Scholar]
- 38.Armijo-Olivo S, Warren S, Magee D. Intention to treat analysis, compliance, drop-outs and how to deal with missing data in clinical research: a review. Phys Ther Rev. 2009;14(1):36–49. [Google Scholar]
- 39.Lipsey M. Design Sensitivity: Statistical Power for Experimental Research. Newbury Park, CA: Sage Publications; 1990. [Google Scholar]


