Abstract
A 77-year-old patient was admitted to our hospital for the further examination of melena. A computed tomography scan detected two submucosal tumors (SMTs) in the stomach and jejunum. Double-balloon endoscopy revealed the presence of a delle on the jejunal SMT, suggesting that the SMT was the origin of the gastrointestinal bleeding. Both tumors were surgically resected and subsequently diagnosed via histology as gastrointestinal stromal tumors (GISTs). Furthermore, the two GISTs had different mutations in the c-kit gene, suggesting that they were derived from different clonal origins. This report depicts an extremely rare case of multiple synchronous sporadic GISTs in the stomach and jejunum.
Keywords: multiple GISTs, c-kit mutation
Introduction
Gastrointestinal stromal tumors (GISTs) are derived from the intestinal cells of Cajal (ICCs) or their stem cell-like precursors (1). GISTs are the most common mesenchymal tumors of the gastrointestinal tract, with an estimated incidence ranging from 10 to 20 per million (2). Multiple GISTs are extremely rare, except for in cases of Carney's syndrome (3), pediatric GISTs (4), type 1 neurofibromatosis-associated GISTs (5-7), and familial GISTs (8,9). These rare cases of multiple GISTs are diagnosed via certain clinicopathological features. Multiple sporadic GISTs derived from different clones in different organs are extremely rare (9-11).
We herein report a rare case of multiple synchronous sporadic GISTs in the stomach and jejunum.
Case Report
A 77-year-old patient was admitted to our hospital for the further examination of melena, dizziness, and fatigue since the preceding night. A physical examination revealed anemia in the conjunctiva and no palpable masses in the abdomen. There was no notable family history. The patient took an anticoagulant agent, as he had a history of cardiogenic cerebral infarction and atrial fibrillation. The blood cell count showed moderate normocytic anemia [mean corpuscular volume (MCV): 95.8 fL and Hb: 7.2 g/dL], and marked reticulocytosis (88.9%). The serum iron and ferritin levels were 27 µg/dL and 18 ng/mL, respectively. The blood urea nitrogen and serum creatinine levels were 12 mg/dL and 0.66 mg/dL, respectively.
Enhanced computed tomography (CT) of the abdomen revealed a submucosal tumor (SMT) with a diameter of 25 mm in the stomach and a second SMT with a diameter of 23 mm in the jejunum (Fig. 1A and B). There was slight enhancement in the stomach lesion but high enhancement in the jejunum lesion. A gastroduodenal endoscopic examination revealed that the SMT in the stomach was located within the posterior wall of the lower body (Fig. 1C). However, the SMT was completely covered with normal mucosa, and there was no evidence of gastrointestinal bleeding. Oral double-balloon endoscopy revealed the presence of a delle in the jejunal SMT (Fig. 1D). A biopsy showed a typical GIST with spindle cell morphology via Hematoxylin and Eosin (H & E) staining and positive KIT expression via immunostaining. Fluorodeoxyglucose (FDG)-positron emission tomography showed an abnormal accumulation of FDG in both tumors (standardized uptake values for stomach and jejunum: 7.76 and 3.48, respectively). Endoscopic and enhanced CT findings suggested that the GIST in the jejunum was the source of the gastrointestinal bleeding and raised the possibility that the SMT in the stomach might also be a GIST.
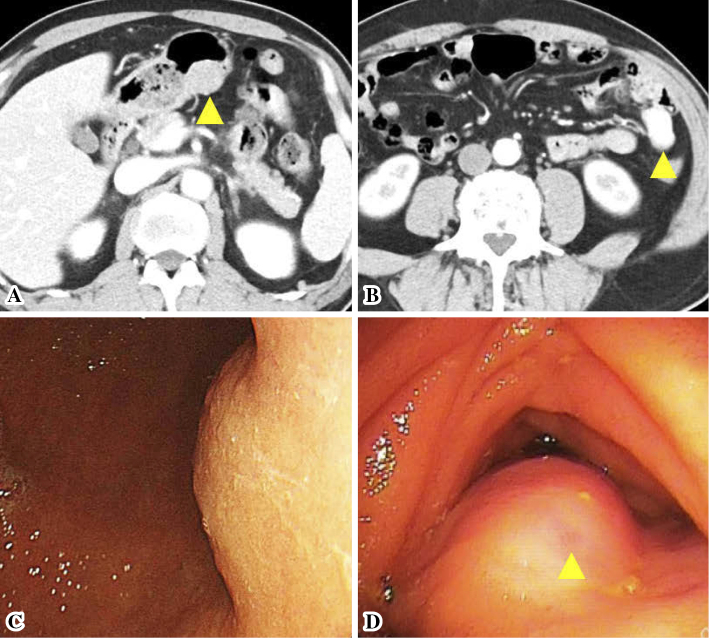
Figure 1.
Enhanced computed tomography and endoscopic findings in the stomach and jejunum. The submucosal tumor (SMT) (arrowhead) in the stomach was slightly enhanced (A), whereas the SMT (arrowhead) in the jejunum was highly enhanced (B). The SMT in the stomach was located in the lower body (C). The SMT in the jejunum had a small delle (arrowhead) (D).
Both tumors were subsequently laparoscopically resected. Upon a gross examination, the gastric tumor was found to be the intramural development type and 25×25 mm in size. The jejunal tumor was also the intramural development type, but it was dumbbell-like in shape and 23×23 mm in size. It also had a delle on the surface of the luminal side. According to the histological data, the H & E staining patterns showed spindle cell morphology and < 5 mitoses per 50 high-power fields (HPFs) in both tumors. The immunohistochemical examination revealed that KIT expression to be positive in both tumors, whereas CD34 was positive only in the gastric GIST (Fig. 2). Based on the tumor size and mitotic count, the patient was ultimately diagnosed with multiple low-risk GISTs. Given the differences in the immunohistochemical staining patterns between the two GISTs, they were thought to be derived from different clonal origins. To address this possibility, mutation analyses were subsequently carried out in both GISTs, focusing on exons 9 and 11 of the c-kit gene. The results showed the presence of the V560D mutation of exon 11 in the gastric GIST, whereas the jejunal GIST was associated with the V559G mutation of exon 11 (Fig. 3).
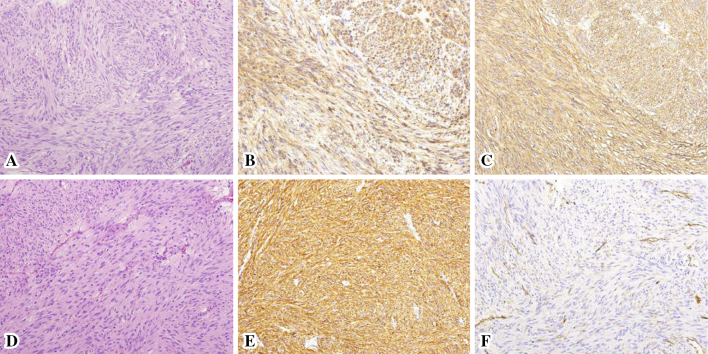
Figure 2.
A histological examination of the resected specimens of the gastric (A, B, and C) and jejunal SMT (D, E, and F). Hematoxylin and Eosin staining (A and D) and immunostaining of KIT (B and E) and CD34 (C and F) were performed (20× objective lens).
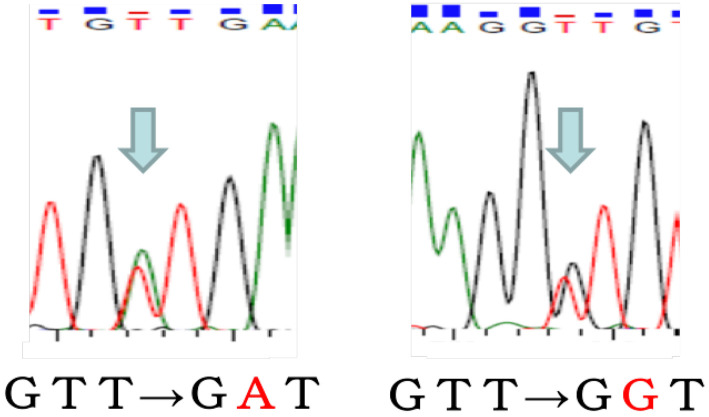
Figure 3.
A sequence analysis of exon 11 of the c-kit gene in the two GISTs. The V560D mutation (GTT→GAT) was present in the gastric GIST (A), whereas the V559G mutation (GTT→GGT) was present in the jejunal GIST (B). The mutations were confirmed by the sequence analysis.
The ultimate diagnosis was multiple synchronous sporadic GISTs in the stomach and jejunum. The patient recovered well after surgery and was discharged nine days later. Given the low risk of recurrence, adjuvant chemotherapy of imatinib was not given. After 12 months of follow-up, the patient is well without evidence of gastrointestinal bleeding or tumor recurrence.
Discussion
This report describes a patient with two GISTs: one in the stomach and one in the jejunum. Of note, the patient was an older man with no family history and no signs suggestive of Carney's triad or type 1 neurofibromatosis. Interestingly, we found that the immunohistochemical staining patterns of the two GISTs were different; while the KIT expression was positive in both GISTs, the CD34 expression was positive in the gastric GIST and negative in the jejunal GIST. Furthermore, the GISTs were associated with separate mutations in the c-kit gene, suggesting that they were derived from different clonal origins.
The standard treatment for localized and low-risk GISTs is complete surgical resection, whereas chemotherapy with imatinib is recommended for advanced or metastatic GISTs (12,13). In the present case, SMTs were detected in the stomach as well as in the jejunum. The SMT in the jejunum was diagnosed as a GIST and was the source of the observed gastrointestinal bleeding. Therefore, surgery was performed for both SMTs. As a result, the SMTs in the stomach and jejunum were both diagnosed as GISTs, and interestingly, they showed different immunohistochemical staining patterns. Furthermore, the two GISTs showed different mutations in the c-kit gene, as confirmed by a sequence analysis.
To date, only six cases of multiple synchronous sporadic GISTs in multiple organs have been reported with mutation analyses (10,11,14-16) (Table). The present reported case was the seventh. Of these, 6 patients were men, and all 7 were older than 60 years of age. Six cases reported GISTs located in the stomach or small intestine, including the present case. The reported tumor sizes ranged from 1 to 90 mm. All of the tumors < 10 mm in size have been detected incidentally. Three cases reported different cytomorphologies between the two GISTs. Given that the mitotic rate was > 5 per HPF in all reported GISTs, no or scant mitotic activity might be a characteristic of multiple sporadic GISTs in multiple organs. There was no consistency in the mutated gene or site among the multiple sporadic GISTs compared with common sporadic GISTs.
Table.
Clinicopathological Characteristics and Mutation Patterns of Reported Multiple Sporadic GISTs.
| Case | Age (years) |
Sex | Location | Size (mm) |
Morphology | Mitoses/ 50 HPF |
Mutated gene |
Mutation site | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | M | Stomach | 5 | Spindle | 0 | None§ | - | 11 |
| Esophagus | 1 | Spindle | 0 | KIT | V560D | ||||
| 2 | 67 | M | Stomach | 55 | Epithelioid | <5 | KIT | 1756ins cod572_585dup | 15 |
| Jejunum | 35 | Spindle | <5 | None¶ | - | ||||
| 3 | 72 | M | Stomach | 13 | Spindle | <5 | PDGFR | R558_I562del | 16 |
| Duodenum | 14 | Spindle | <5 | KIT | A502_Y503dup | ||||
| 4 | 78 | Not mentioned | Stomach | 90 | Epithelioid | <5 | PDGFR | deletion (aa 884-847) | 14 |
| Jejunum | 4 | Spindle | <5 | KIT | V560D | ||||
| 5 | 81 | M | Stomach | 5 | Spindle | 0 | KIT | V559D | 11 |
| Jejunum | 4 | Spindle | 0 | BRAF | V600E | ||||
| 6 | 85 | M | Stomach | 62 | Spindle | <5 | KIT | I478fsX2+W557G | 10 |
| Small intestine | 60 | Mixed | <5 | KIT | V555_I571d2L | ||||
| 7 | 66 | M | Stomach | 25 | Spindle | <5 | KIT | V560D | Present case |
| Jejunum | 23 | Spindle | <5 | KIT | V559G |
§The mutation was analyzed for KIT exons 9, 11, 13, and 17; PDGFR exons 12, 14, and 18; BRAF exon 15; and KRAS exon 2.
¶The mutation was analyzed for KIT exons 9, 11, 13, and 17; and PDGFR exons 12 and 18.
To determine the mechanism underlying GIST development, Ogasawara et al. (17) analyzed the mutations in the c-kit gene in ICCs adjacent to 16 GISTs in the stomach. They found that mutations were frequently detected (17). This result supports the notion that GISTs may develop from a “field defect”. In contrast, Nakajima et al. reported no mutations in ICCs adjacent to 10 GISTs in the small intestine by sequencing the c-kit and the PDGFR genes (18). This result was not consistent with the notion of a “field defect”. One potential explanation for these discrepant findings is that the mechanism underlying GIST development differs between the stomach and small intestine. As such, we speculate that common oncogenic stimuli may have induced GIST development in the stomach and small intestine in the present case.
In conclusion, we herein described an extremely rare case of multiple synchronous sporadic GISTs in the stomach and jejunum, which appeared to originate from different clones.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Hirota S, Isozaki K, Moriyama Y, et al. . Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279: 577-580, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors-definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 438: 1-12, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Carney JA. Carney triad: a syndrome featuring paraganglionic, adrenocortical, and possibly other endocrine tumors. J Clin Endocrinol Metab 94: 3656-3662, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Agaram NP, Laquaglia MP, Ustun B, et al. . Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res 14: 3204-3215, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirashima K, Takamori H, Hirota M, et al. . Multiple gastrointestinal stromal tumors in neurofibromatosis type 1: report of a case. Surg Today 39: 979-983, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Takazawa Y, Sakurai S, Sakuma Y, et al. . Gastrointestinal stromal tumors of neurofibromatosis type I (von Recklinghausen's disease). Am J Surg Pathol 29: 755-763, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Yantiss RK, Rosenberg AE, Sarran L, Besmer P, Antonescu CR. Multiple gastrointestinal stromal tumors in type I neurofibromatosis: a pathologic and molecular study. Mod Pathol 18: 475-484, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Lim SJ, Park K, Yuh YJ, Jang SJ, Choi J. Multiple gastrointestinal stromal tumors with a germline c-kit mutation. Pathol Int 55: 655-659, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Kang DY, Park CK, Choi JS, et al. . Multiple gastrointestinal stromal tumors: clinicopathologic and genetic analysis of 12 patients. Am J Surg Pathol 31: 224-232, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Gasparotto D, Rossi S, Bearzi I, et al. . Multiple primary sporadic gastrointestinal stromal tumors in the adult: an underestimated entity. Clin Cancer Res 14: 5715-5721, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Agaimy A, Markl B, Arnholdt H, et al. . Multiple sporadic gastrointestinal stromal tumours arising at different gastrointestinal sites: pattern of involvement of the muscularis propria as a clue to independent primary GISTs. Virchows Arch 455: 101-108, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Nishida T, Hirota S, Yanagisawa A, et al. . Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 13: 416-430, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Group EESNW.. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3): iii21-iii26, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Miselli F, Conca E, Casieri P, et al. . A sporadic multiple GIST with unusual pathologic, molecular, and genetic features. Am J Surg Pathol 32: 340-341, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Dell'Avanzato R, Carboni F, Palmieri MB, et al. . Laparoscopic resection of sporadic synchronous gastric and jejunal gastrointestinal stromal tumors: report of a case. Surg Today 39: 335-339, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Blandamura S, Alessandrini L, Bertorelle R, et al. . Multiple sporadic gastrointestinal stromal tumors concomitant with ampullary adenocarcinoma: a case report with KIT and PDGFRA mutational analysis and miR-221/222 expression profile. Pathol Res Pract 210: 392-396, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Ogasawara N, Tsukamoto T, Inada K, et al. . Frequent c-Kit gene mutations not only in gastrointestinal stromal tumors but also in interstitial cells of Cajal in surrounding normal mucosa. Cancer Lett 230: 199-210, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima T, Miwa S, Ando T, et al. . Interstitial cells of Cajal do not harbor c-kit or PDGFRA gene mutations in patients with sporadic gastrointestinal stromal tumors. J Gastroenterol 44: 426-431, 2009. [DOI] [PubMed] [Google Scholar]