Abstract
A 35-year-old Japanese man was emergently admitted to our hospital with chief complaints of palpitation and dyspnea. He has been treated for Basedow's disease. He was diagnosed with acute decompensated heart failure, atrial fibrillation and thyrotoxicosis. We started anti-thyroid agents and a treatment for heart failure with beta blockers and diuretics under anti-coagulation therapy. His B-type natriuretic peptide levels remained high, although the heart failure had been compensated and the heart rate was well controlled while hyperthyroidism still existed. We should bear in mind that a discrepancy can exist between the clinical course and the B-type natriuretic peptide level in heart failure patients complicated with hyperthyroidism.
Keywords: BNP, hyperthyroidism, heart failure
Introduction
Heart failure is a complex clinical syndrome caused by systolic or diastolic dysfunction of heart. Heart failure is a global pandemic worldwide and is even increasing in prevalence. Despite advances in treatments and prevention, mortality and morbidity of heart failure are still high. B-type natriuretic peptide (BNP) is an established diagnostic and therapeutic marker of heart failure. BNP is generated from myocardium primarily following mechanical stimulation of the ventricular myocardium. BNP-guided therapy is considered to be useful for managing heart failure. A higher BNP at discharge was found to be related to a higher incidence of re-hospitalization for heart failure (1-5). However, there are miscellaneous causes of elevated levels of BNP, which include both cardiac and non-cardiac causes (6). We herein report a patient with heart failure complicated with hyperthyroidism with a discrepancy between the clinical course and the BNP levels.
Case Report
A 35-year-old Japanese man was emergently admitted to our hospital with chief complaints of palpitation and dyspnea that had started a month prior to presentation. He had been treated medically for Basedow's disease since the age of 28. The patient has discontinued the treatment approximately six months prior to presentation. One week prior to his admission, he had visited a local physician, and the treatment of his hyperthyroidism was resumed, which resulted in no improvement of his symptoms.
A physical examination upon admission revealed the following: height 173.0 cm, body weight 73.3 kg, body mass index 24.5, Glasgow Coma Scale 15, blood pressure 125/89 mmHg, heart rate 183/min, body temperature 36.5℃, and respiratory rate 20/min. Cardiac auscultation revealed irregular heart sounds and a Levine ΙΙ/VΙ systolic murmur with the point of maximum intensity located at the apex. Moist rales were auscultated over the lower lung fields. Edema in the lower extremities was also present.
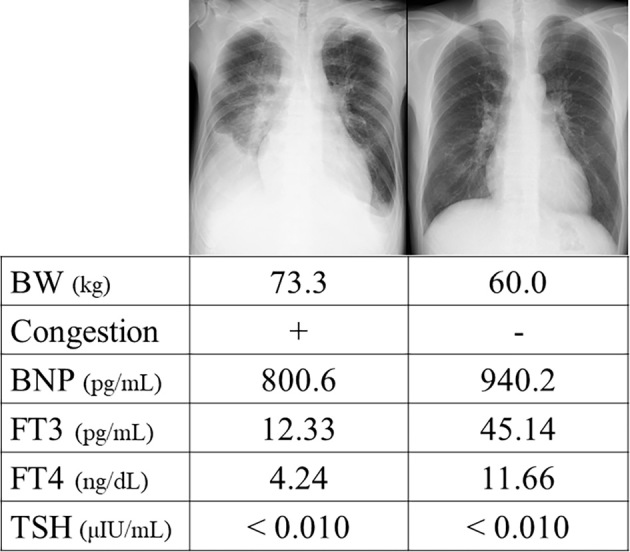
Chest X-ray showed bilateral lung congestion, dullness of the costophrenic angle and cardiomegaly with a cardiothoracic ratio of 58%. His electrocardiogram revealed atrial fibrillation with a heart rate of 175/min and an inverted T wave in V5 and V6. Blood examinations indicated an elevated white blood cell count, slight anemia (hemoglobin concentration 12.4 g/dL; hematocrit 39.4%) and hyperthyroidism (free T3 12.33 pg/mL, free T4 4.24 ng/dL and undetectable levels of thyroid-stimulating hormone). His BNP level was 800.6 pg/mL. Echocardiography revealed a reduced left ventricular systolic function with a left ventricular ejection fraction (LVEF) of 26%, severe functional mitral regurgitation, moderate tricuspid regurgitation, a high estimated right ventricular systolic pressure (RVSP) of 60 mmHg, bilateral atrial enlargement and distended inferior vena cava without any changes in his respiratory status.
Upon admission, he was diagnosed with acute decompensated heart failure, atrial fibrillation with a rapid ventricular response and thyrotoxicosis. We managed his hyperthyroidism with thiamazole accompanied by treatment for heart failure using beta blockers, diuretics and anti-coagulants. On the 14th day of admission, the BNP level remained high despite compensation for the heart failure, improved echocardiographic findings (LVEF 48%, moderate MR and RVSP 49 mmHg) and a well-controlled ventricular rate of atrial fibrillation while hyperthyroidism still existed (Figure). The BNP level and thyroid status along with heart failure were carefully followed during the clinical course after discharge. There were no clinical findings of exacerbated heart failure, and the BNP levels gradually decreased with an accompanying improvement in his hyperthyroidism. At his 9-month follow-up examination, his BNP level was 22.8 pg/mL, and no hyperthyroidism was found, with an improvement in his LVEF from 26% to 53%.
Figure.
Changes in the clinical findings, B-type natriuretic peptide level and thyroid status before and after the initiation of treatment for heart failure and hyperthyroidism. The B-type natriuretic peptide level remained high, accompanied by persistent hyperthyroidism despite an improvement in the patient’s decompensated heart failure.
Discussion
We herein report a patient with heart failure complicated with hyperthyroidism with a discrepancy between the clinical course and the BNP levels. BNP-guided therapy is considered to be useful for managing heart failure. A higher BNP at discharge was found to be related to a higher incidence of re-hospitalization for heart failure (1-5). In our patient, the BNP level remained relatively high despite improvement in his heart failure, including the symptoms and examination findings. However, on the 14th day of admission, the thyroid function was still high although his thyrotoxicosis had improved. Thyrotoxicosis is a life-threatening condition, although its incidence is not very high (7), and it is often accompanied by heart failure (estimated incidence of approximately 40%) (8).
Previous reports have described a relationship between the BNP levels and thyroid hormones. Ertugrul et al. reported that BNP and thyroxine were positively correlated in patients without heart failure or cardiac disease (9). In that report, the BNP level was approximately four-fold higher in patients with hyperthyroidism than in other subjects in a euthyroid state.
The mechanism underlying the association between BNP and thyroid hormones has not been fully elucidated. BNP is generated from myocardium primarily following mechanical stimulation of the ventricular myocardium. BNP is produced rapidly through mRNA synthesis if proper stimuli exist. These stimuli are mainly excessive stretching of the myocytes rather than transmural pressure loading (10,11). However, thyroid hormones can stimulate the secretion of BNP. Previous experimental studies have shown that free T3 hormone directly stimulates the secretion of BNP from myocardial cells via an increase in the gene expression of BNP (12,13). These studies might explain the discrepancy between the clinical improvement of heart failure and the BNP level in our patient.
General management of heart failure usually involves a systemic evaluation, including the severity of symptoms, physical findings, and grade of fluid retention on chest X-ray, as well as an evaluation of the echocardiographic findings and serum BNP level. Nevertheless, in heart failure patients complicated with hyperthyroidism, the relationship between the clinical course and BNP level needs to be carefully interpreted and monitored.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. . Task Force on Clinical Practice Guidelines and the Heart Failure Society of America of Heart Failure: A Report of the American College of Cardiology/American Heart Association 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management. Circulation 136: e137-e161, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Kociol RD, Horton JR, Fonarow GC, et al. . Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 4: 628-636, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng V, Kazanagra R, Garcia A, et al. . A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol 37: 386-391, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Dhaliwal AS, Deswal A, Pritchett A, et al. . Reduction in BNP levels with treatment of decompensated heart failure and future clinical events. J Card Fail 15: 293-299, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Logeart D, Thabut G, Jourdain P, et al. . Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 43: 635-641, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P, Voors AA, Anker SD, et al. . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129-2200, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Dillmann WH. Thyroid storm. Curr Ther Endocrinol Metab 6: 81-85, 1997. [PubMed] [Google Scholar]
- 8.Isozaki O, Satoh T, Wakino S, et al. . Treatment and management of thyroid storm: analysis of the nationwide surveys: the taskforce committee of the Japan Thyroid Association and Japan Endocrine Society for the establishment of diagnostic criteria and nationwide surveys for thyroid storm. Clin Endocrinol (Oxf) 84: 912-918, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Ertugrul DT, Gursoy A, Sahin M, et al. . Evaluation of brain natriuretic peptide levels in hyperthyroidism and hypothyroidism. J Natl Med Assoc 100: 401-405, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Wiese S, Breyer T, Dragu A, et al. . Gene expression of brain natriuretic peptide in isolated atrial and ventricular human myocardium: influence of angiotensin II and diastolic fiber length. Circulation 102: 3074-3079, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno Y, Yoshimura M, Harada E, et al. . Plasma levels of A- and B-type natriuretic peptides in patients with hypertrophic cardiomyopathy or idiopathic dilated cardiomyopathy. Am J Cardiol 86: 1036-1040, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Kohno M, Horio T, Yasunari K, et al. . Stimulation of brain natriuretic peptide release from the heart by thyroid hormone. Metabolism 42: 1059-1064, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Liang F, Webb P, Marimuthu A, Zhang S, Gardner DG. Triiodothyronine increases brain natriuretic peptide (BNP) gene transcription and amplifies endothelin-dependent BNP gene transcription and hypertrophy in neonatal rat ventricular myocytes. J Biol Chem 278: 15073-15083, 2003. [DOI] [PubMed] [Google Scholar]


