Abstract
A 67-year-old woman presented with hematuria and proteinuria 16 and 11 months ago, respectively. She had been followed up as mixed connective tissue disease and Sjögren's syndrome for over 19 years. Blood chemistry showed no elevated serum creatinine or C-reactive protein but did reveal myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA) of 300 U/dL. A kidney biopsy showed pauci-immune focal necrotizing glomerulonephritis. She was treated with prednisolone and rituximab, resulting in normal urinalysis and decreased MPO-ANCA. The complication of ANCA-associated glomerulonephritis should not be overlooked when abnormal urinalysis findings appear in the course of connective tissue disease, irrespective of the presence of rapidly progressive glomerulonephritis.
Keywords: ANCA-associated glomerulonephritis, mixed connective tissue disease, Sjögren's syndrome
Introduction
Connective tissue diseases can be associated with a variety of renal diseases. Renal involvement in primary Sjögren's syndrome (pSS) is common and the most frequent renal lesion in pSS is tubulointerstitial nephritis (1). Glomerulonephritis, such as membranoproliferative glomerulonephritis (MPGN) with or without cryoglobulinemic glomerulonephritis and membranous nephropathy, has been reported to occur in pSS (1-3). Glomerulonephritis was found in 2% of 261 patients with pSS over a 3.6-year follow-up period (4). In mixed connective tissue disease (MCTD), the rate of occurrence of glomerulonephritis, such as membranous glomerulonephritis and mesangial proliferative glomerulonephritis, was reported to range from 0% to 37% (5-8). Patients with MCTD were also found to be frequently associated with secondary Sjögren's syndrome (9).
The rate of antineutrophil cytoplasmic antibody (ANCA) positivity in patients with pSS by an indirect immunofluorescence is reported to range from 3.2% to 16.7% and most cases show P-ANCA positivity (10-12). However, the rate of ANCA positivity in patients with MCTD is not known. Typical renal manifestation of ANCA-associated glomerulonephritis is rapidly progressive glomerulonephritis (RPGN) characterized by a rapid loss of the renal function (usually a 50% decline in the glomerular filtration rate within several weeks to months) with nephritic urinalysis. Although rare, patients with pSS or MCTD have also been reported to be accompanied by RPGN due to ANCA-associated glomerulonephritis (Table).
Table.
Clinical Features of Patients with MCTD Or Sjögren’s Syndrome Presenting with Rapidly Progressive Glomerulonephritis Due to ANCA-associated Glomerulonephritis.
| References | Reported year | Age (years/gender) | MCTD | pSS | Interval (months) | MPO/ PR3-ANCA | Cr(mg/dL) | Proteinuria | Microscopic hematuria | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 2000 | 58/F | + | - | 192 | +/- | 1.5 | 0.77 g/day | + | mPSL, PSL, CYP | imp |
| 26 | 2006 | 47/F | + | - | 228 | +/ | 2.7 | 6.5 g/day | + | mPSL, PSL, CYP | imp |
| 27 | 2006 | 42/F | + | +, # | 168 | +/- | 6.0 | + | + | mPSL, PSL, CYP, PE | dead |
| 28 | 2011 | 68/F | + | - | 48 | *+/ | 0.58 | 2+ | + | PSL | dead |
| 29 | 2013 | 42/F | + | - | 36 | +/- | 0.9 | 11 g/day | + | PSL, CYP | imp |
| 30 | 2014 | 35/F | + | - | 8 | +/ | 0.95 | 4.1 g/day | + | mPSL, PSL, CYP | imp |
| 31 | 1992 | 63/F | - | + | 7 | +/- | 2.3 | 2.07 g/day | + | PSL, mPSL, CYP | imp |
| 32 | 1996 | 74/F | - | + | 36 | +/- | 2.6 | 1.60 g/day | + | mPSL, PSL | imp |
| 33 | 1999 | 67/F | - | + | 7 | +/- | 2.8 | 0.43 g/day | + | mPSL, PSL CYP, PE | imp |
| 34 | 2000 | 49/F | - | + | 24 | +/- | 1.3 | 1.20 g/day | + | mPSL, PSL | imp |
| 35 | 2009 | 49/F | - | + | 12 | +/- | 1.2 | 0.48 g/day | + | mPSL, PSL MMF | imp |
| 36 | 2011 | 86/M | - | + | 0 | +/- | 4.2 | 1.31 g/day | + | PSL, CYP | ESRD |
| 37 | 2014 | 66/F | - | + | 72 | +/- | 2.8 | 2.40 g/day | + | PSL | imp |
| 38 | 2015 | 64/M | - | + | 528 | +/- | 2.22 | 1.97 g/day | + | mPSL, PSL, CYP, AZA | imp |
| 38 | 2015 | 71/F | - | + | 101 | +/- | 2.04 | 1.70 g/day | + | mPSL, PSL, CYP | imp |
| 38 | 2015 | 57/M | - | + | 12 | +/- | 3.77 | 6.50 g/day | + | mPSL, PSL, CYP | death |
| 39 | 2015 | 65/F | - | + | 94 | +/- | 1.6 | 1.6 g/day | + | mPSL, PSL AZA | imp |
| 40 | 2016 | 71/F | - | + | 1 | +/+ | 2.9 | 1.68 g/day | + | mPSL, PSL, CYP | imp |
| Our case | 67/F | + | + | 228 | +/- | 0.82 | 0.58 g/gCr | + | mPS, PSL, RTX | imp |
Interval: interval between the first symptoms of Sjögren’s syndrome or MCTD and presentation of RPGN, +: presence or positive, -: absence or negative. #: secondary Sjögren’s syndrome, *: MPO-ANCA related crescentic glomerulonephritis and immune complex glomerulonephritis. imp: improvement, ESRD: end stage renal disease, mPSL: methylprednisolone pulse, PSL: oral prednisolone, CYP: cyclophosphamide, PE: plasma exchange, MMF: mycophenolate mofetil, AZA: azathioprine, RTX: rituximab
We herein report a patient with ANCA-associated glomerulonephritis during the clinical course of MCTD and Sjögrens's syndrome and summarize a review of the English literature. In contrast to RPGN as the typical renal manifestation of ANCA-associated glomerulonephritis, the patient showed a smoldering clinical course, atypical renal manifestation of ANCA-associated glomerulonephritis with long-term nephritic urinalysis and little or no renal insufficiency. This case may provide an important diagnostic implication of ANCA in patients with MCTD or Sjögren's syndrome presenting with nephritic urinalysis.
Case Report
A 67-year-old woman presented with proteinuria and hematuria. She had a medical history of MCTD and Sjögren's syndrome at the age of 48. At the diagnosis, her subjective symptoms were characterized by skin eruption, a fever, arthralgia, Raynaud's phenomenon, face erythema, swelling of the fingers of both hands and sicca symptom with keratoconjunctivitis. Laboratory tests showed elevated levels of anti-nuclear antibody (speckled pattern), anti-U1-RNP antibody and anti-SS-B antibody and leukopenia. A Schirmer tear test and rose bengal test were found to be positive. A lip biopsy was not performed. After initiation of 20 mg/day of prednisolone (PSL), her clinical condition became stable. She had presented with microscopic hematuria 16 months earlier and 1+ proteinuria 11 months earlier at a dose of 8 mg/day of PSL. Three months before admission, a urinary examination showed increased proteinuria of 1.01 g/g creatinine, red cell casts and alpha1-microglobulin of 16.1 mg/L. Blood chemistry showed serum creatinine (Cr) of 1.03 mg/dL, C-reactive protein (CRP) of 0.11 mg/dL and MPO-ANCA of 300 U/mL. The test of ANCA positivity had not been conducted before. Although MPO-ANCA was positive, the patient did not show RPGN or elevated CRP. Therefore, her nephritic urinalysis was suspected to have been caused by Sjögren's syndrome and/or MCTD-associated glomerulonephritis rather than MPO-ANCA-associated glomerulonephritis.
On admission, she had no objective symptoms and no pulmonary or skin lesions. Laboratory examinations showed urinary protein of 0.58 g/g Cr, RBC 30-40/HPF and RBC casts in urinary sediments, serum Cr of 0.82 mg/dL, CRP of 0.16 mg/dL, anti-nuclear antibody of ×320, anti-U1-RNP antibody of 11.6 U/mL, anti-SS-A antibody of 1.8 U/mL, anti-SS-B antibody of 0.5 U/mL, MPO-ANCA of 262 U/mL and cryoglobulin (-). A kidney biopsy revealed 1/3 to circumferential fibrocellular to fibrous crescents, with segmental fibrinoid necrosis in 11 out of 37 glomeruli (Fig. 1A and B). However, small crescent formation was predominantly found. Tubulointerstitial nephritis and arteritis were not observed (Fig. 1A and C). An immunofluorescence study showed no immune reactant. Pauci-immune focal segmental necrotizing crescentic glomerulonephritis due to MPO-ANCA-associated vasculitis was diagnosed.
Figure 1.
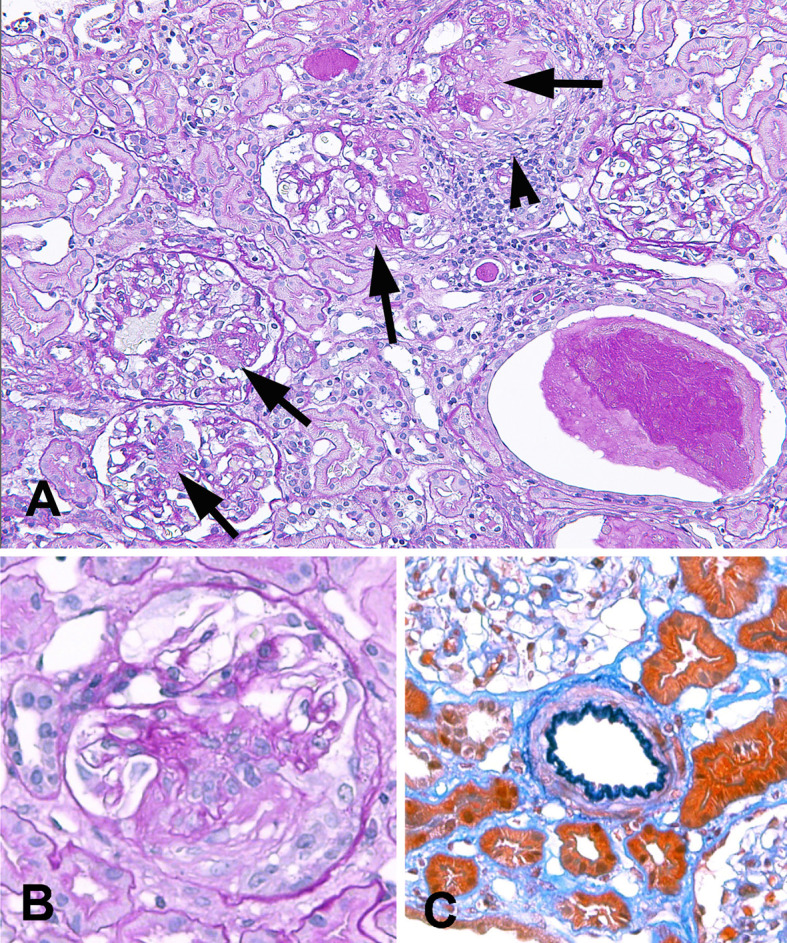
Light microscopic findings of the kidney biopsy. A. Light micrograph shows glomeruli with focal segmental necrotizing lesions (arrows). There are proteinaceous cast formations and mononuclear cell infiltration around a glomerulus with extensive dissolution of the Bowman’s capsule (arrow head). Tubulointerstitial nephritis with lymphoplasmacytic infiltration is not observed. (PAS staining) ×200. B. A glomerulus with fibrinoid necrosis and fibrocellular crescent formation (PAS staining) ×400. C. A small artery shows no vasculitis (Elastica-Masson staining) ×400. PAS: periodic acid-Schiff
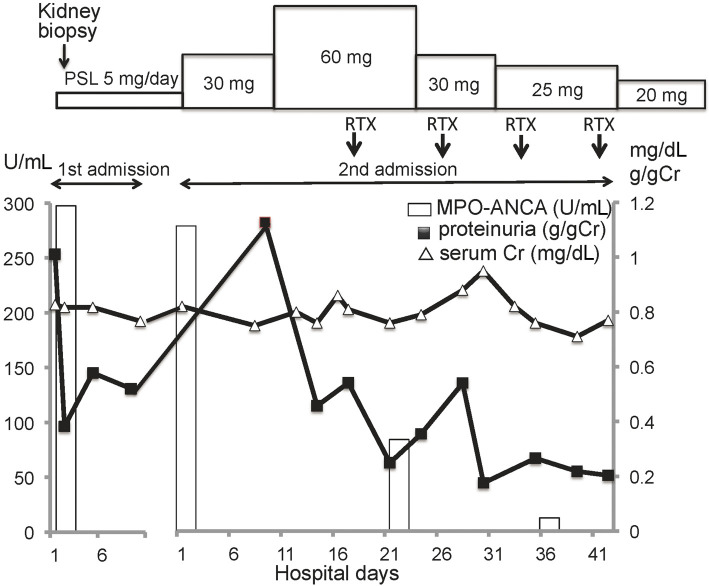
The clinical course is shown in Fig. 2. The patient was treated with 30 mg/day of PSL; however, 1 month after the treatment, her laboratory data had not improved. Thus, the dose of PSL was raised to 60 mg/day in combination with intravenous rituximab 560 mg every week for a total of 4 doses.
Figure 2.
Clinical course. PSL: prednisolone, RTX: 560 mg rituximab, MPO-ANCA: myeloperoxidase-antineutrophil cytoplasmic antibody, Cr: creatinine
The clinical condition was improved 5 weeks after the therapy, and the patient was discharged with proteinuria (-), hematuria (-) and MPO-ANCA of 7.4 U/mL. One year after the discharge, the patient's status was stable, and clinical remission of ANCA-associated glomerulonephritis had been maintained at a dose of 10 mg/day of PSL.
Discussion
We reported a patient with MCTD and secondary Sjögren's syndrome presenting with a smoldering clinical course of ANCA-associated glomerulonephritis. Her nephritic urinalysis was suspected to be caused by glomerulonephritis, such as MPGN or mesangial proliferative glomerulonephritis, which are rarely reported in patients with MCTD or pSS (1-7) before a kidney biopsy, because she showed year-long nephritic urinalysis without elevated serum creatinine and elevated CRP despite MPO-ACNA positivity.
The characteristic feature of ANCA-associated glomerulonephritis is a focal necrotizing glomerulonephritis associated with crescent formation, and the typical renal manifestation is RPGN with hematuria, proteinuria and elevated serum creatinine (13). The renal manifestation in a smaller subset of patients is a smoldering, remitting and relapsing course, and the pathology shows glomerular sclerosis either alone or accompanied by focal active lesions with necrosis and crescents (14,15). Our case may therefore be an instance of the latter renal manifestation, which does not necessarily indicate a favorable prognosis. Extra-renal manifestations, such as vasculitis allergica cutis (16) or arthralgia (17), have been reported to be an opportunity to identify patients with either a smoldering course or a slowly progressive course of ANCA-associated glomerulonephritis. However, if such patients do not show any extra-renal manifestations, then the presence of proteinuria and/or hematuria, as identified by routine urine tests, are thus thought to be symptoms which indicate the early phase of the disease.
The serum Cr level may not have increased in our patient due to there being less glomerular injury at the time of the kidney biopsy than in characteristic ANCA-associated glomerulonephritis. This may be due in part to the low activity of nephritogenic MPO-ANCA and/or the anti-inflammatory action of low-dose corticosteroid treatment.
Connective tissue diseases have been reported to be occasionally associated with ANCA-associated glomerulonephritis during the clinical course. However, no causal relationship between connective tissue diseases and ANCA-associated vasculitis has been found. Various factors have been proposed to be involved in ANCA-associated vasculitis, including heredity, environment, infection and cellular or humoral immunity (18). One explanation for the development of ANCA-associated vasculitis in some MCTD/SS patients may be an infectious trigger caused by the relatively immunosuppressed condition associated with systemic autoimmune diseases and their treatments (19). B-cell depletion therapy is a well-established therapeutic option for inducing and maintaining remission in patients with ANCA-associated vasculitis (20). Recent reports have also shown that B-cell depletion brought about favorable results with regard to reducing the systemic disease activity in patients with MCTD (21,22) and pSS (23,24). Thus, increased B-cell-associated autoimmunity might lead to an excessive production of immunoglobulins and various autoantibodies, which is shared as a common mechanism of pathogenesis between MCTD/SS and ANCA-associated vasculitis. Our patient was successfully treated with high-dose PSL and rituximab as B-cell depletion therapy after insufficient effect of 30 mg/day of PSL.
The reported cases of MCTD or pSS presenting with pauci-immune ANCA-associated glomerulonephritis are listed in the Table. There was only one case report in which ANCA-associated glomerulonephritis occurred in a patient with both MCTD and Sjögren's synderome. All cases showed proteinuria, hematuria, MPO-ANCA positivity and RPGN. The mean interval between the first symptoms of pSS or MCTD and presentation of RPGN was 94.8 months. Three of the 18 cases died of organ failure or infection, and 1 developed end-stage renal disease despite immunosuppressive treatment. None of the reported cases were treated with rituximab. Our case showed smoldering ANCA-associated glomerulonephritis at the later clinical course of MCTD and Sjögren's syndrome and was successfully treated with high-dose PSL and rituximab.
In summary, the determination of ANCA is necessary in patients with MCTD or Sjögren's syndrome who present with nephritic urinalysis even without RPGN, as ANCA-associated glomerulonephritis can be a complication of MCTD or Sjögren's syndrome. In addition, renal pathology is essential for obtaining the correct diagnosis and appropriately managing ANCA-associated glomerulonephritis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Ms. Hiromi Yamaguchi for her superb technical assistance.
References
- 1.Maripuri S, Grande JP, Osborn TG, et al. . Renal involvement in primary Sjögren's syndrome: a clinicopathologic study. Clin J Am Soc Nephrol 4: 1423-1431, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goules A, Masouridi S, Tzioufas AG, Ioannidis JP, Skopouli FN, Moutsopoulos HM. Clinically significant and biopsy-documented renal involvement in primary Sjögren syndrome. Medicine (Baltimore) 79: 241-249, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Ichikawa K, Konta T, Sato H, Ueda Y, Yokoyama H. The clinical and pathological characteristics of nephropathies in connective tissue diseases in the Japan Renal Biopsy Registry (J-RBR). Clin Exp Nephrol 2017. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 4.Skopouli FN, Dafni U, Ioannidis JP, Moutsopoulos HM. Clinical evolution, and morbidity and mortality of primary Sjögren's syndrome. Semin Arthritis Rheum 29: 296-304, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Kitridou RC, Akmal M, Turkel SB, Ehresmann GR, Quismorio FP Jr, Massry SG. Renal involvement in mixed connective tissue disease: a longitudinal clinicopathologic study. Semin Arthritis Rheum 16: 135-145, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida A, Morozumi K, Takeda A, Koyama K. Nephropathy in patients with mixed connective tissue disease. Ryumachi 34: 976-980, 1994. (Japanese, Abstract in English). [PubMed] [Google Scholar]
- 7.Ungprasert P, Crowson CS, Chowdhary VR, Ernste FC, Moder KG, Matteson EL. Epidemiology of mixed connective tissue disease, 1985-2014: a population-based study. Arthritis Care Res (Hoboken) 68: 1843-1848, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdt MA, Hoffman RW, Deutscher SL, Wang GS, Johnson JC, Sharp GC. Long-term outcome in mixed connective tissue disease: longitudinal clinical and serologic findings. Arthritis Rheum 42: 899-909, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Usuba FS, Lopes JB, Fuller R, et al. . Sjögren's syndrome: an underdiagnosed condition in mixed connective tissue disease. Clinics (Sao Paulo) 69: 158-162, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Font J, Ramos-Casals M, Cervera R, et al. . Antineutrophil cytoplasmic antibodies in primary Sjögren's syndrome: prevalence and clinical significance. Br J Rheumatol 37: 1287-1291, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Nishiya K, Chikazawa H, Hashimoto K, Miyawaki S. Antineutrophil cytoplasmic antibody in patients with primary Sjögren's syndrome. Clin Rheumatol 18: 268-271, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Casals M, Nardi N, Brito-Zerón P, et al. . Atypical autoantibodies in patients with primary Sjögren syndrome: clinical characteristics and follow-up of 82 cases. Semin Arthritis Rheum 35: 312-321, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Pettersson EE, Sundelin B, Heigl Z. Incidence and outcome of pauci-immune necrotizing and crescentic glomerulonephritis in adults. Clin Nephrol 43: 141-149, 1995. [PubMed] [Google Scholar]
- 14.Falk RJ, Nachman PH, Hogan SL, Jennette JC. ANCA glomerulonephritis and vasculitis: a Chapel Hill perspective. Semin Nephrol 20: 233-243, 2000. [PubMed] [Google Scholar]
- 15.Hauer HA, Bajema IM, van Houwelingen HC, et al. ; European Vasculitis Study Group (EUVAS).. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int 61: 80-89, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Nakabayashi I, Yoshizawa N, Kubota T, et al. . ANCA associated vasculitis allergica cutis (VAC) and mild proliferative necrotizing glomerulonephritis. Clin Nephrol 40: 265-269, 1993. [PubMed] [Google Scholar]
- 17.Aoyama T, Shimizu T, Matsuo T, et al. . MPO-ANCA-positive slowly progressive glomerulonephritis with focal tuft necrosis and crescents. Intern Med 41: 458-462, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Talarico R, Baldini C, Della Rossa A, et al. . Large- and small-vessel vasculitis: a critical digest of the 2010-2011 literature. Clin Exp Rheumatol 30 (1 Suppl 70): S130-S138, 2012. [PubMed] [Google Scholar]
- 19.Konstantinov KN, Ulff-Møller CJ, Tzamaloukas AH. Infections and antineutrophil cytoplasmic antibodies: triggering mechanisms. Autoimmun Rev 14: 201-203, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Hassan RI, Gaffo AL. Rituximab in ANCA-Associated Vasculitis. Curr Rheumatol Rep 19: 6, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Haroon M, O'Gradaigh D, Foley-Nolan D. A case of Raynaud's phenomenon in mixed connective tissue disease responding to rituximab therapy. Rheumatology (Oxford) 46: 718-719, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Lepri G, Avouac J, Airò P, et al. . Effects of rituximab in connective tissue disorders related interstitial lung disease. Clin Exp Rheumatol 100 (Suppl): 181-185, 2016. [PubMed] [Google Scholar]
- 23.Meijer JM, Meiners PM, Vissink A, et al. . Effectiveness of rituximab treatment in primary Sjögren's syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 62: 960-968, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Verstappen GM, Kroese FG, Meiners PM, et al. . B Cell depletion therapy normalizes circulating follicular Th cells in primary Sjögren syndrome. J Rheumatol 44: 49-58, 2017. [DOI] [PubMed] [Google Scholar]
- 25.Makita N, Katori H, Takemoto F, et al. . A case of mixed connective tissue disease (MCTD) complicated with MPO-ANCA-related necrotizing glomerulonephritis. Clin Nephrol 54: 164-168, 2000. [PubMed] [Google Scholar]
- 26.Hernández-Molina G, Reyes E, Crispín JC. ANCA associated glomerulonephritis in a patient with mixed connective tissue disease. Ann Rheum Dis 65: 410-411, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitaura K, Miyagawa T, Asano K, et al. . Mixed connective tissue disease associated with MPO-ANCA-positive polyangiitis. Intern Med 45: 1177-1182, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Murakami T, Endo S, Moriki T, Doi T, Matsumoto Y. Mixed connective tissue disease developing into MPO-ANCA-positive polyangiitis. Intern Med 50: 591-595, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Konstantinov KN, Harris AA, Barry M, Murata GH, Tzamaloukas AH. Sustained remission of antineutrophil cytoplasmic antibody-mediated glomerulonephritis and nephrotic syndrome in mixed connective tissue disease. J Clin Med Res 5: 316-321, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, He L, Lü X, Mo L, Zhang J. ANCA associated glomerulonephritis in a patient with mixed connective tissue disease. J Cent South Univ (Med Sci) 39: 209-214, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Böttinger EP, Niles JL, Collins AB, McCluskey RT, Arnaout MA. Antineutrophil cytoplasmic autoantibody-associated vasculitis presenting as Sjögren's syndrome. Arthritis Rheum 35: 1373-1376, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Hernández JL, Rodrigo E, De Francisco AL, Val F, González-Macías J, Riancho JA. ANCA-associated pauci-immune crescentic glomerulonephritis complicating Sjögren's syndrome. Nephrol Dial Transplant 11: 2313-2315, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Kamachi M, Migita K, Tominaga M, et al. . Sjögren's syndrome complicated by MPO-ANCA positive crescentic glomerulonephritis. Nephrol Dial Transplant 14: 1033-1034, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Tatsumi H, Tateno S, Hiki Y, Kobayashi Y. Crescentic glomerulonephritis and primary Sjögren's syndrome. Nephron 86: 505-506, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Guillot X, Solau-Gervais E, Coulon A, Debiais F. Sjögren's syndrome with ANCA-associated crescentic extramembranous glomerulonephritis. Joint Bone Spine 76: 188-189, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Wang WJ, Wu HS, Chu TS. Anti-neutrophil cytoplasmic antibody-associated Pauci-immune crescentic glomerulonephritis complicating Sjögren's syndrome. J Formos Med Assoc 110: 473-477, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Shavit L, Grenader T. Clinical manifestations and outcome of ANCA-related pauci-immune glomerulonephritis in patients with Sjögren's syndrome. Clin Exp Rheumatol 32 (3 Suppl 82): S19-25, 2014. [PubMed] [Google Scholar]
- 38.Guellec D, Cornec-Le Gall E, Groh M, et al. . ANCA-associated vasculitis in patients with primary Sjögren's syndrome: detailed analysis of 7 new cases and systematic literature review. Autoimmun Rev 14: 742-750, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Kubota K, Ueno T, Mise K, et al. . ANCA-Associated Vasculitis in a Patient with Systematic Sclerosis and Sjögren's Syndrome: A Case Report. Case Rep Nephrol Dial 5: 113-117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee IH, Kim SK, Kim MK. Dual anti-neutrophil cytoplasmic antibody-related pauci-immune crescentic glomerulonephritis in a patient with Sjögren's syndrome. Rheumatol Int 36: 1327-1334, 2016. [DOI] [PubMed] [Google Scholar]