Abstract
The incidence of Helicobacter pylori-negative gastric cancer is very low. A 60-year-old man was referred to Tokai University Hospital from a local clinic because of eosinophilia. The laboratory data revealed prominent eosinophilia, with a white blood cell count of 7,900 /μL and increased eosinophil granulocyte level of 1,659 /μL. After an examination for secondary eosinophilia, esophagogastroduodenoscopy showed an enlarged gastric fold in the corpus, suggesting type 4 gastric cancer. Repeated esophagogastroduodenoscopy (EGD) and a re-biopsy demonstrated poorly differentiated adenocarcinoma and signet ring cell carcinoma. The patient was negative for Helicobacter pylori infection according to the serum anti-Helicobacter pylori antibody, culture and histopathological findings.
Keywords: Helicobacter pylori-negative gastric cancer, eosinophilia, poorly differentiated adenocarcinoma, signet ring cell carcinoma
Introduction
The incidence of Helicobacter-negative gastric cancer is estimated to be 0.42-0.66% (1,2). Regarding the characteristics of Helicobacter-negative gastric cancer, the undifferentiated type is more frequent than the differentiated type (3). Furthermore, the marked eosinophilia occurs in several important disorders, such as allergic diseases, parasitic infection, and collagen diseases and malignancy (4). There are some eosinophil-derived malignancies (acute and chronic eosinophilic leukemia) as well as malignancies in which eosinophils are increased as part of the overall cellular response.
We herein report a case of Helicobacter pylori-negative gastric carcinoma with peripheral eosinophilia. The patient received chemotherapy with S-1 and cisplatin. After the first course of chemotherapy, the eosinophilia was resolved, with values in the normal range.
Case Report
A 60-year-old man was referred to Tokai University Hospital from a local clinic because of eosinophilia. At the local clinic, stool testing results for ova and parasites were negative, and he had no history of allergic disorder. He had taken no medication that could have caused eosinophilia. At the consultation, the laboratory data revealed prominent eosinophilia, with a white blood cell count of 7,900 /μL and increased eosinophil granulocyte level of 1,659 /μL.
A physical examination on consultation demonstrated no wheezing, rhinitis, or eczema, and a review of the patient's history revealed no abnormalities, or the presence of a pet dog. After the first visit, he lost 1 kg of body weight and developed anorexia within 2 months. Laboratory tests showed elevated CA 19-9 of 179.7 U/mL (normal range, <30 U/mL), and slight elevation of interleukin (IL)-2R was observed (Table 1).
Table 1.
Laboratory Data on Initial Admission.
| Hematology | |||||
| WBC | 7,400 | /μL | Ca | 8.8 | mg/dL |
| RBC | 514×104 | /μL | T.bil | 0.4 | mg/dL |
| Hb | 17.0 | g/dL | AST | 12 | U/L |
| Ht | 50.8 | % | ALT | 10 | U/L |
| Plt | 25.1×104 | ALP | 186 | U/L | |
| γ-GTP | 32 | U/L | |||
| Neu | 51.0 | % | LDH | 171 | U/L |
| Lym | 22.0 | % | Glu | 114 | mg/dL |
| Mono | 2.0 | % | CRP | 0.15 | mg/dL |
| Eos | 23.0 | % | IgE | 253 | IU/mL |
| Baso | 1 | % | antiPR2-ANCA | <1.0 | U/mL |
| Chemistry | antiMPO-ANCA | <1.0 | U/mL | ||
| TP | 6.8 | g/dL | ANA | - | |
| Alb | 3.5 | g/dL | CEA | 4.5 | ng/mL |
| UN | 7 | mg/dL | CA19-9 | 179.7 | U/mL |
| Cre | 0.73 | mg/dL | IL-2R | 695 | U mL |
| UA | 5.1 | mg/dL | IL-3 | <31 | pg/mL |
| Na | 142 | mEq/L | IL-5 | <3.9 | pg/mL |
| K | 4.1 | mEq/L | GM-CSF | <8 | pg/mL |
| Cl | 105 | mEq/L | H. pylori antibody | <3 | U/mL |
To determine the cause of eosinophilia, computed tomography (CT) was performed (Fig. 1). The findings were circumferential thickening of the gastric wall and thickening of the transverse colon, as well as ascites, suggesting gastric carcinoma with colonic invasion. Esophagogastroduodenoscopy (EGD) revealed an enlarged gastric fold in the corpus, suggesting type 4 gastric cancer (Fig. 2). The histopathological results were not neoplastic. Furthermore, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) demonstrated no uptake anywhere in the body, including the stomach (Fig. 3). These findings suggested that the biopsy was a false-negative and that the gastric lesion was type 4 advanced gastric cancer. H. pylori culture, serum anti-H. pylori antibody (E-plate Eiken H. pylori II, Tokyo, Japan), and a histological examination were negative. Repeated EGD and a re-biopsy showed poorly differentiated adenocarcinoma and signet ring cell carcinoma (Fig. 4). Immunohistochemistry for carcinoembryonic antigen (CEA) and CA19-9 showed positive staining in the tumor cells. Periodic acid-Schiff (PAS)/alcian blue staining showed mucin in the signet ring cells. The patient was diagnosed with an inoperable stage of advanced gastric carcinoma and received chemotherapy with S-1 and cisplatin. After the first course of chemotherapy, the eosinophil count returned to a normal range (Table 2).
Figure 1.
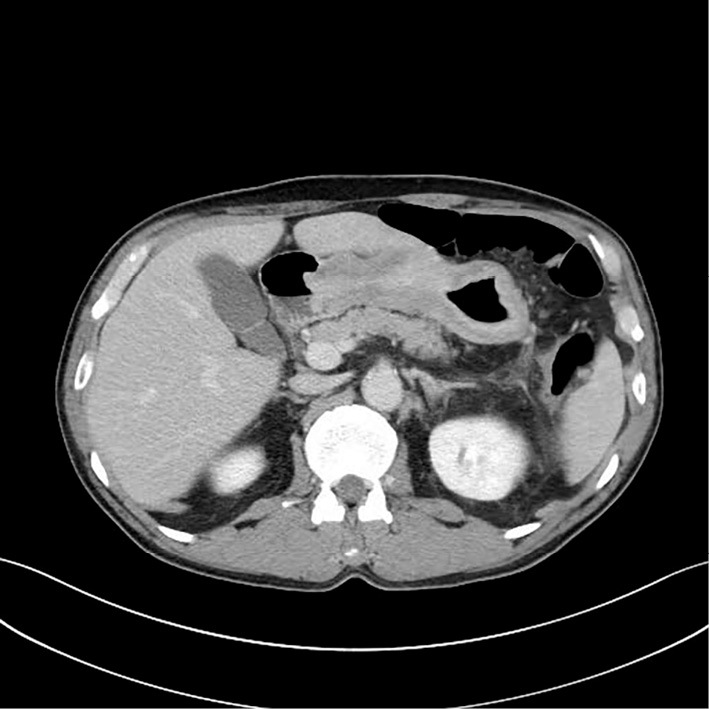
Abdominal CT showed circumferential wall thickening of the whole stomach.
Figure 2.
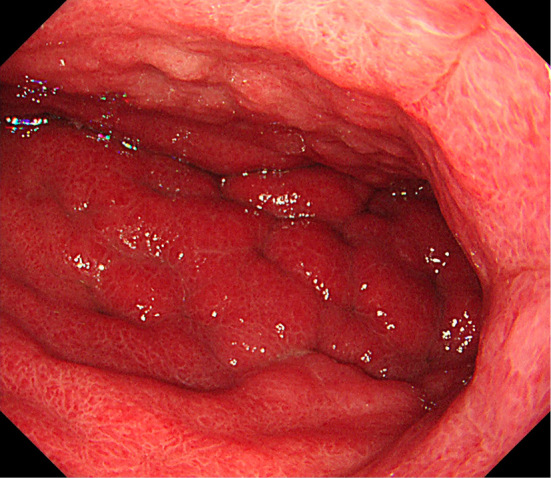
EGD demonstrated an enlarged gastric fold in the corpus, suggesting type 4 gastric cancer.
Figure 3.
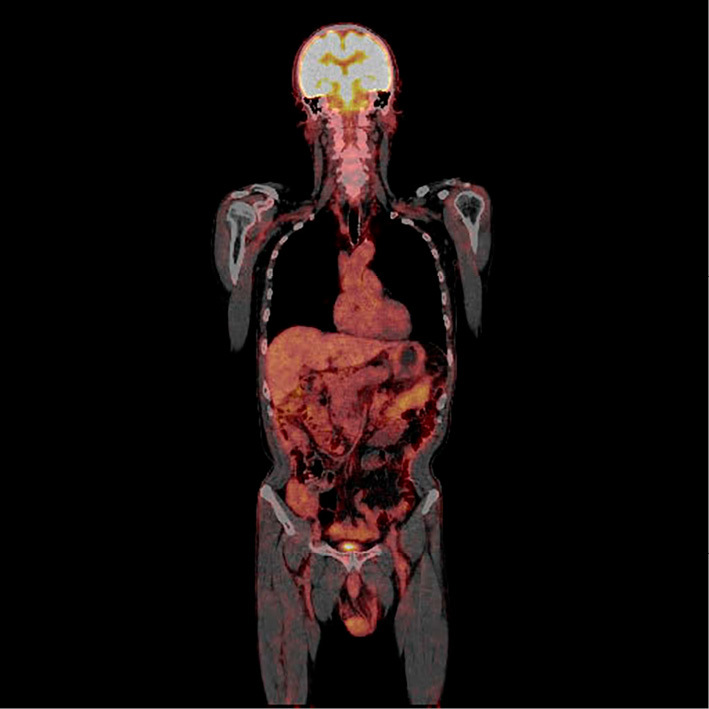
FDG-PET showed no uptake anywhere in the body, including the stomach.
Figure 4.
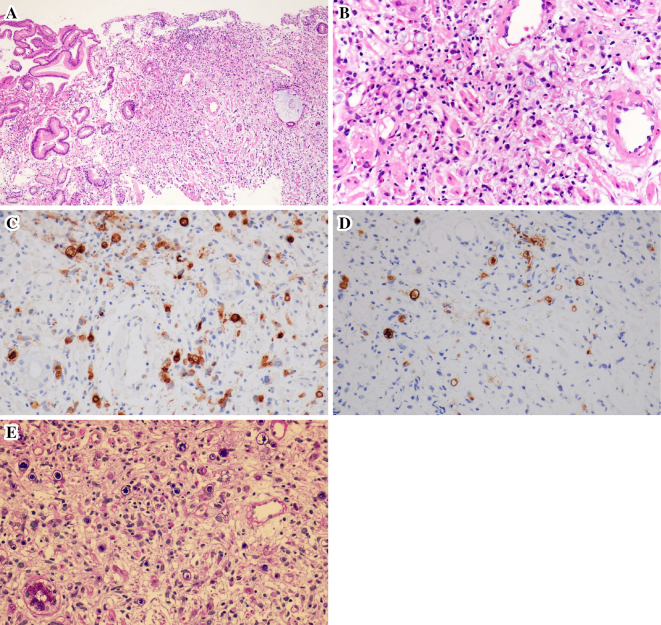
A: A biopsy of the gastric specimens revealed poorly differentiated adenocarcinoma with signet cell carcinoma. Low-power view (original magnification ×40). Proliferation of adenocarcinoma occupies the right side of the figure. B: The adenocarcinoma comprises signet ring cells and poorly differentiated cells with a high N/C ratio. About 10 eosinophils can be seen in the adenocarcinoma lesion in the high-power view. C: Immunohistochemistry for CEA showed positive staining in the tumor cells. D: Immunohistochemistry for CA19-9 showed positive staining in 10% of the tumor cells. E: PAS/alcian blue staining showed mucin in the signet ring cells.
Table 2.
Time Course of Laboratory Data after Chemotherapy.
| TS-1 | TS-1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cis | Cis | ||||||||||
| Day | 1 | 8 | 10 | 12 | 21 | 28 | 35 | 41 | 43 | 45 | |
| WBC | (μL) | 6,400 | 6,600 | 9,400 | 7,800 | 4,000 | 5,400 | 10,200 | 8,900 | ||
| eosinophil | (µL) | 1,376 | 1,518 | 94 | 0 | 172 | 507 | 0 | 0 | ||
| RBC | (μL) | 468×104 | 430×104 | 395×104 | 407×104 | 405×104 | 380×104 | 363×104 | 376×104 | ||
| Hb | (g/dL) | 14.9 | 13.9 | 12.7 | 13.2 | 13.4 | 12.9 | 12.3 | 12.7 | ||
| Ht | (%) | 45.7 | 41.7 | 38.2 | 39.4 | 40.4 | 38.3 | 36.5 | 36.8 | ||
| PLT | (μL) | 23.2×104 | 22.5×104 | 21.1×104 | 20.4×104 | 16.4×104 | 19.4×104 | 20×104 | 22.2×104 | ||
| CEA | (ng/dL) | 4.1 | 4.9 | ||||||||
| CA19-9 | (U/mL) | 45.3 | 24.7 | ||||||||
Discussion
We encountered a case of H. pylori-negative gastric cancer with eosinophilia. The incidence of H. pylori-negative gastric cancer is very low. It is reported to be 0.42-0.66% (1,2). Regarding the characteristics of Helicobacter negative-gastric cancer, the undifferentiated type is more frequent than the differentiated type (3). Repeated EGD and a re-biopsy showed poorly differentiated adenocarcinoma and signet ring cell carcinoma. Immunohistochemistry for CEA and CA19-9 showed positive staining in the tumor cells, and PAS/alcian blue staining showed mucin in the signet ring cells.
The mechanisms underlying H. pylori-negative gastric cancer are not fully understood, although it has been reported that the Epstein-Barr (EB) virus is associated with gastric cancer (5). In the current case, H. pylori culture, serum anti-H. pylori antibody, and the findings of a histological examination were all negative.
Peripheral eosinophilia is associated with a broad range of allergic, infectious, neoplastic, hematological, collagen and idiopathic diseases (4). Among these conditions, malignancy is a well-recognized albeit unusual cause of hypereosinophilia. In the current case, blood and feces examinations ruled out the possibility of allergic diseases, collagen disease, and parasitic infection. A relationship between gastric cancer and hypereosinophilia has been reported (6-8), although the mechanism by which gastric cancer induces hypereosinophilia is not clear. The major growth factors for eosinophils are IL-5, granulocyte-macrophage colony-stimulating factors, and IL-3 (4). Hong et al. suggested that gastric cancer cells produce IL-2, IL-5, and granulocyte-macrophage colony-stimulating factors (9). We detected no elevations in these cytokines in the serum; however, after the first course of chemotherapy, the eosinophilia was resolved, suggesting a relationship between the gastric cancer and eosinophilia.
No previous case studies have analyzed the relationship between H. pylori infection and eosinophilia. Indeed, some reports predate the discovery of H. pylori or recognition of the relationship between H. pylori and gastric cancer (6,7). Interestingly, H. pylori colonization is inversely correlated with asthma and allergy (10,11). Furthermore, H. pylori infection is reported to be associated with a reduced risk of developing eosinophilic esophagitis (12). Although the effects of H. pylori on eosinophilia are unclear, it is possible that, in the current case, the suppression of eosinophilia did not occur without H. pylori infection.
FDG-PET has recently been used in clinical oncology (10). The diagnosis of gastric cancer is made by endoscopy and biopsies. The sensitivity of FDG-PET for the detection locally advanced gastric carcinoma is dependent on the microscopic growth type of the tumor, as an increased uptake of FDG is more commonly seen in the intestinal growth type than non-intestinal types (13). Interestingly, all previously reported cases of gastric cancer with eosinophilia were poorly differentiated carcinoma. This finding suggests that, in the differential diagnosis of eosinophilia, the FDG-PET findings and the presence of negative H. pylori antibodies are considered to be insufficient diagnostic information to rule out gastric cancer.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Ono S, Kato M, Suzuki M, et al. Frequency of Helicobacter-negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion 86: 59-65, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter 16: 415-419, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto Y, Fujisaki J, Omae M, Hirasawa T, Igarashi M. Helicobacter pylori-negative gastric cancer: Characteristics and endoscopic findings. Dig Endosc 27: 551-561, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg ME. Eosinophilia. New Eng J Med 338: 1592-1600, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Chen XZ, Chen H, Castro FA, Hu JK, Brenner H. Epstein-Barr virus infection and gastric cancer. Medicine (Baltimore) 94: 1-9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsutsumi Y, Ohshita T, Yokoyama T. A case of gastric carcinoma with massive eosinophilia. Acta Oathol Jpn 34: 117-122, 1984. [DOI] [PubMed] [Google Scholar]
- 7.Takeda H, Nishikawa H, Tsumura T, Sekikawa A, Maruno T, Okabe Y. Prominent hypereosinophlia with disseminated intravascular coagulation as an unusual presentation of advanced gastric cancer. Intern Med 53: 563-569, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Satoh T, Kawamura M, Katoh H, et al. A case of superficial spreading type of gastric carcinoma associated with eosinophilia. Nihon Shokakibyo Gakkai Zasshi 88: 719-723, 1991. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 9.Hong SW, Cho MY, Park C. Expression of eosinophil chemotactic factors in stomach cancer. Yonsei Med J 40: 131-136, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Chen YC, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med 167: 821-827, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hussain K, Letley D, Greenaway AB, et al. Helicobacter pylori-mediated protection from allergy is associated with IL-10-secreting peripheral blood regulatory T cells. Front Immunol 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Arnim U, Wex T, Link A, et al. Helicobacter pylori infection is associated with a reduced risk of developing eosinophilic oesophagitis. Aliment Pharmacol Ther 43: 825-830, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Stahl A, Ott K, Weber WA, et al. FDG-PET imaging of locally advance gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 30: 288-295, 2003. [DOI] [PubMed] [Google Scholar]