Abstract
The patient was a 23-year-old man who was diagnosed with severe hypoxemia and liver dysfunction after suffering from sudden difficulty breathing. At 2 years of age, he had been diagnosed with hypopituitarism, and had received hormone-replacement until he was 18 years of age. Echocardiography using micro bubbles and pulmonary scintigraphy indicated intrapulmonary shunt and a liver biopsy showed steatohepatitis. He was diagnosed with hepatopulmonary syndrome associated with nonalcoholic steatohepatitis. Hormone-replacement therapy was re-started. After 5 months, a second liver biopsy revealed the amelioration of nonalcoholic steatohepatitis, which improved his respiratory condition. This case suggested that early effective therapy for chronic liver diseases might improve the pathological and clinical conditions of hepatopulmonary syndrome.
Keywords: nonalcoholic steatohepatitis, hepatopulmonary syndrome, hypopituitarism
Introduction
Hepatopulmonary syndrome (HPS) is defined as a defect in arterial oxygenation induced by the intrapulmonary vascular dilations associated with chronic liver disease (1). HPS was observed in 4-32% of the adult patients with end-stage liver disease (2). Previous studies have determined that HPS is an independent risk factor for long-term mortality in patients with liver cirrhosis (3). Orthotopic liver transplantation is the only successful treatment for patients with HPS. However, the postoperative mortality rate of patients with severe hypoxemia before transplantation is high (4).
Nonalcoholic steatohepatitis (NASH) is an emerging progressive hepatic disease. Patients with NASH show demonstrates steatosis, inflammation, and fibrosis; insulin resistance is a common feature in its development (5). Interestingly, NASH demonstrates a striking similarity to the pathologic conditions observed in hypopituitarism, especially adult growth hormone deficiency (AGHD) (6). Hypopituitarism is characterized by decreased lean body mass, increased visceral adiposity, an abnormal lipid profile, and insulin resistance. Moreover, liver dysfunction with hyperlipidemia and nonalcoholic fatty liver disease (NAFLD) is frequently observed in patients with AGHD, and it is often accompanied by metabolic syndrome (7). Takahashi et al. (8) reported that in a patient with AGHD-associated NASH, six months of growth-hormone-replacement therapy drastically ameliorated NASH and the abnormal lipid profile concomitant with a marked reduction in oxidative stress.
We experienced the case of a patient hypopituitarism-associated NASH combined with HPS. He was treated with growth hormone, testosterone, thyroid hormone and corticosteroid replacement. introduce the effects of hormone-replacement therapy as a treatment for NASH and HPS.
Case Report
A 23-year-old man entered our hospital with breathing difficulty. At 2 years of age, he had been diagnosed with functional pituitary hypopituitarism due to pituitary stalk rupture. He received growth hormone and male hormone-replacement therapy until he was 18 years of age (body weight, 55 kg at 18 years of age). At 23 years of age, he suffered sudden difficulty breathing when moving and a medical examination was performed to determine the etiology of this symptom.
The results of arterial blood gas analyses performed on admission were as follows (on room air): pH, 7.421; PaO2, 49.8 mmHg; PaCO2, 39.5 mmHg; and HCO3-, 25.1 mmol/L (Table 1). A physical examination revealed clear bilateral respiratory sounds, cyanotic lips, and clubbed fingers. He was 172 cm tall and weighed 60 kg; he had no history of pulmonary or hepatic disorders or alcohol intake. The medical examination revealed the absence of pubic hair and delayed secondary sexual development. His sexual and physical desire was decreased. His hepatic and renal profiles were as follows (Table 2): total bilirubin, 1.1 mg/dL; albumin, 3.5 g/dL; aspartate aminotransferase (AST), 78 U/L; alanine aminotransferase (ALT), 42 U/L; gamma-glutamyltranspeptidase (γ-GTP), 69 IU/L; prothrombin time (%), 52.6%; platelet count, 13.0×104/μL and creatinine, 0.53 mg/dL (Table 2). The hormonal data were as follows: testosterone, <5.0 ng/mL; adrenocorticotropic hormone (ACTH), <2.0 pg/mL; growth hormone (GH), 0.03 ng/mL; insulin-like growth factors-1 (IGF-1), <4.0 ng/mL. A growth hormone-releasing peptide 2 (GHRP-2) stimulating test showed a low GH response (before 0.008 ng/mL, peak 0.38 ng/mL) and a TRH (thyrotropin-releasing hormone) stimulating test showed a delayed response. We diagnosed the patient with severe adult GH deficiency based on his past history, physical features and the results of the hormone stimulating test.
Table 1.
Respiratory and Hormone Data.
| Arterial blood gas (room air) | Hormone data | ||||
| pH | 7.421 | TSH | 0.879 | μIU/mL | |
| PaCO2 | 39.5 | mmHg | Free T3 | 2.27 | pg/mL |
| PaO2 | 49.8 | mmHg | Free T4 | 1.51 | ng/dL |
| BE | 0.7 | mmol/L | Prolactin | 7.0 | ng/mL |
| HCO3- | 25.1 | mmol/L | LH | <0.2 | mIU/mL |
| Respiratory Examination | FSH | 0.3 | mIU/mL | ||
| FVC | 3.90 | L | Testosterone | <5.0 | ng/mL |
| %VC | 78.2 | % | ACTH | <2.0 | pg/mL |
| FEV1.0 | 3.49 | L | Cortisol | 24.5 | μg/dL |
| FEV1.0% | 99.6 | % | GH | 0.03 | ng/mL |
| %DLCO | 26.3 | % | IGF-1 | <4 | ng/mL |
BE: base excess, FVC: forced vital capacity, VC: vital capacity, FEV: forced expiratory volume, DLCO: diffusing capacity of the lung for carbon monoxide, TSH: thyroid stimulating hormone, LH: luteinizing hormone, FSH: follicle stimulating hormone, ACTH: adrenocorticotropic hormone, GH: growth hormone, IGF-1: insulin-like growth factors-1
Table 2.
Laboratory Data.
| WBC | 4.85×103 | /μL | Cr | 0.53 | mg/dL | HBsAg | (-) | |
| Hb | 11.5 | g/dL | Na | 143 | mEq/L | HBcAb | (-) | |
| Plt | 13.0×104 | /μL | K | 3.7 | mEq/L | HCVAb | (-) | |
| TP | 6.9 | g/dL | Glu | 82 | mg/dL | CMVIgM | (-) | |
| Alb | 3.5 | g/dL | HbA1c | 5.4 | % | EBV-VCAIgG | 20 | |
| T-Bil | 1.1 | mg/dL | CRP | 0.26 | mg/dL | EBV-VCAIgM | (-) | |
| AST | 78 | U/L | EBNA | 40 | ||||
| ALT | 42 | U/L | IgG | 1,429 | mg/dL | |||
| LDH | 222 | U/L | IgA | 239 | mg/dL | PT | 52.6 | % |
| γGTP | 69 | U/L | IgM | 89 | mg/dL | FDP | 1.5> | mg/mL |
| TCHO | 153 | mg/dL | ANA | ×320 | ||||
| TG | 72 | mg/dL | AMA | (-) |
WBC: white blood cell, Hb: hemoglobin, Plt: platelet, TP: total protein, Alb: albumin, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactase dehydrogenase, ALP: alkaline phosphatase, γ-GTP: gamma-glutamyltranspeptidase, TCHO: total cholesterol, TG: triglyceride, Cr: creatinine, Glu: glucose, HbA1c: hemoglobin A1c, CRP: C-reactive protein, ANA: antinuclear antibody, AMA: anti-mitochondrial antibodies, HBsAg: hepatitis B surface antigen, HBcAb: hepatitis B core antibody, HCVAb: anti-hepatitis C antibody, CMVIgM: cytomegalovirus IgM, EBV-VCA: Epstein-Barr virus-viral capsid antigen, EBNA: EBV nuclear antigen, PT: prothrombin time, FDP: fibrin degradation products
Chest X-ray and computed tomography (CT) showed clear lung fields (Fig. 1). A pulmonary function test revealed that the patient's percent vital capacity (%VC) and forced expiratory volume in 1 second (FEV1.0%) were 78.2% and 99.6%, respectively (Table 1). The percentage diffusing capacity of the lung for carbon monoxide (%DLCO) was decreased to 26.3%. This finding suggested a decrease in the extent to which oxygen passed from the air sacs of the lungs into the blood. There was no evidence of an intracardiac shunt on echocardiography; left ventricle (LV) contraction, normal, ejection fraction (EF) 52%, right ventricle systolic pressure (RVSP) 29.4 mmHg. When intravenous micro bubbles were created by the agitation of saline solution, they appeared on the right side of the heart first, then after a few seconds they appeared on the left side of the heart, suggesting right-to-left side intrapulmonary shunting. 99mTc macroaggregated albumin (99mTc MAA) pulmonary scintigraphy showed the intrapulmonary shunt (shunt ratio, 45.5%) (Table 3).
Figure 1.
Chest X ray and CT. No abnormalities were found in the lung field or heart.
Table 3.
99mTc-MAA Perfusion Lung Scan, O2 Saturation and FIB4 Index.
| 99mTc-MAA perfusion lung scan | Shunt ratio 45.5% | Shunt ratio 21.9% | Shunt ratio 6.0% |
|---|---|---|---|
| O2 saturation | 74% (93.9% at O2 6 L) | 84% (93.0% at O2 3 L) | 98% (room air) |
| Fibrosis 4 index (FIB4 index) | 2.13 | 1.06 | 0.76 |
| Date | XXX2/Jun. | XXX2/Nov. | XXX3/Jun. |
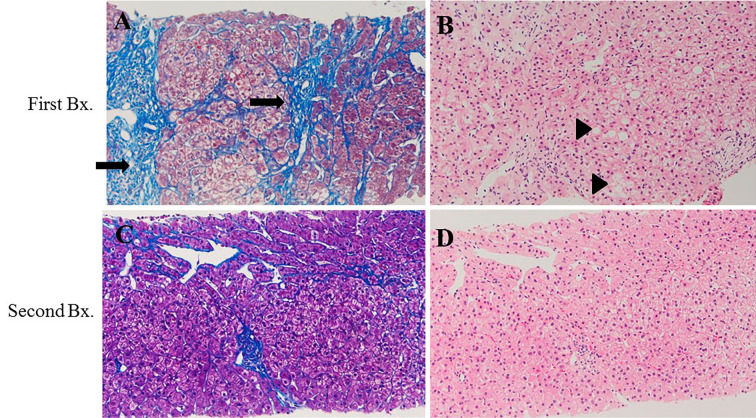
Splenomegaly and atrophy of the left lobe were observed on an abdominal CT scan (Fig. 2). His visceral fat area was 60 cm2 and his subcutaneous fat area was 154 cm2. Liver biopsy indicated stage 3-4 steatohepatitis; grade 1 according to Brunt's classification (Fig. 3A and B). Based on these data, the patient was diagnosed with NASH associated with hypopituitarism combined with HPS.
Figure 2.
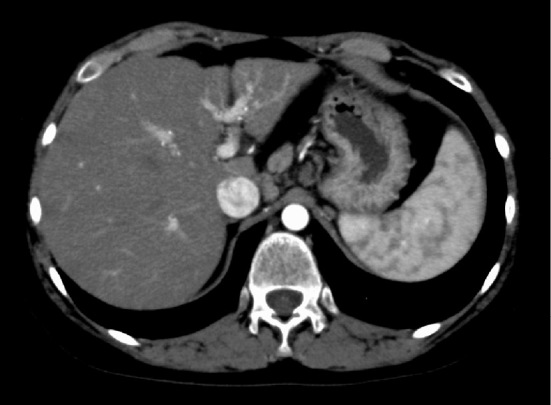
Abdominal CT. Splenomegaly and atrophy of the left lobe were observed.
Figure 3.
Liver biopsy. First biopsy (A) (B) showed steatohepatitis, which was classified as stage 3-4; grade 1 according to Brunt’s classification. The arrow indicates expanded fibrosis. The triangle indicates steatosis. After 6 months, a second biopsy was performed (C) (D). The fibrosis stage was found to have decreased to stage 2-3, and the steatosis and ballooning hepatocytes were diminished. (A) and (C), Mallory Azan staining. (B) and (D), Hematoxylin and Eosin staining.
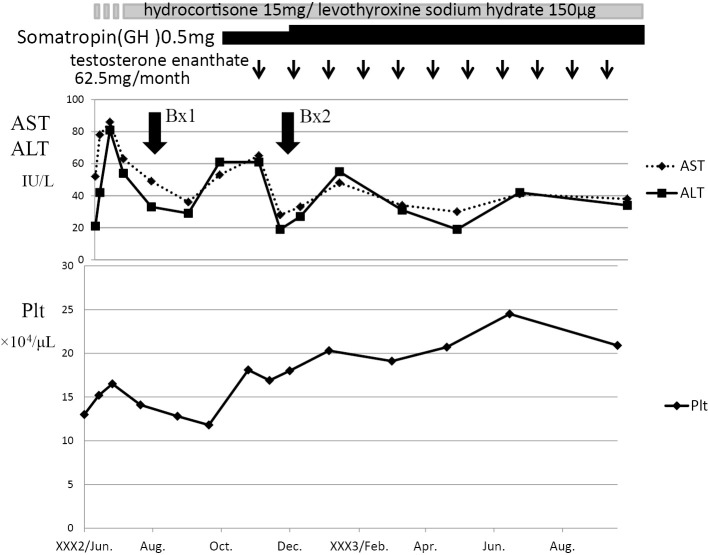
Growth hormone (somatropin, 5 mg), testosterone (testosterone enanthate, 62.5 mg/month), thyroid hormone (levothyroxine Sodium Hydrate, 100 μg/day) and corticosteroid (hydrocortisone, 15 mg/day) replacement therapy were re-started (Fig. 4). The serum AST and ALT levels gradually decreased, and the platelet count increased to 16-20×104/μL (Fig. 4). A second liver biopsy performed at 5 months after re-starting hormone therapy showed the amelioration of NASH (stage 2-3). No steatosis or ballooning hepatocytes were observed (Fig. 3C and D). The fibrosis 4 index (FIB4 index) decreased from 2.1 to 0.76. The intrapulmonary shunt ratio was also decreased from 45.5% to 6.0%, and the O2 saturation was markedly improved to 98% on room air (Table 3). He became able to move without oxygen. His body weight decreased -2 kg after therapy. Before therapy he had left his job and hated being physically active; however, physical activity became much easier and he returned to his desk job.
Figure 4.
The clinical course. Growth hormone, testosterone, thyroid hormone and corticosteroid replacement therapy were re-started. The serum AST and ALT levels gradually decreased, and the platelet count increased to 16-20×104/μL.
Discussion
HPS is a pulmonary gas exchange disorder complicated by advanced chronic liver diseases, and characterized by severe hypoxemia and pulmonary vascular dilatation. The hypoxemia is caused by intrapulmonary vascular dilatation, which increases the alveolar-arterial pressure gradient for oxygen (1,2). The diagnostic criteria for HPS are as follows: chronic liver disease with or without portal hypertension; an alveolar-arterial difference in the partial pressure of oxygen of 20 mmHg and/or a partial pressure of oxygen in arterial blood (PaO2 ) of <70 mmHg; intrapulmonary vascular dilatation; and no primary cardiac or lung disease. Our patient met these criteria, confirming a diagnosis of HPS. There is no effective therapy for HPS other than liver transplantation (4). However, liver transplantation for HPS is associated with a high degree of risk. In addition, a donor and immunosuppressants are needed. In our case, we considered liver transplantation but selected hormone-replacement therapy and expected the patient's liver dysfunction and respiratory condition to improve. His steatohepatitis was not decompensated liver cirrhosis, which suggested the possibility of improvement with hormone therapy.
In our case, hormone-replacement therapy improved the FIB-4 index and liver histological change, including the fibrosis grade and the number of ballooning hepatocytes. Takahashi et al. (8) also reported the amelioration of NASH-associated histological changes. In addition, the improvement of hepatic fibrosis led to the amelioration of the intrapulmonary shunt ratio and O2 saturation, as shown in Table 3. It was reported that pulmonary vascular remodeling induced by circulating mediators, which could not be removed from circulation due to liver cirrhosis, plays an important role in the pathogenesis of HPS (9). The amelioration of the liver condition might remove vasoactive mediators such as tumor necrosis factor-alpha (TNF-α), heme oxygenase-derived carbon monoxide and nitric oxide (10-12). The present case demonstrated that the early diagnosis of HPS and effective therapy for chronic liver disease might improve the pathological and clinical conditions of HPS.
Recently, two cases of HPS were reported in Japan. The first case was a 65-year-old woman who was diagnosed with NASH combined with HPS. Body weight loss and home oxygen therapy improved her FIB4 index, which was linked to the improvement of the HPS-associated respiratory condition (13). The other case was a 69-year-old man who was diagnosed with alcoholic liver cirrhosis with HPS. Discontinuing the patient's alcohol intake and O2 admission improved the liver failure, which led to the amelioration of the patient's respiratory condition, including his O2 saturation (14). These two cases also indicated the possibility that effective therapy for chronic liver disease may improve HPS. However in these of cases, the histological changes were not confirmed by repeated liver biopsy and intrapulmonary shunt was not confirmed by 99Tc MCAA. In our case, we could confirmed the improvement of the liver histology and intrapulmonary shunt by performing these important examinations.
Recently, direct antiviral agents (DAA) were found to cure hepatitis C virus (HCV) and even liver cirrhosis (15). If HPS combined with liver cirrhosis due to HCV can be diagnosed at an early stage, DAA might improve not only liver function but also HPS.
Several papers have reported that HPS is not associated with the severity of liver cirrhosis as reflected by the Child-Pugh score or the model for end-stage liver disease (MELD) scoring system (1,3). Thus, we should consider the possibility of HPS, even in patients with compensated cirrhosis. In this case, not only liver dysfunction but also a hormone imbalance might have influenced the occurrence of HPS.
In conclusion, it is important to diagnose HPS at an early stage. If liver cirrhosis combined with HPS is diagnosed, effective treatments for chronic liver diseases should be administered before liver transplantation is considered.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Rodriguez-Roisin R, Krowka MJ, Herve P, Fallon MB. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 24: 861-880, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Schenk P, Fuhrmann V, Madl C, et al. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut 51: 853-859, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology 41: 1122-1129, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Egawa H, Kasahara M, Inomata Y, et al. Long-term outcome of living related liver transplantation for patients with intrapulmonary shunting and strategy for complications. Transplantation 67: 712-717, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis; summary of an AASLD single topic conference. Hepatology 37: 1202-1219, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology 39: 909-914, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J 82: 315-322, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi Y, Iida K, Takahashi K, et al. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology 132: 938-943, 2007. [DOI] [PubMed] [Google Scholar]
- 9.He J, Yi B, Chen Y, et al. The ET-1-mediated carbonylation and degradation of ANXA1 induce inflammatory phenotype and proliferation of pulmonary artery smooth muscle cells in HPS. PLoS One 12: e0175443, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunes H, Lebrec D, Mazmanian M. Role of nitric oxide in hepatopulmonary syndrome in cirrhotic rats. Am J Respir Crit Care Med 164: 879-885, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Carter EP, Hartsfield CL, Miyazono M, Jakkula M, Morris KG, McMurtry IF. Regulation of heme oxygenase-1 by nitric oxide during hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol 283: L346-L353, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Strassburg CP. Gastrointestinal disorders of the critically ill. Shock liver. Best Pract Res Clin Gastroenterol 17: 369-381, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Miyata H, Miyata S. A case of hepatopulmonary syndrome derived from nonalcoholic fatty liver disease with severe fibrosis, in which hypoxia could be recovered by improvement of liver fibrosis. Kanzo 55: 479-487, 2014. (in Japanese, Abstract in English). [Google Scholar]
- 14.Mastumoto S, Takizawa N, Kodama N, Matsubayashi S. Alcoholic cirrhosis associated with hepatopulmonary syndrome: abstinence from alcohol and long term oxygen therapy improved the hepatic function and ameliorated hypoxia. Kanzo 55: 235-239, 2014. (in Japanese, Abstract in English). [Google Scholar]
- 15.Chang CY, Nguyen P, Le A, et al. Real-world experience with interferon-free, direct acting antiviral therapies in Asian Americans with chronic hepatitis C and advanced liver disease. Medicine (Baltimore) 96: e6128, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]