Abstract
Invasive fungal infections in immunosuppressed transplant patients are associated with significant morbidity and mortality. We present a case of splenic mucormycosis post-double lung transplant, presenting as uncontrolled near-fatal upper gastrointestinal haemorrhage, to remind clinicians of the need to consider pre-transplant invasive fungal infection risk factors if an unexpected fungal infection arises in the post-transplant period. This case also highlights the valuable contribution of molecular technology for fungal identification but also the need for clinical correlation.
Keywords: Gastrointestinal mucormycosis, Splenic mucormycosis, Lung transplantation, Haemorrhage
1. Introduction
Worldwide, lung transplantation programmes continue to report increasing activity leading to an ever-growing population of patients on lifelong maintenance immunosuppressive therapy, with the associated risks of invasive fungal infections (IFI) [1]. Post-transplant IFI are associated with a high morbidity and mortality [2]. Historically, infections due to Candida spp. and Aspergillus spp. predominated in lung transplant recipients, but there is increasing recognition and reporting of IFI caused by a wider spectrum of fungal pathogens, generally considered innocuous in the non-immunosuppressed population, including members of the order Mucorales, Fusarium and Scedosporium [3].
Mucormycosis is a clinical syndrome cause by a number of mould species belonging to the order Mucorales (class Glomeromycetes; previously known as Zygomycetes), mainly by species of the genera Rhizopus, Mucor, Cunninghamella and Absidia [4]. They are ubiquitous saprophytic fungi present in soil and decaying organic matter such as bread, hay, vegetation and animal faeces [4]. Opportunist moulds associated with mucormycosis typically invade the deep tissues via inhalation of airborne spores, percutaneous inoculation of spores or ingestion of spores with resultant rhino-sino-orbito/cerebral or pulmonary, cutaneous, and upper/lower gastrointestinal (GI) infections respectively. Fungal spores subsequently germinate to produce characteristic hyphae, which invade blood vessels with progression to tissue infarction and vascular invasion. Vessel invasion readily facilitates the rapid dissemination of the invading fungus to other organs [4]. A high index of clinical suspicion is required for diagnosis of mucormycosis as clinical signs and imaging findings can be subtle or non-specific leading to misdiagnosis [3].
The incidence of mucormycosis in the non-immunocompromised population is almost 15 times lower than those of invasive Candida or Aspergillus infections, with 1.7 cases per million per year (500 cases average) in the United States [5], [6]. Mucormycosis is estimated to account for only 2% of all fungal infections in solid organ transplant (SOT) recipients with an overall incidence rate of 1–2% at 12-months post-lung transplant [7].
We present a case of splenic mucormycosis at day + 19 post-double lung transplant presenting as a near-fatal massive uncontrolled massive upper gastrointestinal haemorrhage. The case highlights that pre-existing pre-transplant risk factors for IFI are as important as risk factors acquired post-transplant for IFI, discusses the inherent difficulties in making a diagnosis of mucormycosis using both traditional and newer molecular methods and illustrates how multi-disciplinary management with surgical debridement, systemic anti-fungal therapy and prolonged intensive rehabilitation all contributed to our patient's survival.
2. Case
A 46 year old woman underwent a double lung transplant (CMV donor positive/recipient positive) in April 2015 (day 0) for idiopathic pulmonary fibrosis. She had a background history of rheumatoid arthritis (RA). Prior to transplant the patient had no history of diabetes mellitus, gastritis, gastro-oesophageal reflux disease (GORD) or peptic ulcer disease. She was known to have a penicillin allergy. Culture of intra-operative transplant specimens yielded methicillin-sensitive Staphylococcus aureus (MSSA) only. Histology of the explanted lungs did not demonstrate fungal infection. Post-operatively she was commenced on standard antimicrobial and immunosuppressive therapy and discharged home on day + 17.
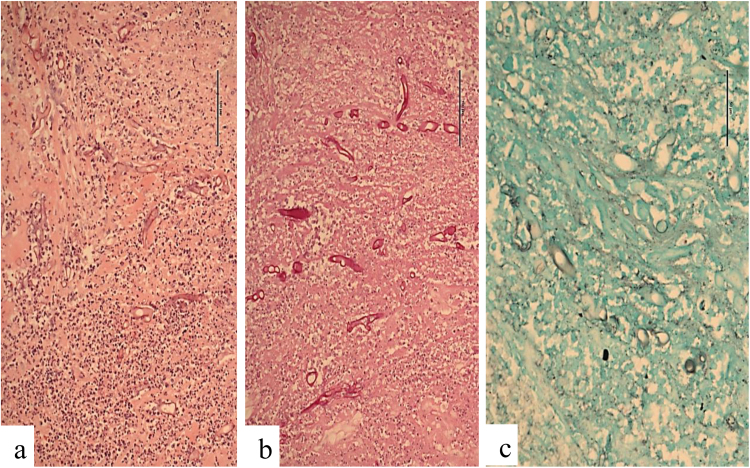
On day + 19 post-transplant, she presented to the emergency department in hypovolaemic shock secondary to massive haematemesis and malaena. She underwent an emergency exploratory laparotomy. A bleeding gastric ulcer eroding into the splenic hilum involving the splenic artery was identified, necessitating gastrostomy, duodenostomy and splenectomy. Intra-operative specimens were culture-negative. Histological review identified a splenic abscess with a fistula of splenic vessels into the gastric fundus. Periodic acid Schiff (PAS) staining subsequently confirmed large broad irregular shaped fungal forms morphologically most consistent with mucormycosis (Fig. 1a, b), with weak staining for Grocott's methenamine silver stain (Fig. 1c). Antifungal therapy with intravenous liposomal amphotericin B and posaconazole was commenced, with doses discussed in consultation with a transplant pharmacist in view of the patient's complex regimen of immunosuppressive medications. Therapy was subsequently switched to posaconazole monotherapy secondary to renal impairment and nephrogenic diabetes insipidus attributed to amphotericin B.
Fig. 1.
Periodic acid Schiff (PAS) staining demonstrating large broad irregular shaped fungal forms morphologically most consistent with mucormycosis (a, b), with weak staining for Grocott's methenamine silver stain (c).
On day + 25 post-transplant, the patient returned to theatre for a subtotal colectomy due to the development of an acute abdomen. Multiple colonic specimens sent for histological examination demonstrated extensive serosal adhesion and serosal abscess formation, similar to that seen in earlier splenic hilum histological specimens, but no fungal forms were seen on multiple stains. Peritoneal fluid samples were sent for additional polymerase chain reaction (PCR) testing as all specimens were culture negative. Of 13 peritoneal samples tested, three were positive on a specific Aspergillus PCR test and three were positive on a PCR panfungal testing panel but no further fungal species identification was possible.
On day + 27 post-transplant, she went back to theatre for an emergency distal gastrectomy for management of a duodenal leak at the previous duodenectomy area. Fluid cultured Staph. epidermidis and vancomycin-resistant Enterococcus faecium (VRE). Histological examination, including PAS and Grocott stains for evidence of fungal forms, were negative. Serosal abscess formation was again noted in duodenal specimens, similar to that in the splenic hilum, with negative fungal staining. On day + 32 post-transplant the patient returned to theatre for the fourth time for repair of a bile leak with culture-negative intra-operative specimens. No fungal forms were seen on PAS or Grocott staining. A weak positive for Aspergillus spp. was identified from a gallbladder tissue specimen using 18S PCR. A sample of left pleural fluid underwent bacterial and fungal culture, 16S and 18S PCR testing plus histological analysis, all of which were negative. Serum beta-D-glucan and galactomannan were also negative.
On day + 60 she underwent a Roux-en-Y intestine-biliary bypass. PAS staining for fungi was negative on all gastric specimens received. Drain fluid samples sent for 18S PCR were negative. No fungal forms were noted on histology. On day + 130 post lung transplant an elective biopsy was performed of the transplanted right lung. All fungal stains were negative.
To date the patient is well and continues on prophylactic posaconazole for an indefinite duration.
3. Discussion
GI mucormycosis accounts for 7% of all mucormycosis cases, with 75% of cases identified at post mortem, indicative of the high mortality and poor prognosis [8]. The stomach is the most common site of GI infection (67%), followed by colon (21%), small intestine (4%) and oesophagus (2%). Necrotising enterocolitis has been described in neutropenic adults [8]. GI mucormycosis can present with either non-specific symptoms of abdominal pain and distension, massive GI haemorrhage due to local angioinvasion or gastric ulceration or rarely peritonitis due to bowel perforation [8]. In presentations with GI symptoms that are nonspecific and associated with pyrexia, a misdiagnosis of an intra-abdominal abscess can erroneously occur.
Reports of splenic mucormycosis, as in our case, are rare in the literature and are predominantly associated to patients with background immunosuppressive states. The death of a 64-year old man with multiple myeloma, who presented with rectal haemorrhage, was reported in 1999, in whom necrotic 4 cm splenic abscesses and colonic nodule with histologic evidence mucormycosis were identified at autopsy [9]. In 2001, a case of disseminated mucormycosis in a 16-year old patient with acute lymphoblastic leukaemia resulting in a splenic psuedoaneurysm was reported, that was successfully treated via splenectomy and therapy with amphotericin B [10]. In 2009 the post-mortem diagnosis of gastric and splenic mucormycosis was made in a middle-aged HIV-positive male who presented with an ileosigmoid knot [11]. A case of splenic and renal mycormycosis in a healthy host with no risk factors was published in 2010; the patient survived and was aggressively treated with radical debridement and amphotericin B [12]. Our case differs from this case in that despite histological evidence of mucor-associated splenic abscess formation, the splenic parenchyma, omentum and pancreatic specimens did not demonstrate evidence of disseminated mucormycosis. A fatal case of invasive gastric and splenic mucormycosis has been described in Saudi Arabia that presented as a massive GI haemorrhage secondary to gastric ulcer perforation, hypothesised, that contiguous spread had occurred via vascular invasion from the stomach to the spleen resulting in widespread splenic infarction [13]. This is also the most likely route of spread in our case with a bleeding gastric ulcer eroding into the splenic artery resulting in massive gastrointestinal haemorrhage despite no histological confirmation of mucor-associated gastric invasion.
Risk factors associated with mucormycosis have been well described and include, but are not limited to, extremes of age, diabetic ketoacidosis, prolonged neutropenia, corticosteroid therapy, immunosuppression post-SOT and haematological malignancies [14]. As part of a maintenance treatment regimen for management of RA in the pre-transplant period, our patient had received six months of maintenance oral prednisolone and mycophenolate mofetil. Corticosteroid therapy is immunomodulatory and impairs the function of macrophages and neutrophils resulting in reduced phagocytic function, thereby increasing the susceptibility of corticosteroid-treated patients to mucormycosis [14]. The patient also worked as an interior designer and as part of her work spent time on construction sites, with potential exposure to spores in decaying organic matter and vegetation. Ingestion of mucor species spores in foods and beverages such as “home-brewed” beer fermented milk, dried bread products, fermented porridge have been reported as possible sources, but none were applicable to our patient [15]. An outbreak of GI mucormycosis, due to contaminated wooden tongue depressors used to mix nasogastric feeds, in a Spanish critical care unit in 2004 has been widely cited, but these tongue depressors were not in use in our critical care unit [16]. Given the short time period between double lung transplant and the presentation with gastrointestinal haemorrhage and the absence of alternative causative risk factors, it is conceivable that environmental occupational acquisition in the pre-transplant period was most likely.
The diagnosis of mucormycosis in the microbiology laboratory remains challenging, as demonstrated in this case, with culture negative and histologically negative samples despite invasive life-threatening disease. Negative cultures does not out rule infection if there is a high index of clinical suspicion [16]. The most definitive histopathological diagnosis is made by observing non-septate or minimally septate broad ribbon-like hyphae (10–20 μm) branching at right angles and invading blood vessels in tissue specimens [17]. Panfungal PCR testing, such as 18S PCR, has been shown to identify a wide spectrum of fungi and has a useful role as an adjunct to culture and histopathology, but false positive results can occur secondary to degradation of DNA, the presence of PCR inhibitors or environmental contamination of specimens and/or PCR master mixture by fungal spores in the laboratory [18]. The identification of Aspergillus spp. from theatre specimens was unexpected. A clinical, microbiological and radiological evaluation for evidence of invasive aspergillosis (IA) was performed with negative results as concomitant clinical cases of invasive mucormycosis and IA had been previously reported [19]. The Infectious Diseases Society of America (IDSA) 2016 guidelines for the diagnosis and management of Aspergillosis recommends that for a diagnosis of IA to be established tissue and fluid specimens should be sent in adequate quantities for simultaneous histopathological/cytologic and culture examination. There is no consensus in the guidelines regarding the utility of PCR in the diagnosis of IA due to the lack of conclusive validation of commercially available assays and the IDSA recommends that PCR results be “considered in conjunction with other diagnostic tests and the clinical context” [20]. Following extensive multi-disciplinary conferences regarding this case, consensus in our centre was that the positive Aspergillus spp. PCR results were false positives.
To the best of our knowledge this is the first reported case of splenic mucormycosis post-double lung transplant with a successful outcome. With regard to the management of this case of mucormycosis in the critical post-transplant period, multidisciplinary care with rapid surgical debridement of necrotic tissue, prompt commencement of antifungal therapy, necessitating close consultation with our transplant pharmacist to ensure no interactions with multiple immunosuppressive medications necessary to prevent organ rejection, and intensive rehabilitation involving physiotherapists, clinical psychologists, nursing staff, dieticians and occupational therapists all contributed to our patient's survival. The psychological consequences of a life-threatening IFI for transplant recipients in the immediate post-transplant period cannot be overstated. This case emphasises the value of histological analysis of theatre specimens and the need to consider carefully in the clinical context results generated from molecular technologies that are unexpected or unexplained.
Acknowledgements
None to declare.
Acknowledgments
Conflict of interest
None to declare.
Funding
This research did not receive any funding.
Ethical approval
No ethical approval was required for this case report. Written informed consent was obtained from the patient for the publication of this case report. A copy of the written consent is available for review.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.mmcr.2018.03.009.
Appendix A. Supplementary material
Supplementary material
References
- 1.Fuller J., Fisher A.J. An update on lung transplantation. Breathe. 2013;9(3):188–200. [Google Scholar]
- 2.Singh N. Fungal infections in the recipients of solid organ transplantation. Infect. Dis. Clin. N. Am. 2003;17(1):113–134. doi: 10.1016/s0891-5520(02)00067-3. (viii. Review) [DOI] [PubMed] [Google Scholar]
- 3.Huprikar S., Shoham S., AST Infectious Diseases Community of Practice Emerging fungal infections in solid organ transplantation. Am. J. Transplant. 2013;13(Suppl. 4):S262–S271. doi: 10.1111/ajt.12118. [DOI] [PubMed] [Google Scholar]
- 4.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012;54(Suppl. 1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 5.Almyroudis N.G., Sutton D.A., Linden P., Rinaldi M.G., Fung J., Kusne S. Zygomycosis in solid organ transplant recipients in a tertiary transplant center and review of the literature. Am. J. Transplant. 2006;6(10):2365–2374. doi: 10.1111/j.1600-6143.2006.01496.x. PMID:16925570. [DOI] [PubMed] [Google Scholar]
- 6.Antachopoulos C., Gea-Banacloche T.C., Walsh T.J. Zygomycosis (mucormycosis) In: Hospental D.R., Rinaldi M.D., editors. Diagnosis and Treatment of Human Mycoses. Springer; New York, NY: 2008. pp. 227–243. [Google Scholar]
- 7.Pappas P.G., Alexander B.D., Andes D.R., Hadley S., Kauffman C.A., Freifeld A. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin. Infect. Dis. 2010;50(8):1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 8.Spellberg B., Edwards J., Jr., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastor E., Esperanza R., Grau E. Splenic mucormycosis. Haematologica. 1999;84(4):375. [PubMed] [Google Scholar]
- 10.Nevitt P.C., Das Narla L., Hingsbergen E.A. Mucormycosis resulting in a pseudoaneurysm in the spleen. Pediatr. Radiol. 2001;31(2):115–116. doi: 10.1007/s002470000383. [DOI] [PubMed] [Google Scholar]
- 11.Islam J., Thomson S.R., Tudor G., Khan Z., Mjoli M. Ileosigmoid knot complicated by gastric and splenic mucormycosis: a lethal combination. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.10.2008.1049. (pii: bcr10.2008.1049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta V., Singh S.K., Kakkar N., Jain S., Kalra N., Sakia U.N. Splenic and renal mucormycosis in a healthy host: successful management by aggressive treatment. Trop. Gastroenterol. 2010;31(1):57–58. [PubMed] [Google Scholar]
- 13.Enani M.A., Alharthi B.N., Dewanjee N., Bhat N.A., Fagih M. Spontaneous gastric ulcer perforation and acute spleen infarction caused by invasive gastric and splenic mucormycosis. J. Glob. Infect. Dis. 2014;6(3):122–124. doi: 10.4103/0974-777X.138509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kontoyiannis D.P., Lewis R.E. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect. Dis. Clin. N. Am. 2006;20(3):581–607. doi: 10.1016/j.idc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Martinello M., Nelson A., Bignold L., Shaw D. “We are what we eat!” Invasive intestinal mucormycosis: a case report and review of the literature. Med. Mycol. Case Rep. 2012;1(1):52–55. doi: 10.1016/j.mmcr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maraví-Poma E., Rodríguez-Tudela J.L., de Jalón J.G. Outbreak of gastric mucormycosis associated with the use of wooden tongue depressors in critically ill patients. Intensive Care Med. 2004;30(4):724–728. doi: 10.1007/s00134-003-2132-1. [DOI] [PubMed] [Google Scholar]
- 17.Ledgard J.P., van Hal S., Greenwood J.E. Primary cutaneous zygomycosis in a burns patient: a review. J. Burn Care Res. 2008;29(2):286–290. doi: 10.1097/BCR.0b013e31816673b1. [DOI] [PubMed] [Google Scholar]
- 18.Sugawara Y., Nakase K., Nakamura A. Clinical utility of a panfungal polymerase chain reaction assay for invasive fungal diseases in patients with haematologic disorders. Eur. J. Haematol. 2013;90(4):331–339. doi: 10.1111/ejh.12078. [DOI] [PubMed] [Google Scholar]
- 19.Gowami S., Vohra R., Raju B.M., Agarwal A. Concomitant mucromycosis and aspergillosis of rhinocerebral region in a renal transplant patient – air cooler being the culprit. Indian J. Case Rep. 2016;5(1):30–34. [Google Scholar]
- 20.Patterson T.F., Thompson G.R., 3rd, Denning D.W. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material