Abstract
Experiments have demonstrated that in mice, the PVT strongly projects to the CeL and participates in the formation of fear memories by synaptic potentiation in the amygdala. Herein, we propose a mathematical model based on a positive feedback loop of BDNF expression and signaling to investigate PVT manipulation of synaptic potentiation. The model is validated by comparisons with experimental observations. We find that a high postsynaptic firing frequency after stimulation is induced by presynaptic when the rates of BDNF secretion from PVT and LA neurons to the CeL are above a threshold value. Moreover, the positive feedback of postsynaptic BDNF production is important for the maintenance of the high excitability of the CeL neuron after stimulation. The model brings insight into the underlying mechanisms of PVT modulation of synaptic potentiation at LA-CeL synapses and provides a framework of understanding other similar processes associated with synaptic plasticity.
Keywords: Fear conditioning, Synaptic potentiation, Positive feedback, Presynaptic , Firing frequency
Introduction
Animal anxiety disorders, dependent on fear learning, are associated with clear modifications of neural network activities (Duvarci and Pare 2014; Shin and Liberzon 2010; Graham and Milad 2011; Mahan and Ressler 2012). Pavlovian conditioning, consisting of the combination of a neutral conditioning stimulus (CS, for example, a tone) with a noxious unconditioning stimulus (US, for example, a foot shock), is often used to experimentally elicit fear responses in animals (Duvarci and Pare 2014; Pape and Pare 2010; Janak and Tye 2015). Fear conditioning can induce widespread synaptic plasticity in many brain regions, including the thalamus, auditory cortex and lateral amygdala (LA) (Pape and Pare 2010; Armony et al. 1998; Sigurdsson et al. 2007; Letzkus et al. 2011; Cho et al. 2011). It is a fundamental issue in neuroscience to uncover the underlying mechanisms of synaptic plasticity.
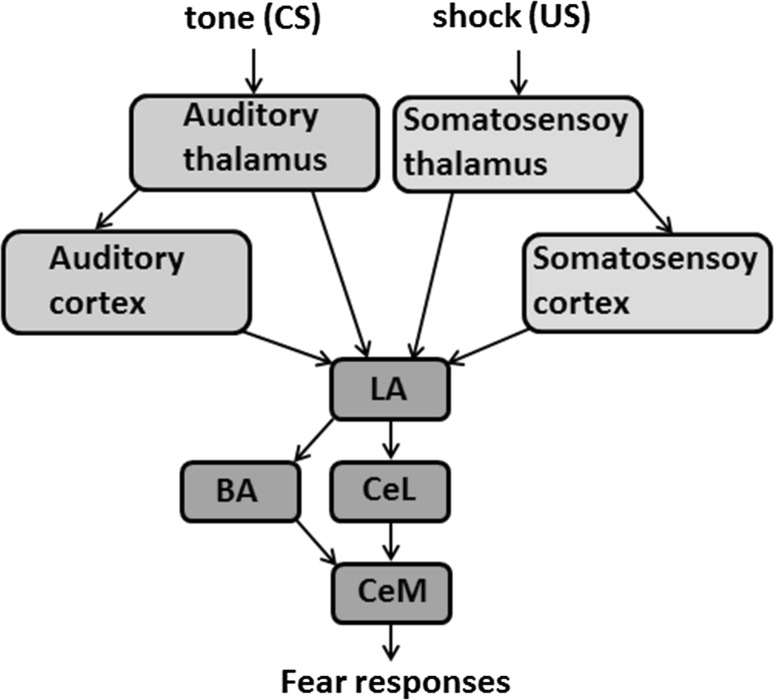
The amygdala is a critical component of the neural circuitry essential for fear learning, including the acquisition, storage, and expression of fear memories (Janak and Tye 2015; LeDoux 2000, 2003; Roozendaal et al. 2009; Tye et al. 2011; Tovote et al. 2015). The LA receives thalamic and cortical inputs that are involved in the process of fear conditioning; the input signals are conveyed to the basal nucleus of the amygdala (BA) and the lateral division of the central amygdala (CeL), which relay the signals to the medial subdivision of the central amygdala (CeM) (see Fig. 1) (Duvarci and Pare 2014; Janak and Tye 2015; Johansen et al. 2011). After fear conditioning, a population of CeL neurons acquires excitatory responses ( neurons), while other neurons display strong inhibitory responses ( neurons) to CS (Ciocchi et al. 2010; Haubensak et al. 2010; Duvarci et al. 2011). These two subtypes of CeL neurons inhibit each other, and the inhibitory microcircuit in the CeL regulates the CeM output to control the level of conditioned freezing (Ciocchi et al. 2010; Haubensak et al. 2010).
Fig. 1.
Schematic of the neural pathways involved in Pavlovian conditioning (see the text for details)
LA-CeL synaptic plasticity is essential in fear learning. Experiments have demonstrated that fear conditioning in mice can induce robust synaptic potentiation at the LA-CeL synapses, and changes in presynaptic release can partly account for the fear conditioning-induced synaptic plasticity in the CeL (Li et al. 2013). At the molecular level, synaptic plasticity involves the activities of postsynaptic excitatory receptors, e.g., N-methyl-d-aspartate (NMDA), and -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors (Soderling and Derkach 2000; Song and Huganir 2002; Lee 2006). Moreover, cascades of second messengers can regulate the activities and expression of key proteins at synapses to interfere with synaptic plasticity (Lisman and Zhabotinsky 2001; Merrill et al. 2005; Zhong et al. 2009).
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family of growth factors that facilitate neuronal survival and development, is a key regulator of synaptic plasticity (McAllister et al. 1999; Lu 2003; Li et al. 2013; Bramham and Messaoudi 2005; Cowansage et al. 2010; Edelmann et al. 2014). Presynaptic depolarization elicits the transcription of the BDNF gene, as well as the synthesis and secretion of BDNF proteins by presynaptic nerve terminals (Lu 2003; Black 1999). Secreted BDNF at synapses may act on both pre- and postsynaptic compartments to alter the efficacy of synaptic transmission and the capacity of activity-induced long-term potentiation (LTP) (Tyler et al. 2002; Park and Poo 2013). Binding of BDNF to the postsynaptic TrkB receptor can phosphorylate the NMDA receptor, which induces the enhancement of influx and the activation of the cyclic AMP response element (CRE)-binding protein (CREB) via intracellular signaling pathways. Activated CREB at the BDNF promoter leads to an increase in BDNF expression and subsequent secretion by the postsynaptic nerve terminals (Black 1999; Tyler et al. 2002).
Recent studies have suggested that the paraventricular nucleus of the thalamus (PVT) is a crucial component of fear-processing circuits (Do-Monte et al. 2015; Penzo et al. 2015). The PVT strongly projects to the CeL and participates in the formation of fear memories via the regulation of fear conditioning-induced plasticity at the LA-CeL synapses (Penzo et al. 2015). PVT neurons preferentially innervate somatostatin-positive () neurons in the CeL, and the stimulation of PVT afferents facilitates neuron activity and promotes intra-CeL inhibition (Penzo et al. 2015). The majority of CeL-projecting PVT neurons synthesize and release BDNF to the CeL to mediate PVT-CeL communication (Penzo et al. 2015). Interestingly, only long-term synaptic potentiation is susceptible to PVT manipulations; short-term synaptic potentiation is not affected when the PVT is inactivated (Do-Monte et al. 2015; Penzo et al. 2015). However, it remains unclear how the PVT regulates the maintenance of fear conditioning-induced plasticity at LA-CeL synapses and how transient stimuli can induce long-term synaptic potentiation.
Many computational models of neural networks have been developed to understand the process of conditioned fear in the amygdala (Armony et al. 1995; Balkenius and MorÉn 2001; Li et al. 2009; Kim et al. 2013a, b). Li et al. (2009) proposed a biophysically realistic network model of LA neuron activity during fear conditioning and extinction. In this model, fear learning is modeled through Hebbian plasticity, which is implemented in excitatory AMPA and inhibitory receptor-mediated synapses (Li et al. 2009). Furthermore, the calcium control hypothesis is introduced in the model, where synaptic potentiation or depression is determined by intracellular calcium levels (Li et al. 2009). The main finding in this study is that LA activity during both acquisition and extinction can be controlled through a balance between pyramidal cell and interneuron activation (Li et al. 2009). Kim et al. (2013b) developed a large-scale biophysical model of the LA to investigate the relative contributions of plasticity in the amygdala versus its afferent pathways to conditioned fear. The model reproduces previous findings regarding the cellular correlates of fear conditioning in the LA (Kim et al. 2013b). Model simulations demonstrate that training-induced increases in the responsiveness of afferent neurons are required for fear memory formation, and the synaptic plasticity between LA neurons only play a minor role in the maintenance of fear memories (Kim et al. 2013b). A subsequent study based on this model demonstrated that LA neurons with high intrinsic excitability are more likely to be integrated into memory traces (Kim et al. 2013a). In these large-scale network models, Hebbian plasticity is implemented to model learning via the adjustment of synaptic weights in the synaptic conductances, and the learning rate in the Hebbian rule is dependent on the calcium level. However, the molecular mechanisms that underlie synaptic plasticity in the amygdala remain elusive. Specifically, the question arises how calcium levels control synaptic potentiation.
In this study, we develop a computational model to investigate the underlying mechanisms of PVT manipulation of synaptic plasticity at LA-CeL synapses. The model consists of a presynaptic vesicle-release process, the postsynaptic membrane potential, and the regulation of BDNF gene expression. We introduce the hypothesis that there is a positive feedback loop of BDNF transcription in the postsynaptic compartment; this positive feedback is important for the persistence of the PVT manipulation. Model simulations were used to examine previous experimental findings regarding how the PVT controls the potentiation of excitatory synapses onto CeL neurons (Penzo et al. 2015). We confirm that there is a threshold for the total secretion rate of BDNF from the PVT and LA neurons to the CeL. When the secretion rate is above the threshold, presynaptic calcium concentrations, as well as postsynaptic firing frequencies, switch from low to high. In addition, our results reveal a linear dependence between the postsynaptic firing frequency and the presynaptic concentration. We further demonstrate that the positive feedback loop of BDNF expression at a certain strength is crucial for the maintenance of the high spontaneous firing frequency of CeL neurons after stimulation. Finally, we find that short-term synaptic potentiation at the LA-CeL synapses can be regulated by the secretion of BDNF from the LA to the CeL.
Model and method
Model description
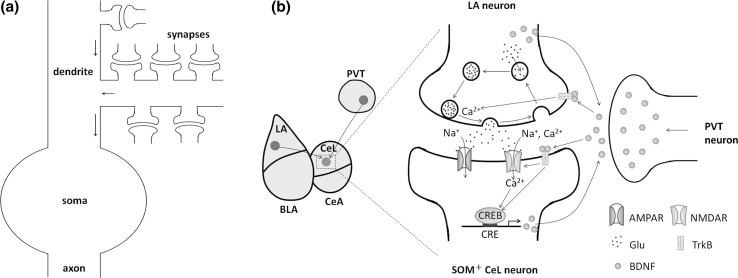
Our model considers LA-CeL synaptic plasticity through the action potentials of a postsynaptic neuron (see Fig. 2a). There are many LA-CeL synapses contained on the dendrite of an CeL neuron, and all synaptic currents are integrated to induce action potentials in the postsynaptic neuron. Axons originating from both LA and PVT neurons enter the CeL to innervate CeL neurons. Fear conditioning induces synaptic potentiation at the LA-CeL synapses, which is associated with increases in both frequency and amplitude of the miniature excitatory postsynaptic currents (mEPSC) (Li et al. 2013; Penzo et al. 2015). The model describes the synaptic potentiation at the LA-CeL synapses, which is measured by the high spontaneous firing frequency of the CeL neuron.
Fig. 2.
Illustration of the model. a The overall connection from synapses to postsynaptic action potentials. b A single LA-CeL synapse considered in the model. The LA and PVT neurons secrete BDNF to the CeL during stimulation. On the presynaptic side (a glutamatergic LA neuron), BDNF binds to TrkB receptors to upregulate the intercellular concentration, which in turn enhances the release of the neurotransmitter glutamate (Glu). On the postsynaptic side (an CeL neuron), TrkB receptors are activated following the binding of BDNF. The activated TrkB receptors phosphorylate NMDAR, increase influx and subsequently activate CREB proteins. BDNF also induces the activation of CREB proteins through other intracellular signaling pathways following TrkB receptor phosphorylation. Activated CREB binds to CRE in the BDNF promoter and increases BDNF expression in the CeL neuron. The produced BDNF molecules are subsequently secreted to the extracellular environment to form a positive feedback loop of BDNF production. Here, BLA refers to the basolateral amygdala, and CeA refers to the central amygdala
Illustration of a single LA-CeL synapse is shown in Fig. 2b. The PVT strongly projects to the CeL and participates in the formation of fear memories via the regulation of fear conditioning-induced plasticity at the LA-CeL synapses (Penzo et al. 2015). The PVT modulates CeL neurons through a circuit that involves BDNF secretion during fear conditioning and activation of the BDNF receptor tropomyosin-related kinase B (TrkB) in CeL neurons (Penzo et al. 2015). Molecular interactions of the circuit are detailed below.
The neurotrophic factor BDNF is important for the modulation of both presynaptic and postsynaptic neurons. Presynaptically, the binding of BDNF to the TrkB receptor activates PLC-, triggers the release of from internal calcium stores, and in turn enhances the release of the neurotransmitter glutamate (Glu) from the presynaptic nerve terminal (Park and Poo 2013; Leal et al. 2014; Yano et al. 2006). Postsynaptically, the neurotransmitter Glu binds to both NMDA and AMPA glutamate receptors (Li et al. 1995; McKernan and Shinnick-Gallagher 1997). Glutamate activates AMPA channels to produce a net inward current of influx, while the EPSC component of NMDA current contains both and influxes (Collingridge and Lester 1989). Binding of BDNF to the postsynaptic TrkB receptor can phosphorylate the NMDA receptor to increase the conductance of the NMDA channel and upregulate the influx and NMDAR-mediated synaptic current (Black 1999; Tyler et al. 2002). The intracellular signal subsequently activates several forms of calcium/calmodulin-dependent kinases (CaMK) (Finkbeiner et al. 1997). Moreover, the phosphorylation of TrkB receptors leads to Ras-dependent activation of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) (Finkbeiner et al. 1997). Both CaMK-regulated and MAPK-dependent pathways converge to CREB (Finkbeiner et al. 1997; Ying et al. 2002). Hence, BDNF induces the phosphorylation and activation of CREB through these signaling cascades. Finally, activated CREB binds to its cognate-binding site, CRE, a BDNF promoter, to promote the transcription of BDNF in the postsynaptic neuron (Johansen et al. 2011; Tyler et al. 2002; Martinowich et al. 2003).
The PVT is a major source of BDNF for the CeL (Penzo et al. 2015). In light-foot shock paired training, fear conditioning induces BDNF gene expression in the LA (Ou and Gean 2006, 2007), which might become a second source of BDNF in the CeL. Therefore, we assume that both the LA and the CeL-projecting PVT neurons can secrete BDNF to the CeL during stimulation. Moreover, normal neural activity can induce BDNF secretion from the dendrites of cultured hippocampal neurons (Matsuda et al. 2009). Thus, we further assume that BDNF is also synthesized and secreted from the CeL neuron.
Mathematical formulations
Formulations of the model include the following three components: a presynaptic vesicle-release process, postsynaptic membrane potential, and BDNF concentration dynamics.
Presynaptic vesicle-release process
The component of presynaptic vesicle-release describes the process of Glu release at a single synapse regulated by presynaptic . Here, we refer to the three-states model proposed in Tsodyks and Markram (1997) and Nadkarni et al. (2008). The presynaptic neurotransmitter resources are partitioned into three states: effective (E), inactive (I), and recovered (R). For a single synapse, the dynamics of the fraction of neurotransmitter resources in each state are given by equations below (Tsodyks and Markram 1997):
| 1 |
| 2 |
where E, I, and R are the fraction of resources at the corresponding state (), and and are the time constants of inactivation and recovery, respectively. The delta function represents the events of spontaneous or stimulated vesicle release at the discrete time series . In each synapse, the neurotransmitter content of a vesicle, uR, is released into the synaptic cleft upon vesicle release. Presynaptic ( for the concentration) binds to the vesicle release machinery to regulate spontaneous vesicle release (Nadkarni et al. 2008; Schneggenburger and Neher 2000; Bollmann et al. 2000). In a single synapse, the events of spontaneous vesicle release are assumed to be a Poisson process, and the rate is given below (Nadkarni et al. 2008):
| 3 |
The presynaptic released from the internal calcium stores includes the basal release (with a rate ) and the stimulated release (with a rate ) induced by TrkB activation when presynaptic TrkB is bound to extracellular BDNF. Thus, the dynamics of presynaptic concentration is formulated as
| 4 |
where is the time constant of clearance, and the rate of BDNF-induced presynaptic release is given by the Michaelis–Menten function:
| 5 |
Postsynaptic membrane potential
The component of postsynaptic membrane potential describes the action potential at the soma in response to the integration of synaptic currents from AMPA and NMDA channels. The cell membrane potential is modeled on the classic Hodgkin–Huxley equations (Hodgkin and Huxley 1952), and the AMPA and NMDA currents are incorporated into the equations through effective integrated synaptic currents (Destexhe et al. 1998).
The equations for the action potential of the postsynaptic CeL neuron are given by:
| 6 |
| 7 |
| 8 |
| 9 |
Here, () are maximum conductances, are equilibrium potentials, and and () are opening and closing rates of the ion channel gates, represents the effective integrated synaptic currents due to AMPA and NMDA channels in the LA-CeL synapses, and is the integration of all other external stimulations onto the postsynaptic neuron.
The rates are similar to the classic Hodgkin–Huxley equation (Hodgkin and Huxley 1952):
| 10 |
| 11 |
| 12 |
| 13 |
| 14 |
| 15 |
The synaptic current consists of and , representing effective currents onto the postsynaptic neuron due to AMPA and NMDA channels from all LA-CeL synapses, respectively:
| 16 |
The effective currents are given by effective conductance and the potential as:
| 17 |
| 18 |
| 19 |
| 20 |
| 21 |
Here, and are maximal effective soma conductances of an LA-CeL synapse through the AMPA and NMDA channels on the dendrite according to Zhou et al. (2013), so that and represent the effective soma currents onto the postsynaptic neuron due to AMPA and NMDA channels from the LA-CeL synapses, respectively. The coefficient l represents the number of effective LA-CeL synapses to produce the synaptic currents, and and are fractions of the open state AMPA receptors (AMPAR) and NMDA receptors (NMDAR), respectively. In Eqs. (20) and (21), and are the opening and closing rates of the gate channels, and is the total concentration of presynaptic neurotransmitters. The equilibrium potentials of AMPAR and NMDAR are assumed to be the same value, (Destexhe et al. 1998; Wang 1999; Kirli et al. 2014). In addition, magnesium blocks the NMDA receptor channel in a voltage-dependent way (Jahr and Stevens 1990; Vargas-Caballero and Robinson 2004; Maio et al. 2016a, b), hence the NMDA current is controlled by the concentration of extracellular magnesium () and the membrane voltage (V).
The AMPA channel conductance is upregulated by postsynaptic via the phosphorylation of the AMPAR GluR1 subunit and the promotion of GluR1 insertion (Lisman et al. 2012). Hence, we assume that depends on postsynaptic concentration, , through the Michaelis–Menten dynamics:
| 22 |
where and are the basal rates of increasing and decreasing AMPA channel maximal effective soma conductance, respectively.
The maximal effective NMDA conductance, , is dependent on the extracellular BDNF concentration () as a result of TrkB activation following BDNF binding and is modeled by the Michaelis–Menten dynamics:
| 23 |
where and are the basal rates of increasing and decreasing NMDA channel maximal conductance, respectively.
BDNF dynamics
The component of BDNF dynamics describes the changes in BDNF concentration. We omit the changes of BDNF concentration in the LA and PVT neurons for simplicity and only consider the dynamics of intracellular BDNF in the CeL neuron () and extracellular BDNF in the CeL ().
In the CeL neuron, the transcription of BDNF is upregulated with active CREB, and CREB activation is dependent on the intracellular signaling pathways that involve postsynaptic and extracellular BDNF through the receptor TrkB. Hence, the intracellular BDNF synthesis rate can be given by a function of , and the CREB activation rate is expressed as a function of and . Moreover, intracellular BDNF is secreted to the extracellular space with a rate . In addition, we assume that the PVT and the LA neurons release BDNF to the CeL during stimulation with the rates and , respectively. These processes lead to the following equations:
| 24 |
| 25 |
| 26 |
where is the inactivation rate of CREB, and are the degradation rates of intracellular and extracellular BDNF, respectively, and is the total basal BDNF secretion rate. The intracellular BDNF synthesis rate is formulated as a Hill-type function:
| 27 |
in which indicates the basal synthesis rate. The CREB activation rate is provided as follows:
| 28 |
in which is the basal activation rate, and the dependence with is provided by the Michaelis–Menten function.
Finally, the sources of postsynaptic include two parts, a basal release (with a rate ) from the calcium stores and an influx through NMDARs that is dependent on the conductance of the NMDA channel (with a rate ). Thus, the dynamics of postsynaptic concentration is formulated as follows:
| 29 |
where is the time constant of clearance.
Equations (1)–(29) provide a set of differential equations for the model. All values of default parameters used in our study are listed in Table 1, in which most parameter values are referred to the published literature, and the others are set to fit the experimental data.
Table 1.
Default parameter values of the model under control condition.
Source: c = Hodgkin and Huxley (1952), d = Destexhe et al. (1998), e = Nadkarni et al. (2008), f = Golomb et al. (2006). Other parameters are adjusted to fit experimental data
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| u | 0.01 | ||
| l | 10000 | ||
| 400 | |||
| 0.002 | |||
| 0.2 | |||
| 0.004 | |||
| 50 | |||
| 0.02 | |||
| 0.001 | |||
| 29 | |||
| 0.006 | 50 | ||
| 70 | |||
| 0 | |||
| 0 | 0.01 |
During stimulation,
During stimulation,
Methods
The differential equations are solved with the Euler scheme. All simulations were performed in MATLAB, and the codes are available upon request. Note that the time scale of the model covers a wide range, over milliseconds of membrane potential dynamics, seconds of gene expression, and hours of simulations for the system to reach a stationary state. Therefore, we performed the simulations with a time step of 0.02 ms and extended the simulation to 24 h in order to compare our model simulations with experimental findings.
In simulations, fear training was mimicked by a 200 s stimulation with the following processes.
The frequency of presynaptic vesicle release following the arrival of presynaptic action potentials is 40 Hz and is independent of ;
The PVT secretes BDNF at a rate of ;
The LA secretes BDNF at a rate of .
We defined as the total rate of BDNF secretion from PVT and LA neurons, and set during stimulation.
In data analysis, the firing frequency is defined as the average number of spikes per second. To eliminate errors in data analysis, the firing frequency at a particular point of time is obtained from dynamic data over a range of 500 s starting from the given time point and averaged over three independent runs. In the following analyses, the simulation results are labeled “Control”, “Normal, stimulated”, “PVT inactivation, stimulated”, “Trkb deletion, stimulated”, and “Bdnf deletion, stimulated”, which represent the following groups, respectively: naive control, stimulated in a normal animal, stimulated under PVT inactivation, stimulated under Trkb deletion in the CeL neuron, and stimulated under Bdnf deletion in the CeL neuron.
Results
Validation of the model for synaptic potentiation via postsynaptic neuron excitability
Many possibilities lead to changes in cell excitability (measured by spontaneous firing rate) (Goosens et al. 2003; Yiu et al. 2014). In our model, increases in excitability of the stimulated CeL neuron are induced by the potentiation of excitatory synaptic transmissions, since we assume that the CeL neuron receives excitatory inputs from only the presynaptic LA neurons.
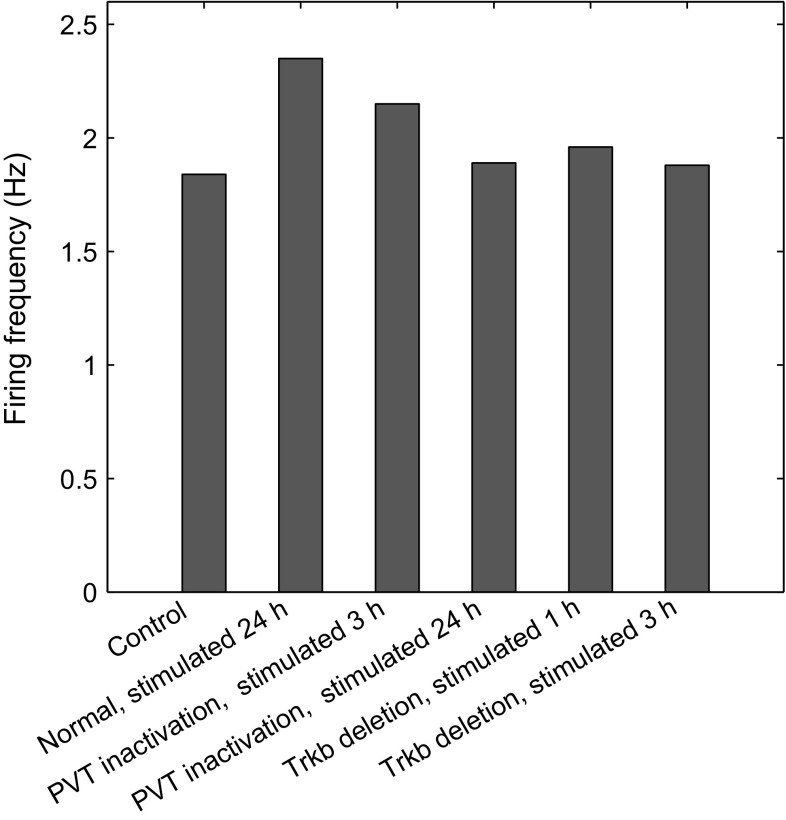
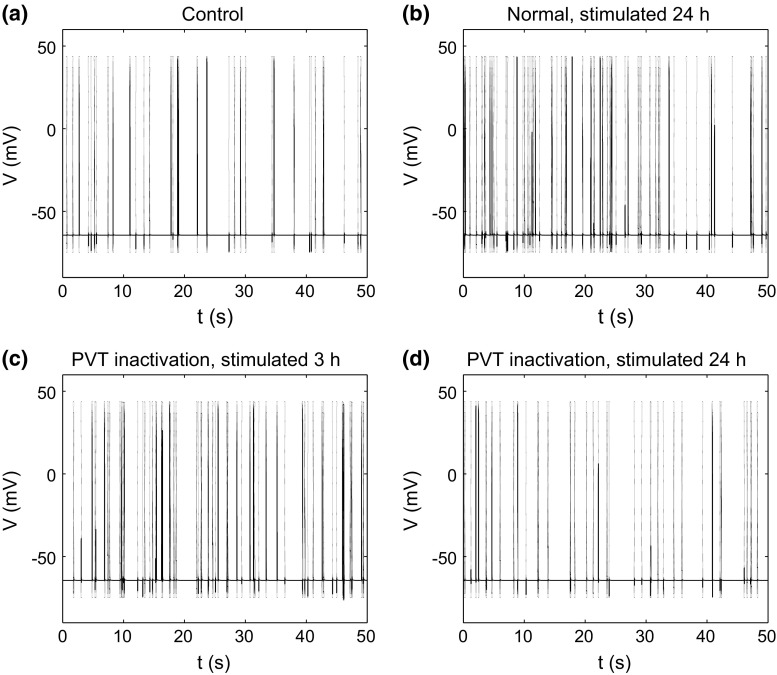
First, we validated our model with the experimental results of cell excitability in response to fear conditioning (Penzo et al. 2015). Experiments in mice have shown that fear conditioning highly elevates the frequency of mEPSC at 3 and 24 h after conditioning for synaptic potentiation, and the inhibition of CeL-projecting PVT neurons did not affect short-term synaptic potentiation at 3 h after conditioning, but completely abolished long-term synaptic potentiation at 24 h after conditioning (Penzo et al. 2015). In model simulations, we set the parameter at and for situations of normal animal and PVT inactivation, respectively. Model simulations showed that the firing frequency of the postsynaptic CeL neuron increased significantly at 24 h after stimulation for the normal animal (Figs. 3, 4b); under PVT inactivation, the firing frequency increased at 3 h but was abolished at 24 h after stimulation (Figs. 3, 4c, d). These results are consistent with the experimental findings that PVT activation is required for the maintenance of the CeL neuron excitability after stimulation (Penzo et al. 2015).
Fig. 3.
Postsynaptic firing frequency of different situations (from left to right): naive control, normal at 24 h after stimulation, PVT inactivation at 3 h after stimulation, PVT inactivation at 24 h after stimulation, Trkb deletion at 1 h after stimulation and Trkb deletion at 3 h after stimulation
Fig. 4.
Time courses of the action potentials of the postsynaptic neuron. a Control situation without stimulation. b Normal at 24 h after stimulation. c PVT inactivation at 3 h after stimulation. d PVT inactivation at 24 h after stimulation
The PVT modulation of CeL neurons is mediated by the signaling of the BDNF receptor TrkB, and the deletion of Trkb in the CeL can impair fear conditioning-induced synaptic plasticity (Penzo et al. 2015). In the model, BDNF regulates synaptic plasticity through TrkB, hence, we simulated the situation of Trkb deletion by setting and . Model simulations showed that in the CeL neuron, the spontaneous firing frequency was at the same level as that of the control state at 3 h after stimulation, in agreement with experimental observations. However, the deletion of Bdnf in the PVT leads to , and so the spontaneous firing frequency under the deletion of Bdnf in the PVT was the same as that under PVT inactivation, seen in Fig. 3. These results confirm that BDNF/TrkB signaling is a mediator of PVT-CeL communication.
Postsynaptic firing frequency is determined by presynaptic concentration
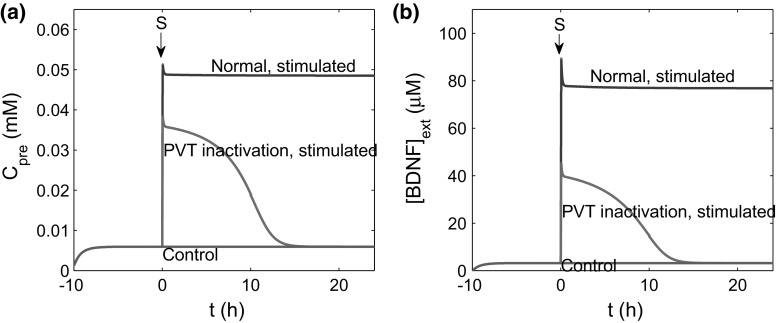
To investigate how stimulation induces the increase and maintenance of the postsynaptic firing frequency, we further examined the system dynamics, focusing on the dynamics of presynaptic and BDNF concentrations. After stimulation, PVT and LA neurons secrete BDNF to the CeL, leading to an increase in presynaptic calcium and triggering the fusion of synaptic vesicles and the release of neurotransmitter (Schneggenburger and Neher 2000). The released neurotransmitter and extracellular BDNF together form input signals to the postsynaptic membrane potential. Figure 5 shows the time courses of presynaptic calcium concentration and after stimulation in the cases of control, stimulated, and stimulated under PVT inactivation. The results demonstrate that stimulation triggered an increase in and immediately after stimulation, regardless of changes in PVT activation. However, persistently high levels of and were maintained only when the PVT was active; in the case of PVT inactivation, stimulation only induced a short-term increase in and , which regained their basal levels approximately 14 h later. These findings suggest that the abolishment of the increase in the CeL neuron excitability under PVT inactivation may be associated with the recovery of or post-stimulation.
Fig. 5.
Time courses of (a) and (b), for control (, red lines), stimulated case (, blue lines), and stimulated under PVT inactivation (, green lines). In simulations, the system is initially trained to reach the stationary state, and a 200 s stimulation is added to the system at . (Color figure online)
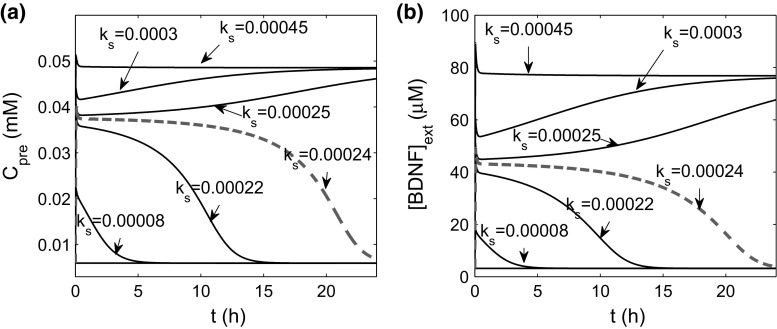
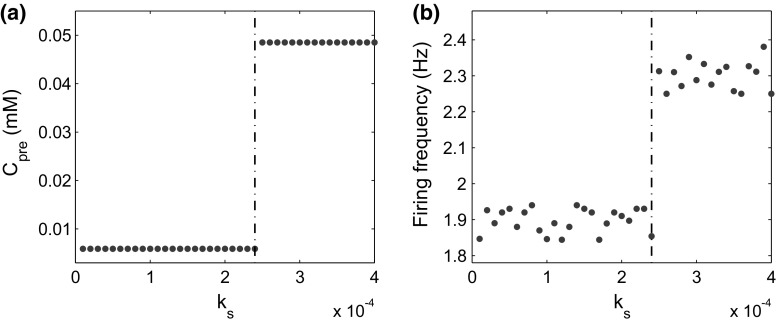
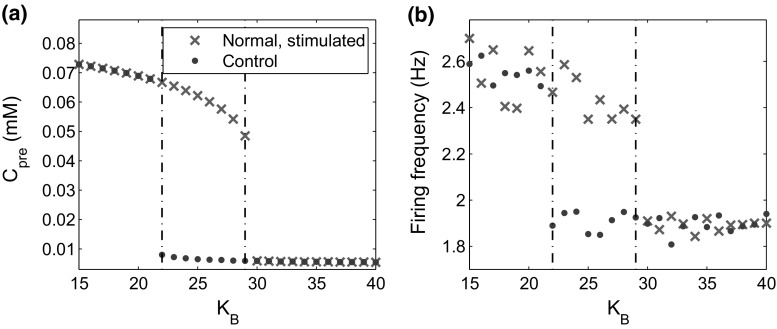
We further investigated how the stimulus induces dynamical changes in or and the postsynaptic firing frequency. For simplicity, we let , the total rate of BDNF secretion from the PVT and the LA neurons. We varied from to , and examined the long-term dynamics of and (Fig. 6). There was a threshold at (red dashed lines), above which both the presynaptic calcium concentration and the postsynaptic firing frequency switched from low to high (Fig. 7). These results indicate that the stimulation induces persistent high excitability of the CeL neuron only when the BDNF secretion rate exceeds a given threshold.
Fig. 6.
Time courses of (a) and (b) in response to stimulations with different values of the total secretion rate () of BDNF from PVT and LA neurons to the CeL
Fig. 7.
Stationary-state presynaptic concentration () and the postsynaptic firing frequency after a substantial amount of time after stimulation for different rates of BDNF secretion (). a The concentration of presynaptic versus . b The postsynaptic firing frequency versus
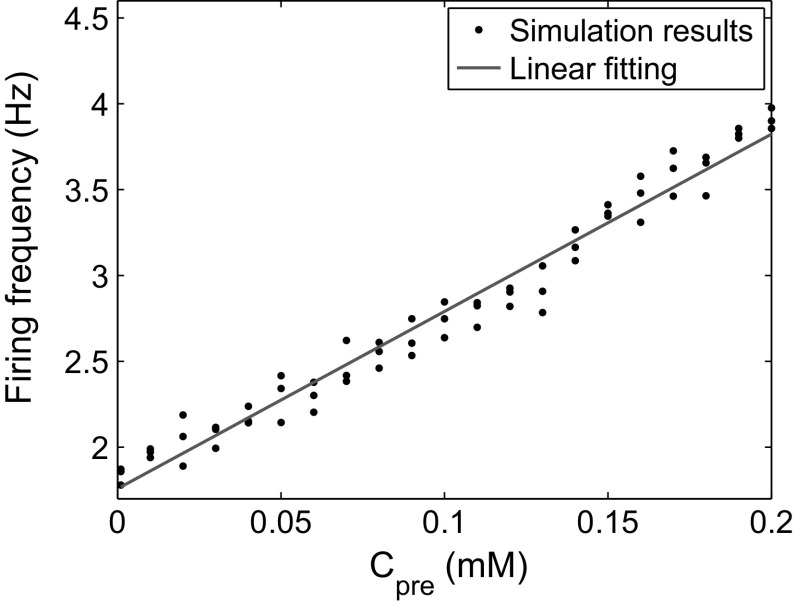
The simultaneous switching of presynaptic and postsynaptic firing frequency (Fig. 7) suggests a potential correlation between these two factors. To further investigate the correlation, we calculated the postsynaptic firing frequency for different concentrations of presynaptic . The results showed that the firing frequency obviously and in a linear fashion depends on (Fig. 8). Biophysically, increases in presynaptic lead to a faster spontaneous release of synaptic vesicles and, hence, more postsynaptic action potentials. These results indicate that presynaptic after stimulation is essential for the rise of the postsynaptic firing frequency, and a proper stimulation over a given threshold is necessary for persistently high levels of presynaptic and postsynaptic firing frequency.
Fig. 8.
Postsynaptic firing frequency versus . (Color figure online)
Positive feedback of postsynaptic BDNF production maintains the high excitability of the CeL neuron after stimulation
The above studies have demonstrated that brief stimulation can induce an increase in the presynaptic calcium concentration and persistent high postsynaptic firing frequency. From our model, extracellular BDNF in the CeL may bind to presynaptic TrkB receptors and lead to an efflux of from presynaptic calcium stores and the release of neurotransmitter (Park and Poo 2013; Leal et al. 2014; Yano et al. 2006); the neurotransmitter triggers a switch in the postsynaptic firing frequency from low to high. Moreover, the binding of extracellular BDNF to postsynaptic TrkB receptors activates the transcription factor CREB and promotes the intracellular expression of BDNF and the secretion of BDNF to the extracellular environment of CeL neurons to form a positive feedback loop of BDNF expression (Johansen et al. 2011; Tyler et al. 2002; Martinowich et al. 2003). We asked whether this positive feedback loop of BDNF expression is able to maintain the long-term excitability of the CeL neuron after a brief stimulation.
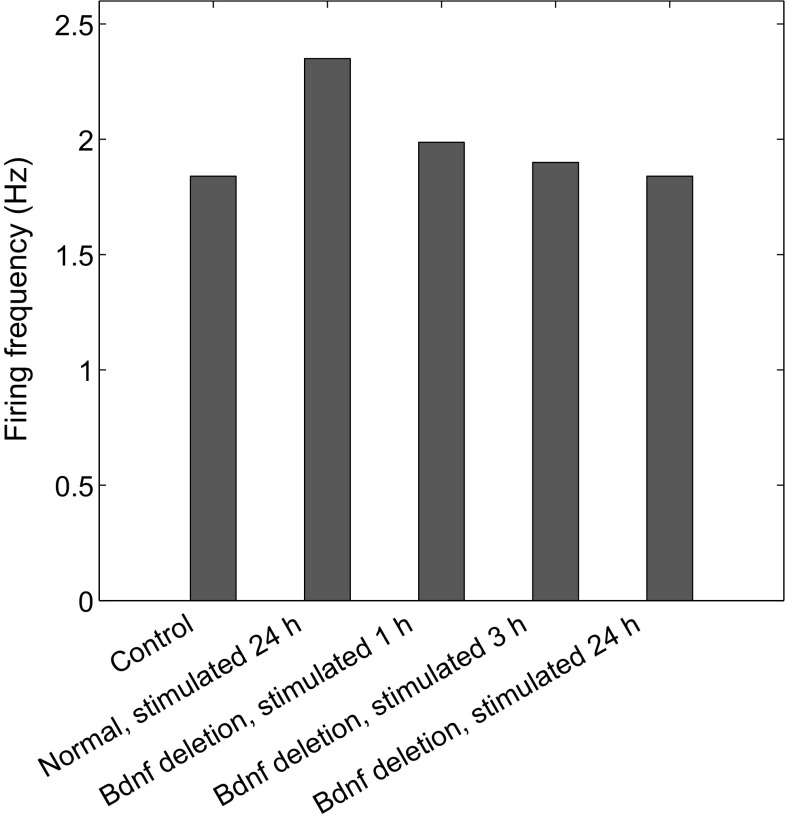
First, experiments have demonstrated that protein synthesis inhibition in the central nucleus of the amygdala (CeA) can impair fear memory consolidation (Wilensky et al. 2006). To determine whether postsynaptic BDNF transcription is required for high excitability, we introduced a loss of Bdnf in the CeL neuron by setting ; the postsynaptic firing frequency weakly increased at 1 h after stimulation, yet decreased to the same level as the control case at 24 h after stimulation (Fig. 9). This indicates that BDNF expression in the CeL neuron is required for the induction and persistence of high-frequency postsynaptic firing following stimulation.
Fig. 9.
Postsynaptic firing frequency of the following groups (from left to right): naive control, normal at 24 h after stimulation, Bdnf deletion in the CeL neuron () at 1 h after stimulation, Bdnf deletion at 3 h after stimulation, and Bdnf deletion at 24 h after stimulation
To investigate the effects of the positive feedback loop of BDNF expression on the CeL neuron, we varied the feedback strength, , to examine the responses of presynaptic calcium dynamics and postsynaptic action potentials. For each value of , we ran the model equations under either control or stimulated conditions until the system reached a stationary state. Figure 10 shows the dependence of the presynaptic concentration and postsynaptic firing frequency on the feedback strength , under conditions either without stimulation or after a 200 s stimulation. Both and postsynaptic firing frequency decreased with the increase in and changed from a high to low level when in the control condition and when after stimulation. Thus, two states of either high or low postsynaptic firing frequency coexisted under proper feedback strength values (); brief stimulation can induce the transition from low to high levels of presynaptic concentration and postsynaptic firing frequency, and the high-level state persists after stimulation. These results verify that the positive feedback loop of postsynaptic BDNF expression with proper feedback strength is essential for the induction and persistence of high CeL neuron excitability after stimulation.
Fig. 10.
Effects of changing the positive feedback loop strength of BDNF expression in the CeL neuron. a The concentration of presynaptic () versus the feedback strength, , in control and stimulated cases. b The postsynaptic firing frequency versus in the control and stimulated case. Here, the data for the stimulated cases were obtained after a substantial amount of time to ensure that the system reached an approximately stationary state. The dash-dotted lines indicate the jumps between low and high-level states
The effects of BDNF secretion from the LA neurons to the CeL on CeL neuron excitability
In the above discussions, the long-term high excitability is supported by PVT activation and the positive feedback loop of BDNF expression in the CeL neuron. Nevertheless, short-term high excitability of the CeL neuron after stimulation can be induced even under PVT inactivation (Fig. 3). In light-foot shock training, fear conditioning upregulates BDNF expression in the LA (Ou and Gean 2006, 2007); it is interesting to know whether the LA plays a role in the short-term CeL neuron excitability.
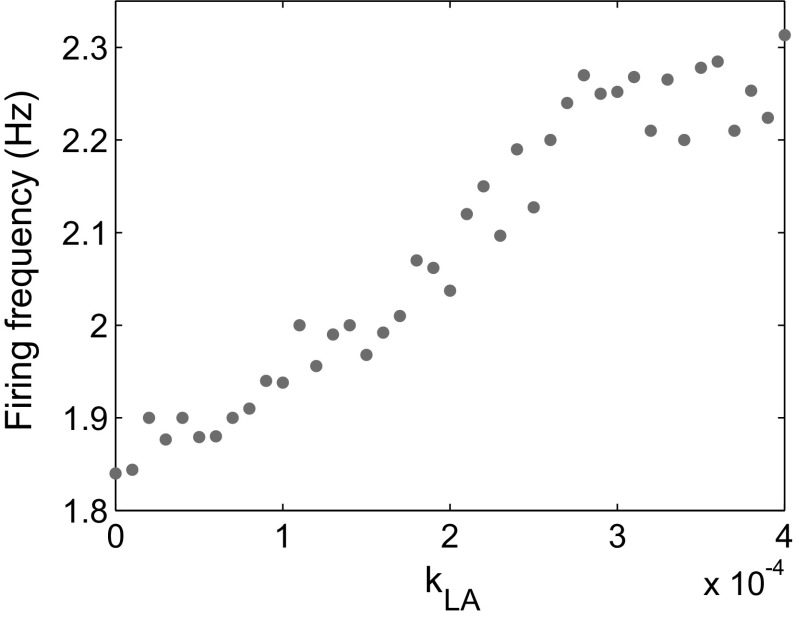
In the model, we assumed that the LA neurons secrete BDNF to the CeL by axons. To discuss how the spontaneous firing frequency of the CeL neuron depends on the secretion of BDNF from the LA, we considered the case of PVT inactivation () and varied the BDNF secretion rate during stimulation. Simulations showed that the postsynaptic firing frequency at 3 h after stimulation continuously increased with the (Fig. 11), from 1.84 Hz, approximately the value of the native control, at to 2.3 Hz, the level of normal controls under stimulation, at . These results suggest that BDNF secreted from the LA during stimulation is essential for the short-term high excitability of the CeL neuron.
Fig. 11.
Postsynaptic firing frequency 3 h after stimulation for PVT inactivation () and various values of
Discussion
Fear conditioning can induce synaptic plasticity at the LA-CeL synapses, which is measured by increases in the frequency and amplitude of miniature excitatory postsynaptic currents. Recent studies suggest that the PVT is a crucial component in the fear-processing circuits (Penzo et al. 2015). Here, we have developed a computational model containing the presynaptic vesicle-release process, the postsynaptic membrane potential, and the regulation of BDNF gene expression to investigate the underlying mechanisms of PVT manipulation of fear conditioning-induced long-term plasticity at LA-CeL synapses (Fig. 2). We assumed that the CeL neuron receives excitatory inputs only from the presynaptic LA neurons and that the excitability can result from changes in excitatory synaptic transmission. The LA-CeL synapses were potentiated when the CeL neuron exhibited increases in spontaneous firing frequency. The model was validated by reproducing experimental findings showing that the inhibition of CeL-projecting PVT neurons impaired fear conditioning-induced synaptic potentiation of CeL neurons 24 h after conditioning but did not affect the synaptic potentiation 3 h after conditioning, as well as the finding that BDNF/TrkB signaling is a mediator of PVT-CeL communication (Figs. 3, 4).
Model simulations indicated that the sustained high level of the postsynaptic firing frequency was associated with a persistently high state of presynaptic concentration (). The postsynaptic firing frequency linearly depends on (Fig. 8). After stimulation, the binding of BDNF to the presynaptic TrkB receptor induced an increase in intracellular concentration, and there was a threshold of the total secretion rate of BDNF from the PVT and LA neurons to the CeL during stimulation for the persistent increase of (Fig. 7). At this threshold level, the postsynaptic firing frequency switched from low to high at a long-term point after stimulation.
In the model, we assumed a positive feedback loop of BDNF expression in the CeL neuron. This positive feedback is mediated by the regulation of BDNF transcription through CREB activation, and CREB activation is induced by TrkB receptors that bind to extracellular BDNF. We demonstrated that both postsynaptic firing frequency and presynaptic concentration decrease with feedback strength, , under either control conditions or long-term after stimulation, and both phenomena exhibit switches between low and high levels with changes in (Fig. 10). The critical values of marked a region for feedback strength at which the stimulation induced a long-term increase in the postsynaptic firing frequency. Moreover, we examined the effect of BDNF deletion in the CeL neuron and demonstrated that postsynaptic firing frequency decreased under BDNF deletion (Fig. 9). Our results support the idea that the positive feedback is crucial for synaptic plasticity and the formation of fear memories. However, this result requires further experimental confirmation.
This paper developed a computational model to investigate the underlying mechanisms of PVT modulation of fear conditioning-induced long-term plasticity at the LA-CeL synapses. The model also provides a framework for understanding other similar processes associated with synaptic plasticity. However, the proposed model only includes synapses connected to a single neuron for simplicity. More precisely, a systematic model of neuronal networks involving molecules, synapses and neurons from various brain regions involved in the fear circuits is required for a more comprehensive understanding of fear memories. A potential approach to developing this network model is to combine the single neuron model in the current study with previous network models (Li et al. 2009; Kim et al. 2013b) to attempt to address network phenomena observed in recent studies. Furthermore, the cell membrane model in this work is based on the Hodgkin–Huxley equations with and leak currents, and more realistic models with voltage-dependent calcium channels should be further incorporated into the model framework.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (91430101, 11272169, and 11372017) and the Academic Excellence Foundation of BUAA for PhD Students.
Contributor Information
Zhuoqin Yang, Email: yangzhuoqin@buaa.edu.cn.
Jinzhi Lei, Email: jzlei@tsinghua.edu.cn.
References
- Armony JL, Servan-Schreiber D, Cohen JD, LeDoux JE. An anatomically constrained neural network model of fear conditioning. Behav Neurosci. 1995;109(2):246–257. doi: 10.1037/0735-7044.109.2.246. [DOI] [PubMed] [Google Scholar]
- Armony JL, Quirk GJ, LeDoux JE. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning. J Neurosci. 1998;18(7):2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkenius C, MorÉn J. Emotional learning: a computational model of the amygdala. Cybern Syst. 2001;32(6):611–636. doi: 10.1080/01969720118947. [DOI] [Google Scholar]
- Black IB. Trophic regulation of synaptic plasticity. J Neurobiol. 1999;41(1):108–118. doi: 10.1002/(SICI)1097-4695(199910)41:1<108::AID-NEU14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JGG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289(5481):953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76(2):99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Cho JH, Bayazitov IT, Meloni EG, Myers KM, Carlezon WA, Jr, Zakharenko SS, Bolshakov VY. Coactivation of thalamic and cortical pathways induces input timing-dependent plasticity in amygdala. Nat Neurosci. 2011;15(1):113–122. doi: 10.1038/nn.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468(7321):277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Lester RAJ. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989;41(2):143–210. [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3(1):12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Mainen ZF, Sejnowski TJ. Kinetic models of synaptic transmission. In: Koch C, Segev I, editors. Methods in neuronal modeling. Cambridge: MIT Press; 1998. [Google Scholar]
- Di Maio V, Ventriglia F, Santillo S. A model of cooperative effect of AMPA and NMDA receptors in glutamatergic synapses. Cogn Neurodyn. 2016;10(4):315–325. doi: 10.1007/s11571-016-9383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio V, Ventriglia F, Santillo S. AMPA/NMDA cooperativity and integration during a single synaptic event. J Comput Neurosci. 2016;41(2):127–142. doi: 10.1007/s10827-016-0609-5. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Quiñones Laracuente K, Quirk GJ. A temporal shift in the circuits mediating retrieval of fear memory. Nature. 2015;519(7544):460–463. doi: 10.1038/nature14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82(5):966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Popa D, Paré D. Central amygdala activity during fear conditioning. J Neurosci. 2011;31(1):289–294. doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann E, Leßmann V, Brigadski T. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology. 2014;76:12–29. doi: 10.1016/j.neuropharm.2013.05.043. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19(5):1031–1047. doi: 10.1016/S0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Golomb D, Yue C, Yaari Y. Contribution of persistent Na current and M-type K current to somatic bursting in CA1 pyramidal cells: combined experimental and modeling study. J Neurophysiol. 2006;96(4):1912–1926. doi: 10.1152/jn.00205.2006. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Hobin JA, Maren S. Auditory-evoked spike firing in the lateral amygdala and pavlovian fear conditioning: mnemonic code or fear bias? Neuron. 2003;40(5):1013–1022. doi: 10.1016/S0896-6273(03)00728-1. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Translational research in the neuroscience of fear extinction: implications for anxiety disorders. Am J Psychiatry. 2011;168(12):1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Lüthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468(7321):270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci. 1990;10(9):3178–3182. doi: 10.1523/JNEUROSCI.10-09-03178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147(3):509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Paré D, Nair SS. Assignment of model amygdala neurons to the fear memory trace depends on competitive synaptic interactions. J Neurosci. 2013;33(36):14,354–14,358. doi: 10.1523/JNEUROSCI.2430-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Paré D, Nair SS. Mechanisms contributing to the induction and storage of Pavlovian fear memories in the lateral amygdala. Learn Mem. 2013;20(8):421–430. doi: 10.1101/lm.030262.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirli KK, Ermentrout G, Cho RY. Computational study of NMDA conductance and cortical oscillations in schizophrenia. Front Comput Neurosci. 2014;8:133. doi: 10.3389/fncom.2014.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76(Part C):639–656. doi: 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4–5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK. Synpatic plasticity and phophorylation. Pharmacol Therapeut. 2006;112(3):810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, Herry C, Lüthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480(7377):331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Li XF, Phillips R, LeDoux JE. NMDA and non-NMDA receptors contribute to synaptic transmission between the medial geniculate body and the lateral nucleus of the amygdala. Exp Brain Res. 1995;105(1):87–100. doi: 10.1007/BF00242185. [DOI] [PubMed] [Google Scholar]
- Li G, Nair SS, Quirk GJ. A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. J Neurophysiol. 2009;101(3):1629–1646. doi: 10.1152/jn.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci. 2013;16(3):332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. A model of synpatic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31(2):191–201. doi: 10.1016/S0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13(3):169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10(2):86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity, and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35(1):24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent Bdnf gene regulation. Science. 2003;302(5646):890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Mm P. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29(45):14,185–14,198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22(1):295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390(6660):607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Merrill MA, Chen Y, Stack S, Hall JW. Activity-driven postsynaptic translocation of CaMKII. Trends Neurosci. 2005;26(12):645–653. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Nadkarni S, Jung P, Levine H. Astrocytes optimize the synaptic transmission of information. PLoS Comput Biol. 2008;4(5):e1000,088. doi: 10.1371/journal.pcbi.1000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology. 2006;31(2):287–296. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol Pharmacol. 2007;72(2):350–358. doi: 10.1124/mol.107.034934. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90(2):419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo M. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He M, Huang ZJ, Li B. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 2015;519(7544):455–459. doi: 10.1038/nature13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen B, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406(6798):889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Doyère V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Nature. 2007;52(1):215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000;23(2):75–80. doi: 10.1016/S0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synpatic plasticity. Trends Neurosci. 2002;25(11):578–588. doi: 10.1016/S0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16(6):317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA. 1997;94(2):719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471(7738):358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9(5):224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Caballero M, Robinson HPC. Fast and slow voltage-dependent dynamics of magnesium block in the NMDA receptor: the asymmetric trapping block model. J Neurosci. 2004;24(27):6171–6180. doi: 10.1523/JNEUROSCI.1380-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J Neurosci. 1999;19(21):9587–9603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26(48):12,387–12,396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Ninan I, Zhang H, Milner TA, Arancio O, Chao MV. BDNF-mediated neurotransmission relies upon a myosin VI motor complex. Nat Neurosci. 2006;9(8):1009–1018. doi: 10.1038/nn1730. [DOI] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TVP, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22(5):1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu AP, Hsiang HLL, Mercaldo V, Yan C, Richards B, Rashid AJ, Pressey J, Mahadevan V, Tran MM, Kushner SA, Woodin MA, Frankland PW, Josselyn SA. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83(3):722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Zhong H, Sia GM, Sato TR, Gray NW, Mao T, Khuchua Z, Huganir RL, Svoboda K. Subcellular dynamics of type II PKA in neurons. Neuron. 2009;62(3):363–374. doi: 10.1016/j.neuron.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Li S, Zhang X, Cai D. Phenomenological incorporation of nonlinear dendritic integration using integrate-and-fire neuronal frameworks. PLoS ONE. 2013;8(1):e53,508. doi: 10.1371/journal.pone.0053508. [DOI] [PMC free article] [PubMed] [Google Scholar]