Abstract
DNA polymerases accommodate various base pair conformations in the event of incorrect insertions. In particular, Watson-Crick-like dG:dTTP base pair has been observed at the insertion site of human DNA polymerase β (pol β). A potential factor contributing to the diverse conformations of base pair mismatches is minor groove interactions. To gain insight into the effect of minor groove interactions on base pair conformations, we generated an Asn279Ala polβ mutant that cannot make minor groove contacts with incoming nucleotide. We conducted structural and kinetic studies of Asn279Ala polβ in complex with incoming dTTP and templating dG or O6-methyl-dG. The crystal structure of the Asn279Ala polβ-G:T complex showed a wobble dG:dTTP base pair, indicating that the previously observed Watson-Crick-like dG:dTTP conformation was induced by the minor groove contact. In contrast, O6-methyl-dG, an analog of the enol tautomer of guanine, formed a Watson-Crick-like base pair with dTTP in the absence of the minor groove contact. These results suggest that the Watson-Crick-like G:T base pair at the insertion site is formed by the rare enol tautomers of G or T, whose population is increased by the minor groove hydrogen bond with Asn279. Kinetic studies showed that Asn279Ala mutation decreased dG:dTTP misincorporation rate 6-fold in the presence of Mg2+ but increased the rate 3-fold in the presence of Mn2+, highlighting the effect of minor groove interactions and metal ions on promutagenic replication by polβ.
INTRODUCTION
DNA polymerases play a critical role in preserving genome integrity by incorporating the correct nucleotide from a pool of structurally similar nucleotides during DNA repair and replication [1]. Kinetic and structural studies of DNA polymerases that incorporate correct nucleotides have provided important mechanistic insights into how these enzymes efficiently and accurately catalyze nucleotidyl transfer [2–5]. Several factors, such as inter-nucleobase hydrogen bonding, shape complementarity, dNTP binding affinity, the induced-fit mechanism, intrinsic proofreading activity, and minor groove interactions, have been identified as contributors to the replication fidelity of DNA polymerases [6,7].
Recent structural studies of the incorporation of incorrect nucleotides by DNA polymerases have provided insights into how these enzymes increase their replication fidelity by deterring misincorporation [8–13]. Crystal structures have shown that most DNA polymerases allow only Watson-Crick base pairing for correct insertions, whereas they accommodate varying base pair conformations for mismatches in the binding pocket. For example, the A-family DNA polymerase Bacillus stearothermophilus DNA polymerase I fragment (BF) accommodates wobble dG:dTTP and dA:dCTP base pairs in the presence of active-site Mg2+ [11,14,15,16]. The B-family DNA polymerase RB69 induces wobble dG:dTTP and dT:dGTP base pairs in the active site [12]. In the catalytic pocket of the Y-family DNA polymerases Sulfolobus solfataricus Dpo4 and human polη, dT:dGTP adopts staggered and wobble base pair conformations, respectively [10,17]. In contrast, in the active site of the X-family DNA polymerase polλ, Watson-Crick-like dT:dGTP base pairs have been observed in the presence of active-site Mg2+ [18]. Interestingly, BF shows a metal-dependent conformational transition for dA:dCTP mismatches [11]: Substituting the active-site Mn2+ for Mg2+ triggers a wobble-to-Watson-Crick-like conformational transition for dA:dCTP via a rare tautomerization mechanism. Recently, we have reported that the X-family DNA polymerase β (polβ) induces the formation of a staggered dG:dTTP base pair in the presence of active-site Mg2+ and a Watson-Crick-like dG:dTTP base pair in the presence of Mn2+ (Figure 1) [19]. Unlike BF, polβ accommodates a staggered dA:dCTP base pair in the presence of either Mg2+ or Mn2+ [20].
Figure 1.
Base-pairing patterns of G:T mismatches. G:T mismatches with wobble and Watson-Crick-like base pairing. Mismatched base pairs with a Watson-Crick-like geometry were observed in the active sites of wild-type polβ, polλ and BF (11, 18, 19).
What explains the wide variety of mismatch base pair conformations observed in DNA polymerase active sites? A potential contributing factor is minor groove hydrogen bonding interactions, which vary widely among DNA polymerases [11,21]. Crystal structures of DNA polymerases in complex with duplex DNA and an incoming nucleotide show significant differences in specific hydrogen bonding interactions between the minor groove edge of the DNA and the amino acid residues of the protein [2,4, 11,22]. Minor groove contacts in the catalytic pocket of DNA polymerases contribute to the replication fidelity and the catalytic efficiency of these enzymes [23–28]. In addition, minor groove interactions can modulate deletion error rates [29], base substitution specificity [24], misincorporation rates [30], mismatch extension [14,30] and lesion bypass [26]. However, the effect of minor groove hydrogen bonding interactions on mismatched base pair conformations in the catalytic pocket remains poorly understood.
We selected polβ as a model DNA polymerase to gain insights into the effect of minor groove interactions on the conformations of base pair mismatches. Polβ, an error-prone enzyme that lacks intrinsic 3′-to-5′ exonuclease activity, is the smallest mammalian DNA polymerase and has been extensively used as a model enzyme to study the replication-fidelity and nucleotidyl-transfer mechanisms of DNA polymerases [31]. Polβ plays a critical role in the base-excision-repair pathway by filling in short nucleotide gaps [31]. During correct nucleotide insertion, Tyr271, Arg283, and Asn279 make hydrogen bond contacts with the minor groove edges of the primer terminus, templating base, and incoming nucleotide, respectively [22,32]. Disrupting the minor groove hydrogen bonding to the primer terminus by using a Tyr271Phe mutation has minimal effects on catalytic efficiency and fidelity [33]. However, introducing an Arg283Lys mutation to disturb minor groove contact to the templating base drastically decreases both the catalytic efficiency and the replication fidelity of the enzyme [25,33–35]. Intriguingly, the disrupted minor groove interactions in the Asn279Ala mutant leads to a decrease in replication fidelity for non-gapped DNA, whereas replication fidelity increases 5-fold for short-nucleotide-gapped DNA [33, 36].
To evaluate the effect of minor groove interactions and metal ions on promutagenic replication by polβ, we conducted kinetic studies of Asn279Ala polβ for correct and incorrect insertions in the presence of Mg2+ or Mn2+. In addition, we determined four ternary complex structures of Asn279Ala polβ. The four structures contained the mutant enzyme in complex with dG:dTTP in the presence of the active-site Mg2+ or Mn2+, dG:dCTP in the presence of Mg2+, and O6MeG:dTTP in the presence of Mn2+. These studies provide new insights into how minor groove hydrogen bonding interactions and metal ions affect promutagenic replication by DNA polymerases.
EXPERIMENTAL
Preparation of the Asn279Ala polβ mutant
The Asn279Ala mutant polβ construct was generated by using oligonucleotide-directed mutagenesis, according to the manufacturer’s protocol (QuikChange Mutagenesis System, Stratagene). A pair of complementary mutagenic oligonucleotides, (forward) 5′-CACTGGGAGTGATATTTTCGCTAAGAATATGAGGGCTCATG-3′ and (reverse) 5′-CATGAGCC CTCATATTCTTAGCGAAAATATCACTCCCAGTG-3′, were designed to introduce a mutant codon (underlined) encoding the Asn279Ala mutation in the polβ sequence. PCR was performed using a pET32 vector containing human POL B as a template, the mutagenic primers, dNTP mixture, and Pfu turbo DNA polymerase in the manufacturer’s buffer under the following conditions: 95 °C for 2 min, followed by 28 cycles of 95 °C for 30 s, 50–65 °C for 1 min, and 68 °C for 8 min. DpnI was then added to the reaction mixture and incubated for 30 min at 37 °C to digest the methylated parent plasmid. The mixture was used to transform competent XL-10 Gold Escherichia coli cells, and the colonies were selected on LB agar plates supplemented with kanamycin (30 μg/mL). The plasmids were purified from an overnight culture, and the desired DNA sequence was confirmed by DNA sequencing (DNA Core Facility, The University of Texas at Austin). The Asn279Ala polβ mutant was expressed and purified according to previously described protocols [19], with minor modifications.
Steady-state kinetics of nucleotide insertion opposite the templating G
The steady-state kinetic parameters for insertion opposite the templating guanine by polβ were measured as described previously [20]. Briefly, the DNA oligonucleotides for the kinetic assays (upstream primer, 5′-FAM-CTGCAGCTGATGCG-3′, downstream primer, 5′-phosphate/CGTACGGATCCCCGGGTAC-3′, and template, 5′-GTACCCGGGGATCCGTACGGCGCATCAGCTGCAG-3′) were synthesized by Midland Certified Reagent company (Midland, TX) and Integrated DNA Technologies (Coralville, IA). They were purified by the manufacturers and confirmed by MALDI-TOF mass spectrometry. Each oligonucleotide was annealed in hybridization buffer (10 mM Tris-HCl, pH 7.5, and 1 mM EDTA) to prepare a DNA substrate for polβ containing a single-nucleotide gap opposite guanine. Enzyme activities were determined using a reaction mixture containing 50 mM Tris-HCl, pH 7.5, 100 mM KCl, 5 mM MgCl2 or MnCl2, 80 nM single-nucleotide gapped DNA, and different concentrations of incoming dNTP.
To prevent end-product inhibition and substrate depletion from interfering with accurate velocity measurements, reaction times and enzyme concentrations (either the wild type or Asn279Ala polβ mutant) were optimized for each experiment (less than 20% insertion product formed). The reactions were initiated by the addition of the enzyme and stopped with gel-loading buffer (95% formamide with 20 mM EDTA, 45 mM Tris-borate, 0.1% bromophenol blue, and 0.1% xylene cyanol). The quenched samples were separated on 18 or 20% denaturing urea polyacrylamide gels. The gels were analyzed using a PhosphorImager (Molecular Dynamics) to quantify product formation. The kcat and Km were determined by fitting the reaction rates at various dCTP concentrations to the Michaelis-Menten equation. Each experiment was repeated at least three times to measure the average of the kinetic results. The efficiency of nucleotide insertion was calculated as kcat/Km. The relative frequency of dNTP incorporation opposite the templating guanine was determined as f = (kcat/Km) [dNTP:dG]/(kcat/Km) [dCTP:dG].
Co-crystallization and structure determination of binary and ternary polβ-DNA complexes
To obtain the binary complex of Asn279Ala polβ-DNA complex, polβ was incubated with a single-nucleotide gapped DNA containing a 16-mer template (5′-CCGAC[G or O6-methylguanine]GCGCATCAGC-3′), a complementary 10-mer upstream primer (5′-GCTGATGCGC-3′), and a 5-mer downstream primer (5′-pGTCGG-3′). Subsequently, a 10-fold molar excess of nonhydrolyzable dTMPNPP or dCMPNPP (Jena Bioscience) was added to the binary complex. Ternary Asn279Ala polβ-DNA complex co-crystals with nonhydrolyzable dNTP analogs paired with templating G or O6-methylguanine (O6MeG) were grown in a buffer solution containing 50 mM imidazole, pH 7.5, 14–23% PEG3400, and 350 mM sodium acetate as described previously [19]. The diffraction data were collected at 100 K at the beamline 5.0.3 at the Advanced Light Source, Lawrence Berkeley National Laboratory. All diffraction data were processed using HKL2000. The structures were solved by molecular replacement using a ternary complex structure with a closed conformation (PDB ID 1BPY) as the search model [22]. The model was built using COOT and refined using Phoenix, and the figures were generated using PyMOL.
RESULTS
The Asn279Ala mutation of polβ modulates the catalytic efficiency of the enzyme
To assess the effect of the Asn279Ala mutation on catalytic efficiency, we first determined the kinetic parameters for single nucleotide incorporation opposite the templating dG by polβ in the presence of active-site Mg2+ or Mn2+ (Table 1). DNA containing a single-nucleotide gap and a 5′-phosphorylated downstream primer was used for the kinetic study because this type of DNA has been shown to be an ideal substrate for polβ-mediated nucleotidyl transfer [37]. Mn2+ was chosen because this metal ion increased the misincorporation rate and promote the formation of a closed conformation [38].
Table 1.
Steady-state kinetics for nucleotide incorporation opposite templating dG by wild-type or Asn279Ala polβ.
| template-dNTP (metal ion) | Km (μM) | kcat (10−3s−1) | kcat/Km (10−3s−1μM−1) | F ins | |
|---|---|---|---|---|---|
| wild type polβ | G-C (Mg2+) | 0.6 ±0.10 | 212.0 ±19.87 | 353.3 | 1 |
| G-T (Mg2+) | 56.1±4.59 | 2.77 ±0.35 | 0.049 | 1.4×10−4 | |
| G-C (Mn2+) | 0.08±0.01 | 30.3 ±1.51 | 383.7 | 1 | |
| G-T (Mn2+) | 11.2±0.47 | 19.1 ±0.78 | 1.71 | 4.4×10−3 | |
| Asn279Ala polβ | G-C (Mg2+) | 0.79±0.04 | 730±17.52 | 924.1 | 1 |
| G-T (Mg2+) | 110.9±5.29 | 2.38±0.17 | 0.021 | 2.3×10−5 | |
| G-C (Mn2+) | 0.39±0.03 | 31.52±0.90 | 80.8 | 1 | |
| G-T (Mn2+) | 7.93±0.38 | 10.28±0.55 | 1.29 | 1.6×10−2 |
A comparison of the kinetic parameters between Asn279Ala polβ and wild-type polβ indicated that the Asn279Ala mutation significantly affected catalytic efficiency. In the presence of Mg2+, the Asn279Ala mutation yielded an ~3-fold increase in the catalytic efficiency for dG:dCTP (924.1 vs. 353.3 s−1μM−1) compared with wild-type polβ. This result contrasted with previous work in which an Asn279Ala polβ mutant showed a 17-fold decrease in catalytic efficiency for correct base pairing when non-gapped DNA was used [36], thus highlighting the substrate dependence of the catalytic efficiency in Asn279Ala polβ. The enhanced catalytic efficiency for correct nucleotide insertion resulted from an increased kcat rather than a decreased Km. The Asn279Ala mutation decreased the insertion efficiency for dG:dTTP ~2-fold (0.021 vs. 0.049 s−1μM−1) compared with the wild-type enzyme, owing to an increased Km for the incorrect nucleotide (110.9 vs. 56.1 μM). The increased catalytic efficiency for correct nucleotide insertion and the decreased efficiency for incorrect insertion decreased the dG:dTTP misincorporation frequency (Fins) ~6-fold (2.3×10−5 vs. 1.4×10−4) compared with wild-type polβ (Table 1).
Published reversion-frequency studies with Asn279Ala and wild-type polβ have shown that the mutation increases the replication fidelity ~5-fold [33], which is consistent with our observed 6-fold decrease (2.3×10−5 vs. 1.4×10−4) in the error rate for dG:dTTP incorporation by Asn279Ala polβ. Together, the results suggest that the increased replication fidelity of the Asn279Ala polβ mutant [33] is a consequence of increased catalytic efficiency for correct insertion and reduced incorrect insertion efficiency. The decreased misincorporation rate for Asn279Ala polβ is interesting because previous studies of polβ with mutations at Tyr265, Ile260, Lys289, Lys280, and Arg283 have shown an increase in the misincorporation rate [24, 33, 36, 39–41].
The effect of metal cofactor on promutagenic replication of polβ
When Mn2+ was used as metal cofactor in place of Mg2+, the Asn279Ala mutation increased the dG:dTTP misincorporation frequency 3-fold (4.4×10−3 vs. 1.6×10−2, Table 1), exhibiting the opposite phenotype. In the case of wild-type polβ, replacing Mg2+ with Mn2+ did not significantly change the correct nucleotide insertion efficiency (353.3 vs. 383.7 s−1μM−1) but increased the efficiency for incorrect insertion by 34-fold (0.049 vs. 1.71 s−1μM−1), thereby greatly promoting misinsertion. The Mn2+-mediated increase in misincorporation rate suggests significant conformational differences between the Mg2+- and Mn2+-bound mismatched (dG:dTTP) complexes. In the case of the Asn279Ala mutant, the use of Mn2+ decreased the efficiency of correct insertion by 12-fold (924.1 vs. 80.8 s−1μM−1) but increased the efficiency of incorrect nucleotide insertion by 60-fold (0.021 vs. 1.29 s−1μM−1), thereby dramatically (720-fold) increasing the misincorporation rate. The Mn2+-induced decrease in correct insertion efficiency (dG:dCTP) mainly results from decreased kcat (730 vs. 31.5).
The Asn279Ala mutation alters the protein and base-pair conformations in G:T mismatches
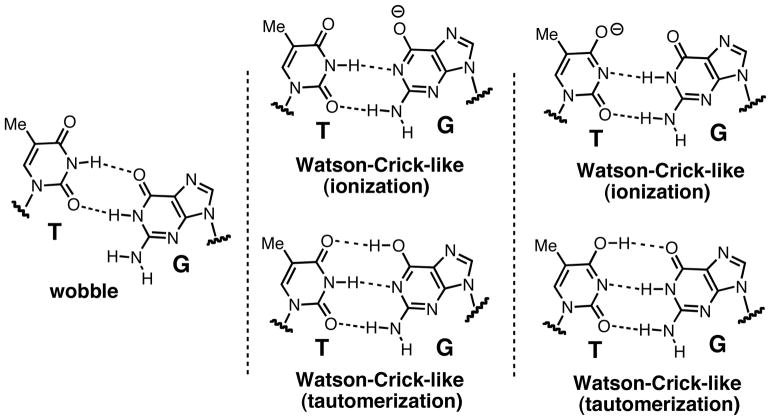
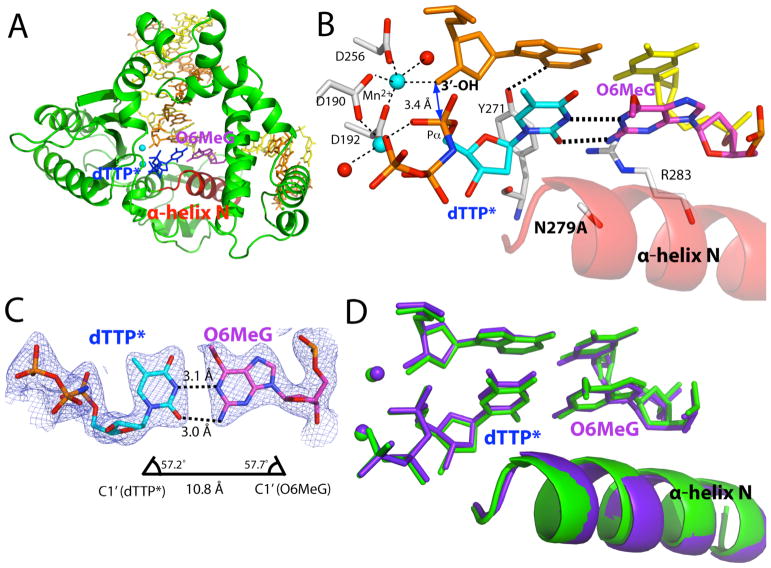
We solved the ternary structure of Asn279Ala polβ in complex with the dG:dTTP mismatch and Mg2+ to investigate the effects of Asn279-mediated minor groove interactions on the conformation of a G:T base pair. The Asn279Ala polβ-G:T-Mg2+ ternary structure, which was refined to 2.51 Å, shows that the Asn279Ala mutation significantly alters the protein and DNA conformations (Figure 2D). The mutant-G:T-Mg2+ ternary structure shows a semi-closed conformation, a coplanar G:T base-pair conformation, and one active-site metal ion (Figure 2A). This structure differs from the published wild-type polβ-G:T-Mg2+ ternary structure, which exhibits an open conformation, a pseudo-propeller twist base-pair conformation and two active-site metal ions (Figure 2D). The templating dG forms a wobble base pair with the incoming dTTP* (Figure 2C). The O6 and N1 of the templating dG engage in hydrogen bonding interactions with N3 and O2 of dTTP*, respectively (Figure 2B). Notably, Tyr271 forms a bifurcated hydrogen bond with N2 of the templating dG and O2 of the incoming dTTP*. The formation of a hydrogen bond network among the templating dG, incoming dTTP*, and Tyr271 stabilizes a wobble dG:dTTP base-pair conformation and a semi-closed conformation.
Figure 2.
Ternary structure of Asn279Ala polβ in complex with the dG:dTTP* mismatch in the presence of active-site Mg2+ (PDB ID 4Z6D). (A) Overall structure of Asn279Ala polβ with the templating dG paired with nonhydrolyzable dTMPNPP (dTTP*). The incoming dTTP* is shown in blue. The templating dG is shown in magenta, whereas the rest of templating strand bases are shown in yellow. The upstream and downstream primers are shown in orange. The α-helix N is shown in red. (B) Close-up view of the active site of the Asn279Ala dG:dTTP-Mg2+ ternary structure. The catalytic aspartates and amino acid residues involved in minor groove edge recognition are indicated. (C) Base pairing between the templating dG and the incoming dTTP*. The C1′ distance and λ angles are shown. A 2Fo-Fc map contoured at 1σ around dG:dTTP* is shown. Distances for O6(dTTP*)-O6(dG), N1(dTTP*)-N1(dG), and O2(dTTP*)-N2(dG) are 3.8Å, 3.9Å, and 3.9Å, respectively, indicating a wobble dG:dTTP* base pair. (D) Comparison of the active site of the Asn279Ala polβ-dG:dTTP*-Mg2+ structure (shown in green) with that of the published wild-type polβ-dG:dTTP*-Mg2+ structure (shown in blue, PDB ID 4PGQ) (19).
Minor groove contact by Asn279 is required for the formation of a Watson-Crick-like dG:dTTP base-pair conformation
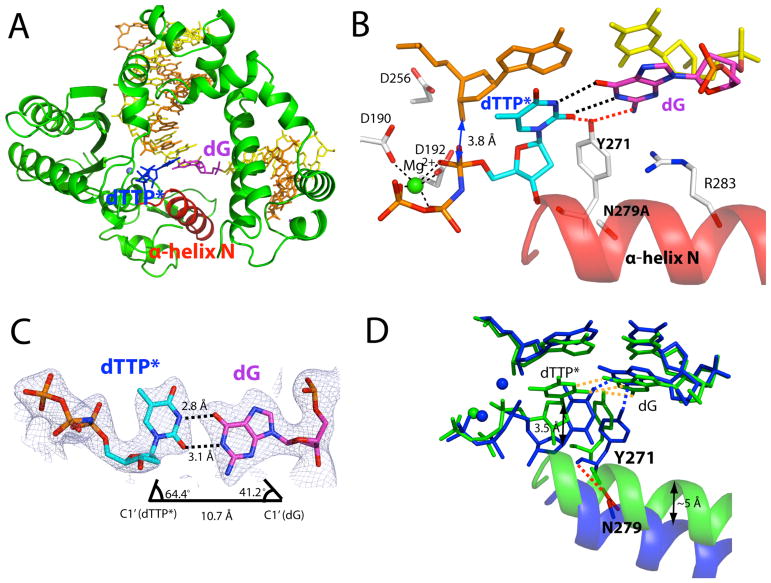
The Asn279Ala polβ-G:T-Mg2+ structure with a semi-closed conformation does not represent a catalytically competent state. To gain structural insights into the pre-chemistry state of the Asn279Ala polβ-G:T complex, we determined the ternary structure of Asn279Ala polβ in complex with a dG:dTTP mismatch and Mn2+ (Figure 3). Using Mn2+ in place of Mg2+ has been shown to promote the formation of a closed polβ conformation [19,38]. The Asn279Ala polβ-G:T-Mn2+ structure, which was solved to 2.75 Å resolution, shows a closed conformation and two active-site metal ions rather than the semi-closed conformation and one active-site metal ion that were observed in the Asn279Ala polβ-G:T-Mg2+ structure. This result indicates that substituting Mn2+ for Mg2+ facilitated the formation of a closed conformation in addition to catalytic metal ion coordination (Figures 3B and 3D). The large conformational difference between the Asn279Ala polβ-G:T-Mn2+ and the Asn279Ala polβ-G:T-Mg2+ complexes is consistent with the ~60-fold difference in catalytic efficiency (Table 1). The overall structure of the Asn279Ala polβ-G:T-Mn2+ complex is essentially identical to that of the wild-type polβ-G:T-Mn2+ structure (RMSD = 0.356 Å, Figure 3E).
Figure 3.
Ternary structure of Asn279Ala polβ with the dG:dTTP* mismatch and active-site Mn2+ (PDB ID 4Z6E). (A) Overall structure of the Asn279Ala polβ-dG:dTTP*-Mn2+ ternary complex. Polβ adopts a closed conformation. (B) Close-up view of the active site of the Asn279Ala polβ-dG:dTTP*-Mn2+ ternary structure. Mn2+ ions are shown as cyan spheres. (C) dG:dTTP* base pairing observed in the Asn279Ala polβ active site. Distances for N1(dTTP*)-O6(dG) and O2(dTTP*)-N1(dG) are indicated by dashed lines. Distances for O6(dTTP*)-O6(dG), N1(dTTP*)-N1(dG), and O2(dTTP*)-N2(dG) are 3.8Å, 3.4Å, and 3.1Å, respectively. The λ angles for dG and dTTP* are 45.8° and 65.8°, respectively, indicating a wobble base pair geometry. A 2Fo-Fc map contoured at 1σ around dG:dTTP* is shown. (D) Comparison of the Asn279Ala polβ-dG:dTTP*-Mg2+ (green) and the Asn279Ala polβ-dG:dTTP*-Mn2+ (purple) structures. (E) Published ternary structure of wild-type polβ with the dG:dTTP* mismatch and active-site Mn2+ (PDB ID 4PGX). Hydrogen bond contacts by Asn279 are indicated by dotted lines. O2 of dTTP* and the backbone carbonyl oxygen of Tyr271 engage in hydrogen bond interactions with Asn279. (F) Base pairing properties of dG:dTTP* in the published structure with the wild-type polβ and Mn2+ (PDB ID 4PGX)19. The λ angles for dG and dTTP* are 50.1° and 56.2°, respectively, indicating Watson-Crick-like base pair geometry. A 2Fo-Fc map contoured at 1σ around dG:dTTP* is shown. Distances of N1(dTTP*)-O6(dG) and O2(dTTP*)-N1(dG) are 3.1Å and 3.2Å, respectively. (G) Comparison of the Asn279Ala polβ-dG:dTTP*-Mn2+ (green) and the polβ-dG:dTTP*-Mn2+ (black) structures. Both structures contain two Mn2+ ions at the insertion site. (H). Comparison of dG:dTTP base pairing in the active site of wild-type polβ (black) and Asn279Ala polβ (green). Hydrogen bonds are indicated by dashed lines.
The Asn279Ala mutation significantly affects the base-pair conformation of dG:dTTP in the enzyme active site (Figures 3C and 3F). The major structural difference between the Asn279Ala polβ-G:T-Mn2+ and wild-type polβ-G:T-Mn2+ complexes is the base-pair conformation of the G:T mismatch. While the published wild-type polβ-G:T-Mn2+ structure shows a Watson-Crick-like G:T conformation in the active site (Figure 1), the Asn279Ala polβ-G:T-Mn2+ structure shows a wobble G:T base-pair conformation, thus signifying that the formation of a Watson-Crick-like G:T conformation requires hydrogen bonding between the O2 of dTTP and the NH2 of Asn279. The hydrogen bonding between the O2 of dTTP and NH2 of Asn279 appears to increase the population of the enol tautomeric or ionized species of dTTP (Figure 1), thereby promoting the formation of a Watson-Crick-like G:T base pair.
The presence of a Watson-Crick-like G:T base pair in the active site of wild-type polβ, as opposed to the wobble base pair in the mutant, suggests that the catalytic pocket is ideally equipped to accommodate Watson-Crick or Watson-Crick-like conformations in a closed conformation. This result contrasts with the characteristics of other DNA polymerases, such as the high-fidelity DNA polymerases BF and RB69, which allow wobble-mismatched base pairs in a closed conformation [11,12]. Differences in the mismatched base-pair conformations among DNA polymerases may result from differences in the geometric selection mechanisms employed by these enzymes. While BF and RB69 enforce the A-DNA conformation in their catalytic sites, polβ promotes the B-DNA conformation, which is narrower than A-DNA [31]. The strict geometric selection of polβ may be largely influenced by the minor groove interactions of Asn279. Disrupting these interactions through the Asn279Ala mutation appears to mitigate the geometric restraints imposed by the enzyme active site, thereby allowing a relaxed wobble G:T base pair.
The Asn279Ala mutation does not affect the protein and nascent base-pair conformations for correct nucleotide insertion
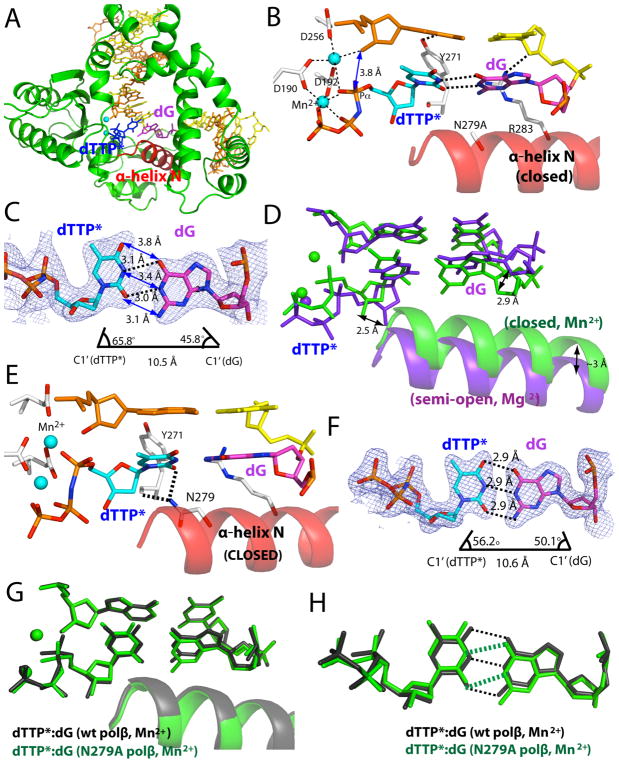
To evaluate whether the minor groove hydrogen bonding by Asn279 also affects protein and base-pair conformations during correct nucleotide insertion, we determined the crystal structure of Asn279Ala polβ in complex with nonhydrolyzable dCMPNPP (hereafter dCTP*) opposite the templating dG in the presence of Mg2+. The Asn279Ala polβ-G:C ternary structure, which was refined to 2.7 Å, shows a closed conformation and a coplanar Watson-Crick base pair (Figure 4). The protein and base-pair conformations are essentially indistinguishable from those observed in the published polβ ternary structures with correct insertions, thus indicating that Asn279 has a minimal effect on the polβ ternary complex with correct base pairing. The Asn279Ala polβ-G:C structure indicates that the minor groove contact by Asn279 is not required for the formation of a closed polβ conformation.
Figure 4.
Ternary structure of Asn279Ala polβ with dG:dCTP* (PDB ID 4Z6C) in the presence of active-site Mg2+. (A) Overall structure of the Asn279Ala polβ-dG:dCTP* complex. (B) Close-up view of the active site of the Asn279Ala polβ-dG:dCTP* complex. The α-helix N adopts a closed conformation. (C) Base pairing of dG:dCTP* in the active site of Asn279Ala polβ. A 2Fo-Fc map contoured at 1σ around dG:dCTP* is shown. (D) Comparison of the active site structures of the Asn279Ala polβ-dG:dCTP*-Mg2+ (green) and wild-type polβ-dG:dTTP*-Mn2+ (blue) complexes.
Minor groove hydrogen bonding by Asn279 is not required for the formation of the Watson-Crick-like O6-methylguanine:dTTP base pair
The observation of a Watson-Crick-like G:T base pair in the presence of the minor groove contact with the incoming nucleotide in the polβ active site and the formation of a wobble G:T base pair in the absence of the minor groove contact strongly suggests that the formation of Watson-Crick-like G:T base pairs involves the ionization or enol tautomerization of dTTP, which is promoted by the minor groove hydrogen bonding with Asn279. To evaluate the possible involvement of ionized or tautomeric bases in Watson-Crick-like G:T base pairing, we determined the crystal structure of polβ in complex with O6-methylguanine (O6MeG) and incoming dTTP in the presence of active-site Mn2+. We chose O6MeG because it is a close analog of the enol tautomeric species of guanine and because an analog of the tautomeric species of dTTP was not commercially available. Active-site Mn2+ was used because this metal ion is known to facilitate the formation of a closed polβ conformation [20]. We expected O6MeG and dTTP to form a Watson-Crick-like base pair in the absence of the minor groove contact with dTTP. The cocrystal structure of Asn279Ala polβ in complex with templating O6MeG and incoming dTTP* in the presence of Mn2+ was refined to 2.44 Å.
The structure of the Asn279Ala polβ-O6MeG:dTTP* complex shows a closed conformation and a Watson-Crick-like O6MeG:dTTP* base pair, which is virtually identical to that of the published wild-type polβ-O6MeG:dTTP* complex [20] (Figure 5A). The N1 and N2 positions of O6MeG form hydrogen bonds with the N3 and O2 positions of dTTP, respectively (Figures 5B and 5C). The O6-methyl group does not form a C-H••O hydrogen bond with O4 of dTTP. The base-pair geometry of O6MeG:dTTP* in the mutant polβ structure is indistinguishable from that in the wild-type polβ structure (Figure 5D). This result contrasts with the large conformational difference that was observed in the dG:dTTP base-pairing geometry of the polβ-G:T and Asn279Ala polβ-G:T complexes [20]. Unlike the Watson-Crick-like dG:dTTP base pair, the formation of the Watson-Crick-like O6MeG:dTTP base pair in the polβ active site does not require a minor groove contact with Asn279, probably because O6MeG, which is structurally analogous to the enol tautomeric form of guanine, can form a Watson-Crick-like base pair with the keto tautomer of dTTP (Figure 1). The formation of a Watson-Crick-like O6MeG:dTTP* base pair in the absence of the minor groove contact thus supports the hypothesis that the ionized or enol tautomeric species of guanine or thymine is involved in Watson-Crick-like G:T base pairing.
Figure 5.
Structure of Asn279Ala polβ with the O6MeG:dTTP* mismatch in the presence of active site Mn2+ (PDB ID 4Z6F). (A) Overall structure of the Asn279Ala polβ-O6MeG:dTTP* complex. (B) Closeup view of the active site of the Asn279Ala polβ-O6MeG:dTTP* complex. The α-helix N adopts a closed conformation, and O6MeG:dTTP* adopts coplanar conformation. (C) Base pairing properties of O6MeG:dTTP* in the active site of Asn279Ala polβ. O6MeG:dTTP* forms a Watson-Crick-like base pair with two hydrogen bonds. A 2Fo-Fc map contoured at 1σ around O6MeG:dTTP* is shown. (D) Comparison of the active site structures of the Asn279Ala polβ-O6MeG:dTTP* (shown in green) and published wild-type polβ-O6MeG:dTTP* (shown in purple, PDB ID 4NXZ) complexes (20).
DISCUSSION
The effect of Asn279Ala mutation and Mn2+ on polβ and base pair conformations
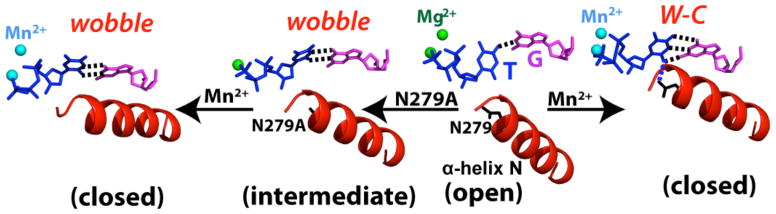
Our structural studies reveal that the Asn279Ala mutation and metal cofactor replacement significantly alters conformations of polβ and dG:dTTP base pair (Figure 6). In the presence of Mg2+ and wild-type polβ, the nascent dG:dTTP base pair adopts a staggered conformation with an interbase hydrogen bond (between N1 of dG and O4 of dTTP) and the protein is in an open conformation. When Mn2+ is used as a cofactor, the mismatched base pair takes on Watson-Crick-like conformation and the protein now assumes a closed conformation, which is structurally competent for nucleotide incorporation. The Mn2+-induced conformational change of polβ and dG:dTTP base pair is consistent with the 35-fold increase in catalytic efficiency (Table 1). The introduction of Asn279Ala of polβ in the presence of Mg2+ results in the formation of wobble base pair with two interbase hydrogen bonds (between dG and dTTP) and a semi-open protein conformation, where only nucleotide-binding metal ion is present. Under the combined influence of Asn279Ala mutation and Mn2+ cofactor, the nascent dG:dTTP remains in the same wobble base pair but the protein undergoes semi-open to closed conformational reorganization. When Mn2+ is used, the catalytic efficiencies of dG:dTTP insertion for wild-type and Asn279Ala pol polβ are similar (1.71 vs. 1.29) despite the difference in base pair conformation (Watson-Crick-like vs. wobble), indicating that protein conformation contributes more to the catalytic efficiency than the base pair conformation. Overall, these results clearly show that Asn279Ala mutation and Mn2+ exert significant impact on conformations of polβ and dG:dTTP mismatch.
Figure 6.
The effect of Asn279Ala mutation and metal cofactor substitution on the conformations of polβ and base pair. Key hydrogen bonds involving Asn279 and base pairs are indicated in dotted lines. Mg2+ and Mn2+ are shown in green and cyan spheres, respectively.
Our results provide insight into the mechanisms of manganese mutagenesis. The trace element Mn2+ is used as a cofactor for wide range of cellular enzymes such as superoxide dismutase. Although Mg2+ is physiologically relevant cofactor for DNA polymerases, other metal ions such as Mn2+ can substitute for Mg2+ at higher concentrations: The higher level of Mn2+, which can be arisen by disruption of Mn2+ homeostasis, is toxic and causes higher mutagenicity and a central nervous system disorder. When Mg2+ is used as a cofactor, polβ misinserts dTTP opposite templating dG about every 7,000 nucleotides incorporated (Table 1). The utilization of Mn2+ cofactor for nucleotide incorporation facilitates mutagenic replication, making an error for about every 230 nucleotides incorporated (Table 1). This manganese-promoted mutagenesis is likely caused, in part, by stabilization of mismatched nascent base pair in the active site.
Minor groove interactions may facilitate the formation of Watson-Crick-like mismatched base pairs in DNA polymerase active sites
In the absence of protein contacts, mismatched base pairs with Watson-Crick-like geometry are energetically unfavorable and exist transiently in low abundance [13,42, 43,44]. The enol tautomer of guanine has been calculated to be ~one million-fold less abundant than the keto tautomer in water [45]. Thus, the observation of Watson-Crick-like mismatched base pairs in the active site of various DNA polymerases indicates that the microenvironments within the active site of DNA polymerases promote the formation of Watson-Crick-like mispairs. The wild-type and Asn279Ala polβ-G:T structures provide insights into the effect of minor groove interactions on mismatched base pair conformations (Figure 6). The formation of a Watson-Crick-like dG:dTTP base pair in the presence of the minor groove hydrogen bond donor Asn279 and a wobble dG:dTTP base pair in the presence of Asn279Ala suggest that the minor groove contact with an incoming nucleotide promotes Watson-Crick-like G:T mispairs. In principle, Watson-Crick-like dG:dTTP base pairs can occur through either tautomerization or ionization. Hydrogen bonding to the minor groove edge of an incoming nucleotide may increase the population of the tautomeric or ionic form of the nucleotide, thereby facilitating the formation of Watson-Crick-like mismatches.
Minor groove interactions between DNA polymerase and an incoming nucleotide may contribute to the formation of a wide variety of mispair conformations at the insertion site. To date, mismatches with a Watson-Crick-like geometry have been observed in the structures of A- and X-family DNA polymerases. In the case of the A-family DNA polymerase BF, dA:dCTP adopts a wobble conformation in the absence of minor groove contact with the incoming nucleotide. In contrast, the same base pair adopts a Watson-Crick-like conformation in the presence of Mn2+-induced minor groove contact [11], illustrating the effect of minor groove interactions on mispair conformations. In the case of the X-family DNA polymerase polλ, dT:dGTP adopts a Watson-Crick-like conformation in the presence of minor groove contact with the incoming nucleotide [18]. The B-family DNA polymerase RB69, which does not engage in minor groove hydrogen bonding with the incoming nucleotide, accommodates wobble conformations for both dG:dTTP and dT:dGTP base pairs [12]. These observations highlight that minor groove contact with the incoming nucleotide may significantly influence mismatched base pair conformations.
A highly stringent geometric selection mechanism for polβ
Although polβ lacks an intrinsic proofreading exonuclease activity, this enzyme possesses several characteristics of high-fidelity DNA polymerases, such as an open-to-closed conformational change and a strict geometric selection mechanism [31]. Polβ uses a rigorous geometric selection mechanism to discriminate between correct and incorrect base pairs within the nascent base-pair binding pocket. Although many DNA polymerases, including the A-, B-, and Y-family DNA polymerases, allow a wobble G:T base pair with two hydrogen bonds in the incipient base-pair site, the X-family DNA polymerase polβ does not allow such base pair. Instead, this enzyme enforces a staggered G:T base pair with a hydrogen bond and adopts an open conformation in the presence of active-site Mg2+. When the mutagenic Mn2+ is used in place of Mg2+, the enzyme induces the formation of a Watson-Crick-like G:T base pair and adopts a closed conformation [19]. In the case of A:C mismatches, even the presence of Mn2+ does not induce the formation of a Watson-Crick-like mispair, and the protein adopts a semi-open conformation [19]. The observed conformational differences indicate that in the closed conformation, the nascent base-pair binding pocket of polβ is highly stringent and strongly discriminates between base pairs with Watson-Crick and non-Watson-Crick geometry. The rigid geometric constraints imposed by the polβ active site may enforce the B-DNA conformation rather than the A-DNA conformation, with a widened minor groove within the active site, thereby deterring the formation of a relaxed, non-Watson-Crick conformation in the pocket.
The minor groove contacts of polβ with the incoming nucleotide and templating base may contribute to the strict geometric selection of this enzyme. A DNA polymerase that engages in minor groove contacts with both the incoming nucleotide and templating base is rare. The A-, C- and Y-family DNA polymerases (e.g., BF, PolC, Dpo4, and polη) make contact with only the minor groove edge of incoming nucleotide. On the other hand, the B-family DNA polymerase RB69 probes the minor groove edges of the replicating base pair via only Van der Waals interactions. In the case of the X-family polymerases polβ and polλ, the minor groove edges of the incoming nucleotide and templating base are contacted by Asn and Arg residues, respectively. Polβ, which possesses some characteristics of high-fidelity DNA polymerases but lacks an intrinsic proofreading function, appears to increase its replication fidelity by extensively probing minor groove edges and imposing tight geometric constraints at the insertion site. The Asn279Ala-mediated abrogation of the minor groove contact with the incoming nucleotide alleviated the strict geometric selection of polβ, thereby allowing a wobble G:T base pair at the insertion site. This result highlights the effect of minor groove contact on the geometric selection mechanism and implies that perturbing minor groove interactions in other DNA polymerases may alter their geometric selection mechanisms.
The tight geometric constraints of the polβ active site may enable this enzyme to display a distinct mismatch discrimination strategy. First, polβ strongly discriminates between correct and incorrect base pairs when it is in an open conformation. In particular, in the open-conformation state, polβ does not allow coplanar wobble G:T and A:C base pairs. Instead, the enzyme induces staggered conformations for G:T and A:C mispairs. Second, polβ allows only Watson-Crick and Watson-Crick-like geometries when in the closed conformation, thereby precluding the formation of mispairs with non-Watson-Crick geometry at the insertion site. In the closed conformation induced by active site Mn2+, the G:T mismatch evades the mismatch discrimination of polβ by adopting a Watson-Crick-like geometry, but other mismatches cannot readily escape such discrimination. This result partially explains why the G:T mismatch is the major (~60%) spontaneous replication error made by polβ [46]. Third, the nascent base-pair binding pocket of polβ is highly sensitive to structural and electronic perturbations caused by nucleobase lesions, particularly alkylated lesions. For example, polβ incorporates dCTP opposite N7-methylguanine 300-fold less efficiently than opposite guanine [47]. In addition, although N7-methylguanine:T forms a Watson-Crick-like conformation in the absence of protein contact [48], polβ does not permit the formation of a Watson-Crick-like N7-methylguaine:T base pair at the insertion site and induces a staggered base-pair conformation [47]. The enzyme’s active site architecture appears to sense the steric and/or electronic perturbation of the N7-methylguanine moiety. Fourth, while the A-family polymerase BF accommodates a coplanar relaxed O6-methylguanine:C base pair in a closed conformation [49], polβ enforces a staggered O6-methylguanine:C base pair and adopts an open conformation, even in the presence of the misincorporation-promoting Mn2+ [20]. In the case of the O6-methylguanine:T base pair, polβ fails to discriminate the mismatch in the closed conformation, allowing a Watson-Crick-like conformation [20]. These results highlight differences in the mismatch discrimination strategies used by DNA polymerases.
The effect of minor groove interaction on replication fidelity and catalytic efficiency
Crystal structures and studies with nucleoside analogs have indicated that minor groove interactions between DNA polymerases and the N3 of purines or O2 of pyrimidines may play important roles in probing for correct base pairs in the nascent base-pair binding pocket [23, 27]. In the case of polβ, minor groove contacts with the incoming nucleotide and templating base exert opposing effects on replication fidelity and catalytic efficiency. An alanine substitution at Arg283, which interacts with the minor groove edge of the incoming nucleotide, dramatically decreases both replication fidelity (~2,000-fold) and catalytic efficiency (~30,000-fold) [33]. On the other hand, substituting alanine for Asn279 reduces misinsertion efficiency and increases correct insertion efficiency. The abrogation of the minor groove contact with the incoming nucleotide by the Asn279Ala mutation increases the kcat (~3-fold) for correct nucleotide incorporation but had minimal effects on Km. The Asn279Ala mutation of polβ has been shown to increase the replication fidelity ~5-fold [33].
Asn279 of polβ has been shown to stimulate 8-oxo-dGTP incorporation opposite templating dA by stabilizing syn conformation of the oxidized nucleotide [50, 51]. While wild-type polβ more efficiently incorporates 8-oxo-dGTP opposite dA over dC (24:1), Asn279Ala mutation leads to the preferential 8-oxo-dGTP insertion opposite dC over dA (14:1), highlighting that minor groove interactions of Asn279 with syn-8-oxo-dGTP facilitates mutagenic replication.
An X-family DNA polymerase η from Thermus thermophiles (Tth PolX) contains Ser266, which is equivalent to Asn279 of polβ [52]. Mutation of Ser266 of Tth PolX into Asn, thereby mimicking polβ, greatly (~20-fold) accelerates 8-oxo-dGTP opposite dA compared to the wild-type enzyme, suggesting that the minor groove interaction at the nucleotide-binding site promotes mutagenic 8-oxo-dGTP insertion opposite dA in the active site of the X-family DNA polymerases.
Minor groove interactions with the incoming nucleotide are observed in most DNA polymerases, including A-, C-, X-, and Y-family DNA polymerases. In the case of the Y-family DNA polymerase η, removal of the minor groove interaction with 3-deaza-dGTP decreases the catalytic efficiency for correct insertion ~120-fold and increases incorrect insertion ~12-fold, thereby reducing replication fidelity ~1,800-fold, thus illustrating the effect of the minor groove interaction with the incoming nucleotide on fidelity [26]. Determining whether the elimination or addition of minor groove contacts at the insertion sites of DNA polymerases significantly influences replication fidelity and mismatched base-pair conformations would be of interest. Such studies would provide insights into how DNA polymerases utilize insertion site-minor groove interactions to perform their functions in replication, DNA repair and translesion synthesis.
Table 2.
Data collection and refinement statistics.
| PDB code | Asn279Ala dG:dCTP* Mg2+ (4Z6C) | Asn279Ala dG:dTTP* Mg2+ (4Z6D) | Asn279Ala dG:dTTP* Mn2+ (4Z6E) | Asn279Ala O6MeG:dTTP* Mn2+ (4Z6F) |
|---|---|---|---|---|
| Data Collection | ||||
|
| ||||
| Detector | Rigaku RAxis IV++ imaging plate area detector | Rigaku RAxis IV++ imaging plate area detector | Rigaku RAxis IV++ imaging plate area detector | 3 × 3 CCD array (ADSC Q315R) |
|
| ||||
| Wavelength | 1.5418 | 1.5418 | 1.5418 | 0.97648 |
|
| ||||
| Space Group | P21 | P21 | P21 | P21 |
|
| ||||
| Cell constants | ||||
| a (Å) | 48.180 | 54.283 | 50.562 | 50.753 |
| b | 79.274 | 79.382 | 79.567 | 79.558 |
| c | 54.372 | 54.859 | 55.143 | 55.204 |
| α (°) | 90.00 | 90.00 | 90.00 | 90.00 |
| β | 105.96 | 110.18 | 107.64 | 107.70 |
| γ | 90.00 | 90.00 | 90.00 | 90.00 |
| Resolution (Å)a | 20-2.68 (2.73-2.68) | 20-2.51 (2.55-2.51) | 20-2.75 (2.80-2.75) | 20-2.44 (2.48-2.44) |
| Rmerge b (%) | 0.110 (0.380) | 0.138 (0.525) | 0.111 (0.385) | 0.125 (0.478) |
| <I/σ> | 13.3 (2.22) | 12.7 (2.04) | 13.8 (2.46) | 13.3 (2.00) |
| Completeness (%) | 99.8 (98.4) | 99.9 (99.7) | 99.3 (93.9) | 99.6 (96.9) |
| Redundancy | 4.0 (3.4) | 4.4 (4.2) | 4.4 (3.9) | 4.6 (4.1) |
|
| ||||
| Refinement | ||||
| Rwork c/Rfree d (%) | 20.4/27.2 | 19.3/27.1 | 18.1/24.8 | 19.5/24.8 |
| Unique reflections | 11111 | 15005 | 10785 | 15517 |
| Mean B factor (Å2) | ||||
| Protein | 31.4 | 32.0 | 31.1 | 29.6 |
| Ligand | 30.3 | 38.6 | 27.9 | 28.2 |
| Solvent | 32.6 | 29.7 | 30.0 | 29.8 |
| Ramachandran plot | ||||
| Most favored (%) | 96.9 | 97.8 | 96.0 | 97.5 |
| Additional allowed (%) | 3.1 | 2.2 | 4.0 | 2.5 |
| RMSD | ||||
| Bond lengths (Å) | 0.005 | 0.004 | 0.004 | 0.004 |
| Bond angles (°) | 1.178 | 1.063 | 1.034 | 1.065 |
Values in parentheses are for the highest resolution shell.
Rmerge = Σ|I–<I>|/ΣI where I is the integrated intensity of a given reflection.
Rwork = Σ|F(obs)−F(calc)|/ΣF(obs).
Rfree = Σ|F(obs)−F(calc)|/ΣF(obs), calculated using 5% of the data.
Acknowledgments
Instrumentation and technical assistance for this work were provided by the Macromolecular Crystallography Facility, with financial support from the College of Natural Sciences, the Office of the Executive Vice President and Provost, and the Institute for Cellular and Molecular Biology at the University of Texas at Austin. The Berkeley Center for Structural Biology is supported in part by the National Institutes of Health, National Institute of General Medical Sciences, and the Howard Hughes Medical Institute. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Funding
This work was supported by grants from the Robert Welch Foundation (F-1741) and the National Institutes of Health (ES26676).
Abbreviations
- BF
Bacillus stearothermophilus DNA polymerase I fragment
- O6MeG
O6-Methylguanine
- Tth
Thermus thermophiles
Footnotes
Author Contribution
M.K conducted protein expression, kinetic experiments, and structure determination experiments and wrote the paper; S.L. designed the experiments and wrote the paper.
Competing Interests
The authors declare that there are no competing interests.
References
- 1.Hoeijmakers J. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 2.Doublie S, Tabor S, Long AM, Richardson CC, Ellenberger T. Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 A resolution. Nature. 1998;391:251–258. doi: 10.1038/34593. [DOI] [PubMed] [Google Scholar]
- 3.Sawaya MR, Pelletier H, Kumar A, Wilson SH, Kraut J. Crystal structure of rat DNA polymerase beta: evidence for a common polymerase mechanism. Science. 1994;264:1930–1935. doi: 10.1126/science.7516581. [DOI] [PubMed] [Google Scholar]
- 4.Kiefer JR, Mao C, Braman JC, Beese LS. Visualizing DNA replication in a catalytically active Bacillus DNA polymerase crystal. Nature. 1998;391:304–307. doi: 10.1038/34693. [DOI] [PubMed] [Google Scholar]
- 5.Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994;264:1891–1903. doi: 10.1126/science.7516580. [DOI] [PubMed] [Google Scholar]
- 6.Kunkel TA. DNA replication fidelity. J Biol Chem. 2004;279:16895–16898. doi: 10.1074/jbc.R400006200. [DOI] [PubMed] [Google Scholar]
- 7.Kunkel TA, Bebenek K. DNA replication fidelity. Annu Rev Biochem. 2000;69:497–529. doi: 10.1146/annurev.biochem.69.1.497. org/10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]
- 8.Gregory MT, Park GY, Johnstone TC, Lee YS, Yang W, Lippard SJ. Structural and mechanistic studies of polymerase η bypass of phenanthriplatin DNA damage. Proc Natl Acad Sci USA. 2014;111:9133–8. doi: 10.1073/pnas.1405739111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin P, Batra VK, Pedersen LC, Beard WA, Wilson SH, Pedersen LG. Incorrect nucleotide insertion at the active site of a G:A mismatch catalyzed by DNA polymerase. Proc Natl Acad Sci USA. 2008;105:5670–5674. doi: 10.1073/pnas.0801257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaisman A, Ling H, Woodgate R, Yang W. Fidelity of Dpo4: effect of metal ions, nucleotide selection and pyrophosphorolysis. EMBO J. 2005;24:2957–2967. doi: 10.1038/sj.emboj.7600786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Hellinga HW, Beese LS. Structural evidence for the rare tautomer hypothesis of spontaneous mutagenesis. Proc Natl Acad Sci USA. 2011;108:17644–17648. doi: 10.1073/pnas.1114496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia S, Wang J, Konigsberg WH. DNA Mismatch Synthesis Complexes Provide Insights into Base Selectivity of a B Family DNA Polymerase. J Am Chem Soc. 2013;135:193–202. doi: 10.1021/ja3079048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimsey IJ, Petzold K, Sathyamoorthy B, Stein ZW, Al-Hashimi HM. Visualizing transient Watson-Crick-like mispairs in DNA and RNA duplexes. Nature. 2015;519:315–320. doi: 10.1038/nature14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson SJ, Beese LS. Structures of mismatch replication errors observed in a DNA polymerase. Cell. 2004;116:803–816. doi: 10.1016/S0092-8674(04)00252-1. [DOI] [PubMed] [Google Scholar]
- 15.Wu EY, Beese LS. The Structure of a high fidelity DNA polymerase bound to a Mismatched Nucleotide Reveals an “Ajar” Intermediate Conformation in the Nucleotide Selection Mechanism. J Biol Chem. 2011;286:19758–19767. doi: 10.1074/jbc.M110.191130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren JJ, Forsberg LJ, Beese LS. The structural basis for the mutagenicity of O(6)-methyl-guanine lesions. Proc Natl Acad Sci USA. 2006;103:19701–19706. doi: 10.1073/pnas.0609580103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Gregory MT, Biertümpfel C, Hua YJ, Hanaoka F, Yang W. Mechanism of somatic hypermutation at the WA motif by human DNA polymerase η. Proc Natl Acad Sci USA. 2013;110:8146–8151. doi: 10.1073/pnas.1303126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bebenek K, Pedersen LC, Kunkel TA. Replication infidelity via a mismatch with Watson-Crick geometry. Proc Natl Acad Sci USA. 2011;108:1862–1867. doi: 10.1073/pnas.1012825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koag MC, Nam K, Lee S. The spontaneous replication error and the mismatch discrimination mechanisms of human DNA polymerase β. Nucleic Acids Res. 2014;42:11233–11245. doi: 10.1093/nar/gku789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koag MC, Lee S. Metal-Dependent Conformational Activation Explains Highly Promutagenic Replication across O6-Methylguanine by Human DNA Polymerase β. J Am Chem Soc. 2014;136:5709–5721. doi: 10.1021/ja500172d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales JC, Kool ET. Functional hydrogen-bonding map of the minor groove binding tracks of six DNA polymerases. Biochemistry. 2000;39:12979–12988. doi: 10.1021/bi001578o. [DOI] [PubMed] [Google Scholar]
- 22.Pelletier H, Sawaya MR, Wolfle W, Wilson SH, Kraut J. Crystal structures of human DNA polymerase beta complexed with DNA: implications for catalytic mechanism, processivity, and fidelity. Biochemistry. 1996;35:12742–12761. doi: 10.1021/bi952955d. [DOI] [PubMed] [Google Scholar]
- 23.Morales JC, Kool ET. Minor groove interactions between polymerase and DNA: more essential to replication than Watson-Crick hydrogen bonds? J Am Chem Soc. 1999;121:2323–2324. doi: 10.1021/ja983502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osheroff WP, Beard WA, Wilson SH. Base substitution specificity of DNA polymerase β depends on interactions in the DNA minor groove. J Biol Chem. 1999;274:20749–20752. doi: 10.1074/jbc.274.30.20749. [DOI] [PubMed] [Google Scholar]
- 25.Freudenthal BD, Beard WA, Wilson SH. DNA polymerase minor groove interactions modulate mutagenic bypass of a templating 8-oxoguanine lesion. Nucleic Acids Res. 2013;41:1848–1858. doi: 10.1093/nar/gks1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Washington MT, Wolfle WT, Spratt TE, Prakash L, Prakash S. Yeast DNA polymerase eta makes functional contacts with the DNA minor groove only at the incoming nucleoside triphosphate. Proc Natl Acad Sci USA. 2003;100:5113–5118. doi: 10.1073/pnas.0837578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales JC, Kool ET. Minor Groove Interactions between Polymerase and DNA: More Essential to Replication than Watson-Crick Hydrogen Bonds? J Am Chem Soc. 1999;121:2323–2324. doi: 10.1021/ja983502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia S, Christian TD, Wang J, Konigsberg WH. Probing Minor Groove Hydrogen Bonding Interactions between RB69 DNA Polymerase and DNA. Biochemistry. 2012;51:4343–4353. doi: 10.1021/bi300416z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osheroff WP, Beard WA, Yin S, Wilson SH. Minor groove interactions at the DNA polymerase β active site modulate single-base deletion error rates. J Biol Chem. 2000;275:28033–28038. doi: 10.1074/jbc.M003462200. [DOI] [PubMed] [Google Scholar]
- 30.McCain MD, Meyer AS, Schultz SS, Glekas A, Spratt TE. Fidelity of mispair formation and mispair extension is dependent on the interaction between the minor groove of the primer terminus and Arg668 of DNA polymerase I of Escherichia coli. Biochemistry. 2005;44:5647–5659. doi: 10.1021/bi047460f. [DOI] [PubMed] [Google Scholar]
- 31.Beard WA, Wilson SH. Structure and Mechanism of DNA Polymerase β. Chem Rev. 2006;106:361–382. doi: 10.1021/cr0404904. [DOI] [PubMed] [Google Scholar]
- 32.Freudenthal BD, Beard WA, Shock DD, Wilson SH. Observing a DNA Polymerase Choose Right from Wrong. Cell. 2013;154:157–168. doi: 10.1016/j.cell.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beard WA, Osheroff WP, Prasad R, Sawaya MR, Jaju M, Kraut J, Kunkel TA, Wilson SH. Enzyme-DNA Interactions Required for Efficient Nucleotide Incorporation and Discrimination in Human DNA Polymerase beta. J Biol Chem. 1996;271:12141–1214. doi: 10.1074/jbc.271.21.12141. [DOI] [PubMed] [Google Scholar]
- 34.Batra VK, Shock DD, Beard WA, McKenna CE, Wilson SH. Binary complex crystal structure of DNA polymerase β reveals multiple conformations of the templating 8-oxoguanine lesion. Proc Natl Acad Sci USA. 2012;109:113–118. doi: 10.1073/pnas.1112235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freudenthal BD, Beard WA, Wilson SH. Structures of dNTP Intermediate States during DNA Polymerase Active Site Assembly. Structure. 2012;20:1829–1837. doi: 10.1016/j.str.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraynov VS, Werneburg BG, Zhong X, Lee H, Ahn J, Tsai MD. DNA polymerase beta: analysis of the contributions of tyrosine-271 and asparagine-279 to substrate specificity and fidelity of DNA replication by pre-steady-state kinetics. Biochem J. 1997;323:103–111. doi: 10.1042/bj3230103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chagovetz AM, Sweasy JB, Preston BD. Increased activity and fidelity of DNA polymerase beta on single-nucleotide gapped DNA. J Biol Chem. 1997;272:27501–27504. doi: 10.1074/jbc.272.44.27501. [DOI] [PubMed] [Google Scholar]
- 38.Batra VK, Beard WA, Shock DD, Pedersen LC, Wilson SH. Structures of DNA polymerase beta with active-site mismatches suggest a transient abasic site intermediate during misincorporation. Mol Cell. 2008;30:315–324. doi: 10.1016/j.molcel.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah AM, Li SX, Anderson KS, Sweasy JB. Y265H mutator mutant of DNA polymerase beta. Proper teometric alignment is critical for fidelity. J Biol Chem. 2001;276:10824–10831. doi: 10.1074/jbc.M008680200. [DOI] [PubMed] [Google Scholar]
- 40.Beard WA, Shock DD, Yang XP, DeLauder SF, Wilson SH. Loss of DNA polymerase beta stacking interactions with templating purines, but not pyrimidines, alters catalytic efficiency and fidelity. J Biol Chem. 2002;277:8235–8242. doi: 10.1074/jbc.M107286200. [DOI] [PubMed] [Google Scholar]
- 41.Lang T, Maitra M, Starcevic D, Li SX, Sweasy JB. A DNA polymerase beta mutant from colon cancer cells induces mutations. Proc Natl Acad Sci USA. 2004;101:6074–6079. doi: 10.1073/pnas.0308571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson JD, Crick FH. Genetical implications of the structure of deoxyribonucleic acid. Nature. 1953;171:964–967. doi: 10.1038/171964b0. [DOI] [PubMed] [Google Scholar]
- 43.Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a. [DOI] [PubMed] [Google Scholar]
- 44.Eoff RL, Irimia A, Egli M, Guengerich FP. Sulfolobus solfataricus DNA polymerase Dpo4 is partially inhibited by “wobble” pairing between O6-methylguanine and cytosine, but accurate bypass is preferred. J Biol Chem. 2007;282:1456–1467. doi: 10.1074/jbc.M609661200. [DOI] [PubMed] [Google Scholar]
- 45.Jang YH, Goddard WA, III, Noyes KT, Sowers LC, Hwang S, Chung DS. pKa values of guanine in water: Density functional theory calculations combined with Poisson-Boltzmann continuum-solvation model. J Phys Chem B. 2003;107:344–357. doi: 10.1021/jp020774x. [DOI] [Google Scholar]
- 46.Osheroff WP, Jung HK, Beard WA, Wilson SH, Kunkel TA. The fidelity of DNA polymerase beta during distributive and processive DNA synthesis. J Biol Chem. 1999;274:3642–3650. doi: 10.1074/jbc.274.6.3642. [DOI] [PubMed] [Google Scholar]
- 47.Koag MC, Kou Y, Ouzon-Shubeita H, Lee S. Transition-state destabilization reveals how human DNA polymerase β proceeds across the chemically unstable lesion N7-methylguanine. Nucleic Acids Res. 2014;42:8755–8766. doi: 10.1093/nar/gku554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kou Y, Koag M-C, Lee S. N7 methylation alters hydrogen-bonding patterns of guanine in duplex DNA. J Am Chem Soc. 2015;137:14067–14070. doi: 10.1021/jacs.5b10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wareen JJ, Forsberg LJ, Beese LS. The structural basis for the mutagenicity of O(6)-methyl-guanine lesions. Proc Natl Acad Sci U S A. 2006;103:19701–19706. doi: 10.1073/pnas.0609580103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller H, Prasad R, Wilson SH, Johnson F, Grollman AP. 8-OxodGTP incorporation by DNA polymerase β is modified by active sites residue Asn279. Biochemistry. 2000;39:1029–1033. doi: 10.1021/bi991789x. [DOI] [PubMed] [Google Scholar]
- 51.Batra VK, Beard WA, Hou EW, Pederson LC, Prasad R, Wilson SH. Mutagenic conformation of 8-oxo-7,8-dihydro-2′-dGTP in the confines of a DNA polymerase active site. Nat Struct Mol Biol. 2010;17:889–890. doi: 10.1038/nsmb.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garrido P, Mejia E, Garcia-Diaz M, Blanco L, Picher AJ. The active site of TthPolX is adapted to prevent 8-oxo-dGTP misincorporation. Nucleic Acids Res. 2014;42:534–543. doi: 10.1093/nar/gkt870. [DOI] [PMC free article] [PubMed] [Google Scholar]