Abstract
Exercise physiology is different in males and females. Females are poorly studied due to the complexity of the estrous cycle and this bias has created an exercise sex gap. Here, we evaluated the impact of sexual dimorphism and of the estrous cycle on muscle strength and running power of C57BL/6 mice. Like men, male mice were stronger and more powerful than females. Exercise-induced increase of O2 consumption (O2) and CO2 production (CO2) were equal between sexes, indicating that running economy was higher in males. Thermoregulation was also more efficient in males. In females, proestrus increased exercise O2 and CO2 at low running speeds (30–35% female O2max) and estrus worsened thermoregulation. These differences translated into different absolute and relative workloads on the treadmill, even at equal submaximal O2 and belt speeds. In summary, our results demonstrate the better muscle strength, running power and economy, and exercise-induced thermoregulation of males compared to females. Proestrus and estrus still undermined the running economy and exercise-induced thermoregulation of females, respectively. These results demonstrate an important exercise sex gap in mice.
Introduction
The importance of differences between sexes/genders is recognized in biology and medicine. Sex describes biological differences, while gender includes social, cultural and economic aspects1. The historical gender differences in motivation/opportunity to practice physical activity (including physical exercise and training) limited the best women exercise/sport performance, a phenomenon known as exercise gender gap in humans2. For instance, women are more prone to physical inactivity3, a risk factor for many diseases4,5. The historical evolution of exercise gender gap in modern Olympic Games (World Record and 10 best performances) also reveals a systematically lower sport performance of females compared to males; nowadays, the differences varies between 10.7% for running and 36.8% for weightlifting2. The exercise gender gap is greatest in sports that require running economy, muscle strength, and exercise power2. Running economy is the energy demand for a submaximal running speed6, higher in men7,8 but it is unknown if this sex difference is also present in laboratory animals.
A review of ≈1400 manuscripts involving more than 6 million people revealed an under-representation of women in studies of exercise and sports (35–37%)9. However, sex is a major determinant of exercise performance through the impact of anthropometry (height, weight, body fat, and muscle mass), aerobic power and anaerobic threshold, besides genetic and hormonal factors2–4,10. The minor representation of females also translates into less knowledge about the biology of exercise in this sex. So far, the main features of sexual dimorphism important for exercise described in rodents are differences in skeletal muscle kinetics and fiber-type composition10 and energy metabolism11,12. In fact, the biological mechanisms underlying the benefits of exercise were investigated in numerous animal studies in a laboratory setting, with a strong tendency to only use males probably to avoid dealing with the possible influence of the menstrual/estrous cycle9,13. Exercise-induced thermoregulation, submaximal and maximal O2 and CO2, and running economy are gold physiological indexes for exercise, but have never been studied in females at different phases of the estrous cycle.
In humans, the exercise sex gap is greatest in sports that require running economy, strength, and power. Similarly, we investigated the role of sex and estrous cycle in maximum (and submaximal) muscle strength and running power/economy of mice. We also evaluated exercise-induced thermoregulation. This knowledge is essential to advance the knowledge of exercise physiology in female sex. We will demonstrate that the simple extrapolation of male knowledge is not correct.
Results
Mouse morphology and the estrous cycle
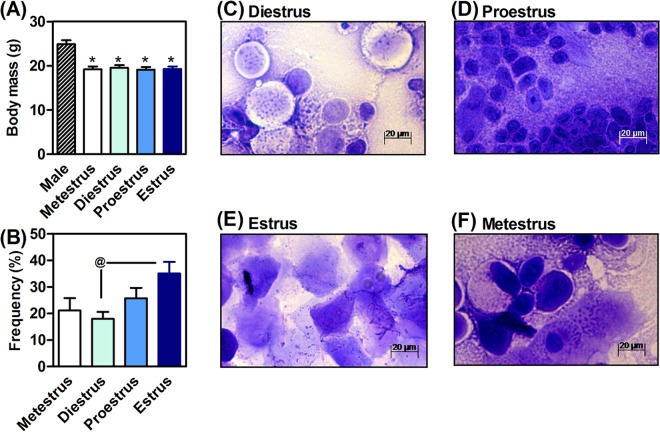
The body mass of males was 22.7 ± 1.1% higher than females (F4,51 = 12.6, P < 0.05, Fig. 1A). Rodent tails are important for regulating body temperature14–16. The length of the tail of the males (6.5 ± 0.2 cm) was shorter than females (8.1 ± 0.3 cm, t27 = 4.5, P < 0.05). These parameters were independent of the estrous cycle, which was devoid of effects on body weight (F3,43 = 0.1, P > 0.05; Fig. 1A) and tail length (F4.51 = 0.4, P > 0.05; data not shown). The prominent estrous cycle was estrus (H2 = 8.1, P < 0.05, Fig. 1B), where vaginal smears were marked by clusters of cornified squamous epithelial cells (Fig. 1E). The vaginal smears also allowed the morphological identification of diestrus, proestrus and metestrus, as exemplified in Fig. 1C,D and F, respectively.
Figure 1.
Impact of sexual dimorphism on body mass (A) and analysis of the estrous cycle based on a morphological analysis of vaginal smears (C–F) that revealed that the prominent estrous cycle was estrus (B). Values are expressed as mean ± standard error of the mean (SEM). N = 8–10 animals/group. *P < 0.05 vs. male (ANOVA, Bonferroni post hoc test). @P < 0.05 (Kruskal-Wallis test).
Male are stronger and more powerful than females
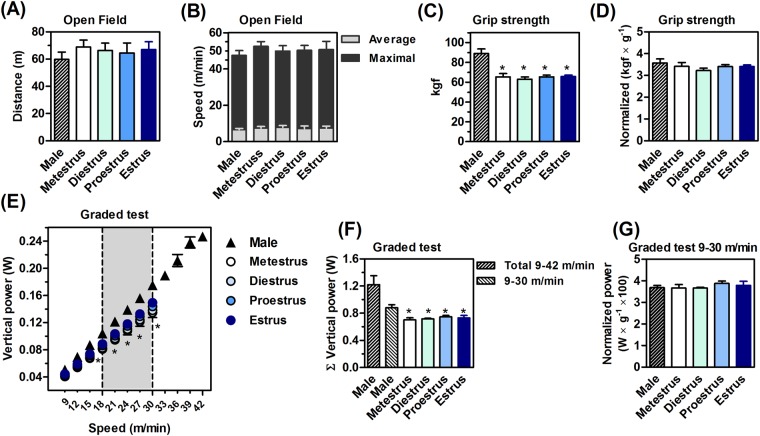
Figure 2 shows the basal motor behavior and ergometric performance of male and female mice. The open field test did not reveal significant differences in locomotion (F4,53 = 0.39, P > 0.05; Fig. 2A), average (F4,53 = 0.38, P > 0.05; Fig. 2B) and maximum speed of males and females, independently of their estrous cycle (F4,53 = 0.43, P > 0.05; Fig. 2B).
Figure 2.
Motor and ergometric data. Sex and estrous cycle did not influence the basal locomotion (A) and speed (B) of mice. Males were stronger (C) and more powerful on the treadmill (E,F) than females, regardless of the phase of the estrous cycle. The normalization of the performance per body mass dissipated the sexual dimorphism (D and G). Values are expressed as mean ± standard error of the mean (SEM). N = 8–10 animals/group. *P < 0.05 vs. male (ANOVA, Bonferroni post hoc test).
Absolute exercise performance of females was curtailed in relation to males, being 27.2 ± 1.1% (F4,32 = 14.2, P < 0.05; Fig. 2C) and 40.5 ± 0.9% lower (F4,32 = 9.9, P < 0.05; Fig. 2F) in the absolute grip strength and treadmill power test, respectively. Moreover, the absolute exercise performance of females was independent of the estrous cycle in the two tests (grip strength F3,27 = 0.27, P > 0.05; Fig. 2C) (treadmill power test F3,27 = 0.19, P > 0.05; Fig. 2F).
Although the absolute exercise performance of males was higher, the submaximal comparisons indicated a different conclusion. The ergometric test applied progressive running speeds for males and females through serial acceleration (F21,310 = 3.2, P < 0.05; Fig. 2E). The treadmill running power in males and females was statistically similar up to 15 m/min (F21,310 = 3.2, P > 0.05; Fig. 2E), when the relative intensity was 50 ± 3.7% of the maximum power for females, and 35 ± 3.9%% for males. The lower running power of females appeared at speeds 18 → 30 m/min (F28,252 = 18.1, P < 0.05; Fig. 2E, gray area). At 30 m/min, the maximum overload of females (100 ± 5.7%) corresponded to a relative overload of males (71 ± 2.2% of maximum). Males reached maximum overload at speeds 39 → 42 m/min (Fig. 2E).
The normalization of exercise performance by body mass eliminates sexual dimorphism
We then normalized the exercise performance by the body mass. This transformation eliminated the sex differences for muscle strength (F4,32 = 0.78, P > 0.05; Fig. 2D) and running power at speeds 15 → 30 m/min (F4,32 = 0.63, P > 0.05; Fig. 2G).
Males show a better running economy
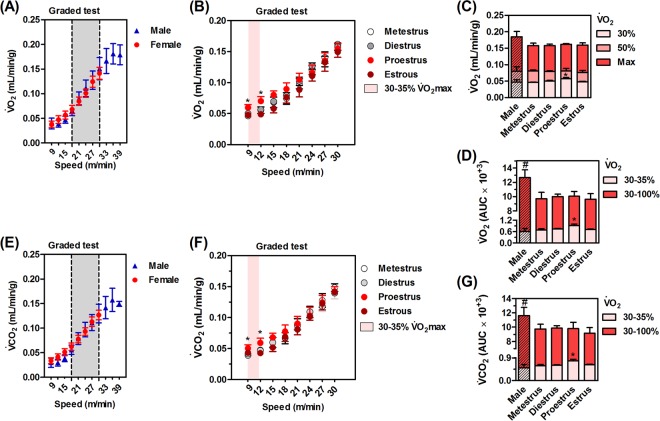
There were no differences in O2 and CO2 kinetics between sexes. The progressive running speeds of the ergospirometry increased the O2 (F7,126 = 2.8, P < 0.05; Fig. 3A) and CO2 (F7,126 = 2.4, P < 0.05; Fig. 3E) of males and females at all comparative intensities (9 → 30 m/min, Fig. 3A and E). Thus, the higher submaximal running power developed at speeds 18 → 30 m/min, associated to the same submaximal O2, showed a better running economy in males compared to females at (Fig. 3A and E, gray area).
Figure 3.
Respiratory gases during an incremental test. Running similarly increased general O2 consumption (O2, A,B) and CO2 production (CO2, E,F) in males and females at different speeds up to 33 m/min. O2max was similar between the sexes (C) total O2 (D) and CO2 (G) was only higher in males due to higher running speeds. Proestrus increased submaximal O2 (B and D) and CO2 (G) at lighter intensities of ergospirometry (30–35% O2max). Values are expressed as mean ± standard error of mean (SEM). N = 8–10 animals/group. *P < 0.05 vs. male, #P < 0.05 vs. females (ANOVA, Bonferroni post hoc test).
Importantly, males ran up to higher speeds (33 → 42 m/min; Fig. 2E), which resulted in a higher O2 (F4,33 = 2.7, P < 0.05; Fig. 3D) and CO2 (F4,33 = 2.7, P < 0.05; Fig. 3G), but not O2max in relation to females.
Proestrus increases O2 and CO2 during submaximal exercise testing
The submaximal O2 (F18,192 = 2.5, P < 0.05; Fig. 3B) and CO2 (F18,192 = 2.3, P < 0.05; Fig. 3C) of females during proestrus were significantly larger at lower exercise intensities (30–35% O2max females. We also detected these differences in total O2 (F3,35 = 3.8, P < 0.05; Fig. 3D) and CO2 (F3,35 = 3.2, P < 0.05; Fig. 3G) for females at proestrus during these low exercise intensities (30–35% O2max females). The higher intensities (30–100% O2max females) presented similar kinetics for O2 and CO2 in the different phases of the estrous cycle.
Exercise-induced thermoregulation is less effective in estrus females
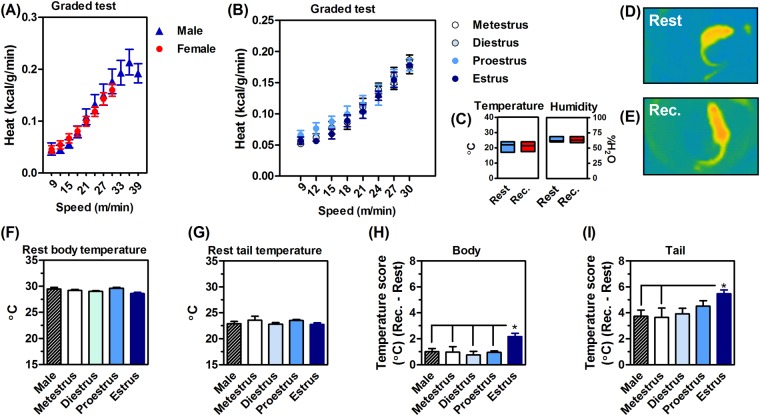
Thermoregulation requires the dissipation of heat produced during exercise. Exercise increased the heat production of males and females (F7,126 = 264, P < 0.05; Fig. 4A), without influence of the estrous cycle (F7,94 = 0.32, P > 0.05; Fig. 4B). Environment temperature and humidity did not interfere in the thermography results, since they were similar before and after the exercise test session (Fig. 4C). The thermal image (Fig. 4D) shows a female at rest, with the body and tail heated after a maximum exercise test (Fig. 4E).
Figure 4.
Exercise heat production and dissipation, or thermoregulation. Upon exercise, male and female mice consumed similar calories during the incremental test (A) without any evident impact of the estrous cycle. (B) Experiments were conducted in a controlled temperature and humidity environment. (C) The thermal IR image shows an evident tail heating after the maximum exercise (or recovery time, REC, E) in relation to rest. (D) The body and tail temperature was not different at rest (F and G, respectively). Exercise warmed the body of females at estrus (H) and the tails of all groups of mice (I). Again, female tail heating was larger at estrous (I). Values are expressed as mean ± standard error of the mean (SEM). N = 8–10 animals/group. *P < 0.05 (ANOVA, Bonferroni post hoc test).
Resting body and tail infrared temperatures did not differ between sexes or in females at different phases of the estrous cycle (body, F4,19 = 0.53, P > 0.05; Fig. 4F) (tail, F4,19 = 2.01, P > 0.05; Fig. 4G). The maximum exercise was not enough to heat the body of males and females on metestrus, diestrus and proestrus cycle (F4,43 = 3.4, P < 0.05; Fig. 4H). Moreover, all males and females (all cycles) presented significant tail warm up after maximal exercise (F4,43 = 2.8, P < 0.05).
The temperature scores (Fig. 4H and I) reinforced the prominent exercise-induced hyperthermia of females at estrus. Estrus female body heating was larger than that of males and females in other cycles (F4,43 = 3.3, P < 0.05; Fig. 4H). The tail warming of estrus females was superior to males and females at metestrus after exercise (F4,43 = 2.3, P < 0.05; Fig. 4H).
Discussion
Sex matters
Sexual dimorphism and the estrous cycle influenced exercise performance and metabolism of mice implying that these factors should be considered in experimental designs and data interpretation involving exercise biology. We showed that males were stronger and more powerful than females at moderate-high intensities of exercise, when evaluating strength and running. Since submaximal and maximum overloads of exercise were different for males and females, but submaximal O2 and CO2 were similar, this means that the running economy of females was lower than that of males. The estrous cycle did not influence muscle strength, but undermined the running economy and exercise-induced thermoregulation.
Size matters
The sex-related exercise differences disappeared after normalization of exercise performance by size (body mass). This had already been described for muscle strength17–19, but not for running power and economy. However, body size and muscle strength are well-known secondary sexual characteristics, influenced primarily by the anabolic action of the hormone testosterone, a major determinant of sexual dimorphism20.
Skeletal muscle mass and strength are lower in females19,21. Likewise, normalization of exercise performance by specific muscle mass (rather than body mass) makes sexual dimorphism disappear19,21. Male mammals are larger, with larger cross-sectional muscle area8,10. Several studies also showed that muscle length (and the length of the long bones) is also higher in male mammals, important for greater tetanic strength of the anterior masseter muscle8. Larger levers determine higher torques and muscle strength. Sex is also important for muscle fiber-type composition, especially the myosin IIB gene (fast muscle fiber)10. Evidence shows threefold more IIB muscle fibers in the masseter of male mice8,22. In addition, testosterone signals hypertrophy in this musculature20. Conversely, estrogen decreases muscle contractile force in female mice23,24. Thus, muscle strength and running power depends on size and sex: males have large muscles and bones, responsible for great muscle strength; this difference is further amplified by the anabolic effects of testosterone, resulting in larger muscle strength, speed and power.
The testosterone also seems to influence running endurance, but not the running economy. Castration of mouse testicles deplete blood testosterone and impair running wheel endurance (10–30% males with intact gonads)25, a model of submaximal physical activity. Testosterone replacement completely reversed this impairment25. The antiandrogen Flutamide decreases the treadmill endurance of rats, but does not change O2max and running economy26. Here, the exercise-induced submaximal O2 and CO2 up to O2max were similar between sexes, as previously described11,12,27. Only one study demonstrated increased female submaximal O2 during treadmill test, which further reinforces the hypothesis of females’ worst running economy27. These testosterone evidences support the best physical performance (power and endurance) of running male mice, but not the best running economy.
On the other hand, estrogen seems to influence O2 and possibly the running economy of mouse. Similar to our results, submaximal O2 was higher in female rats during the estrogen-dominant proestrus at low treadmill speeds 5–12 m/min (6° grade, without acceleration)17, which may be considered as a low intensity exercise. We also found these differences at near speeds 9–12 m/min. A possibility is the effect of estradiol in the lung gas-exchange surface area (GSA). O2 is directly proportional to GSA28,29; which increases during proestrus with high estradiol levels28,29. Estradiol also increases lung’s GSA and O2 in ovariectomized rats29. Our results suggest that estrogen can increase O2 during exercise, and worsen the running economy, especially at proestrus.
Exercise-induced hyperthermia is a biological response due to greater muscle activation, mitochondrial uncoupling and proton leak30. We now report that sex and the estrous cycle do not modify the calories consumed by exercise, another important variable for running economy; however, our results showed that male thermoregulation was more efficient, since the infrared dissipation of males was more effective. Literature suggests two important points for mouse thermoregulation: body surface area (BSA) and tail dry heat loss. BSA is estimated by the Meeh’s formula (BSA = body weight0.667)25. The greater body mass of males assists in better heat dissipation during/after exercise. Moreover, tail size seems to be related to thermal stress14–16,31, with animals that live in warm environments having longer tails15,32. Female tails, even longer, warmed up more during exercise than that of males. The tail length of C57BL/6 female mice was similar to that described in female BALB/c mice15. Thus, a longer tail length in female mice is suggestive of a required adaptation to compensate for their lower body mass (and area).
Sanchez-Alavez33 demonstrated that body warming during exercise was higher in female mice at estrous. We saw it in the tails. Progesterone promotes heat conservation and higher body temperatures at rest34,35. Bilateral ovariectomy eliminated this estrous-associated change14,33. We suggest that this may apply to body temperature of running female mice during estrus, characterized by high progesterone levels. Thus, sex seems to be a crucial factor also for the exercise-induced thermoregulation of mice.
Some of our results are similar to those reported in humans, since the physical performance of women is generally lower than in men, in accordance with the exercise gender gap2,36,37. The woman’s menstrual cycle is divided into three phases: follicular, ovulation and luteal. The follicular phase can be divided into initial and late, corresponding to metestrus and diestrus, respectively. Ovulation corresponds to proestrus, and the luteal phase to estrus. The woman’s follicular and luteal menstrual cycle does not seem to influence muscle strength, power, and O21,38–40. Human studies still allow evaluating rate of perceived effort (RPE), which also does not differ in the different menstrual phases38,41. However, the differences we found are close to ovulation, virtually impossible to evaluate in women. We demonstrated that the mouse proestrus (or human ovulation) increased O2 and heat production at light exercise.
In summary, our results highlight differences in exercise performance and metabolism between male and female mice. Sex influences size, which appear to be the main factor for mice exercise sex gap. Mouse sexual dimorphism also influenced exercise workload, but not O2 and CO2, implying a finest running economy in males. Males also presented better thermoregulation after exercise. The estrous cycle played a subtle role in mouse physical performance: proestrus impaired running economy and estrus impaired exercise heat loss. This implies that the impact of the estrous cycle on the performance of females should not be considered a limiting factor for their use in experimental designs. In fact, size is the main factor that should be considered in the construction of experimental designs involving exercising male and female mice. For running, a light-intensity exercise seems similar between the sexes (except proestrus), but the performance of females at moderate-intensity running corresponded to the performance of males at low-moderate intensity; the performance of females at high-intensity running corresponded to the performance of males at moderate-high for males, and male high-intensity running was supra-maximal for females. Failure to consider these differences by measuring only the running speed, as done in most studies, introduces an error to compare performance between sexes. These results are of particular interest to counteract the underrepresentation of females in exercise experimental designs.
Methods
Animals
Male and female C57BL/6 mice (10–12 weeks old) were obtained from Charles River (Barcelona, Spain). Mice were housed under controlled environment (12 h light-dark cycle, lights on at 7:00 AM, and room temperature of 21 ± 1 °C) with ad libitum access to food and water. Animals were housed and handled according to European Union guidelines and the study was approved by the Ethical Committee of the Center for Neuroscience and Cell Biology (University of Coimbra).
The animals were accustomed to the treadmill for 3 days. The open field or grip strength test was performed on the 4th day in independent groups of animals. Ergospirometry was performed on the 5th day. All tests were carried out between 9:00 and 17:00 hours in a sound-attenuated and temperature controlled observation room under low-intensity light (≈10 lux), where mice had been habituated for at least 1 hour. The apparatuses were cleaned with 10% ethanol between animals. Within the time window of the tests, we did not record any significant impact of the time of day (morning vs. afternoon) on the treadmill vertical power, O2max and temperature of the tail at rest in either males or females (data not showed).
Vaginal cytology
We evaluated the estrous cycle immediately after the behavioral and exercise experiments, through 4–5 consecutive vaginal lavages (with 40–50 μL of distillated H2O) then mounted on gelatinized slides (76 × 26 mm). These procedures lasted no more than 3–5 minutes, and there were no major temporal delays between behavioral experiments and fluid collection for vaginal cytology.
The vaginal smear were desiccated at room temperature and covered with 0.1% crystal violet for 1 min, then twice washed with 1 mL H2O and desiccated at room temperature. The slides were mounted with Eukitt medium (Sigma-Aldrich) and evaluated under an optical microscope at 1x, 5x and 20x (Zeiss Axio Imager 2). The characterization of the estrous cycle was performed according to literature20,42. Females were categorized for initial (metestrus) or late (diestrus) follicular phase, ovulation (proestrus), or luteal phase (estrus)20,42.
Open field
The exploration of an open field (38 × 38 cm) was analyzed for 15 min using the ANY-maze™ video tracking system (Stoelting Co.)41.
Grip strength
The animal was hung with its forepaws to the central position of a 300 g metal grid and the grip strength was determined as the weight pushed (in grams)41. The computed result was the average of 3 trials, expressed in kgf.
Ergospirometry
Mice were accustomed with a single-lane treadmill (Panlab LE8710, Harvard apparatus) for 3 consecutive days (speed 15 cm/s, 10 min, slope 8.7%, 0.2 mA), with 24 h interval between each habituation session.
The ergospirometry test was carried out on 5th day, 48 hours after the last habituation session. The incremental protocol started at 15 cm/s with an increment of 5 cm/s every 2 min, with a constant inclination of 8.7% (5° for the LE8710 model). The exercise test lasted until running exhaustion, defined by the inability of the animal to leave the electrical grid for 5 seconds43,44. We estimated the power output for treadmill running based on a standard conversion of the vertical work, body weight and running speed45,46. Power is the 1st derivative of work relative to time (run time at each stage).
Oxygen uptake (O2) and carbon dioxide production (CO2) were estimated during treadmill running in a metabolic chamber47 (Gas Analyzer ML206, 23 × 5 × 5 cm, AD Instruments, Harvard) coupled to treadmill. The animals remained in the chamber for 15 min prior to exercise testing. Atmospheric air (≈21% O2, ≈0.03% CO2) was renewed at a rate 120 mL/min, using the same sampling rate for the LASER oxygen sensor (Oxigraf X2004, resolution 0.01%) and infrared carbon dioxide sensor (Servomex Model 15050, resolution 0.1%). Heat (calories) was estimated according to the equations of Lusk48.
Thermal imaging
An infrared (IR) camera (FLiR C2, emissivity of 0.95, FLiR Systems) placed overtop (25 cm height) of a plastic tube (25 cm diameter) was used to acquire a static dorsal thermal image49. IR images were taken immediately before and after exercise tests, namely at rest (Fig. 4D) and recovery (REC, Fig. 4E) periods, respectively. IR images were analyzed with FLiR Tools software (Flir, Boston).
Tail length
The FLiR C2 camera also captures digital pictures (640 × 480 pixels) that were loaded and calibrated (plastic tube, 25 cm diameter) in the ImageJ software (v1.51j8, NIH, USA) for tail length measurement of live animals (ImageJ software).
Statistics
Data are presented as mean ± Standard Error of the Mean (SEM). A test for normality was performed by Kolmogorov–Smirnov test. For each test, the experimental unit was an individual animal. The frequency of the estrous cycle was assessed using the Kruskal-Wallis test. The role of sex and estrous cycle in the dependent variables body mass, open field, grip strength and vertical power, O2 and CO2, and body and tail temperature was evaluated using on-way ANOVA. The repeated measures of ANOVA were performed to evaluate the effect of different treadmill speeds, sex and estrous cycle on the vertical power, O2 and CO2, and heat. The Bonferroni post hoc test was applied for significant F values. The accepted level of significance was p < 0.05. Statistics were performed using Dell Statistica (data analysis software system), version 13.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The work was supported by Prémio Maratona da Saúde, CAPES-FCT (039/2014), FCT (PTDC/NEU-NMC/4154) and ERDF through Centro 2020 (project CENTRO-01-0145-FEDER-000008:BrainHealth 2020). A.S.A.Jr is a CNPq fellow. We would like to acknowledge Flávio N.F. Reis and Frederico C. Pereira (IBILI - Institute for Biomedical Imaging and Life Sciences, University of Coimbra) for making available the treadmill and gas analyzer.
Author Contributions
A.S.A. Jr. designed and performed the experiments, prepared the figures, and wrote the manuscript. A.E.S. and I.A. performed the experiments. P.M.C. designed the experiments and wrote the manuscript. R.A.C. designed the experiments and wrote the manuscript. All authors revised the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oertelt-Prigione S, Gohlke BO, Dunkel M, Preissner R, Regitz-Zagrosek V. GenderMedDB: an interactive database of sex and gender-specific medical literature. Biol Sex Differ. 2014;5:7. doi: 10.1186/2042-6410-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thibault V, et al. Women and Men in Sport Performance: The Gender Gap has not Evolved since 1983. J Sports Sci Med. 2010;9:214–223. [PMC free article] [PubMed] [Google Scholar]
- 3.Althoff T, et al. Large-scale physical activity data reveal worldwide activity inequality. Nature. 2017;547:336–339. doi: 10.1038/nature23018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of Inactivity in Chronic Diseases: Evolutionary Insight and Pathophysiological Mechanisms. Physiol Rev. 2017;97:1351–1402. doi: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokkinos P. Physical activity, health benefits, and mortality risk. ISRN Cardiol. 2012;2012:718789. doi: 10.5402/2012/718789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders PU, Pyne DB, Telford RD, Hawley JA. Factors affecting running economy in trained distance runners. Sports Med. 2004;34:465–485. doi: 10.2165/00007256-200434070-00005. [DOI] [PubMed] [Google Scholar]
- 7.Helgerud J. Maximal oxygen uptake, anaerobic threshold and running economy in women and men with similar performances level in marathons. Eur J Appl Physiol Occup Physiol. 1994;68:155–161. doi: 10.1007/BF00244029. [DOI] [PubMed] [Google Scholar]
- 8.Daniels JT. A physiologist’s view of running economy. Med Sci Sports Exerc. 1985;17:332–338. [PubMed] [Google Scholar]
- 9.Costello JT, Bieuzen F, Bleakley CM. Where are all the female participants in Sports and Exercise Medicine research? Eur J Sport Sci. 2014;14:847–851. doi: 10.1080/17461391.2014.911354. [DOI] [PubMed] [Google Scholar]
- 10.Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda) 2015;30:30–39. doi: 10.1152/physiol.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brager AJ, et al. Homeostatic effects of exercise and sleep on metabolic processes in mice with an overexpressed skeletal muscle clock. Biochimie. 2017;132:161–165. doi: 10.1016/j.biochi.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezende EL, Kelly SA, Gomes FR, Chappell MA, Garland T., Jr. Effects of size, sex, and voluntary running speeds on costs of locomotion in lines of laboratory mice selectively bred for high wheel-running activity. Physiol Biochem Zool. 2006;79:83–99. doi: 10.1086/498187. [DOI] [PubMed] [Google Scholar]
- 13.Bruinvels G, et al. Sport, exercise and the menstrual cycle: where is the research? Br J Sports Med. 2017;51:487–488. doi: 10.1136/bjsports-2016-096279. [DOI] [PubMed] [Google Scholar]
- 14.Gordon CJ. Influence of heating rate on control of heat loss from the tail in mice. Am J Physiol. 1983;244:R778–784. doi: 10.1152/ajpregu.1983.244.6.R778. [DOI] [PubMed] [Google Scholar]
- 15.Gordon CJ, et al. Behaviorally mediated, warm adaptation: a physiological strategy when mice behaviorally thermoregulate. J Therm Biol. 2014;44:41–46. doi: 10.1016/j.jtherbio.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Serrat MA. Allen’s rule revisited: temperature influences bone elongation during a critical period of postnatal development. Anat Rec (Hoboken) 2013;296:1534–1545. doi: 10.1002/ar.22763. [DOI] [PubMed] [Google Scholar]
- 17.Daniels DW, Tian Z, Barton ER. Sexual dimorphism of murine masticatory muscle function. Arch Oral Biol. 2008;53:187–192. doi: 10.1016/j.archoralbio.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean, A. C., Valenzuela, N., Fai, S. & Bennett, S. A. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp, e4389, 10.3791/4389 (2012). [DOI] [PMC free article] [PubMed]
- 19.Ueberschlag-Pitiot V, et al. Gonad-related factors promote muscle performance gain during postnatal development in male and female mice. Am J Physiol Endocrinol Metab. 2017;313:E12–E25. doi: 10.1152/ajpendo.00446.2016. [DOI] [PubMed] [Google Scholar]
- 20.Bardin CW, Catterall JF. Testosterone: a major determinant of extragenital sexual dimorphism. Science. 1981;211:1285–1294. doi: 10.1126/science.7010603. [DOI] [PubMed] [Google Scholar]
- 21.MacLean HE, et al. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008;22:2676–2689. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- 22.Deasy BM, et al. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73–86. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol (1985) 2006;100:548–559. doi: 10.1152/japplphysiol.01029.2005. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Yamamuro T. Long-term effects of estrogen on rat skeletal muscle. Exp Neurol. 1985;87:291–299. doi: 10.1016/0014-4886(85)90219-5. [DOI] [PubMed] [Google Scholar]
- 25.Bowen RS, et al. Effects of Supraphysiological Doses of Sex Steroids on Wheel Running Activity in Mice. J Steroids Horm Sci. 2012;3:110. doi: 10.4172/2157-7536.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgieva KN, et al. The effect of flutamide on the physical working capacity and activity of some of the key enzymes for the energy supply in adult rats. Asian J Androl. 2017;19:444–448. doi: 10.4103/1008-682X.177842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molinero A, et al. Role of muscle IL-6 in gender-specific metabolism in mice. Plos One. 2017;12:e0173675. doi: 10.1371/journal.pone.0173675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massaro GD, Mortola JP, Massaro D. Sexual dimorphism in the architecture of the lung’s gas-exchange region. Proc Natl Acad Sci USA. 1995;92:1105–1107. doi: 10.1073/pnas.92.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massaro GD, Mortola JP, Massaro D. Estrogen modulates the dimensions of the lung’s gas-exchange surface area and alveoli in female rats. Am J Physiol. 1996;270:L110–114. doi: 10.1152/ajplung.1996.270.1.L110. [DOI] [PubMed] [Google Scholar]
- 30.Gaesser GA, Brooks GA. Metabolic bases of excess post-exercise oxygen consumption: a review. Med Sci Sports Exerc. 1984;16:29–43. [PubMed] [Google Scholar]
- 31.Conley KE, Porter WP. Heat loss regulation: role of appendages and torso in the deer mouse and the white rabbit. J Comp Physiol B. 1985;155:423–431. doi: 10.1007/BF00684671. [DOI] [PubMed] [Google Scholar]
- 32.Harrison GA. The adaptability of mice to high environmental temperatures. J. Exper. Bio. 1958;35:10. [Google Scholar]
- 33.Sanchez-Alavez M, Alboni S, Conti B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age (Dordr) 2011;33:89–99. doi: 10.1007/s11357-010-9164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charkoudian N, Hart ECJ, Barnes JN, Joyner MJ. Autonomic control of body temperature and blood pressure: influences of female sex hormones. Clin Auton Res. 2017;27:149–155. doi: 10.1007/s10286-017-0420-z. [DOI] [PubMed] [Google Scholar]
- 35.Opas EE, Gentile MA, Kimmel DB, Rodan GA, Schmidt A. Estrogenic control of thermoregulation in ERalphaKO and ERbetaKO mice. Maturitas. 2006;53:210–216. doi: 10.1016/j.maturitas.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Cureton K, et al. Sex difference in maximal oxygen uptake. Effect of equating haemoglobin concentration. Eur J Appl Physiol Occup Physiol. 1986;54:656–660. doi: 10.1007/BF00943356. [DOI] [PubMed] [Google Scholar]
- 37.Maldonado-Martin S, Mujika I, Padilla S. Physiological variables to use in the gender comparison in highly trained runners. J Sports Med Phys Fitness. 2004;44:8–14. [PubMed] [Google Scholar]
- 38.De Souza MJ, Maguire MS, Rubin KR, Maresh CM. Effects of menstrual phase and amenorrhea on exercise performance in runners. Med Sci Sports Exerc. 1990;22:575–580. doi: 10.1249/00005768-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Grucza R, Pekkarinen H, Titov EK, Kononoff A, Hanninen O. Influence of the menstrual cycle and oral contraceptives on thermoregulatory responses to exercise in young women. Eur J Appl Physiol Occup Physiol. 1993;67:279–285. doi: 10.1007/BF00864229. [DOI] [PubMed] [Google Scholar]
- 40.Williams TJ, Krahenbuhl GS. Menstrual cycle phase and running economy. Med Sci Sports Exerc. 1997;29:1609–1618. doi: 10.1097/00005768-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson LA, Kolka MA, Wilkerson JE. Perceived exertion and anaerobic threshold during the menstrual cycle. Med Sci Sports Exerc. 1982;14:218–222. [PubMed] [Google Scholar]
- 42.Caligioni, C. S. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4, Appendix 4I, 10.1002/0471142301.nsa04is48 (2009). [DOI] [PMC free article] [PubMed]
- 43.Ayachi M, Niel R, Momken I, Billat VL, Mille-Hamard L. Validation of a Ramp Running Protocol for Determination of the True VO2max in Mice. Front Physiol. 2016;7:372. doi: 10.3389/fphys.2016.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee-Young RS, et al. Obesity impairs skeletal muscle AMPK signaling during exercise: role of AMPKa2 in the regulation of exercise capacity in vivo. Int J Obes (Lond) 2011;35:982–989. doi: 10.1038/ijo.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbato JC, et al. Spectrum of aerobic endurance running performance in eleven inbred strains of rats. J Appl Physiol. 1998;85:530–536. doi: 10.1152/jappl.1998.85.2.530. [DOI] [PubMed] [Google Scholar]
- 46.Workman JM, Armstrong BW. Oxygen cost of treadmill walking. J Appl Physiol. 1963;18:798–803. doi: 10.1152/jappl.1963.18.4.798. [DOI] [PubMed] [Google Scholar]
- 47.Kemi OJ, Loennechen JP, Wisloff U, Ellingsen O. Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol. 2002;93:1301–1309. doi: 10.1152/japplphysiol.00231.2002. [DOI] [PubMed] [Google Scholar]
- 48.Lusk, G. Animal csalorimetry. Twenty-fourth paper. Analysis of the oxidation of mixtures of carbohydrate and fat. A correction. J Biol Chem59, 41–42 (1924).
- 49.Crane JD, Mottillo EP, Farncombe TH, Morrison KM, Steinberg GR. A standardized infrared imaging technique that specifically detects UCP1-mediated thermogenesis in vivo. Mol Metab. 2014;3:490–494. doi: 10.1016/j.molmet.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.