Abstract
Mitochondria are established essential regulators of cellular function and metabolism. Mitochondria regulate redox homeostasis, maintain energy (ATP) production through oxidative phosphorylation, buffer calcium levels, and control cell death through apoptosis. In addition to these critical cell functions, recent evidence supports a signaling role for mitochondria. For example, studies over the past few years have established that peptides released from the mitochondria mediate stress responses such as the mitochondrial unfolded protein response (UPRMT) through signaling to the nucleus. Mitochondrial damage or danger associated molecular patterns (DAMPs) provide a link between mitochondria, inflammation and inflammatory disease processes. Additionally, a new class of peptides generated by the mitochondria affords protection against age-related diseases in mammals. In this short review, we highlight the role of mitochondrial signaling and regulation of cellular activities through the mitochondrial UPRMT that signals to the nucleus to affect homeostatic responses, DAMPs, and mitochondrial derived peptides.
Keywords: mitochondria, stress response, longevity, signaling peptides, retrograde response
Introduction
Mitochondria are essential double membrane cellular organelles that provide the energy to the cell through oxidative phosphorylation. Mitochondria also play a key role in cellular function and homeostasis through adenosine triphosphate (ATP) production, calcium homeostasis, apoptosis signaling, and fatty acid oxidation. In addition, mitochondria modulate redox signaling through the production of reactive oxygen species (ROS). While the role of mitochondrial ROS signaling in modulation of cellular processes has been established, recently several studies have highlighted additional signaling roles for mitochondria that are not initiated by ROS. The idea that mitochondria can regulate cellular metabolism through mitochondrial derived signaling was first described in yeast. Using mitochondrial DNA depleted rho° yeast cells, Ron Butow discovered yeast mitochondria have adapted a mitochondria-to-nucleus signal transduction pathway termed the retrograde response to induce the transcription of nuclear-encoded mitochondrial genes and alleviate mitochondrial stress (Parikh et al., 1987). Higher organisms have adapted a similar retrograde signaling response known as the mitochondrial unfolded protein response (UPRMT). This response is initiated by the accumulation of unfolded proteins in the mitochondria resulting in the induction of UPRMT components (Benedetti et al., 2006). In addition, mitochondria can regulate cell function and metabolism by signaling pathways that involve mitochondrial-derived peptides and other mitochondrial signals such as mitochondrial damage-associated molecular patterns (mito-DAMPs). In this review, we highlight the role of mitochondrial signaling and regulation of cellular activities through the mitochondrial UPRMT that signals to the nucleus to affect homeostatic responses, damage or danger associated molecular patterns (DAMPs), and mitochondrial derived peptides (MDPs) (Figure 1).
FIGURE 1.
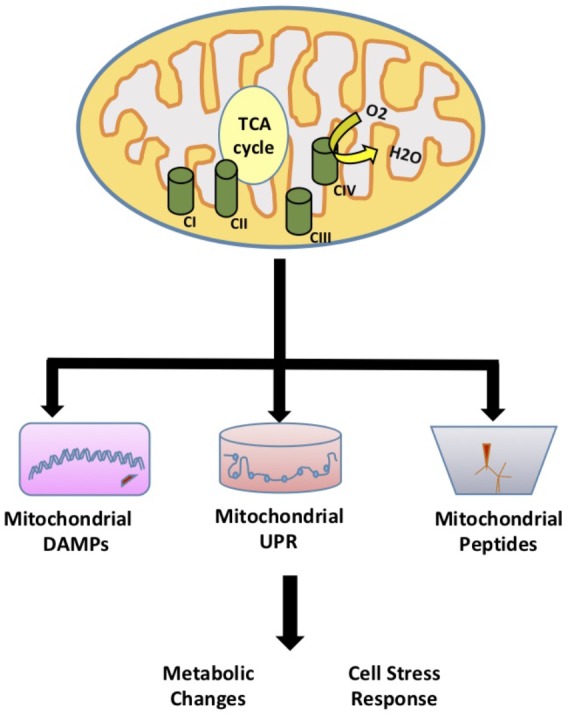
Mitochondrial Signaling. The role of mitochondria in signaling and regulation of cellular activities through the mitochondrial unfolded protein response (UPRMT) that signals to the nucleus to affect homeostatic responses, damage or danger associated molecular patterns (DAMPs), and mitochondrial derived peptides (MDPs).
Mitochondrial Unfolded Protein Response (UPRMT)
The UPRMT signaling pathway was first elucidated in C. elegans. Mitochondrial stress generated from misfolded proteins was shown to activate the adenosine triphosphate (ATP)-dependent mitochondrial protease, ClpP, to cleave misfolded proteins (Benedetti et al., 2006; Haynes et al., 2007). The exported peptides in turn activate the nuclear translocation of ATFS-1 (Activating Transcription Factor associated with Stress) where it subsequently activates UBL-5 to form a complex with transcription factor DVE-1 to transcriptionally activate UPRMT genes, heat shock protein 6 (hsp-6) and hsp-10 (Martinus et al., 1996; Haynes et al., 2007, 2010). Subsequent studies found that under normal conditions ATFS-1 accumulates in the mitochondria, but during mitochondrial stress (ETC dysfunction, ROS production, proteotoxic stress, etc.) ATFS-1 accumulates in the cytosol and is transported to the nucleus to induce the expression of mitochondrial stress proteins (Nargund et al., 2012). A recent study in C. elegans showed that the H3K27 demethylases jmjd-1.2 and jmjd-3.1 are required for the UPRMT suggesting that the UPRMT can also be regulated at the epigenetic level (Merkwirth et al., 2016). In another recent report in C. elegans, the UPRMT was proposed to promote the propagation of deleterious mtDNA rather than eliminating deleterious mtDNA (Lin et al., 2016). These findings suggest that the UPRMT could be used as a therapeutic target for mtDNA disorders and associated diseases (further reviewed in Tian et al., 2016).
The regulation of the UPRMT in mammals is not fully understood, but a few key components have been identified in mammals with the first report by Zhao et al. (2002) showing that UPRMT components are elevated in mitochondrial DNA (mtDNA) depleted mammalian cells. The mammalian transcription factors, CHOP and C/EBPβ were implicated as putative transcription factors for this pathway based on conserved regulatory element in promoters of the UPRMT related genes (Aldridge et al., 2007). In this model, mitochondrial proteotoxic stress mediates CHOP transcriptional activation through the Jnk/c-Jun pathway (Weiss et al., 2003; Jaeschke et al., 2006). This pathway is conserved in mammals as the Jnk/c-Jun pathway is required for transcriptional activation of CHOP and UPRMT induction in mitochondrial ornithine transcarbamylase (OTC) mutant Cos-7 cells under proteotoxic stress (Rath et al., 2012). A recent study by Fiorese et al. (2016) identified the mammalian transcription factor ATF-5 to be the functional ortholog of the C. elegans ATFS-1 transcription factor. The transcription factor, ATF-4 has also been recently shown to be an important mediator of mitochondrial stress response (Quiros et al., 2017). However, ATF-4 was not shown to regulate UPRMT genes but cytoprotective genes that regulate cellular metabolism. Studies in mouse tissue show a consistent correlation between H3K27 demethylases and UPRMT transcripts supporting the notion that UPRMT gene regulation is through demethylases proceeding the retrograde signaling from the mitochondria (Merkwirth et al., 2016).
Studies from our laboratory have shown that mice harboring a null mutation in the SURF1 gene, an electron transport chain complex IV assembly factor, show an induction of the UPRMT in several tissues that is associated with a number of beneficial phenotypes including increased insulin sensitivity, mitochondrial biogenesis, and increased resistance to oxidative stress in cultured fibroblasts (Deepa et al., 2013; Lin et al., 2013; Pulliam et al., 2014; Pharaoh et al., 2016). Shpilka and Haynes (2018) have extensively reviewed the implications of the UPRMT in aging and age-related diseases. These studies suggest a potential link between the UPRMT, metabolism and stress resistance in mammals.
Signaling Peptides Associated With the Mitochondrial Unfolded Protein Response
The mitochondrial UPRMT is of interest as a direct form of communication between the mitochondria and the nucleus. However, a study by Durieux et al. (2011) suggested that mitochondrial stress could be signaled through cell non-autonomous mechanisms. Specifically, studies in C. elegans showed that mitochondrial proteotoxic stress restricted to neuronal tissue induced the mitochondrial UPRMT in a heterologous tissue, the intestine (Durieux et al., 2011). These findings suggest that the UPRMT can be activated in a cell non-autonomous fashion. Interestingly, components of the UPRMT, ATFS-1, and DVE-1 in the neurons are required to induce the UPRMT in the intestine. Further studies have been conducted to determine the signaling factor released by the neurons to induce the UPRMT in the intestine. One study suggested that distal activation of the UPRMT requires UNC-31 mediated secretion of serotonin (Berendzen et al., 2016). However, this study used a general proteotoxic stress model, PolyQ40, and not a mitochondrial specific model. In another study, neuropeptides were investigated to determine if they mediate the UPRMT in a cell non-autonomous fashion using a neuronal specific CRISPR-Cas9 approach to manipulate mitochondrial stress (Shao et al., 2016). Neuropeptides are released from dense core vesicles derived at the synapse and may function as hormones to systemically alter cellular activities. This study showed that deletion of 6 out of the 103 reported neuropeptides blocked the neuronal-specific mitochondrial stress induction of the UPRMT in the intestine. These neuropeptides include INS-17, INS-34, FLP-2, FLP-15, NLP-10, and NLP-28. Subsequently, only constitutive overexpression of flp-2 was shown to induce the UPRMT on its own. These studies suggest that FLP-2 is released by neurons during mitochondrial stress to signal for the induction of the UPRMT in the periphery. Interestingly, overexpression of flp-2 activates the UPRMT but does not extend lifespan. This supports other studies where the UPRMT was shown to not have a casual effect on lifespan (Bennett et al., 2014). The identification of these signaling molecules could have significant implications in the development of therapies to target mitochondrial disease and improve healthspan.
Mitochondrial Damage Associated Molecular Patterns
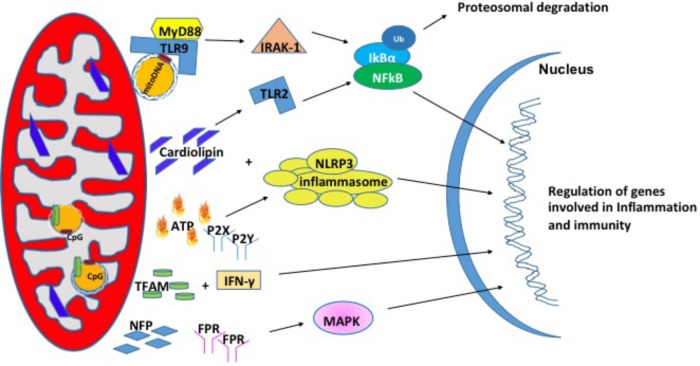
In recent years, studies have shown that mitochondria can modulate inflammation and the immune response through the release of signaling factors known as Mito-DAMPs (Zhang et al., 2010). Under physiological conditions, damage associated molecular patterns (DAMPs) are not recognized by immune system. During cellular stress or tissue injury, these molecules can be released from dying cells or damaged extracellular matrix in to the extracellular environment (Wenceslau et al., 2014; Land, 2015). During pathological insults, Mito-DAMPs have consequences on the innate immune response and inflammation (Krysko et al., 2011). Mito-DAMPs include mtDNA, TFAM, cardiolipin, ATP, and N-formyl peptides (NFPs) (Nakahira et al., 2015) (Figure 2).
FIGURE 2.
Mitochondrial damage-associated molecular patterns (Mito-DAMPs). Mitochondrial damage associated molecular patterns are in the form of mitochondrial DNA (mtDNA), cardiolipin, ATP, TFAM, and NFP. In response to stress, mitochondria release these small molecules to signal for the nuclear transcription of genes involved in inflammation and immunity.
Mitochondrial DNA (mtDNA)
Mitochondrial DNA encodes essential protein subunits of the oxidative phosphorylation system that play primary role in respiration and ATP production. Oxidized or fragmented mtDNA is released from damaged mitochondria and evokes an immune response (Garcia and Chavez, 2007; Nakahira et al., 2011). The unmethylated CpG site of the mtDNA binds to TLR9 and activates a downstream cascade of reactions by recruiting the adaptor proteins MyD88; IL-1R-associated kinase (IRAK4), interferon regulatory factor-7 (IRF7), tumor-necrosis factor-alpha receptor activated factor-6 (TRAF6) (Hacker et al., 2000; Huang and Yang, 2010). These events are followed by activation of ERK, p38, and IkB pathways that finally culminate in the transcription of inflammatory genes (Yi and Krieg, 1998; Deguine and Barton, 2014). mtDNA levels in the plasma of the elderly (age 90 years) correlated with pro-inflammatory cytokines like TNF-α, IL-1β, IL-6, IL-1Rα, and RANTES (Pinti et al., 2014) in glomerular kidney disease (Bao et al., 2016), in heart (Bliksoen et al., 2016), and in sporadic ALS (Vielhaber et al., 2000). In addition, circulating mtDNA levels are increased in acute liver injury (Marques et al., 2012), hypertension (McCarthy et al., 2015), acute kidney injury (Tsuji et al., 2016). Apart from the increased levels in the circulation, mtDNA copy number has also been shown to be increased in aging skeletal muscle (Masser et al., 2016), lung tissue (Lee et al., 2000), in cancer (Cormio et al., 2012) and the level of mtDNA in the synovial fluid was shown to correlate with the severity of rheumatoid arthritis (Hajizadeh et al., 2003). A recent study from Sandler et al. (2018) suggests that mtDNA may be an early biomarker in post-operative complications such as cardiopulmonary bypass surgery (Sandler et al., 2018) and in traumatic brain injury (Walko et al., 2014). Thus, mtDNA is associated with a number of diseases and may also play a role in pathologies of aging.
TFAM
Mitochondrial transcription factor, TFAM is an abundant protein that plays an important role in regulating mtDNA content. TFAM is tightly bound to mtDNA and amplifies the mtDNA induced TLR9 immune response (Julian et al., 2012). TFAM can act directly as a DAMP. For example, treatment of THP1 monocytic cells with TFAM resulted in increased expression IL-1β, IL-6, and IL-8 (Little et al., 2014). In another study, serum TFAM levels were doubled after hemorrhagic shock and resuscitation in Sprague-Dawley rats (Chaung et al., 2012), which in turn induces inflammatory responses. The impact of TFAM in neurodegeneration diseases has been reviewed briefly in Kang et al. (2018).
Cardiolipin
Cardiolipin is a phospholipid present in the inner mitochondrial membrane that is a central player in various processes like mitochondrial calcium uniporter, mitochondrial protein kinase C signaling, mitophagy, cytochrome c release during apoptosis and inflammasome activation (Dudek, 2017). Upon translocation to the outer membrane, cardiolipin activates NLRP3 promoting inflammation (Iyer et al., 2013). During cell injury, cardiolipin undergoes oxidation, and released into the extracellular environment where it acts as a mito-DAMPs (Claypool and Koehler, 2012). In support of this, patients with pneumonia show high concentration of cardiolipin in lung fluid (Ray et al., 2010) and patients with mitochondrial disease showed higher cardiolipin content in the skeletal muscle (Schlame et al., 1999). There are several reports elucidating the mechanism by which cardiolipin acts as mito-DAMP under different pathological conditions. In a study with autoimmune disease patients, cardiolipin signals through TLR2-PI3K-PKN1-AKT-p38MAPK-NFkB pathway to activate antigen presenting cells (Cho et al., 2018). In another study involving pneumonia, cardiolipin inhibits interleukin (IL)-10 production by inducing the SUMOylation of the nuclear receptor PPAR gamma. PPAR gamma SUMOylation results in binding of the repressive complex NCOR/HDAC3 to IL-10 promotor but not the TNF promoter thereby efficiently inhibiting IL-10 production (Chakraborty et al., 2017).
Adenosine Triphosphate (ATP)
Adenosine triphosphate is the currency of intracellular energy. ATP plays an important role in glycolysis, TCA cycle, and beta oxidation. Apart from being used as source of energy, it plays a crucial role in biochemical signaling pathways including DNA and RNA synthesis and protein synthesis. Under physiological conditions, there remains a balance between ATP secretion and its extracellular concentration. When this balance is lost, extracellular ATP (eATP) plays a toxic role. eATP is upregulated in diabetic nephropathy (Chen et al., 2013), hypertension (Ji et al., 2012), induces vascular inflammation, atherosclerosis (Stachon et al., 2016), and lung inflammation (Riteau et al., 2010). eATP can be fueled by stimuli such as membrane damage, mechanical stress, excitation of neural tissue (Fields, 2011). eATP binds to the purinergic receptor subtype P2X or P2Y receptor (Surprenant and North, 2009; Erlinge, 2011) and can play a key role in regulation of vascular endothelium, pain and inflammatory responses. Ectonucleotidases CD39 and CD73 can degrade eATP to ADP, AMP and adenosine and each of these molecules can bind to P2 receptors and activate responses related to tissue damage and inflammation (Wilkin et al., 2001; Bours et al., 2006). eATP has been shown to activate the NLRP3 inflammasome that results in the release of IL-18 (Amores-Iniesta et al., 2017) through P2X7 in allograft rejection (Amores-Iniesta et al., 2017). Importantly, P2X7 -receptor inhibitors CE224, AZD9056, and GSK1482160 are available in clinical use as immunomodulatory agents (Vergani et al., 2014).
N-Formyl Peptides (NFPs)
N-Formyl peptides are a class of peptides that are produced by bacterial cells and mitochondria suggesting their involvement in host defense against bacterial infection and the clearance of damaged cells. NFPs have a high affinity binding site for FPR, NFP (Boulay et al., 1990; Rabiet et al., 2005). FPRs are a class of transmembrane G protein-coupled receptors that have three isoforms (FPR1, FPR2, and FPR3) and are highly expressed on monocytes and neutrophils suggesting their involvement in immune cell response (Waller et al., 2004). NFPs have been shown to drive neutrophil activation through MAPK and ERK1/2 signaling pathways (Hazeldine et al., 2015). In cell culture models, the addition of mito-DAMPs initiated chemotaxis, production of TNFα as well as a rapid release of ROS (Rabiet et al., 2005; Friedenberg et al., 2016). The induction of chemotaxis via NFPs is dependent on calcium influx through FPRs (Le et al., 2002).
A number of studies have shown that NFPs are elevated in response to post-traumatic injury and diseased models (Zhang et al., 2010; Dorward et al., 2017). These elevated levels lead to systemic inflammatory response syndrome (SIRS) (Hazeldine et al., 2015). For example, elevated NFPs lead to airway contraction and lung inflammation (Wenceslau et al., 2016). On the other hand, the loss of NFP binding receptor, FPR increases the susceptibility to L. monocytogenes in mice (Liu et al., 2012). Inflammation is positively correlated with aging and this relationship is coined ‘inflamm-aging’ (Franceschi et al., 2000). Therefore, NFP signaling may be a therapeutic target to reduce systemic inflammation and increase healthspan in humans.
Mitochondrial Derived Peptides (MDPs)
Mitochondrial derived peptides are a novel class of mitochondrial signaling peptides that are encoded by short open reading frames (sORFs) in the mitochondrial genome. MDPs regulate a wide range of cellular signaling pathways and have an implicated role in aging. In this section, we review known MDPs and their role in cellular activities.
Humanin
Humanin was the first discovered MDP using a functional expression screen for peptides that could suppress neuronal cell death induced by Aβ (Hashimoto et al., 2001). Humanin is a 24-amino acid peptide that’s encoded by the mitochondrial 16S rRNA. Humanin has gained significant recognition for its cytoprotective cellular activities and protection against a wide range of pathologies. A number of studies implicate humanin as a potential therapeutic target for a range of diseases. For example, synthetic humanin improves memory deficits and reduced Aβ plaques in rodent models of Alzheimer’s disease (Niikura et al., 2011; Zhang et al., 2012; Chai et al., 2014). Studies have shown that humanin protects against stroke in mice (Xu et al., 2006), ameliorates atherosclerotic plaque formation (Oh et al., 2011), improves insulin sensitivity in rodent models of diabetes (Hoang et al., 2010), and affords cardioprotection against myocardial ischemia (Thummasorn et al., 2016). Additionally, humanin restores cellular ATP levels in cells isolated from human patients affected by the mitochondrial disease, mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (Kariya et al., 2005). The cytoprotective activities of humanin provide this protection in a number of disease models.
Humanin elicits cytoprotection inter- and intracellular via anti-apoptotic effects and binding to two plasma membrane receptors. Humanin interacts via binding with IGFBP-3, Bax, and tBid (Guo et al., 2003; Ikonen et al., 2003; Zhai et al., 2005). In cell culture studies, humanin shows BAX dependent anti-apoptotic effects against serum deprivation and tumor necrosis factor (Guo et al., 2003; Zhai et al., 2005). Humanin also exhibits anti-apoptotic effects by binding to IGFBP-3 to block nuclear translocation required to induce apoptosis (Ikonen et al., 2003). More recently, humanin is shown to prevent endoplasmic reticulum (ER)-stress induced apoptosis by mediating ER-mitochondrial cross talk for cell survival (Matsunaga et al., 2016; Sreekumar et al., 2017). As a result, humanin suppresses apoptosis and promotes cell survival during oxidative and ER stress. Humanin also protects from apoptosis through the preservation of mitochondrial homeostasis in responses to cellular stress (Klein et al., 2013; Thummasorn et al., 2016; Cui et al., 2017). In addition, circulating humanin binds to two plasma membrane receptors to initiate a number of cytoprotective activities. One of the two receptors is the trimeric CNTFR/gp130/WSX-1 receptor (Hashimoto et al., 2001, 2009; Ying et al., 2004). Humanin induces the hetero-oligomerization of CNTFR, gp130, and WSX-1 and subsequently binds to the CNTFR/gp130/WSX-1 receptor to activate the JAK-STAT pathway (Kim et al., 2016). Humanin-induced neuroprotection requires the activation of Stat3 via the CNTFR/gp130/WSX-1 receptor (Hashimoto et al., 2005). Activation of Stat3 by humanin also improves diabetes in a mouse model by inhibiting pancreatic β-cell apoptosis and improving glucose tolerance (Hoang et al., 2010). Humanin protects against damage from Aβ42 by binding to the formyl peptide receptor-like 1/2 (FRPL1/2) receptor (Ying et al., 2004). It’s hypothesized that humanin exerts its neuroprotection by competing with Aβ42 for the binding to FRPL1/2 since Aβ42 accumulation is linked to FRPL1/2 binding.
Due to the universal cytoprotective activities of humanin, it has been proposed to have therapeutic potential to enhance human healthspan. In fact, humanin has been linked to aging. Humanin levels significantly decline with age in humans and rodent models (Muzumdar et al., 2009; Bachar et al., 2010; Hoang et al., 2010). Offspring of centenarians have been shown to have higher levels of humanin compared to the rest of the aging population (Muzumdar et al., 2009). Together, these studies suggest that retaining humanin levels with age may promote healthy aging.
MOTS-c (Mitochondrial Open Reading Frame of the 12S rRNA-c)
MOTS-c is a 16-amino-acid peptide encoded by 12S rRNA sORF in the mtDNA that has been proposed to play a role in regulation of metabolism (Lee et al., 2015). The MOTS-c transcript is polyadenylated and subsequently exported to the cytoplasm for translation. Phylogenetic studies show that MOTS-c is highly conserved across species (Lee et al., 2015). In mice, MOTS-c is present in a number of high-energy demanding tissues with the main targets being skeletal muscle and adipose tissue. MOTS-c is also present in the circulation of humans and mice (Lee et al., 2015).
Microarray analysis in two different mammalian cell culture lines demonstrated that MOTS-c regulates global gene expression specifically affecting metabolic and inflammatory related genes (Lee et al., 2015). The alterations in global metabolic gene expression translate to changes in the metabolite profile. Metabolomic studies showed that MOTS-c reduces metabolites involved in purine and dipeptide metabolism and increases metabolites involved in acylcarnitine and methionine metabolism. MOTS-c also promotes the biosynthesis of the AMPK activator, AICAR (Lee et al., 2015). These alterations in the metabolomic profile induced by MOTS-c suggest a potential target for metabolic disease as well as aging.
Studies have shown that MOTS-c can protect against a number of pathologies. MOTS-c treated mice have reduced body weight, food intake, and blood glucose. MOTS-c promotes insulin sensitivity and protects from high-fat diet induced insulin resistance and obesity in mice (Lee et al., 2015). Specifically, MOTS-c promotes insulin sensitivity in the skeletal muscle by stimulating glucose clearance as measured with the hyperinsulinemic-euglycemic clamp technique (Lee et al., 2015). MOTS-c also has a protective role in ovariectomy-induced bone loss. MOTS-c treatment inhibited receptor activator of nuclear factor-κB ligand (RANK) induced osteoclast differentiation (Ming et al., 2016). The protective effects of MOTS-c on bone loss are partially dependent on MOTS-C mediated AMPK activation since an AMPK inhibitor partially reversed these effects (Ming et al., 2016). Additionally, MOTS-c administration reduced basal circulating levels of IL-6 and TNFα (Lee et al., 2015).
Analyses of the mitochondrial genome from an exceptionally long-lived Japanese population suggest a role for MOTS-c in human longevity (Fuku et al., 2015a). Mitochondrial genome analysis from a Northeast Asian population identified a m.1382A > C polymorphism located in the MOTS-c encoding mtDNA (Fuku et al., 2015a,b). This single nucleotide polymorphism results in a single amino acid substitution predicted to have function consequences on the small peptide. This alteration in MOTS-C could contribute to the exceptional longevity observed in the Northeast Asian population. Together, these data suggest that MOTS-c may be a potential therapeutic target to improve healthspan.
Small Humanin-Like Peptides (SHLPs)
An in silico prediction analyses of sORFs identified six novel peptides named small humanin-like peptides (SHLPs) 1–6 (Cobb et al., 2016). The existence of these novel SHLPs were validated by mRNA and peptide expression levels in cells, tissues, and plasma. The origin of SHLPs 1–6 has been determined by RT-PCR. SHLPs 1, 4, 5, and 6 were confirmed to be mitochondrial in origin. However, SHLPs 2 and 3 were amplified from both mitochondrial and nuclear cDNA, which leaves the possibility that SHLPs 2 and 3 are not exclusively mitochondrial in origin.
Cell viability and apoptosis studies revealed that SHLPs 2 and 3 promotes cell viability and protection against cellular apoptosis in NIT-1 and 22Rv1, mouse beta and human prostate cancer cells, respectively (Cobb et al., 2016). One method in which SHLPs 2 and 3 promote cell viability is through the reduction of ROS production. The peptides, SHLPs 2 and 3 increase basal oxygen consumption and ATP production (Cobb et al., 2016). Furthermore, SHLPs 2 and 3 promotes adipocyte differentiation of 3T3L pre-adipocytes. SHLPs 2 and 3 activate ERK and STAT-3 signaling (Cobb et al., 2016).
Similar to humanin and MOTS-c, SHLP 2 circulating levels decline with age making it a potential therapeutic target for age-related diseases (Cobb et al., 2016). In fact, SHLP 2 has similar effects on insulin sensitivity as humanin and MOTS-c. In Sprague-Dawley rats, SHLP 2 enhanced the exogenous glucose infusion rate by 50% and promoted glucose uptake by peripheral tissues measured with the hyperinsulinemic-euglycemic clamp technique (Cobb et al., 2016). These findings suggest that SHLP 2 is an insulin sensitizer and implicates a therapeutic potential for SHLP 2 for diabetes. Additionally, SHLP 2 supplementation prevented Aβ-induced neuronal cell death (Cobb et al., 2016). SHLP 2 may exert neuroprotection similar to humanin via the activation of STAT-3. Future studies should investigate the effects of SHLP 2 in Alzheimer’s disease and diabetic mammalian models. Furthermore, it is important to establish the role of SHLP 2 in modulating healthspan as well as characterize the biological and physiological effects of the other SHLPs.
Summary
Since the initial finding of mitochondrial nuclear signaling in yeast, our understanding of the potential for mitochondrial to act in a cell signaling capacity has expanded significantly. A similar mitochondrial-nuclear signaling pathway, the UPRMT, was demonstrated in C. elegans and more recently studies have demonstrated that mitochondrial stress restricted to neuronal tissue induced the UPRMT in a heterologous tissue, the intestine. These findings suggest that there is not only intracellular signaling resulting in UPRMT activation, but that there is also signaling between/among tissues resulting in UPRMT activation. This is a novel concept that has important implications for extending our understanding of the role of mitochondria in cellular function and resistance to stress. Mitochondrial peptides have also been shown to regulate cytoprotective activities and mitochondrial stress responses in mammals. For example, a recently discovered MDP, humanin, is released during mitochondrial dysfunction resulting in an increase of cytoprotective activities and providing protection against a range of pathologies. Since the discovery of humanin, MOTS-C and a class of six other mitochondrial derived peptides (SHLPs) have been identified. Similar to humanin, these peptides have shown to afford cytoprotection but not much is known. Future work will likely continue to demonstrate exciting new signaling and regulatory functions for the mitochondria.
Author Contributions
SH, KS, and HVR wrote the text. KS prepared the figures.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank members of the Van Remmen laboratory for rich discussions and their comments throughout the preparation of this mini-review.
Footnotes
Funding. HVR is supported by a Senior Research Career Scientist Award from the Department of Veterans Affairs and SH was supported by a Ruth L. Kirschstein National Research Service Award for Individual Predoctoral Fellows (F31AG047764-03).
References
- Aldridge J. E., Horibe T., Hoogenraad N. J. (2007). Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS One 2:e874. 10.1371/journal.pone.0000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores-Iniesta J., Barbera-Cremades M., Martinez C. M., Pons J. A., Revilla-Nuin B., Martinez-Alarcon L., et al. (2017). Extracellular ATP activates the NLRP3 inflammasome and is an early danger signal of skin allograft rejection. Cell Rep. 21 3414–3426. 10.1016/j.celrep.2017.11.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachar A. R., Scheffer L., Schroeder A. S., Nakamura H. K., Cobb L. J., Oh Y. K., et al. (2010). Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc. Res. 88 360–366. 10.1093/cvr/cvq191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W., Xia H., Liang Y., Ye Y., Lu Y., Xu X., et al. (2016). Toll-like receptor 9 can be activated by endogenous mitochondrial DNA to induce podocyte apoptosis. Sci. Rep. 6:22579. 10.1038/srep22579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti C., Haynes C. M., Yang Y., Harding H. P., Ron D. (2006). Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics 174 229–239. 10.1534/genetics.106.061580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. F., Vander Wende H., Simko M., Klum S., Barfield S., Choi H., et al. (2014). Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat. Commun. 5:3483. 10.1038/ncomms4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendzen K. M., Durieux J., Shao L. W., Tian Y., Kim H. E., Wolff S., et al. (2016). Neuroendocrine coordination of mitochondrial stress signaling and proteostasis. Cell 166 1553.e10–1563.e10. 10.1016/j.cell.2016.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliksoen M., Mariero L. H., Torp M. K., Baysa A., Ytrehus K., Haugen F., et al. (2016). Extracellular mtDNA activates NF-kappaB via toll-like receptor 9 and induces cell death in cardiomyocytes. Basic Res. Cardiol. 111:42. 10.1007/s00395-016-0553-6 [DOI] [PubMed] [Google Scholar]
- Boulay F., Tardif M., Brouchon L., Vignais P. (1990). The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry 29 11123–11133. 10.1021/bi00502a016 [DOI] [PubMed] [Google Scholar]
- Bours M. J., Swennen E. L., Di Virgilio F., Cronstein B. N., Dagnelie P. C. (2006). Adenosine 5’-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 112 358–404. 10.1016/j.pharmthera.2005.04.013 [DOI] [PubMed] [Google Scholar]
- Chai G. S., Duan D. X., Ma R. H., Shen J. Y., Li H. L., Ma Z. W., et al. (2014). Humanin attenuates Alzheimer-like cognitive deficits and pathological changes induced by amyloid beta-peptide in rats. Neurosci. Bull. 30 923–935. 10.1007/s12264-014-1479-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K., Raundhal M., Chen B. B., Morse C., Tyurina Y. Y., Khare A., et al. (2017). The mito-DAMP cardiolipin blocks IL-10 production causing persistent inflammation during bacterial pneumonia. Nat. Commun. 8:13944. 10.1038/ncomms13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaung W. W., Wu R., Ji Y., Dong W., Wang P. (2012). Mitochondrial transcription factor A is a proinflammatory mediator in hemorrhagic shock. Int. J. Mol. Med. 30 199–203. 10.3892/ijmm.2012.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Zhang J., Zhang W., Zhang J., Yang J., Li K., et al. (2013). ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int. J. Biochem. Cell Biol. 45 932–943. 10.1016/j.biocel.2013.02.009 [DOI] [PubMed] [Google Scholar]
- Cho J. A., Kim T. J., Moon H. J., Kim Y. J., Yoon H. K., Seong S. Y. (2018). Cardiolipin activates antigen-presenting cells via TLR2-PI3K-PKN1-AKT/p38-NF-kB signaling to prime antigen-specific naive T cells in mice. Eur. J. Immunol. 48 777–790. 10.1002/eji.201747222 [DOI] [PubMed] [Google Scholar]
- Claypool S. M., Koehler C. M. (2012). The complexity of cardiolipin in health and disease. Trends Biochem. Sci. 37 32–41. 10.1016/j.tibs.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb L. J., Lee C., Xiao J., Yen K., Wong R. G., Nakamura H. K., et al. (2016). Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging 8 796–809. 10.18632/aging.100943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormio A., Guerra F., Cormio G., Pesce V., Fracasso F., Loizzi V., et al. (2012). Mitochondrial DNA content and mass increase in progression from normal to hyperplastic to cancer endometrium. BMC Res. Notes 5:279. 10.1186/1756-0500-5-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui A. L., Zhang Y. H., Li J. Z., Song T., Liu X. M., Wang H., et al. (2017). Humanin rescues cultured rat cortical neurons from NMDA-induced toxicity through the alleviation of mitochondrial dysfunction. Drug Des. Devel. Ther. 11 1243–1253. 10.2147/DDDT.S133042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa S. S., Pulliam D., Hill S., Shi Y., Walsh M. E., Salmon A., et al. (2013). Improved insulin sensitivity associated with reduced mitochondrial complex IV assembly and activity. FASEB J. 27 1371–1380. 10.1096/fj.12-221879 [DOI] [PubMed] [Google Scholar]
- Deguine J., Barton G. M. (2014). MyD88: a central player in innate immune signaling. F1000Prime Rep. 6:97. 10.12703/P6-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward D. A., Lucas C. D., Doherty M. K., Chapman G. B., Scholefield E. J., Conway Morris A., et al. (2017). Novel role for endogenous mitochondrial formylated peptide-driven formyl peptide receptor 1 signalling in acute respiratory distress syndrome. Thorax 72 928–936. 10.1136/thoraxjnl-2017-210030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek J. (2017). Role of Cardiolipin in Mitochondrial Signaling Pathways. Front. Cell. Dev. Biol. 5:90 10.3389/fcell.2017.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J., Wolff S., Dillin A. (2011). The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144 79–91. 10.1016/j.cell.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinge D. (2011). P2Y receptors in health and disease. Adv. Pharmacol. 61 417–439. 10.1016/B978-0-12-385526-8.00013-8 [DOI] [PubMed] [Google Scholar]
- Fields R. D. (2011). Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron-glia signaling. Semin. Cell Dev. Biol. 22 214–219. 10.1016/j.semcdb.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorese C. J., Schulz A. M., Lin Y. F., Rosin N., Pellegrino M. W., Haynes C. M. (2016). The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr. Biol. 26 2037–2043. 10.1016/j.cub.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E., et al. (2000). Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908 244–254. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- Friedenberg S. G., Strange H. R., Guillaumin J., Vangundy Z. C., Crouser E. D., Papenfuss T. L. (2016). Effect of disrupted mitochondria as a source of damage-associated molecular patterns on the production of tumor necrosis factor alpha by splenocytes from dogs. Am. J. Vet. Res. 77 604–612. 10.2460/ajvr.77.6.604 [DOI] [PubMed] [Google Scholar]
- Fuku N., He Z. H., Sanchis-Gomar F., Pareja-Galeano H., Tian Y., Arai Y., et al. (2015a). Exceptional longevity and muscle and fitness related genotypes: a functional in vitro analysis and case-control association replication study with SNPs THRH rs7832552, IL6 rs1800795, and ACSL1 rs6552828. Front. Aging Neurosci. 7:59. 10.3389/fnagi.2015.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuku N., Pareja-Galeano H., Zempo H., Alis R., Arai Y., Lucia A., et al. (2015b). The mitochondrial-derived peptide Mots-c: a player in exceptional longevity? Aging Cell 14 921–923. 10.1111/acel.12389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia N., Chavez E. (2007). Mitochondrial DNA fragments released through the permeability transition pore correspond to specific gene size. Life Sci. 81 1160–1166. 10.1016/j.lfs.2007.08.019 [DOI] [PubMed] [Google Scholar]
- Guo B., Zhai D., Cabezas E., Welsh K., Nouraini S., Satterthwait A. C., et al. (2003). Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423 456–461. 10.1038/nature01627 [DOI] [PubMed] [Google Scholar]
- Hacker H., Vabulas R. M., Takeuchi O., Hoshino K., Akira S., Wagner H. (2000). Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J. Exp. Med. 192 595–600. 10.1084/jem.192.4.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajizadeh S., Degroot J., Tekoppele J. M., Tarkowski A., Collins L. V. (2003). Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res. Ther. 5 R234–R240. 10.1186/ar787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Kurita M., Matsuoka M. (2009). Identification of soluble WSX-1 not as a dominant-negative but as an alternative functional subunit of a receptor for an anti-Alzheimer’s disease rescue factor Humanin. Biochem. Biophys. Res. Commun. 389 95–99. 10.1016/j.bbrc.2009.08.095 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Niikura T., Tajima H., Yasukawa T., Sudo H., Ito Y., et al. (2001). A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc. Natl. Acad. Sci. U.S.A. 98 6336–6341. 10.1073/pnas.101133498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Suzuki H., Aiso S., Niikura T., Nishimoto I., Matsuoka M. (2005). Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci. 77 3092–3104. 10.1016/j.lfs.2005.03.031 [DOI] [PubMed] [Google Scholar]
- Haynes C. M., Petrova K., Benedetti C., Yang Y., Ron D. (2007). ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev. Cell 13 467–480. 10.1016/j.devcel.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Haynes C. M., Yang Y., Blais S. P., Neubert T. A., Ron D. (2010). The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol. Cell 37 529–540. 10.1016/j.molcel.2010.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeldine J., Hampson P., Opoku F. A., Foster M., Lord J. M. (2015). N-Formyl peptides drive mitochondrial damage associated molecular pattern induced neutrophil activation through ERK1/2 and P38 MAP kinase signalling pathways. Injury 46 975–984. 10.1016/j.injury.2015.03.028 [DOI] [PubMed] [Google Scholar]
- Hoang P. T., Park P., Cobb L. J., Paharkova-Vatchkova V., Hakimi M., Cohen P., et al. (2010). The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism 59 343–349. 10.1016/j.metabol.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Yang Y. (2010). Targeting the TLR9-MyD88 pathway in the regulation of adaptive immune responses. Expert Opin. Ther. Targets 14 787–796. 10.1517/14728222.2010.501333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen M., Liu B., Hashimoto Y., Ma L., Lee K. W., Niikura T., et al. (2003). Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc. Natl. Acad. Sci. U.S.A. 100 13042–13047. 10.1073/pnas.2135111100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S. S., He Q., Janczy J. R., Elliott E. I., Zhong Z., Olivier A. K., et al. (2013). Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39 311–323. 10.1016/j.immuni.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke A., Karasarides M., Ventura J. J., Ehrhardt A., Zhang C., Flavell R. A., et al. (2006). JNK2 is a positive regulator of the cJun transcription factor. Mol. Cell 23 899–911. 10.1016/j.molcel.2006.07.028 [DOI] [PubMed] [Google Scholar]
- Ji X., Naito Y., Weng H., Endo K., Ma X., Iwai N. (2012). P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am. J. Physiol. Renal Physiol. 303 F1207–F1215. 10.1152/ajprenal.00051.2012 [DOI] [PubMed] [Google Scholar]
- Julian M. W., Shao G., Bao S., Knoell D. L., Papenfuss T. L., Vangundy Z. C., et al. (2012). Mitochondrial transcription factor A serves as a danger signal by augmenting plasmacytoid dendritic cell responses to DNA. J. Immunol. 189 433–443. 10.4049/jimmunol.1101375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang I., Chu C. T., Kaufman B. A. (2018). The mitochondrial transcription factor TFAM in neurodegeneration: emerging evidence and mechanisms. FEBS Lett. 592 793–811. 10.1002/1873-3468.12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya S., Hirano M., Furiya Y., Sugie K., Ueno S. (2005). Humanin detected in skeletal muscles of MELAS patients: a possible new therapeutic agent. Acta Neuropathol. 109 367–372. 10.1007/s00401-004-0965-5 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Guerrero N., Wassef G., Xiao J., Mehta H. H., Cohen P., et al. (2016). The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus. Oncotarget 7 46899–46912. 10.18632/oncotarget.10380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L. E., Cui L., Gong Z., Su K., Muzumdar R. (2013). A humanin analog decreases oxidative stress and preserves mitochondrial integrity in cardiac myoblasts. Biochem. Biophys. Res. Commun. 440 197–203. 10.1016/j.bbrc.2013.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysko D. V., Agostinis P., Krysko O., Garg A. D., Bachert C., Lambrecht B. N., et al. (2011). Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 32 157–164. 10.1016/j.it.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Land W. G. (2015). The role of damage-associated molecular patterns in human diseases: part i - promoting inflammation and immunity. Sultan Qaboos Univ. Med. J. 15 e9–e21. [PMC free article] [PubMed] [Google Scholar]
- Le Y., Murphy P. M., Wang J. M. (2002). Formyl-peptide receptors revisited. Trends Immunol. 23 541–548. 10.1016/S1471-4906(02)02316-5 [DOI] [PubMed] [Google Scholar]
- Lee C., Zeng J., Drew B. G., Sallam T., Martin-Montalvo A., Wan J., et al. (2015). The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 21 443–454. 10.1016/j.cmet.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. C., Yin P. H., Lu C. Y., Chi C. W., Wei Y. H. (2000). Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem. J. 348(Pt 2), 425–432. 10.1042/bj3480425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. L., Pulliam D. A., Deepa S. S., Halloran J. J., Hussong S. A., Burbank R. R., et al. (2013). Decreased in vitro mitochondrial function is associated with enhanced brain metabolism, blood flow, and memory in Surf1-deficient mice. J. Cereb. Blood Flow Metab. 33 1605–1611. 10.1038/jcbfm.2013.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-F., Schulz A. M., Pellegrino M. W., Lu Y., Shaham S., Haynes C. M. (2016). Maintenance and propagation of a deleterious mitochondrial genome by the mitochondrial upr. Nature 533 416–419. 10.1038/nature17989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. P., Simtchouk S., Schindler S. M., Villanueva E. B., Gill N. E., Walker D. G., et al. (2014). Mitochondrial transcription factor A (Tfam) is a pro-inflammatory extracellular signaling molecule recognized by brain microglia. Mol. Cell. Neurosci. 60 88–96. 10.1016/j.mcn.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Liu M., Chen K., Yoshimura T., Liu Y., Gong W., Wang A., et al. (2012). Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci. Rep. 2:786. 10.1038/srep00786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques P. E., Amaral S. S., Pires D. A., Nogueira L. L., Soriani F. M., Lima B. H., et al. (2012). Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 56 1971–1982. 10.1002/hep.25801 [DOI] [PubMed] [Google Scholar]
- Martinus R. D., Garth G. P., Webster T. L., Cartwright P., Naylor D. J., Hoj P. B., et al. (1996). Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur. J. Biochem. 240 98–103. 10.1111/j.1432-1033.1996.0098h.x [DOI] [PubMed] [Google Scholar]
- Masser D. R., Clark N. W., Van Remmen H., Freeman W. M. (2016). Loss of the antioxidant enzyme CuZnSOD (Sod1) mimics an age-related increase in absolute mitochondrial DNA copy number in the skeletal muscle. Age 38 323–333. 10.1007/s11357-016-9930-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga D., Sreekumar P. G., Ishikawa K., Terasaki H., Barron E., Cohen P., et al. (2016). Humanin protects RPE cells from endoplasmic reticulum stress-induced apoptosis by upregulation of mitochondrial glutathione. PLoS One 11:e0165150. 10.1371/journal.pone.0165150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. G., Wenceslau C. F., Goulopoulou S., Ogbi S., Baban B., Sullivan J. C., et al. (2015). Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc. Res. 107 119–130. 10.1093/cvr/cvv137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkwirth C., Jovaisaite V., Durieux J., Matilainen O., Jordan S. D., Quiros P. M., et al. (2016). Two conserved histone demethylases regulate mitochondrial stress-induced longevity. Cell 165 1209–1223. 10.1016/j.cell.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming W., Lu G., Xin S., Huanyu L., Yinghao J., Xiaoying L., et al. (2016). Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem. Biophys. Res. Commun. 476 412–419. 10.1016/j.bbrc.2016.05.135 [DOI] [PubMed] [Google Scholar]
- Muzumdar R. H., Huffman D. M., Atzmon G., Buettner C., Cobb L. J., Fishman S., et al. (2009). Humanin: a novel central regulator of peripheral insulin action. PLoS One 4:e6334. 10.1371/journal.pone.0006334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K., Haspel J. A., Rathinam V. A., Lee S. J., Dolinay T., Lam H. C., et al. (2011). Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12 222–230. 10.1038/ni.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K., Hisata S., Choi A. M. (2015). The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid. Redox Signal. 23 1329–1350. 10.1089/ars.2015.6407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargund A. M., Pellegrino M. W., Fiorese C. J., Baker B. M., Haynes C. M. (2012). Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337 587–590. 10.1126/science.1223560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura T., Sidahmed E., Hirata-Fukae C., Aisen P. S., Matsuoka Y. (2011). A humanin derivative reduces amyloid beta accumulation and ameliorates memory deficit in triple transgenic mice. PLoS One 6:e16259. 10.1371/journal.pone.0016259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y. K., Bachar A. R., Zacharias D. G., Kim S. G., Wan J., Cobb L. J., et al. (2011). Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis 219 65–73. 10.1016/j.atherosclerosis.2011.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V. S., Morgan M. M., Scott R., Clements L. S., Butow R. A. (1987). The mitochondrial genotype can influence nuclear gene expression in yeast. Science 235 576–580. 10.1126/science.3027892 [DOI] [PubMed] [Google Scholar]
- Pharaoh G., Pulliam D., Hill S., Sataranatarajan K., Van Remmen H. (2016). Ablation of the mitochondrial complex IV assembly protein Surf1 leads to increased expression of the UPR(MT) and increased resistance to oxidative stress in primary cultures of fibroblasts. Redox Biol. 8 430–438. 10.1016/j.redox.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti M., Cevenini E., Nasi M., De Biasi S., Salvioli S., Monti D., et al. (2014). Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging”. Eur. J. Immunol. 44 1552–1562. 10.1002/eji.201343921 [DOI] [PubMed] [Google Scholar]
- Pulliam D. A., Deepa S. S., Liu Y., Hill S., Lin A. L., Bhattacharya A., et al. (2014). Complex IV-deficient Surf1(-/-) mice initiate mitochondrial stress responses. Biochem. J. 462 359–371. 10.1042/BJ20140291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros P. M., Prado M. A., Zamboni N., D’Amico D., Williams R. W., Finley D., et al. (2017). Multi-omics analysis identifies Atf4 as a key regulator of the mitochondrial stress response in mammals. J. Cell Biol. 216 2027–2045. 10.1083/jcb.201702058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiet M. J., Huet E., Boulay F. (2005). Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur. J. Immunol. 35 2486–2495. 10.1002/eji.200526338 [DOI] [PubMed] [Google Scholar]
- Rath E., Berger E., Messlik A., Nunes T., Liu B., Kim S. C., et al. (2012). Induction of dsRNA-activated protein kinase links mitochondrial unfolded protein response to the pathogenesis of intestinal inflammation. Gut 61 1269–1278. 10.1136/gutjnl-2011-300767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray N. B., Durairaj L., Chen B. B., Mcverry B. J., Ryan A. J., Donahoe M., et al. (2010). Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat. Med. 16 1120–1127. 10.1038/nm.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riteau N., Gasse P., Fauconnier L., Gombault A., Couegnat M., Fick L., et al. (2010). Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 182 774–783. 10.1164/rccm.201003-0359OC [DOI] [PubMed] [Google Scholar]
- Sandler N., Kaczmarek E., Itagaki K., Zheng Y., Otterbein L., Khabbaz K., et al. (2018). Mitochondrial DAMPs are released during cardiopulmonary bypass surgery and are associated with postoperative atrial fibrillation. Heart Lung Circ. 27 122–129. 10.1016/j.hlc.2017.02.014 [DOI] [PubMed] [Google Scholar]
- Schlame M., Shanske S., Doty S., Konig T., Sculco T., Dimauro S., et al. (1999). Microanalysis of cardiolipin in small biopsies including skeletal muscle from patients with mitochondrial disease. J. Lipid Res. 40 1585–1592. [PubMed] [Google Scholar]
- Shao L. W., Niu R., Liu Y. (2016). Neuropeptide signals cell non-autonomous mitochondrial unfolded protein response. Cell Res. 26 1182–1196. 10.1038/cr.2016.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilka T., Haynes C. M. (2018). The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 19 109–120. 10.1038/nrm.2017.110 [DOI] [PubMed] [Google Scholar]
- Sreekumar P. G., Hinton D. R., Kannan R. (2017). Endoplasmic reticulum-mitochondrial crosstalk: a novel role for the mitochondrial peptide humanin. Neural Regen. Res. 12 35–38. 10.4103/1673-5374.198970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachon P., Geis S., Peikert A., Heidenreich A., Michel N. A., Unal F., et al. (2016). Extracellular ATP induces vascular inflammation and atherosclerosis via purinergic receptor Y2 in mice. Arterioscler. Thromb. Vasc. Biol. 36 1577–1586. 10.1161/ATVBAHA.115.307397 [DOI] [PubMed] [Google Scholar]
- Surprenant A., North R. A. (2009). Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71 333–359. 10.1146/annurev.physiol.70.113006.100630 [DOI] [PubMed] [Google Scholar]
- Thummasorn S., Apaijai N., Kerdphoo S., Shinlapawittayatorn K., Chattipakorn S. C., Chattipakorn N. (2016). Humanin exerts cardioprotection against cardiac ischemia/reperfusion injury through attenuation of mitochondrial dysfunction. Cardiovasc. Ther. 34 404–414. 10.1111/1755-5922.12210 [DOI] [PubMed] [Google Scholar]
- Tian Y., Merkwirth C., Dillin A. (2016). Mitochondrial UPR: a double-edged sword. Trends Cell Biol. 26 563–565. 10.1016/j.tcb.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Tsuji N., Tsuji T., Ohashi N., Kato A., Fujigaki Y., Yasuda H. (2016). Role of mitochondrial DNA in septic AKI via toll-like receptor 9. J. Am. Soc. Nephrol. 27 2009–2020. 10.1681/ASN.2015040376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani A., Tezza S., Fotino C., Visner G., Pileggi A., Chandraker A., et al. (2014). The purinergic system in allotransplantation. Am. J. Transplant. 14 507–514. 10.1111/ajt.12567 [DOI] [PubMed] [Google Scholar]
- Vielhaber S., Kunz D., Winkler K., Wiedemann F. R., Kirches E., Feistner H., et al. (2000). Mitochondrial DNA abnormalities in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis. Brain 123(Pt 7), 1339–1348. 10.1093/brain/123.7.1339 [DOI] [PubMed] [Google Scholar]
- Walko T. D., III, Bola R. A., Hong J. D., Au A. K., Bell M. J., Kochanek P. M., et al. (2014). Cerebrospinal fluid mitochondrial DNA: a novel DAMP in pediatric traumatic brain injury. Shock 41 499–503. 10.1097/SHK.0000000000000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller A., Sutton K. L., Kinzer-Ursem T. L., Absood A., Traynor J. R., Linderman J. J., et al. (2004). Receptor binding kinetics and cellular responses of six N-formyl peptide agonists in human neutrophils. Biochemistry 43 8204–8216. 10.1021/bi035335i [DOI] [PubMed] [Google Scholar]
- Weiss C., Schneider S., Wagner E. F., Zhang X., Seto E., Bohmann D. (2003). JNK phosphorylation relieves Hdac3-dependent suppression of the transcriptional activity of c-Jun. EMBO J. 22 3686–3695. 10.1093/emboj/cdg364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenceslau C. F., Mccarthy C. G., Szasz T., Spitler K., Goulopoulou S., Webb R. C., et al. (2014). Mitochondrial damage-associated molecular patterns and vascular function. Eur. Heart J. 35 1172–1177. 10.1093/eurheartj/ehu047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenceslau C. F., Szasz T., Mccarthy C. G., Baban B., Nesmith E., Webb R. C. (2016). Mitochondrial N-formyl peptides cause airway contraction and lung neutrophil infiltration via formyl peptide receptor activation. Pulm. Pharmacol. Ther. 37 49–56. 10.1016/j.pupt.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin F., Duhant X., Bruyns C., Suarez-Huerta N., Boeynaems J. M., Robaye B. (2001). The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J. Immunol. 166 7172–7177. 10.4049/jimmunol.166.12.7172 [DOI] [PubMed] [Google Scholar]
- Xu X., Chua C. C., Gao J., Hamdy R. C., Chua B. H. (2006). Humanin is a novel neuroprotective agent against stroke. Stroke 37 2613–2619. 10.1161/01.STR.0000242772.94277.1f [DOI] [PubMed] [Google Scholar]
- Yi A. K., Krieg A. M. (1998). Rapid induction of mitogen-activated protein kinases by immune stimulatory CpG DNA. J. Immunol. 161 4493–4497. [PubMed] [Google Scholar]
- Ying G., Iribarren P., Zhou Y., Gong W., Zhang N., Yu Z. X., et al. (2004). Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J. Immunol. 172 7078–7085. 10.4049/jimmunol.172.11.7078 [DOI] [PubMed] [Google Scholar]
- Zhai D., Luciano F., Zhu X., Guo B., Satterthwait A. C., Reed J. C. (2005). Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J. Biol. Chem. 280 15815–15824. 10.1074/jbc.M411902200 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., et al. (2010). Circulating mitochondrial Damps cause inflammatory responses to injury. Nature 464 104–107. 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhang W., Li Z., Hao J., Zhang Z., Liu L., et al. (2012). S14G-humanin improves cognitive deficits and reduces amyloid pathology in the middle-aged APPswe/PS1dE9 mice. Pharmacol. Biochem. Behav. 100 361–369. 10.1016/j.pbb.2011.09.012 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Wang J., Levichkin I. V., Stasinopoulos S., Ryan M. T., Hoogenraad N. J. (2002). A mitochondrial specific stress response in mammalian cells. EMBO J. 21 4411–4419. 10.1093/emboj/cdf445 [DOI] [PMC free article] [PubMed] [Google Scholar]