Abstract
Introduction: Plague and tularemia are zoonoses and their causative bacteria are circulating in certain regions of Iran. This study was conducted to investigate potential disease reservoirs amongst small wildlife species in different regions of Iran.
Methods: Rodents, insectivores and hares from 17 different provinces of the country were collected in 2014 and 2015. Samples were taken from the spleens of the animals and Real-time PCR was applied to detect nucleic acid sequences that are specific to Francisella tularensis and Yersinia pestis, respectively.
Results: Among 140 collected rodents, 25 distinct species were identified out of which five were the most common: Microtus paradoxus (21% out of 140 rodents), Apodemus witherbyi (12%), Microtus irani (11%), Mus musculus (11%) and Microtus socialis (10%). Seventeen insectivores were collected and identified as Crocidura suaveolens (82%) and C. leucodon (18%). Fifty-one hares were collected and identified as Lepus europaeus (57%), Lepus tolai (14%) and Lepus sp. (29%). Three out of 140 explored rodents (1.91%) were positive for F. tularensis, an A. witherbyi, a Mus musculus domesticus, and a Chionomys nivalis collected from Golestan, Khuzestan and Razavi Khorasan provinces, respectively. Two hares (3.92%) were F. tularensis-positive, a L. europaeus from Khuzestan and a Lepus sp. from the Sistan and Baluchistan province. None of the tested animals were positive for Y. pestis.
Conclusion: This is the first report of direct detection of F. tularensis in mammals of Iran and the first-time observation of the agent in a snow vole, C. nivalis worldwide. The results indicate that tularemia is more widespread in Iran than previously reported including the Northeast and Southwestern parts of the country. Future studies should address genetic characterization of F. tularensis positive DNA samples from Iran to achieve molecular subtyping and rule out assay cross-reactivity with near neighbor Francisella species.
Keywords: tularemia, plague, hares, rodentia, insectivora
Introduction
Emerging and reemerging infectious diseases stand amongst the most challenging problems for public health entities around the world and more than half of them are zoonotic (Parhizgari et al., 2017). Changes in social, economic, environmental, and ecological factors may precipitate the conditions for the reemergence of these infectious diseases (Gupta et al., 2012). Plague and tularemia are two zoonotic diseases that are reported from Iran (Karimi et al., 1981; Esamaeili et al., 2013; Zargar et al., 2015).
Plague is a lethal zoonotic disease that historically has caused pandemics around the world. The causative agent of this disease is the bacterium Yersinia pestis. Plague is still endemic in certain regions of Africa, Asia, and North and South America (Dubyanskiy and Yeszhanov, 2016). Since 1990, most of human plague infections are reported from African countries (Williamson, 2016). Wild rodents and fleas on their bodies are regarded as the main reservoirs of Y. pestis in nature (Bitam et al., 2010); although other wild animals such as mammalian carnivores and insectivores can also play a role as the reservoir of the infection (Poland and Dennis, 1999). Infection of rodents lead to severe damages in liver and spleen, and demise in a period less than 3 days (Bevins et al., 2012). Rabbits and hares can also be infected with Y. pestis and may like rodents transmit the infection to humans by direct contact or indirectly via infected flea bites (Fratini et al., 2017). From 1943 to 1965, nine plague outbreaks were reported amongst humans in the western areas of Iran. In these outbreaks, rodents and hares were regarded as the main reservoirs (Shahraki et al., 2016). The last report of plague among rodents in Iran was in 1978 in the Eastern Azerbaijan province, northwest of Iran (Karimi, 1980). The studies on plague in wildlife in Iran were discontinued for a while but were restarted in 2011 and 2012 (Mostafavi and Keypour, 2017) when antibodies against Y. pestis were found in rodents and dogs of northwest Iran (Esamaeili et al., 2013). Over recent years, several plague outbreaks have been reported from Iran's neighboring countries such as Afghanistan, Saudi Arabia and Jordan (Saeed et al., 2005; Leslie et al., 2011).
Tularemia is caused by the bacterium Francisella tularensis and a vast range of rodents, hares, insectivores, ticks, flies and mosquitoes have been implicated as potential disease reservoirs (Goethert and Telford, 2009; Ulu-Kilic et al., 2013; Zargar et al., 2015). The disease is typically rapidly progressing in small mammals resulting in necrosis distributed in multiple organs including the spleen and death usually follows within a few days, alternatively, a chronic disease type with granulomas in the liver have been described (Maurin and Gyuranecz, 2016). There is incomplete knowledge of the worldwide tularemia burden among humans but generally, it is assumed to be a disease of the Northern hemisphere only. Between 2000 and 2012, 250 to 2500 human cases were reported from countries of Europe and from Turkey (Hestvik et al., 2015; Sanyaolu et al., 2016). Human mortality is generally low but disease and its symptoms including fever and fatigue may be long-lasting (Erdem et al., 2014). Outbreaks in proximity to rivers in Russia, Europe, and in Turkey points toward an important role of water in the survival of the bacterium and that aquatic rodents may serve as disease reservoirs (Sjöstedt, 2007; Kaysser et al., 2008; Clark et al., 2012). Turkey, one of Iran's neighboring countries, is accounted as an endemic region for this disease (Sahin et al., 2007). Humans can be infected via direct contact with infected animals or via bites of infected arthropods, intake of contaminated water or food, or via inhalation of F. tularensis-contaminated aerosols. There are several different clinical forms of disease in humans depending on the infectious route of the bacterium, all of which includes swelling of lymph nodes and may progress to septic disease (Barker and Klose, 2007). The first report of possible tularemia in Iran goes back to 1973 when antibodies against F. tularensis were found in domestic livestock and one hedgehog in the northwest and east of Iran, respectively (Arata et al., 1973). The first and thus far only report of a human case of tularemia was from the Marivan district, Kurdistan province of west Iran, in 1980 (Karimi et al., 1981). Studies that are more recent have shown a relatively high prevalence of F. tularensis-specific antibodies in the blood of humans living in the west, southeast and southwest parts of Iran. The results of these studies suggest that tularemia may be underdiagnosed in Iran and that enhanced disease surveillance would be valuable (Esmaeili et al., 2014a,b; Khoshdel et al., 2014; Zargar et al., 2015). In 2013, F. tularensis-specific antibodies were found in rodents in the southeast and west of Iran indicating that small mammals may be involved in disease transmission (Pourhossein et al., 2015; Mostafavi et al., 2017).
This study was conducted to evaluate the infection of small mammals including rodents, insectivores and hares with Y. pestis and F. tularensis in order to gain better information regarding the status of these agents in the wildlife of Iran.
Materials and methods
In this cross-sectional study, small mammals were captured alive in different provinces (central, northern, southern, eastern and western) of Iran in 2014 and 2015. The captured animals were sprayed with a pesticide in order to destruct the ectoparasites on their bodies; they were then dispatched to predetermined stations and euthanized. The captured small mammals were identified according to standard identification keys (Corbet, 1978; Kryštufek et al., 2005). Splenic samples were collected for molecular studies and preserved in microtubes containing 70% alcohol before being sent to the diagnostic laboratory.
DNA extraction from tissue samples was completed using the QIAamp DNA Mini Kit. In order to trace and identify F. tularensis and Y. pestis in tissue samples, the extracted DNA was subjected to pathogen-specific Real-time PCR assays using a Rotor-Gene 6600 (Corbett Life Science). The gene targets for Y. pestis were the chromosomal yihN gene and the plasmid genes caf1 and pla (Table 1). The same genes cloned into the plasmid pUC57 were used as positive control (provided by the Pasteur Institute of Iran). For Francisella spp. we used the ISFtu2 gene for a first screening step and the fopA gene for confirmation of the presence of F. tularensis as a second step. The DNA of F. tularensis subsp holarctica NCTC 10857 was used as a positive control. The DNA amplification was done in a volume of 25μl for 40 cycles after an initial denaturation at 95°C for 10 min. The cycling for Y. pestis was 95°C for 15 s, 58°C for 30 s, and 72°C for 30 s whereas for F. tularensis it was 95°C for 15 s, 58°C for 60 s, and 72°C for 60 s. As an internal control, the β-actin gene (Qiagen company) was used (Emanuel et al., 2003; Versage et al., 2003; Stewart et al., 2008; Bushon et al., 2010).
Table 1.
Primers and probes used for detection of F. tularensis and Y. pestis.
| Agent | Gene target | Primer and probe | Sequence (5′ to 3′) | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|
| F. tularensis | ISFtu2 (chromosome) | ISFtu2F | TTGGTAGATCAGTTGGTAGGATAACC | 97 | Versage et al., 2003 |
| ISFtu2R | TGAGTTTTATCCTCTGACAACAATATTTC | ||||
| Probe | FAM-AAAATCCATGCTATGACTGATGCTTTAGGTAATCCA-TAMRA | ||||
| fopA (chromosome) | fopA-F | AACAATGGCACCTAGTAATATTTCTGG | 87 | Bushon et al., 2010 | |
| fopA-R | CCACCAAAGAACCATGTTAAACC | ||||
| Probe | FAM-TGGCAGAGCGGGTACTAACATGATTGGT-5-TAMRA | ||||
| Y. pestis | yihN (chromosome) | Chrom F | CGCTTTACCTTCACCAAACTGAAC | 128 | Stewart et al., 2008 |
| Chrom R | GGTTGCTGGGAACCAAAGAAGA | ||||
| Probe | Texas Red-TAAGTACATCAATCACACCGCGACCCGCTT-BHQ-2 | ||||
| caf1 (plasmid) | pMT1 F | CCGTTATCGCCATTGCATTATTTGG | 194 | ||
| pMT1 R | GCCAAGAGTAAGCGTACCAACAAG | ||||
| Probe | FAM-AAGCACCACTGCAACGGCAACTCTT-BHQ-1 | ||||
| pla (plasmid) | pPCP1 F | ATTGGACTTGCAGGCCAGTATC | 144 | ||
| pPCP1 R | ATAACGTGAGCCGGATGTCTTC | ||||
| Probe | FAM-AAATTCAGCGACTGGGTTCGGGCACA-BHQ-1 |
All procedures performed in this study involving capturing and euthanizing of the animals were in accordance with international ethical standards. The institutional animal and human ethical committee of the Pasteur institute of Iran approved the project. Gloves, mask, face shield and gown were worn by personnel handling animals in the field and by laboratory personnel handling animal specimens. Personnel specifically trained in handling pathogenic agents performed the laboratory work. Procedures involving potentially infectious material were performed within a class II plus biological safety cabinet.
Results
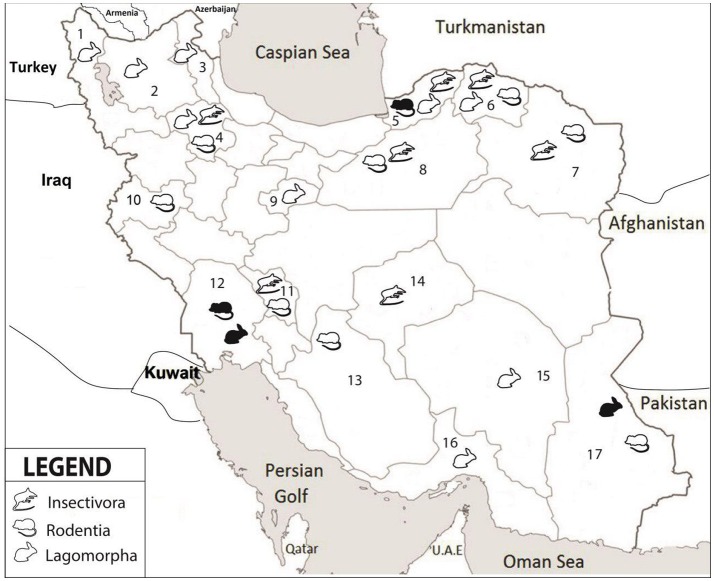
Altogether, 208 specimens from 140 rodents, 17 insectivores and 51 hares were collected from 17 different provinces (Figure 1, Table 2). The rodents were from 11 provinces including Northern Khorasan, Razavi Khorasan, Golestan, Fars, Zanjan, Chaharmahal and Bakhtiari, Semnan, Sistan and Baluchistan, Khuzestan, Kerman and Kermanshah. Of the 25 studied rodent species, Microtus paradoxus, Apodemus witherbyi, Microtus irani, Mus musculus, and Microtus socialis were most common with a frequency of 30 (21.4%), 17 (12.1%), 16 (11.4%), 15 (10.7%), and 14 (10.0%), respectively. The insectivores were identified as 14 (82.4%) Crocidura suaveolens and 3 (17.6%) Crocidura leucodon. C. suaveolens was collected from different parts of the country (Golestan, North Khorasan, Zanjan, Chaharmahal and Bakhtiari, while C. leucodon was collected from a restricted area in northern Iran (Semnan and North Khorasan). The captured hares were 29 (56.9%) Lepus europaeus, 7 (13.7%) Lepus tolai and 29 (29.4%) other Lepus sp. L. europaeus was collected in Zanjan, Khuzestan, Ardebil, Eastern Azerbaijan, Western Azerbaijan and Qom whereas the Lepus sp. were from Sistan and Baluchistan, Southern Khorasan, Kerman and Hormozgan. L. tolai was collected from the Golestan province (Table 2).
Figure 1.
Collection locations in seventeen provinces of Iran. 1-West Azerbaijan, 2-East Azerbaijan, 3-Ardabil, 4-Zanjan, 5-Golestan, 6-North Khorasan, 7-Razavi Khorasan, 8-Semnan, 9-Qom, 10-Kermanshah, 11- Chaharmahal and Bakhtiari, 12-Khuzestan, 13-Fars, 14-Yazd, 15-Kerman, 16-Hormozgan, 17-Sistan and Baluchistan. Different groups of small mammals are indicated by symbols; white color refers to negative and black color to positive samples.
Table 2.
Sampling locations of wild-caught rodents, insectivores, and hares.
| Animal group | Province (no. of collected animals) | Sampling site | Species (no.) |
|---|---|---|---|
| Rodents and insectivores | Golestan (30) | Gorgan, Toskestan, Aliabad-e Katul | Apodemus uralensis*(5), Microtus paradoxus (10), Mus musculus (9), Crocidura suaveolens (4), Rattus rattus(1), Apodemus witherbyi (1) |
| North Khorasan (32) | Bojnord (Darkesh, Dasht, Gachranlo) | M. paradoxus (19), M. musculus (4), C. suaveolens (1), A. witherbyi (3), Meriones persicus (1), Crocidura leucodon (2) | |
| Razavi Khorasan (21) | Mashhad, Moghan, Dargaz | Microtus transcaspicus (3), Chionomys nivalis*(4), A. witherbyi (9), M. musculus (1), Blanfordimys afghanus (1), M. persicus (1), C. suaveolens (1), M. paradoxus (1) | |
| Zanjan (16) | Soltanieh, Mahneshan, Anguran | C. suaveolens (7), Microtus mystacinus (1), Microtus qazvinensis (5), Mus macedonicus (3) | |
| Semnan (9) | Shahmirzad, Jashlobar | M. mystacinus (5), A. witherbyi (3), C. leucodon (1) | |
| Chaharmahal and Bakhtiari (15) | Lordegan, Shahrekord | Microtus socialis (14), C. suaveolens (1) | |
| Kermanshah (1) | Songhor | M. qazvinensis (1) | |
| Fars (17) | Shiraz, Mamasani | Microtus irani (16), A. witherbyi (1) | |
| Kerman (6) | Kerman | Microtus kermanensis (5), M. musculus (1) | |
| Khuzestan (7) | Izeh | Calomyscus bailwardi (3), M. musculus domesticus* (3), M. persicus (1) | |
| Sistan and Baluchistan (3) | Iran Shahr | Jaculus branfordi (3) | |
| Hares | East Azerbaijan (2) | Shabestar, Mianeh | Lepus europaeus (2) |
| West Azerbaijan (1) | Urmia | L. europaeus (1) | |
| Ardabil (5) | Parsabad Moghan, Bilesovar, Meshkinshahr, Khalkhal | L. europaeus (5) | |
| South Khorasan (4) | Khusf, Shosf | L. europaeus (4) | |
| Golestan (7) | Gorgan, Incheh Borun, Gonbad, Kordkuy | Lepus tolai (7) | |
| Zanjan (8) | Sohrein, Tarom, Zarinabad, Mahneshan, Khodabandeh, Dandi | L. europaeus (8) | |
| Kerman (4) | Anbarabad, Kahnuj | Lepus sp. (4) | |
| Khuzestan (8) | Shushtar, Ramhormoz | L. europaeus* (8) | |
| Hormozgan (2) | Sardasht, Rudan | Lepus sp. (2) | |
| Sistan and Baluchistan (9) | Mirjaveh, Maskutan, Bazman | Lepus sp.* (9) | |
| Qom (1) | Kahak | L. europaeus |
F. tularensis was detected.
Out of all wild-caught animals, three rodents and two hares were positive for F. tularensis. The rodents were one each of Apodemus uralensis (Toskestan, Golestan province), Mus musculus domesticus (Izeh, Khuzestan), and Chionomys nivalis (Zoshk village, Mashhad, Razavi Khorasan province). The hares were a L. europaeus from Khuzestan and a Lepus sp. from the Sistan and Baluchistan province. There was no detection of Y. pestis in any of the animals (Table 2).
Discussion
This study is the first report of direct detection of F. tularensis in rodents and hares in Iran and to the best of our knowledge, the first report of infection in the rodent species C. nivalis worldwide. Our study also expands the known geographic distribution of F. tularensis in Iran. In contrast, Y. pestis was not detected in any of the wild-caught animals examined.
The F. tularensis positive samples from animals in Northeast and Southwestern parts of Iran suggests that tularemia is widely distributed in Iran. An early study performed 1969 to 1970 using sampling with serology of >4,600 small mammals and 200 cattle and sheep showed that tularemia existed in the northwest and at one location at the very east of the country (Arata et al., 1973). Over the years, additional serology findings have verified that tularemia exists also in other parts of Iran. Recent studies of rodents in the southeast and west of Iran showed the presence of antibodies against F. tularensis (Pourhossein et al., 2015; Mostafavi et al., 2017). In another study of humans in 2014 in the Sistan and Baluchistan province southeast of Iran, the seroprevalence of tularemia among butchers and workers of slaughterhouses was estimated to be 6.5% (Esmaeili et al., 2014b). In addition, a study on humans with risk factors for acquiring tularemia reported a 14.4% prevalence of antibodies against tularemia in Kurdistan, west Iran (Esmaeili et al., 2014a). Finally, a study of rural children in the Chaharmahal and Bakhtiari province, southwest of Iran, showed that the prevalence of antibodies to F. tularensis was 6% (Khoshdel et al., 2014).
Given our findings of F. tularensis in rodents and hares in several areas of the country including in the Northeast and the Southwest and several previous studies with findings of F. tularensis antibodies in humans and animals, it is noticeable that there have been no human cases or tularemia reported in Iran since 1980 (Karimi et al., 1981). Tularemia in other countries, however, is often a diagnostic challenge to the practicing doctor, especially in areas where it seldom appears (Eliasson et al., 2005). Because the clinical diagnosis is based on awareness of the disease, giving rise to a clinical suspicion, and that it may resemble other diseases, we think that tularemia may be underdiagnosed in Iran. Recent publications from Turkey illustrate that the diagnosis may easily be overlooked because it often mimics other conditions of fever such as tuberculosis and other diseases that may cause enlarged lymph nodes (Karabay et al., 2013; Erdem et al., 2014; Yildirim et al., 2014). A presence of tularemia among humans in Iran's neighboring countries such as Azerbaijan (Clark et al., 2012), Armenia (Melikjanyan et al., 1996), and Turkey (Helvaci et al., 2000; Sahin et al., 2007; Balci et al., 2014) suggests that tularemia may be underdiagnosed in Iran.
In the present study, positive samples of F. tularensis-infection were observed in three rodents (A. uralensis, M. musculus domesticus, and C. nivalis) and two hares (L. europaeus and Lepus sp.) in the north, southeastern and southwestern Iran. The House mouse and the pygmy field mouse (A. uralensis) have previously been reported as a source of F. tularensis (Sakiyev et al., 2013; Unal et al., 2014). However, the natural infection of the European snow vole (C. nivalis) with F. tularensis was to the best of our knowledge not reported. More than 200 species of mammals have been identified as reservoirs of tularemia and many of them are rodents. Aquatic rodents are thought to play a crucial role in disease maintenance and the connection between this bacterium and natural waters (Christova and Gladnishka, 2005; Keim et al., 2007). The significance of rodents in the transmission of this disease is also strengthened by the observation that several tularemia outbreaks in humans were following outbreaks among rodents (Mörner, 1992; Johansson et al., 2014; Rodríguez-Pastor et al., 2017). Those rodents which have been described to play a role in the transmission of tularemia throughout the world were mostly from the genera Microtus (vole), Arvicola (water vole), Apodemus (field mice), and Myodes (Red Vole) (Kaysser et al., 2008; Gyuranecz et al., 2010). The observation of F. tularensis in hares is also in accordance with previous knowledge of that Lepus (hares) are important hosts, contributing significantly to the maintenance of the natural cycle of the agent and carrying a potential to produce infection in humans (Hopla and Hopla, 1994). Our findings of F. tularensis in a L. europaeus from Khuzestan and a Lepus sp. from the Sistan and Baluchistan province can be put into the context that infected rabbits and hares were reported in the tularemia epidemics in 1983, 1990–1992, 2005, and 2007 in Germany (Runge et al., 2011; Stalb et al., 2017). Another example is that in 1988, one case of tularemia was reported in a hare from the genus Lepus in Sudan (Mörner et al., 1988).
Two main bacterial subtypes may cause tularemia, F. tularensis subsp. tularensis (type A) and F. tularensis subsp. holarctica (type B) which are traditionally connected with different disease ecologies. Type A is extensively seen in the USA and is said to be mainly connected with a terrestrial cycle of the disease (rabbits and hares are mammal hosts; arthropods including ticks serve as vectors). Type B bacteria are related with an aquatic cycle and have mostly been found in outbreaks associated with rivers, lakes, ponds and brooks (semi-aquatic rodents are mammal hosts and mosquitoes or flies serve as vectors) (Hopla, 1974; Hopla and Hopla, 1994; Helvaci et al., 2000; Ulu et al., 2011; Maurin and Gyuranecz, 2016). Because F. tularensis subsp. holarctica with connections to water has been repeatedly detected in the neighboring country Turkey (Helvaci et al., 2000; Sahin et al., 2007; Balci et al., 2014), it is probable that F. tularensis identified in this study would be of this subtype. In Turkey, multiple phylogenetic groups of F. tularensis subsp. holarctica have been found indicating that much genetic diversity of this subspecies exists in proximity to Iran (Özsürekci et al., 2015). The presence of F. tularensis subsp. holarctica would also fit with the observation that the European brown hare (L. europaeus) typically serves as host for F. tularensis subsp. holarctica as verified in studies in countries such as Germany (Runge et al., 2011; Stalb et al., 2017) and Sweden (Mörner et al., 1988). Another possibility is that the F. tularensis subsp. mediasiatica exists in Iran, as this subspecies has been found in Kazakhstan, Turkmenistan, Uzbekistan and in the Altai region of Russia (Champion et al., 2009). The current knowledge, however, suggests that subsp. mediasiatica is rare in these geographical areas, which are located relatively distant to Iran (Timofeev et al., 2017).
The last reported human case of plague in Iran goes back to 1965 in the Kurdistan region, west of Iran; despite identification of active foci of plague in the wildlife of western and northwestern parts of the country there have been no human cases ever since. In our study, there was no positive case of Y. pestis amongst the studied rodents and hares although several of the captured rodents such as M. persicus, M. musculus, and Rattus rattus were previously identified as potential reservoirs of plague (Meyer et al., 1965; Saunders and Giles, 1977; Karimi, 1980). The role of hares in the transmission of plague has also been proposed (Hopkins and Gresbrink, 1982).
In natural foci of plague in Iran, four species of the genus Meriones have been shown to play a crucial role in the transmission of this disease (Shahraki et al., 2016). Recent studies performed in 2011 and 2012 identified antibodies against Y. pestis in rodents (1%) and in dogs (3.5%) of the Kurdistan-Hamadan border (Esamaeili et al., 2013); this would imply an ongoing infection cycle in these regions. We suspect that the number of animals studied originating in the west of Iran, the area previously described to be a focus of plague, may have been too small to identify positive samples. In light of the resurgence of plague after long time periods in other countries, e.g., after 50 years in Algeria in 2008, the health system of Iran must continue the surveillance of this disease.
A limitation of our study is that bacterial culture was not performed; this was not feasible with a study design including participation of multiple provinces and demands of in-time dispatch of samples to multiple laboratories. Another limitation is that we have not subtyped the F. tularensis. With the current results at hand, we suggest that future studies of tularemia in Iran should include comprehensive sampling from habitats and regions where positive samples were identified in this study and differentiation of the F. tularensis subtype. Although we used a PCR assay targeting the fopA gene that according to the literature should be specific to F. tularensis and the closely related pathogen F. novicida, there is a possibility that other Francisella species may have cross-reacted with the primers and probes used. The Francisella genus contains much diversity that was only recently discovered (Emanuel et al., 2003; Challacombe et al., 2017). A recent study from the Iberian Peninsula of Europe, e.g., found a F. hispaniensis-like DNA sequence in the wood mouse Apodemus sylvaticus (de Carvalho et al., 2016).
Conclusion
This study reports the first molecular detection of F. tularensis amongst rodents and hares in Iran and the first reported detection in the snow vole, C. nivalis, worldwide. Future studies should include additional characterization of the infectious agent. The present study and several previously conducted studies, indicate that tularemia is an endemic infectious disease of Iran. The current knowledge could be used to motivate information and educational activities among physicians and healthcare workers to increase disease awareness and diagnostic skills.
Author contributions
EM had role in design of the study, receiving the funds, managing the study and writing and finalizing the draft of the manuscript. AG, SE, and MR had the role in laboratory testing, writing and finalizing the draft of the manuscript. AM, ZM, and MA had role in sampling, writing and finalizing the draft of the manuscript. LM and AJ had role in analysis of the data, writing and finalizing the draft of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer RP and handling Editor declared their shared affiliation.
Acknowledgments
The authors would like to sincerely thank Pasteur Institute of Iran and the center for communicable disease control of Ministry of Health (Grant No. 810), Ferdowsi University of Mashhad (Project No. 32482), and National Institute for Medical Research Development (Grant No. 957145) for their financial support. We would also like to express our gratitude to the Rodentology department of research at Ferdowsi University of Mashhad for conducting a part of the sampling. We would like to remark Professor Jamshid Darvish, who had a great role in design and performance of this study. He established the field of Rodentology in Iran, he was the head of the Institute of Applied Zoology at Ferdowsi University of Mashhad, Iran and recently passed away on November 15, 2017. The sampling of this study was conducted in parallel with running two PhD projects (AM and ZM) at the Ferdowsi University of Mashhad.
References
- Arata A., Chamsa M., Farhang-Azad A., Meščerjakova I., Neronov V., Saidi S. (1973). First detection of tularaemia in domestic and wild mammals in Iran. Bull. World Health Organ. 49, 597–603. [PMC free article] [PubMed] [Google Scholar]
- Balci E., Borlu A., Kilic A. U., Demiraslan H., Oksuzkaya A., Doganay M. (2014). Tularemia outbreaks in Kayseri, Turkey: an evaluation of the effect of climate change and climate variability on tularemia outbreaks. J. Infect. Public Health. 7, 125–132. 10.1016/j.jiph.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Barker J. R., Klose K. E. (2007). Molecular and genetic basis of pathogenesis in Francisella tularensis. Ann. N. Y. Acad. Sci. 1105, 138–159. 10.1196/annals.1409.010 [DOI] [PubMed] [Google Scholar]
- Bevins S. N., Baroch J. A., Nolte D. L., Zhang M., He H. (2012). Yersinia pestis: examining wildlife plague surveillance in China and the USA. Integr. Zool. 7, 99–109. 10.1111/j.1749-4877.2011.00277.x [DOI] [PubMed] [Google Scholar]
- Bitam I., Dittmar K., Parola P., Whiting M. F., Raoult D. (2010). Fleas and flea-borne diseases. Int. J. Infect. Dis. 14, e667–e676. 10.1016/j.ijid.2009.11.011 [DOI] [PubMed] [Google Scholar]
- Bushon R. N., Kephart C. M., Koltun G. F., Francy D. S., Schaefer F. W., Alan Lindquist H. D. (2010). Statistical assessment of DNA extraction reagent lot variability in real-time quantitative PCR. Lett. Appl. Microbiol. 50, 276–282. 10.1111/j.1472-765X.2009.02788.x [DOI] [PubMed] [Google Scholar]
- Challacombe J. F., Petersen J. M., Hodge D., Pillai S., Kuske C. R. (2017). Whole-genome relationships among Francisella bacteria of diverse origins define new species and provide specific regions for detection. Appl. Environ. Microbiol. 83:e02589–16. 10.1128/AEM.02589-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion M. D., Zeng Q., Nix E. B., Nano F. E., Keim P., Kodira C. D., et al. (2009). Comparative genomic characterization of Francisella tularensis strains belonging to low and high virulence subspecies. PLoS Pathog. 5:e1000459. 10.1371/journal.ppat.1000459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christova I., Gladnishka T. (2005). Prevalence of infection with Francisella tularensis, Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in rodents from an endemic focus of tularemia in Bulgaria. Ann. Agric. Environ. Med. 12, 149–152. [PubMed] [Google Scholar]
- Clark D. V., Ismailov A., Seyidova E., Hajiyeva A., Bakhishova S., Hajiyev H., et al. (2012). Seroprevalence of tularemia in rural Azerbaijan. Vector-Borne and Zoonotic Dis. 12, 558–563. 10.1089/vbz.2010.0081 [DOI] [PubMed] [Google Scholar]
- Corbet G. B. (1978). Mammals of the Palaearctic Region: London, UK; Ithaca, NY: British Museum (Natural History); Cornell University Press. [Google Scholar]
- de Carvalho I., Toledo A., Carvalho C., Barandika J., Respicio-Kingry L., Garcia-Amil C., et al. (2016). Francisella species in ticks and animals, Iberian Peninsula. Ticks Tick Borne Dis. 7, 159–165. 10.1016/j.ttbdis.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Dubyanskiy V. M., Yeszhanov A. B. (2016). Ecology of Yersinia pestis and the Epidemiology of Plague. Adv. Exp. Med. Biol. 918, 101–170. [DOI] [PubMed] [Google Scholar]
- Eliasson H., Sjöstedt A., Bäck E. (2005). Clinical use of a diagnostic PCR for Francisella tularensis in patients with suspected ulceroglandular tularaemia. Scand. J. Infect. Dis. 37, 833–837. 10.1080/00365540500400951 [DOI] [PubMed] [Google Scholar]
- Emanuel P. A., Bell R., Dang J. L., McClanahan R., David J. C., Burgess R. J., et al. (2003). Detection of Francisella tularensis within infected mouse tissues by using a hand-held PCR thermocycler. J. Clin. Microbiol. 41, 689–693. 10.1128/JCM.41.2.689-693.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem H., Ozturk-Engin D., Yesilyurt M., Karabay O., Elaldi N., Celebi G., et al. (2014). Evaluation of tularaemia courses: a multicentre study from Turkey. Clin. Microbiol. Infect. 20, O1042–O1051. 10.1111/1469-0691.12741 [DOI] [PubMed] [Google Scholar]
- Esamaeili S., Azadmanesh K., Naddaf S. R., Rajerison M., Carniel E., Mostafavi E. (2013). Serologic survey of plague in animals, Western Iran. Emerg. Infect. Dis. 19, 1549–1551. 10.3201/eid1909.121829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili S., Esfandiari B., Maurin M., Gouya M. M., Shirzadi M. R., Amiri F. B., et al. (2014a). Serological survey of tularemia among butchers and slaughterhouse workers in Iran. Trans. R. Soc. Trop. Med. Hyg. 108, 516–518. 10.1093/trstmh/tru094 [DOI] [PubMed] [Google Scholar]
- Esmaeili S., Gooya M. M., Shirzadi M. R., Esfandiari B., Amiri F. B., Behzadi M. Y., et al. (2014b). Seroepidemiological survey of tularemia among different groups in western Iran. Int. J. Infect. Dis. 18, 27–31. 10.1016/j.ijid.2013.08.013 [DOI] [PubMed] [Google Scholar]
- Fratini F., Verin R., Ebani V. V., Ambrogi C., Bertelloni F., Turchi B., et al. (2017). Experimental infection with Yersinia pseudotuberculosis in European brown hare (Lepus europaeus, Pallas). Asian Pac. J. Trop. Med. 10, 285–291. 10.1016/j.apjtm.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Goethert H. K., Telford III S. R. (2009). Nonrandom distribution of vector ticks (Dermacentor variabilis) infected by Francisella tularensis. PLoS Pathog. 5:e1000319. 10.1371/journal.ppat.1000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Gupta P., Sharama P., Shrivaastava A. K., Soni S. K. (2012). Emerging and re-emerging infectious diseases, future challenges and strategy. J. Clin. Diagn. Res. 6, 1095–1100. [Google Scholar]
- Gyuranecz M., Dénes B., Dán Á., Rig,ó K., Földvári G., Szeredi L., et al. (2010). Susceptibility of the common hamster (Cricetus cricetus) to Francisella tularensis and its effect on the epizootiology of tularemia in an area where both are endemic. J. Wildl. Dis. 46, 1316–1320. 10.7589/0090-3558-46.4.1316 [DOI] [PubMed] [Google Scholar]
- Helvaci S., Gedikoglu S., Akalin H., Oral H. (2000). Tularemia in Bursa, Turkey: 205 cases in ten years. Eur. J. Epidemiol. 16, 271–276. 10.1023/A:1007610724801 [DOI] [PubMed] [Google Scholar]
- Hestvik G., Warns-Petit E., Smith L. A., Fox N. J., Uhlhorn H., Artois M., et al. (2015). The status of tularemia in Europe in a one-health context: a review. Epidemiol. Infect. 143, 2137–2160. 10.1017/S0950268814002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D. D., Gresbrink R. A. (1982). Surveillance of sylvatic plaque in Oregon by serotesting carnivores. Am. J. Public Health 72, 1295–1297. 10.2105/AJPH.72.11.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopla C. (1974). Ecology of tularemia. Adv. Vet. Sci. Comp. Med. 18, 25–53. [PubMed] [Google Scholar]
- Hopla C. E., Hopla A. K. (1994). Tularemia, in Handbook of Zoonoses, eds Steele G. W. B., Steele J. H. (Boca Raton, FL: CRC Press Inc.), 113–126. [Google Scholar]
- Johansson A., Lärkeryd A., Widerström M., Mörtberg S., Myrtännäs K., Öhrman C., et al. (2014). An outbreak of respiratory tularemia caused by diverse clones of Francisella tularensis. Clin. Infect. Dis. 59, 1546–1553. 10.1093/cid/ciu621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabay O., Kilic S., Gurcan S., Pelitli T., Karadenizli A., Bozkurt H., et al. (2013). Cervical lymphadenitis: tuberculosis or tularaemia? Clin. Microbiol. Infect. 19, E113–E117. 10.1111/1469-0691.12097 [DOI] [PubMed] [Google Scholar]
- Karimi P. (1980). [Discovery of a new focus of zoonotic plague in the eastern Azarbaidjan region of Iran]. Bull. Soc. Pathol. Exot. Filiales 73, 28–35. [PubMed] [Google Scholar]
- Karimi Y., Salarkia F., Ghasemi M. (1981). Tularemia: first human case in Iran. J. Med. Coun. Iran 8, 134–141. [Google Scholar]
- Kaysser P., Seibold E., Mätz-Rensing K., Pfeffer M., Essbauer S., Splettstoesser W. D. (2008). Re-emergence of tularemia in Germany: Presence of Francisella tularensis in different rodent species in endemic areas. BMC Infect. Dis. 8:157. 10.1186/1471-2334-8-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim P., Johansson A., Wagner D. M. (2007). Molecular epidemiology, evolution, and ecology of Francisella. Ann. N. Y. Acad. Sci. 1105, 30–66. 10.1196/annals.1409.011 [DOI] [PubMed] [Google Scholar]
- Khoshdel A., Saedi Dezaki E., Ganji F., Habibian R., Imani R., Taheri E., et al. (2014). First seroprevalence survey of children with tularemia infection in Chaharmahal va Bakhtiari province, Iran. Iranian. J. Pathol. 9, 23–27. [Google Scholar]
- Kryštufek B., Vohralík V., JanŽekovič F. (2005). Mammals of Turkey and Cyprus: Rodentia I: Sciuridae, Dipodidae, Gliridae, Arvicolinae. Koper: Zgodovinsko društvo za južno Primorsko. [Google Scholar]
- Leslie T., Whitehouse C., Yingst S., Baldwin C., Kakar F., Mofleh J., et al. (2011). Outbreak of gastroenteritis caused by Yersinia pestis in Afghanistan. Epidemiol. Infect. 139, 728–735. 10.1017/S0950268810001792 [DOI] [PubMed] [Google Scholar]
- Maurin M., Gyuranecz M. (2016). Tularaemia: clinical aspects in Europe. Lancet Infect. Dis. 16, 113–124. 10.1016/S1473-3099(15)00355-2 [DOI] [PubMed] [Google Scholar]
- Melikjanyan S., Vanyan A., Avetisyan A., Avetisyan A., Bakunts N. (1996). GIS analysis of tularemia outbreaks in Armenia, 1996-2013. Online J. Public Health Inform. 6:e45 10.5210/ojphi.v6i1.5108 [DOI] [Google Scholar]
- Meyer K., McNeill D., Wheeler C. (1965). Results of a preliminary serological survey of small mammal populations for plague on the island of Hawaii. Bull. World Health Organ. 33, 809–815. [PMC free article] [PubMed] [Google Scholar]
- Mörner T. (1992). The ecology of tularaemia. Rev. Sci. Tech. 11, 1123–1130. 10.20506/rst.11.4.657 [DOI] [PubMed] [Google Scholar]
- Mörner T., Sandström G., Mattsson R., Nilsson P.-O. (1988). Infections with Francisella tularensis biovar palaearctica in hares (Lepus tumidus, Lepus europaeus) from Sweden. J. Wildl. Dis. 24, 422–433. 10.7589/0090-3558-24.3.422 [DOI] [PubMed] [Google Scholar]
- Mostafavi E., Keypour M. (2017). History of Plague Research Center of Pasteur Institute of Iran (1952–2016). J. Res. His Med. 6, 139–158. [Google Scholar]
- Mostafavi E., Shahraki A. H., Japoni-Nejad A., Esmaeili S., Darvish J., Sedaghat M. M., et al. (2017). A field study of plague and tularemia in rodents, Western Iran. Vector Borne Zoonotic Dis. 17, 247–253. 10.1089/vbz.2016.2053 [DOI] [PubMed] [Google Scholar]
- Özsürekci Y., Birdsell D. N., Çelik M., Karadag-Öncel E., Johansson A., Forsman M., et al. (2015). Diverse Francisella tularensis Strains and Oropharyngeal Tularemia, Turkey. Emerg. Infect. Dis. 21, 173–175. 10.3201/eid2101.141087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhizgari N., Gouya M. M., Mostafavi E. (2017). Emerging and re-emerging infectious diseases in Iran. Iranian J. Microbiol. 9, 122–142. [PMC free article] [PubMed] [Google Scholar]
- Poland J. D., Dennis D. T. (1999). Plague manual: epidemiology, distribution, surveillance and control, in Treatment Plague (Geneva: World Health Organization; ), 55–62. [Google Scholar]
- Pourhossein B., Esmaeili S., Gyuranecz M., Mostafavi E. (2015). Tularemia and plague survey in rodents in an earthquake zone in southeastern Iran. Epidemiol. Health 37:e2015050. 10.4178/epih/e2015050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Pastor R., Escudero R., Vidal D., Mougeot F., Arroyo B., Lambin X., et al. (2017). Density-dependent prevalence of Francisella tularensis in fluctuating vole populations, northwestern Spain. Emer. Infect. Dis. 23, 1377–1379. 10.3201/eid2308.161194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge M., von Keyserlingk M., Braune S., Voigt U., Grauer A., Pohlmeyer K., et al. (2011). Prevalence of Francisella tularensis in brown hare (Lepus europaeus) populations in Lower Saxony, Germany. Eur. J. Wildl. Res. 57, 1085–1089. 10.1007/s10344-011-0522-1 [DOI] [Google Scholar]
- Saeed A. A., Al-Hamdan N. A., Fontaine R. E. (2005). Plague from eating raw camel liver. Emerg. Infect. Dis. 11, 1456–1457. 10.3201/eid1109.050081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M., Atabay H. I., Bicakci Z., Unver A., Otlu S. (2007). Outbreaks of tularemia in Turkey. Kobe J. Med. Sci. 53, 37–42. [PubMed] [Google Scholar]
- Sakiyev K., Ospanova S., Abdrakhmanova K. (2013). Current state and consistent pattern of distribution of a tularemia in the territory of the pavlodar region (North Kazakhstan). Sci. J. Pavlodar State Pedagogical Institute. 1, 35–41. [Google Scholar]
- Sanyaolu A., Okorie C., Mehraban N., Ayodele O., Tshitenge S. (2016). Epidemiology of Zoonotic Diseases in the United States: a comprehensive review. J. Infect. Dis. Epidemiol. 2:021 10.23937/2474-3658/1510021 [DOI] [Google Scholar]
- Saunders G., Giles J. (1977). A relationship between plagues of the house mouse, Mus musculus (Rodentia: Muridae) and prolonged periods of dry weather in south-eastern Australia. Wildl. Res. 4, 241–247. 10.1071/WR9770241 [DOI] [Google Scholar]
- Shahraki A. H., Carniel E., Mostafavi E. (2016). Plague in Iran: its history and current status. Epidemiol. Health. 38:e2016033 10.4178/epih.e2016033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstedt A. (2007). Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 1105, 1–29. 10.1196/annals.1409.009 [DOI] [PubMed] [Google Scholar]
- Stalb S., Polley B., Danner K.-J., Reule M., Tomaso H., Hackbare A., et al. (2017). Detection of tularemia in European brown hares (Lepus europaeus) and humans reveals endemic and seasonal occurrence in Baden-Wuerttemberg, Germany. Berliner und Münchener Tierärztliche Wochenschrift. 130, 293–239. 10.2376/0005-9366-16079 [DOI] [Google Scholar]
- Stewart A., Satterfield B., Cohen M., O'Neill K., Robison R. (2008). A quadruplex real-time PCR assay for the detection of Yersinia pestis and its plasmids. J. Med. Microbiol. 57, 324–331. 10.1099/jmm.0.47485-0 [DOI] [PubMed] [Google Scholar]
- Timofeev V., Bakhteeva I., Titareva G., Kopylov P., Christiany D., Mokrievich A., et al. (2017). Russian isolates enlarge the known geographic diversity of Francisella tularensis subsp. mediasiatica. PLoS ONE 12:e0183714. 10.1371/journal.pone.0183714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulu K. A., Kiliç S., Sencan I., Ciçek S. G., Gürbüz Y., Tütüncü E., et al. (2011). [A water-borne tularemia outbreak caused by Francisella tularensis subspecies holarctica in Central Anatolia region]. Mikrobiyoloji Bulteni 45, 234–247. [PubMed] [Google Scholar]
- Ulu-Kilic A., Gulen G., Sezen F., Kilic S., Sencan I. (2013). Tularemia in central Anatolia. Infection 41, 391–399. 10.1007/s15010-012-0355-1 [DOI] [PubMed] [Google Scholar]
- Unal Yilmaz G., Gurcan S., Ozkan B., Karadenizli A. (2014). Investigation of the presence of Francisella tularensis by culture, serology and molecular methods in mice of Thrace Region, Turkey. Mikrobiyoloji Bulteni 48, 213–222. 10.5578/mb.7028 [DOI] [PubMed] [Google Scholar]
- Versage J. L., Severin D. D., Chu M. C., Petersen J. M. (2003). Development of a multitarget real-time TaqMan PCR assay for enhanced detection of Francisella tularensis in complex specimens. J. Clin. Microbiol. 41, 5492–5499. 10.1128/JCM.41.12.5492-5499.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E. (2016). Pathogenesis of Yersinia pestis in Humans. Human Emerging and Re-emerging Infections: Viral and Parasitic Infections. New Jersey: John Wiley & Sons, Inc. [Google Scholar]
- Yildirim S., Turhan V., Karadenizli A., Önem Y., Karagöz E., Eroglu C., et al. (2014). Tuberculosis or tularemia? A molecular study in cervical lymphadenitis. Int. J. Infect. Dis. 18, 47–51. 10.1016/j.ijid.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Zargar A., Maurin M., Mostafavi E. (2015). Tularemia, a re-emerging infectious disease in Iran and neighboring countries. Epidemiol. Health 37:e2015011 10.4178/epih/e2015011 [DOI] [PMC free article] [PubMed] [Google Scholar]