Abstract
The aim of the present study was to characterize sporadic cases and an outbreak of NDM-like-producing Enterobacteriaceae recovered from hospital settings, in Czechia. During 2016, 18 Entrobacteriaceae isolates including 10 Enterobacter cloacae complex (9 E. xiangfangensis and 1 E. asburiae), 4 Escherichia coli, 1 Kluyvera intermedia, 1 Klebsiella pneumoniae, 1 Klebsiella oxytoca, and 1 Raoultella ornithinolytica that produced NDM-like carbapenemases were isolated from 15 patients. Three of the patients were colonized or infected by two different NDM-like producers. Moreover, an NDM-4-producing isolate of E. cloacae complex, isolated in 2012, was studied for comparative purposes. All isolates of E. cloacae complex, except the E. asburiae, recovered from the same hospital, were assigned to ST182. Additionally, two E. coli belonged to ST167, while the remaining isolates were not clonally related. Thirteen isolates carried blaNDM−4, while six isolates carried blaNDM−1 (n = 3) or blaNDM−5 (n = 3). Almost all isolates carried blaNDM-like-carrying plasmids being positive for the IncX3 allele, except ST58 E. coli and ST14 K. pneumoniae isolates producing NDM-1. Analysis of plasmid sequences revealed that all IncX3 blaNDM-like-carrying plasmids exhibited a high similarity to each other and to previously described plasmids, like pNDM-QD28, reported from worldwide. However, NDM-4-encoding plasmids differed from other IncX3 plasmids by the insertion of a Tn3-like transposon. On the other hand, the ST58 E. coli and ST14 K. pneumoniae isolates carried two novel NDM-1-encoding plasmids, pKpn-35963cz, and pEsco-36073cz. Plasmid pKpn-35963cz that was an IncFIB(K) molecule contained an acquired sequence, encoding NDM-1 metallo-β-lactamase (MβL), which exhibited high similarity to the mosaic region of pS-3002cz from an ST11 K. pneumoniae from Czechia. Finally, pEsco-36073cz was a multireplicon A/C2+R NDM-1-encoding plasmid. Similar to other type 1 A/C2 plasmids, the blaNDM−1 gene was located within the ARI-A resistance island. These findings underlined that IncX3 plasmids have played a major role in the dissemination of blaNDM-like genes in Czech hospitals. In combination with further evolvement of NDM-like-encoding MDR plasmids through reshuffling, NDM-like producers pose an important public threat.
Keywords: NDM, metallo-β-lactamases, Enterobacter xiangfangensis, ST182, IncX3
Introduction
Acquired carbapenem-hydrolyzing β-lactamases are resistance determinants of increasing clinical importance in Gram-negative pathogens. Of these, NDM-1 metallo-β-lactamase (MβL) was first described in Klebsiella pneumoniae and Escherichia coli isolated in Sweden in 2008 from an Indian patient transferred from a New Delhi hospital (Yong et al., 2009). Since then, NDM-1-producing bacteria, including clinical isolates of Enterobacteriaceae and Acinetobacter baumannii, have been reported from the Indian subcontinent but also worldwide (Nordmann et al., 2011).
In Czechia, the occurrence of NDM-producing bacteria was rare, with only three sporadic cases being detected during 2011–2013. These cases included an NDM-1-producing A. baumanni isolated from a patient repatriated from Egypt (Hrabák et al., 2012), an NDM-4-producing strain of Enterobacter cloacae complex from a patient previously hospitalized in Sri Lanka (Papagiannitsis et al., 2013b) and a ST11 K. pneumoniae isolate carrying two NDM-1-encoding plasmids, from Slovakia (Studentova et al., 2015). However, an increase in the isolation frequency of NDM-like-producing Enterobacteriaceae from Czech hospitals was observed, during 2016.
Thus, the aim of the present study was to characterize the NDM-like producers detected in Czech hospitals, during 2016. Also, we describe the complete nucleotide sequences of representative blaNDM-like-carrying plasmids harbored by the studied isolates.
Materials and methods
Bacterial isolates and confirmation of carbapenemase production
In 2016, Czech hospitals referred a total of 410 Enterobacteriaceae isolates with a meropenem MIC of >0.125 μg/ml (EUCAST, 2012) to the National Reference Laboratory for Antibiotics. Species identification was confirmed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) using MALDI Biotyper software (Bruker Daltonics, Bremen, Germany). All isolates were tested for carbapenemase production by the MALDI-TOF MS meropenem hydrolysis assay (Rotova et al., 2017). Isolates that were positive by the MALDI-TOF MS meropenem hydrolysis assay were subjected to metallo-β-lactamase, KPC, and OXA-48 detection using the double-disc synergy test with EDTA, the phenylboronic acid disc test, and the temocillin disc test (Lee et al., 2003; Doi et al., 2008; Glupczynski et al., 2012), respectively. Additionally, carbapenemase genes (blaKPC, blaVIM, blaIMP, blaNDM, and blaOXA−48-like) were detected by PCR amplification (Poirel et al., 2004; Ellington et al., 2007; Naas et al., 2008; Yong et al., 2009). PCR products were sequenced as described below. Isolates positive for blaNDM-like genes were further studied. Moreover, the NDM-4-producing isolate of E. cloacae complex, recovered at the University Hospital Pilsen (Pilsen, Czechia) during 2012 (Papagiannitsis et al., 2013b), was included in this study for comparative purposes.
Susceptibility testing
The MICs of piperacillin, piperacillin-tazobactam, cefotaxime, ceftazidime, cefepime, aztreonam, meropenem, ertapenem, gentamicin, amikacin, chloramphenicol, tetracycline, trimethoprim-sulfamethoxazole, ciprofloxacin, colistin, and tigecycline were determined by the broth dilution method (EUCAST, 2003). Data were interpreted according to the criteria of the European Committee on Antimicrobial Susceptibility Testing (EUCAST; www.eucast.org).
Typing
All isolates were typed by multilocus sequence typing (MLST) (Diancourt et al., 2005; Wirth et al., 2006; Miyoshi-Akiyama et al., 2013; Herzog et al., 2014). The databases at https://pubmlst.org/ecloacae/, http://mlst.warwick.ac.uk/mlst/dbs/Ecoli, http://bigsdb.web.pasteur.fr/klebsiella and https://pubmlst.org/koxytoca/ were used to assign STs.
Detection of β-lactamases
The β-lactamase content of all blaNDM-like-positive isolates was determined by isoelectric focusing (IEF). Bacterial extracts were obtained by sonication of bacterial cells suspended in 1% glycine buffer and clarified by centrifugation. Sonicated cell extracts were analyzed by IEF in polyacrylamide gels containing ampholytes (pH 3.5–9.5; AP Biotech, Piscataway, NJ). The separated β-lactamases were visualized by covering the gel with the chromogenic cephalosporin nitrocefin (0.2 mg/ml; Oxoid Ltd., Basingstoke, United Kingdom; Papagiannitsis et al., 2015).
On the basis of the IEF data, PCR detection of various bla genes was performed by the use of primers specific for blaTEM−1, blaOXA−1, blaSHV, blaCTX−M, and blaCMY, as reported previously (Pałucha et al., 1999; Pérez-Pérez and Hanson, 2002; Woodford et al., 2006; Coque et al., 2008). Both strands of the PCR products were sequenced using an ABI 377 sequencer (Applied Biosystems, Foster City, CA).
Transfer of blaNDM-like genes
Conjugal transfer of blaNDM-like genes from the clinical strains was carried out in mixed broth cultures (Vatopoulos et al., 1990), using the rifampin-resistant E. coli A15 laboratory strain as a recipient. Transconjugants were selected on MacConkey agar plates supplemented with rifampin (150 mg/l) and ampicillin (50 mg/l). Plasmid DNA from clinical isolates, which failed to transfer blaNDM-like by conjugation, was extracted using a Qiagen Maxi kit (Qiagen, Hilden, Germany) and used to transform E. coli DH5α cells. The preparation and transformation of competent E. coli cells were done using calcium chloride (Cohen et al., 1972). Transformants were selected on Luria-Bertani agar plates with ampicillin (50 mg/l). Transconjugants or transformants were confirmed to be NDM-like producers by PCR (Yong et al., 2009) and the MALDI-TOF MS meropenem hydrolysis assay (Rotova et al., 2017).
Plasmid analysis
To define the genetic units of the blaNDM-like genes, the plasmid contents of all NDM-producing clinical and recombinant strains were analyzed by pulsed-field gel electrophoresis (PFGE) of total DNA digested with S1 nuclease (Promega, Madison, WI, USA; Barton et al., 1995). Following PFGE, the DNA was transferred to a BrightStar-Plus positively charged nylon membrane (Applied Biosystems, Foster City, CA) and hybridized with digoxigenin-labeled blaNDM-like probe.
Plasmid incompatibility (Inc) groups were determined by the PCR-based replicon typing (PBRT) method (Carattoli et al., 2005; Johnson et al., 2012), using total DNA from transconjugants or transformants. Additionally, the IncR replicon was detected as described previously (García-Fernández et al., 2009).
Detection of characteristic regions
Based on the results from Illumina sequencing (see below), six PCRs targeting characteristic regions of NDM-4-encoding IncX3 plasmids and genomes of ST182 isolates of E. cloacae complex sequenced during this study were designed. The selected regions included: (i) a Tn3-like transposon found in NDM-4-encoding IncX3 plasmids, and (ii) four insertions identified in the genome of Encl-922 (see section Comparative Analysis of Enterobacter Isolates). All NDM-producing clinical or recombinant strains were screened for the presence of the regions described above by the use of specific primers (see Table S1).
Plasmid and chromosome sequencing
Ten plasmids were selected for complete sequencing. These plasmids were selected as representatives of different origins, plasmid sizes and hospitals. Additionally, clinical isolates Encl-922 and Encl-44578 were also selected for whole genome sequencing. The selected isolates were isolated 4-year apart (2012 and 2016).
Plasmid DNAs from transconjugants or transformants were extracted using a Qiagen Large-Construct kit (Qiagen, Hilden, Germany). Additionally, the genomic DNAs of clinical Encl-922 and Encl-44578 isolates were extracted using a DNA-Sorb-B kit (Sacace Biotechnologies S.r.l., Como, Italy). Multiplexed DNA libraries were prepared, using the Nextera XT Library Preparation kit, and 300-bp paired-end sequencing was performed on the Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA) using the MiSeq v3 600-cycle Reagent kit. Initial paired-end reads were quality trimmed using the Trimmomatic tool v0.33 (Bolger et al., 2014) with the sliding window size of 4 bp, required average base quality ≥17 and minimum read length of 48 bases. Genomic DNA reads of clinical isolates of E. cloacae complex were consequently assembled using the de Bruijn graph-based de novo assembler SPAdes v3.9.1 (Bankevich et al., 2012), using k-mer sizes 21, 33, 55, 77, 99, and 127. For assembly of the plasmids, reads were mapped to the reference E. coli K-12 substrain MG 1655 genome (GenBank accession no. U00096) using the BWA-MEM algorithm (Li, 2013), in order to filter out the chromosomal DNA. Then, all the unmapped reads were assembled in the same way as described above. The sequence gaps were filled by a PCR-based strategy and Sanger sequencing. For sequence analysis and annotation, the BLAST algorithm (www.ncbi.nlm.nih.gov/BLAST), the ISfinder database (www-is.biotoul.fr/), and the open reading frame (ORF) finder tool (www.bioinformatics.org/sms/) were utilized. Comparative genome alignments were performed using the Mauve v2.3.1 program (Darling et al., 2010).
Antibiotic resistance genes were identified using the ResFinder 2.1 tool (https://cge.cbs.dtu.dk/services/ResFinder/) with an identity threshold of >90% (Zankari et al., 2012).
Comparative analysis of clinical isolates of E. cloacae complex
Comparative genomic analysis of clinical isolates of E. cloacae complex was based on statistics calculated by QUAST v4.5 (Gurevich et al., 2013) and VarScan v2.3.9 (Koboldt et al., 2012) tools. All quality trimmed Illumina reads of Encl-922 were mapped to contigs of Encl-44578, employing BWA-MEM algorithm v0.7.12 (Li, 2013) and SAMtools v1.3 (Li et al., 2009), for the format conversions and analysis of the results. Then, single nucleotide polymorphisms (SNPs) and indels were detected employing VarScan with parameters set as follows: minimum read depth at a position = 6, minimum base quality at a position = 20 and minimum variant allele frequency threshold of 0.45. Moreover, SNPs and indels located in a region within 127 bp from any edge of a contig, as well as SNPs and indels harbored by contigs smaller than 2 kb were excluded from further analysis. Remaining SNPs and indels were also manually checked and refined by visualization of mapped data via Tablet v1.14.04.10 (Milne et al., 2013). Differences in assembly of E. cloacae complex genomes were inspected using QUAST's Icarus viewer (Mikheenko et al., 2016). In order to examine whether SNPs and indels were located in intergenic or coding regions, as well as to find out what are the differences in genetic information between studied isolates, contigs of clinical strains were annotated using Prokka v1.10 (Seemann, 2014). Genes harboring SNPs were compared against NCBI's conserved domain database (Marchler-Bauer et al., 2017) via CD-Search (Marchler-Bauer and Bryant, 2004) to identify conserved domain hits. Finally, sequencing data of clinical strains were examined for the presence of prophage sequences using PHAST web server (Zhou et al., 2011).
Nucleotide sequence accession numbers
The nucleotide sequences of the pEsco-5256cz, pEncl-922cz, pRor-30818cz, pKpn-35963cz, pEsco-36073cz, pEncl-44578cz, pEnas-80654cz, pEnin-51781cz, pEsco-4382cz, and pKlox-45574cz plasmids have been deposited in GenBank under accession numbers MG252891, MG252892, MG252893, MG252894, MG252895, MG833402, MG833403, MG833404, MG833405, and MG833406, respectively. Whole genome assemblies of isolates of E. cloacae complex were deposited in NCBI under accession number PRJNA432167.
Results
Carbapenemase-producing Enterobacteriaceae
A total of 40 Enterobacteriaceae isolates showing carbapenemase activity on MALDI-TOF MS meropenem hydrolysis assay were recovered from Czech hospitals during 2016. PCR screening showed that 18 of the isolates were positive for blaNDM, 14 isolates were positive for blaOXA−48, while the remaining 8 isolates were positive for blaKPC.
NDM-like-producing isolates
Altogether, 18 nonrepetitive isolates producing NDM-like carbapenemases were isolated from 15 patients in 2016. Among them, 10 were presumptively identified as belonging to E. cloacae complex, 4 were identified to be E. coli, while the remaining isolates belonged to unique species (Enterobacter intermedius, K. pneumoniae, Klebsiella oxytoca, and Raoultella ornithinolytica). A previous study showed that 16S rRNA gene sequence of E. intermedius was included within the cluster of the genus Kluyvera, and therefore, the transfer of E. intermedius to the genus Kluyvera as Kluyvera intermedia was proposed (Pavan et al., 2005). Three of the patients were colonized or infected by two different NDM-like producers (Table 1).
Table 1.
Characteristics of NDM-like-producing Enterobacteriaceae.
| Isolatea | Isolation mn/yr (hospital) | Material (infection/colonization) | ST | β-Lactamase content | Size of NDM-encoding plasmid (kb)b | Replicon of NDM-encoding plasmid | Additional resistance markers |
|---|---|---|---|---|---|---|---|
| E. xiangfangensis | |||||||
| Encl-922 | 09/2012 (B1) | Rectal swab(colonization) | ST182 | NDM-4, CTX-M-15, OXA-1, TEM-1 | ~55 (53.683) | IncX3 | |
| Encl-66918 | 04/2016 (B1) | Rectal swab(colonization) | ST182 | NDM-4, CTX-M-15, OXA-1, TEM-1 | ~55 | IncX3 | |
| Encl-89040 | 06/2016 (B1) | Bile(infection) | ST182 | NDM-4, CTX-M-15, OXA-1, TEM-1 | ~55 | IncX3 | |
| Encl-44578 | 07/2016 (B1) | Venous catheter(infection) | ST182 | NDM-4, CTX-M-15, OXA-1, TEM-1 | ~55 (53.683) | IncX3 | |
| Encl-89485° | 07/2016 (B1) | Bile(infection) | ST182 | NDM-4, CTX-M-15, OXA-1, TEM-1 | ~55 | IncX3 | |
| Encl-91221 | 09/2016 (B1) | Throat swab(colonization) | ST182 | NDM-4, CTX-M-15, OXA-1, TEM-1 | ~55 | IncX3 | |
| Encl-93141 | 10/2016 (B1) | Peritoneal catheter(infection) | ST182 | NDM-4, CTX-M-15, OXA-1 | ~55 | IncX3 | |
| Encl-98042 | 11/2016 (B1) | Rectal swab(colonization) | ST182 | NDM-4, CTX-M-15, OXA-1 | ~55 | IncX3 | |
| Encl-98047■ | 11/2016 (B1) | Rectal swab(colonization) | ST182 | NDM-4, CTX-M-15, OXA-1, TEM-1 | ~55 | IncX3 | |
| Encl-98546 | 12/2016 (B1) | Rectal swab(colonization) | ST182 | NDM-4, CTX-M-15, OXA-1, TEM-1 | ~55 | IncX3 | |
| E. asburiae | |||||||
| Enas-80654° | 07/2016 (B1) | Bile(infection) | NA | NDM-4, CTX-M-15 | ~55 (53.683) | IncX3 | |
| K. intermedia | |||||||
| Enin-51781 | 10/2016 (B1) | Rectal swab(colonization) | NA | NDM-4, CTX-M-15, OXA-1 | ~55 (53.683) | IncX3 | |
| E. coli | |||||||
| Esco-14290 | 06/2016 (B2) | Nasal swab(colonization) | ST167 | NDM-5, CTX-M-15, TEM-1 | ~45 | IncX3 | |
| Esco-5256▴ | 07/2016 (B2) | Bronchoalveolar lavage(infection) | ST167 | NDM-5, CTX-M-15, TEM-1 | ~45 (46.161) | IncX3 | |
| Esco-36073 | 09/2016 (A1) | Urine(infection) | ST58 | NDM-1, CMY-16, OXA-10, CTX-M-15, TEM-1 | ~300 (300.958) | IncR, IncA/C2 | floR, tet(A), strAB, sul2, aacA4, aphA7, dfrA14, arr-2, cmlA1, aadA1, aphA6, sul1 |
| Esco-4382■ | 12/2016 (B1) | Rectal swab(colonization) | ST69 | NDM-4, CTX-M-15, TEM-1 | ~55 (53.683) | IncX3 | |
| K. oxytoca | |||||||
| Klox-45574▴ | 07/2016 (B2) | Rectal swab(colonization) | ST2 | NDM-5 | ~45 (46.161) | IncX3 | |
| K. pneumoniae | |||||||
| Kpn-35963 | 09/2016 (A2) | Urine catheter(infection) | ST14 | NDM-1, SHV-12, CTX-M-15, OXA-1 | ~150 (161.324) | IncFIB | aacA4, dfrA14, mph(A) |
| Raoultella ornithinolytica | |||||||
| Ror-30818 | 09/2016 (C) | Rectal swab(colonization) | NA | NDM-1, SHV-12, CTX-M-15, OXA-1, TEM-1 | ~55 (53.051) | IncX3 | |
NA, not applicable.
White circles, black squares, and black triangles each indicate the NDM-like-producing isolates recovered from the same patient.
Data for plasmids found in transconjugants are shown in bold; data for plasmids observed in transformants are underlined.
NDM-like producers were collected from five Czech hospitals located in three different Czech cities. In hospital B1, an outbreak that included ten patients diagnosed with NDM-like-producing Enterobacteriaceae lasted the studied period. Additionally, two patients colonized or infected with NDM-like producers were reported in hospital B2. The three remaining cases were identified in three different hospitals. None of the patients, treated in hospital B1, had recently traveled abroad or had been previously hospitalized. The patient treated in hospital C was directly repatriated from a hospital in China, while clinical data weren't available for the remaining patients.
Additionally, the NDM-4-producing isolate of E. cloacae complex identified in 2012 (Papagiannitsis et al., 2013b), was studied.
All 19 NDM-like producers exhibited resistance to piperacillin, piperacillin-tazobactam, cephalosporins, and ertapenem (Table S2), while the observed variations in the MICs of aztreonam might reflect the presence of additional resistance mechanisms in some of the isolates. Seventeen of the NDM-like producers also exhibited resistance to ciprofloxacin; 15 were resistant to gentamicin, 13 were resistant to trimethoprim-sulfamethoxazole, 1 was resistant to amikacin, and 1 was resistant to colistin, whereas all isolates were susceptible to tigecycline.
The population structure of NDM-like-producing isolates studied by MLST is shown in Table 1. All isolates of E. cloacae complex, except the E. asburiae strain, which were recovered from hospital B1, belonged to ST182. Of note was that the NDM-4-producing isolate of E. cloacae complex that was isolated, in 2012, from the patient previously hospitalized in Sri Lanka (Papagiannitsis et al., 2013b) was also assigned to ST182. Two of E. coli, both of which were from hospital B2, belonged to ST167. E. coli ST167 was recently found among NDM-5-producing isolates from different healthcare institutions in China (Yang et al., 2014; Zhang et al., 2016). The two remaining E. coli isolates were not clonally related and belonged to different STs (ST58 and ST69). The K. pneumoniae isolate was assigned to the high risk clone ST14 (Woodford et al., 2011), while the K. oxytoca isolate was classified into ST2 that belongs to a growing international clonal complex (CC2) (Izdebski et al., 2015).
Sequencing of the PCR products revealed three blaNDM-type genes encoding the NDM-1, NDM-4, and NDM-5 enzymes (Table 1; Yong et al., 2009; Hornsey et al., 2011; Nordmann et al., 2012). NDM-5 is an NDM-1-related MβL variant that differs from NDM-1 by two amino-acid substitutions, Val88Leu and Met154Leu, the former one being its only change with NDM-4. Thirteen of the isolates, all of which were from hospital B1, were found to produce the NDM-4 MβL (Table 1). The three isolates from hospital B2 produced the NDM-5 enzyme, while the three remaining isolates that were recovered from sporadic cases in three different hospitals expressed NDM-1 carbapenemase. Additionally, most of blaNDM-like-positive isolates were confirmed to coproduce the extended-spectrum β-lactamase CTX-M-15 (n = 18) either alone or along with TEM-1 (n = 13) and/or OXA-1 (n = 13), whereas the K. pneumoniae and R. ornithinolytica isolates also expressed the SHV-12 enzyme. The ST58 NDM-1-producing E. coli isolate coproduced CMY-16, CTX-M-15, OXA-10, and TEM-1 β-lactamases.
blaNDM-like-carrying plasmids
The blaNDM-like genes from all clinical strains were transferred by conjugation (n = 14) or transformation (n = 5) (Table 1). All blaNDM-like-positive recombinants exhibited resistance to piperacillin, piperacillin-tazobactam, cephalosporins, and ertapenem, while they remained susceptible to meropenem (Table S2). The three NDM-1-producing recombinants also exhibited resistance to aztreonam. Additionally, most of blaNDM-like-positive recombinants (n = 18) were susceptible to non-β-lactam antibiotics.
Plasmid analysis of NDM-4-producing donor and transconjugant strains revealed the transfer of plasmids, all of which were ~55 kb in size (Table 1). The three NDM-5-producing transformants harbored plasmids of ~45 kb, whereas the three remaining recombinants carried blaNDM−1-positive plasmids of different sizes (~55, ~150, and ~300 kb). Replicon typing showed seventeen of the plasmids, including those sizing ~45 and ~55 kb, were positive for the IncX3 allele. The blaNDM−1-positive plasmid of ~300 kb was positive for replicons R and A/C, whereas the one remaining blaNDM−1-carrying plasmid was non-typeable by the PBRT method (Carattoli et al., 2005; Johnson et al., 2012).
Structure of blaNDM-like-carrying plasmids
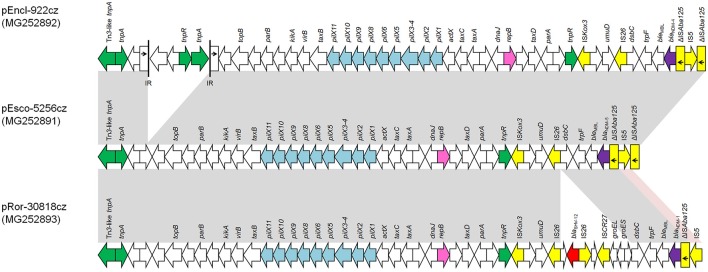
The complete sequence of blaNDM-like-carrying plasmids representative of different plasmid sizes, replicons, and resistance genes (n = 10) was determined (Table 1). Sequence analysis revealed that all IncX3 blaNDM-like-carrying plasmids exhibited a high similarity to each other and to previously described NDM-like-encoding plasmids, belonging to IncX3 group, reported from worldwide (Krishnaraju et al., 2015; Zhu et al., 2016; Pál et al., 2017). The blaNDM−5-positive plasmids, pEsco-5256cz and pKlox-45574cz, were almost identical to NDM-5-encoding plasmid pNDM-QD28 (100% coverage, 99% identity) (GenBank accession no. KU167608) that was characterized from a ST167 E. coli in China (Zhu et al., 2016). Differences among these plasmids consisted in few SNPs (n = 5), almost all located in mobile elements. Similar to pNDM-QD28, no other resistance genes were detected in these plasmids. Compared to other IncX3 NDM-encoding plasmids, all blaNDM−4-encoding plasmids differed by the insertion of a Tn3-like transposon (nt 7108-14624 in pEncl-44578cz) downstream topB gene (Figure 1). The Tn3-like sequence was composed by the 38-bp inverted repeats (IR) of the transposon, tnpA, tnpR, and two ORFs encoding hypothetical proteins. Target site duplications of 5 bp (GTACC) at the boundaries of the Tn3-like element indicated insertion by transposition. Of note was that the sequence of pEncl-922cz, isolated in 2012 (Papagiannitsis et al., 2013b), was identical to the respective sequences of NDM-4-encoding plasmids recovered in the same hospital, during 2016. PCR screening confirmed the presence of the Tn3-like transposon in all NDM-4-encoding IncX3 plasmids, isolated in hospital B1, while Tn3-like wasn't detected in the remaining blaNDM-like-positive plasmids that belonged to IncX3 group. Furthermore, the blaNDM−1-positive plasmid, pRor-30818cz, harbored an additional 7875-bp sequence (nt 40617-48491 in pRor-30818cz) encoding the extended-spectrum β-lactamase SHV-12 (Figure 1). A similar SHV-12-encoding region was found in the IncX3 blaNDM−1-positive plasmid pKP04NDM (100% coverage, 99% identity) (GenBank accession no. KU314941) described from a K. pneumoniae isolate in China.
Figure 1.
Comparison of linear maps of the NDM-like-encoding IncX3 plasmids pEncl-922cz, pEsco-5256cz, and pRor-30818cz. Arrows show the direction of transcription of open reading frames (ORFs), while truncated ORFs appear as rectangles (arrows within rectangles indicate the direction of transcription). Replicons of the plasmids are shown in pink. blaNDM-like genes are shaded purple, while other resistance genes are shown in red. IS elements and transposases are shown in yellow and green, respectively. Light blue arrows indicate genes responsible for the conjugative transfer of the plasmids. The remaining genes, including plasmid scaffold regions, are shown in white. Homologous segments (representing ≥99% sequence identity) are indicated by light gray shading, while pink shading shows inverted homologous segments.
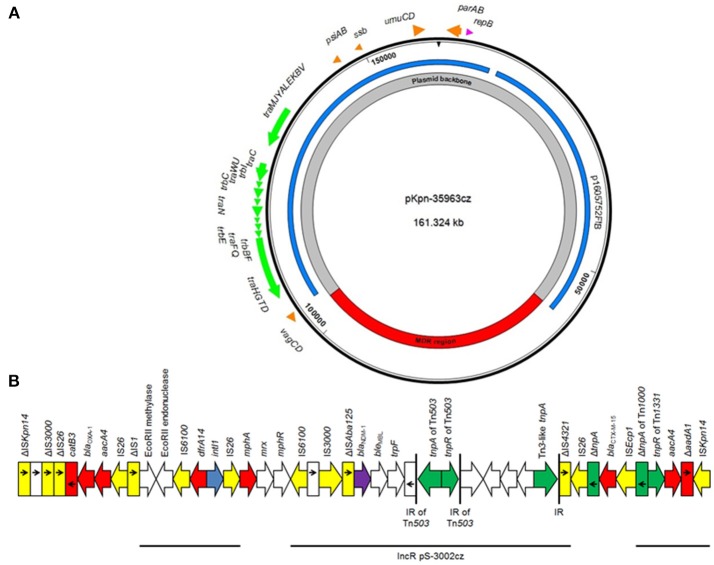
The NDM-1-encoding plasmid pKpn-35963cz that was nontypeable by the PBRT method (Carattoli et al., 2005) was 161,324 bp in size. Plasmid pKpn-35963cz was composed of two distinct parts: a contiguous plasmid backbone of 115,998 bp (nt 1–58,655 and 103,982–161,324) and an acquired sequence of 45,326 bp (nt 58,656–103,981). The plasmid backbone, which shared similarities with the respective regions of plasmid p1605752FIB (GenBank accession no. CP022125) recovered from a pan-resistant isolate of K. pneumoniae from the United States, harbored regions responsible for replication [repB gene; IncFIB(K) replicon], conjugative transfer (tra and trb genes) and plasmid maintenance (vagCD, psiAB, umuCD and parAB operons, and ssb gene; Figure 2). The acquired sequence of pKpn-35963cz contained a 17,836-bp segment (nt 77,360–95,195) encoding NDM-1, which was similar to the mosaic region of pS-3002cz (99% identity) (Studentova et al., 2015). The acquired sequence of pKpn-35963cz contained two additional segments that have also been described in pS-3002cz. The first segment (nt 65,518–72,935) included genes encoding an EcoRII methylase and EcoRII endonuclease, and the class 1 integron In191 carrying the dfrA14 resistance gene. The second segment (nt 101,342–103,981) contained fragments of transposons Tn1000 (ΔTn1000) and Tn1331 (ΔTn1331). ΔTn1331 comprised tnpR and aacA4 resistance gene. Furthermore the acquired sequence of pKpn-35963cz carried a macrolide resistance operon [mph(A)], and regions encoding OXA-1 and CTX-M-15 β-lactamases (Figure 2). In the acquired sequence of pKpn-35963cz, intact and truncated copies of several mobile elements that may have been implicated in the formation of this region were found.
Figure 2.
(A) Overview of the plasmid pKpn-35963cz. The innermost circles show the main regions of the plasmids. Similarities with other plasmids are shown in the next circle; each color represents a unique plasmid. In the outer circle, indicative genes and the direction of transcription are shown by arrows. Replicons of the plasmid are indicated as pink arrows. Genes responsible for plasmid transfer and maintenance are shown in green and orange, respectively. (B) Linear map of the multidrug resistance region (MDR) of the plasmid pKpn-35963cz. Arrows show the direction of transcription of open reading frames (ORFs), while truncated ORFs appear as rectangles (arrows within rectangles indicate the direction of transcription). blaNDM-like genes are shaded purple, while other resistance genes are shown in red. IS elements and transposases are shown in yellow and green, respectively. intI1 genes are shaded blue. The remaining genes are shown in white. Thin lines below the map correspond to highly similar sequences from other plasmids.
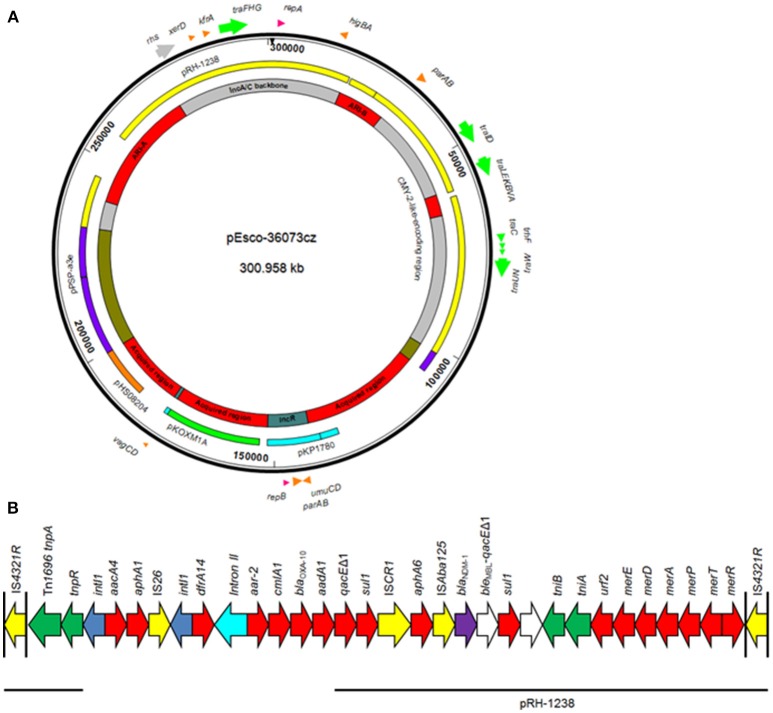
The plasmid pEsco-36073cz, which encoded the NDM-1 carbapenemase, is 300,958 bp in size. The plasmid showed a complex structure, being composed of sequences of diverse origin (Figure 3). A 170,314-bp sequence (nt 232,204–300,958 and 1–101,559) resembled the type 1 A/C2 plasmid pRH-1238 (94% coverage, 99% identity; Figure 3), characterized from a Salmonella enterica serovar Corvallis strain isolated from a migratory wild bird in Germany (Villa et al., 2015). Analysis of A/C2-associated sequence by the core gene PMLST (cgPMLST) scheme (Hancock et al., 2017) indicated that it belonged to cgST3.4. The A/C2 backbone was composed of regions responsible for replication (repA gene), conjugative transfer (Tra1 and Tra2 regions), and plasmid maintenance (higBA and parAB operons and xerD- and kfrA-like genes). Apart from the backbone, pEsco-36073cz carried the blaCMY−2-like-containing region, and the ARI-B and ARI-A resistance islands, as previously described in other type 1 A/C2 MDR plasmids (Harmer and Hall, 2015; Papagiannitsis et al., 2016). The blaNDM−1 gene was located within ARI-A, in a genetic environment similar to those previously identified in pRH-1238 (Villa et al., 2015). However, unlike in pRH-1238, the ARI-A of pEsco-36073cz lacked the macrolide resistance determinant mphA-mel-repAciN. Furthermore, a class 1 integron with aacA4 and aphA1 gene cassettes was located between resI and resII sites of the Tn1696 module. The ARI-A of pEsco-36073cz also carried a new integron, In1459, whose variable region comprised the dfrA14, arr-2, cmlA1, blaOXA−10, aadA1 cassettes. Additionally, pEsco-36073cz included fragments resembling the backbone of the recently described IncR plasmid pKP1780 (Papagiannitsis et al., 2013a), and sequences previously found in the plasmid pPSP-a3e (Conlan et al., 2014) and in the chromosomes of several Gram-negative rods. Genes encoding for resistance to arsenate, cooper and mercury were identified in the three remaining acquired regions of pEsco-36073cz.
Figure 3.
(A) Overview of the plasmid pEsco-36073cz. The innermost circles show the main regions of the plasmids. Similarities with other plasmids are shown in the next circle; each color represents a unique plasmid. In the outer circle, indicative genes and the direction of transcription are shown by arrows. Replicons of the plasmid are indicated as pink arrows. Genes responsible for plasmid transfer and maintenance are shown in green and orange, respectively. (B) Linear map of the ARI-A resistance island of the plasmid pEsco-36073cz. Arrows show the direction of transcription of open reading frames (ORFs), while truncated ORFs appear as rectangles (arrows within rectangles indicate the direction of transcription). blaNDM-like genes are shaded purple, while other resistance genes are shown in red. IS elements and transposases are shown in yellow and green, respectively. intI1 genes are shaded blue; teal blue arrow indicates the group II intron. The remaining genes are shown in white. Thin lines below the map correspond to highly similar sequences from other plasmids.
Comparative analysis of Enterobacter isolates
“In silico” hsp60 typing of the genome sequences (Hoffmann and Roggenkamp, 2003) showed that both isolates belonged to the recently recognized E. xiangfangensis species (Gu et al., 2014).
Since all isolates of E. cloacae complex, except the E. asburiae isolate, belonged to the same ST and carried the same IncX3 blaNDM−4-carrying plasmid, the WGS data of clinical strains Encl-922 and Encl-44578 were compared, using QUAST and VarScan tools, in order to examine the phylogenetic relationship of the isolates recovered in 2012 and 2016.
Comparative analysis of clinical isolates revealed that the genome of Encl-922 exhibited extensive similarity (99.87% identity) to the genome of Encl-44578. Sixteen SNPs were identified in the genome of Encl-922, compared to that of Encl-44578, five of which were located within prophage regions (Table 2). Interestingly, Encl-922 harbored three large insertions of 8,933 bp (nt 439,392–448,324 in node 2), of 17,903 bp (nt 17,786–35,688 in node 32) and of 13,165 bp (nt 1–13,165 in node 27; prophage sequence PHAGE_Salmon_SPN3UB_NC_019545). Additionally, Encl-922 harbored an insertion of 33-bp sequence (AACCCTCTCCCCAAAGGGGAGAGGGGACGATTA) located in an intergenic region. Moreover, Encl-922 showed a single nucleotide (G) deletion leading to CDS annotation change of general stress protein 39 to putative oxidoreductase YghA. Analysis of whole genome sequencing (WGS) data by PHAST web server found five intact prophage sequences (PHAGE_Haemop_HP2_NC_003315, PHAGE_Salmon_SPN3UB_NC_019545, PHAGE_Entero_mEp390_NC_019721, PHAGE_Pseudo_PPpW_3_NC_023006, and PHAGE_Salmon_SP_004_NC_021774) and one questionable prophage region (PHAGE_Entero_SfI_NC_027339), in both E. xiangfangensis isolates. However, Encl-922 included one additional incomplete prophage region (PHAGE_Salmon_SPN3UB_NC_019545), which was absent from the Encl-44578 genome.
Table 2.
Summary table of 16 SNPs found between the genomes of E. xiangfangensis isolates Encl-44578 (reference) and Encl-922 (query).
| PROKKA name | Conserved domain classification | Enzyme commision number | Contig | SNP | Gene length (aa) | aa substitution |
|---|---|---|---|---|---|---|
| –a | – | – | 2 | T64623G | – | – |
| –a | – | – | 7 | T88097G | – | – |
| Methyl viologen resistance protein SmvA | MFS transporter | – | 8 | T51220C | 496 | M293T |
| D-amino acid dehydrogenase small subunit | D-amino acid dehydrogenase | 1.4.99.1 | 23 | A46893G | 432 | S395S |
| NADP-dependent malic enzyme | NADP-dependent malic enzyme | 1.1.1.40 | 2 | A296564G | 759 | N584N |
| Glyoxylate/hydroxypyruvate reductase A | Glyoxylate/hydroxypyruvate reductase A | 1.1.1.79 | 4 | G113111A | 312 | R267H |
| Ribonuclease E | Ribonuclease E | 3.1.26.12 | 4 | T156395C | 1035 | H685R |
| Hypothetical protein | – | – | 38 | C784A | 369 | T239N |
| Hypothetical protein | Similar to protein YjaG | – | 39 | A24170G | 196 | I61V |
| Low-affinity gluconate transporter | Low-affinity gluconate transporter | – | 6 | T100479C | 421 | S277P |
| Arabinose operon regulatory protein | DNA-binding transcriptional regulator | – | 12 | A66284G | 281 | N193S |
| Anaerobic dimethyl sulfoxide reductase chain B | DMSO_dmsB family protein | – | 35 | T1976G | 205 | K120Q |
| Tail length tape measure protein | COG5281 and Phage_HK97_TLTM domain-containing protein | – | 5 | G24809A | 1154 | L824L |
| Tail length tape measure protein | COG5281 and Phage_HK97_TLTM domain-containing protein | – | 5 | T24845C | 1154 | A836A |
| Tail length tape measure protein | COG5281 and Phage_HK97_TLTM domain-containing protein | – | 5 | C24893A | 1154 | G852G |
| Terminase-like family protein | P family protein | – | 26 | G7615T | 589 | R485L |
The first two SNPs are located in intergenic regions.
Screening by PCR and sequencing identified that all E. xiangfangensis isolates, recovered during 2016, didn't harbor any of the four mentioned insertions. Thus, this finding indicated that Enterobacter isolates from 2016 differed from Encl-922.
Discussion
The present study investigated sporadic cases and an outbreak of NDM-like-producing Enterobacteriaceae recovered from Czech hospitals, during 2016. Specifically, 12 NDM-4-producing isolates, which belonged to E. xiangfangensis (n = 9), E. asburiae (n = 1), K. intermedius (n = 1), and E. coli (n = 1) species, 3 NDM-5 producers of E. coli (n = 2) and K. oxytoca (n = 1) species, and one E. coli, one K. pneumoniae and one R. ornithinolytica producing NDM-1 MβL were characterized.
The setting that was most affected was hospital B1, in which an outbreak of NDM-4-producing ST182 E. xiangfangensis isolates took place. ST182 isolates of E. cloacae complex were previously identified in Mexico and were associated with the production of NDM-1 enzyme (Torres-González et al., 2015; Bocanegra-Ibarias et al., 2017). Of note was that the isolate of E. cloacae complex, isolated in 2012 from a patient who had been previously hospitalized in Sri Lanka (Papagiannitsis et al., 2013b), also belonged to ST182 and harbored an IncX3 blaNDM−4-positive plasmid being identical to respective plasmids characterized from isolates recovered from patients treated in hospital B1 (Table 1), during 2016. However, comparative genome analysis revealed the presence of four insertions in the genome of E. xiangfangensis Encl-922 isolate. These insertions were not found in the genomic DNA of E. xiangfangensis isolates from 2016, suggesting a second insertion event of NDM-4-producing E. xiangfangensis isolates in Czech hospitals.
In three of the patients, two different NDM-like producers were identified during their hospitalization, supposing the in vivo horizontal transfer of blaNDM-like-carrying plasmids. Sequencing and PCR screening data revealed the presence of the same blaNDM−4- or blaNDM−5-carrying plasmid in these isolates (Table 1). These results confirmed the hypothesis of the in vivo horizontal transfer of blaNDM-like-carrying plasmids.
Results from Illumina sequencing showed that IncX3 plasmids have played a major role in the dissemination of blaNDM-like genes in Czech hospitals, which is in agreement with the findings from previous studies from worldwide (Krishnaraju et al., 2015; Zhu et al., 2016; Pál et al., 2017). In the current study, three blaNDM-type genes, encoding the NDM-1, NDM-4, and NDM-5 enzymes, were associated with IncX3 plasmids exhibiting high similarity to each other. Considering also the fact that NDM-1, NDM-4, and NDM-5 differ by one or two amino-acid substitutions may indicate the possibility that blaNDM-like genes encoding NDM-1-related variants have evolved in the same plasmid type. Additionally, Illumina data showed the presence of a unique sequence, a Tn3-like transposon, in sequenced blaNDM−4-carrying plasmids. PCR confirmed the presence of the Tn3-like sequence in all transconjugants, carrying blaNDM−4-positive plasmids. Thus, the PCR targeting of the Tn3-like sequence was able to distinguish blaNDM−4-positive plasmids from other IncX3 plasmids carrying blaNDM−1 or blaNDM−5. On the other hand, two of the sporadic isolates carried novel NDM-1-encoding plasmids. Plasmid pKpn-35963cz that was an IncFIB(K) molecule contained an acquired sequence, encoding NDM-1 MβL, which exhibited high similarity to the mosaic region of pS-3002cz. pS-3002cs was characterized from an ST11 K. pneumoniae isolate, producing NDM-1 carbapenemase, identified in Czechia (Studentova et al., 2015). Whereas plasmid pEsco-36073cz was a multireplicon A/C2+R NDM-1-encoding plasmid, being a fusion derivative of sequences of diverse origin. Similar to other type 1 A/C2 plasmids (Harmer and Hall, 2015; Villa et al., 2015), the blaNDM−1 gene was located within the ARI-A resistance island.
In conclusion, the data presented here contribute to the current knowledge of NDM-like-producing Enterobacteriaceae. In agreement with previous studies, our findings punctuate that NDM-like producers constitute an important public threat, mainly due to the rapid horizontal transfer of IncX3 blaNDM-carrying plasmids but, also, due to further evolvement of NDM-like-encoding MDR plasmids via reshuffling.
Author contributions
CP and JH played an important role in interpreting the results and in writing the manuscript. VJ, TB, and HZ helped to acquired data. VP, MM, AS, KC, and IB carried out experimental work. CP supervised the experiments and revised the final manuscript, which was approved by all authors.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are very thankful to Dana Kralova for technical assistance during the present study. The authors also thank Thomas Jové for curating integron sequencing data.
Footnotes
Funding. This work was supported by the Medical Research Foundation of the Czech Republic (grant numbers 15-28663A and 17-29239A); by the National Sustainability Program I (NPU I; grant number LO1503) provided by the Ministry of Education Youth and Sports of the Czech Republic; and the Charles University Research Fund- PROGRES (grant number Q39).
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01549/full#supplementary-material
References
- Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comp. Biol. 19,455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton B. M., Harding G. P., Zuccarelli A. J. (1995). A general method for detecting and sizing large plasmids. Anal. Biochem. 226, 235–240. 10.1006/abio.1995.1220 [DOI] [PubMed] [Google Scholar]
- Bocanegra-Ibarias P., Garza-González E., Morfín-Otero R., Barrios H., Villarreal-Treviño L., Rodríguez-Noriega E., et al. (2017). Molecular and microbiological report of a hospital outbreak of NDM-1-carrying Enterobacteriaceae in Mexico. PLoS ONE 12:e0179651. 10.1371/journal.pone.0179651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M., Usadel B. (2014). Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30, 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A., Bertini A., Villa L., Falbo V., Hopkins K. L., Threlfall E. J. (2005). Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63, 219–228. 10.1016/j.mimet.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Y., Hsu L. (1972). Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc. Natl. Acad. Sci. U.S.A. 69, 2110–2114. 10.1073/pnas.69.8.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan S., Thomas P. J., Deming C., Park M., Lau A. F., Dekker J. P., et al. (2014). Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae. Sci. Transl. Med. 6:254ra126. 10.1126/scitranslmed.3009845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coque T. M., Novais A., Carattoli A., Poirel L., Pitout J., Peixe L., et al. (2008). Dissemination of clonally related Escherichia coli strains expressing extended-spectrum β-lactamase CTX-M-15. Emerging Infect. Dis. 14, 195–200. 10.3201/eid1402.070350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling A. E., Mau B., Perna N. T. (2010). Progressivemauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 5:e11147. 10.1371/journal.pone.0011147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diancourt L., Passet V., Verhoef J., Grimont P. A., Brisse S. (2005). Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43, 4178–4182. 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y., Potoski B. A., Adams-Haduch J. M., Sidjabat H. E., Pasculle A. W., Paterson D. L. (2008). Simple disk-based method for detection of Klebsiella pneumoniae carbapenemase-type beta-lactamase by use of a boronic acid compound. J. Clin. Microbiol. 46, 4083–4086. 10.1128/JCM.01408-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington M. J., Kistler J., Livermore D. M., Woodford N. (2007). Multiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamases. J. Antimicrob. Chemother. 59, 321–322. 10.1093/jac/dkl481 [DOI] [PubMed] [Google Scholar]
- EUCAST (2003). European Committee on Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), (2003). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 9, ix–xv. 10.1046/j.1469-0691.2003.00790.x [DOI] [Google Scholar]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing) (2012). EUCAST Guidelines for Detection of Resistance Mechanism and Specific Resistances of Clinical and/or Epidemiological Importance. Växjö: European Committee on Antimicrobial Susceptibility Testing. Available online at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Consultation/EUCAST_guidelines_detection_of_resistance_mechanisms_121222.pdf
- García-Fernández A., Fortini D., Veldman K., Mevius D., Carattoli A. (2009). Characterization of plasmids harbouring qnrS1, qnrB2 and qnrB19 genes in Salmonella. J. Antimicrob. Chemother. 63, 274–281. 10.1093/jac/dkn470 [DOI] [PubMed] [Google Scholar]
- Glupczynski Y., Huang T. D., Bouchahrouf W., Rezende de Castro R., Bauraing C., Gérard M., et al. (2012). Rapid emergence and spread of OXA-48-producing carbapenem-resistant Enterobacteriaceae isolates in Belgian hospitals. Int. J. Antimicrob. Agents 39, 168–172. 10.1016/j.ijantimicag.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Gu C. T., Li C. Y., Yang L. J., Huo G. C. (2014). Enterobacter xiangfangensis sp. nov., isolated from Chinese traditional sourdough, and reclassification of Enterobacter sacchari Zhu et al. 2013 as Kosakonia sacchari comb. nov. Int. J. Syst. Evol. Microbiol. 64, 2650–2656. 10.1099/ijs.0.064709-0 [DOI] [PubMed] [Google Scholar]
- Gurevich A., Saveliev V., Vyahhi N., Tesler G. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075. 10.1093/bioinformatics/btt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock S. J., Phan M. D., Peters K. M., Forde B. M., Chong T. M., Yin W. F., et al. (2017). Identification of IncA/C plasmid replication and maintenance genes and development of a plasmid multilocus sequence typing scheme. Antimicrob. Agents Chemother. 61, e01740–e01816. 10.1128/AAC.01740-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer C. J., Hall R. M. (2015). The A to Z of A/C plasmids. Plasmid 80, 63–82. 10.1016/j.plasmid.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Herzog K. A., Schneditz G., Leitner E., Feierl G., Hoffmann K. M., Zollner-Schwetz I., et al. (2014). Genotypes of Klebsiella oxytoca isolates from patients with nosocomial pneumonia are distinct from those of isolates from patients with antibiotic-associated hemorrhagic colitis. J. Clin. Microbiol. 52, 1607–1616. 10.1128/JCM.03373-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H., Roggenkamp A. (2003). Population genetics of the nomenspecies Enterobacter cloacae. Appl. Environ. Microbiol. 69, 5306–5318. 10.1128/AEM.69.9.5306-5318.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsey M., Phee L., Wareham D. W. (2011). A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob. Agents Chemother. 55, 5952–5954. 10.1128/AAC.05108-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak J., Stolbová M., Studentová V., Fridrichová M., Chudacková E., Zemlickova H. (2012). NDM-1 producing Acinetobacter baumannii isolated from a patient repatriated to the Czech Republic from Egypt, July 2011. Euro Surveill. 17:20085. [PubMed] [Google Scholar]
- Izdebski R., Fiett J., Urbanowicz P., Baraniak A., Derde L. P., Bonten M. J., et al. (2015). Phylogenetic lineages, clones and β-lactamases in an international collection of Klebsiella oxytoca isolates non-susceptible to expanded-spectrum cephalosporins. J. Antimicrob. Chemother. 70, 3230–3237. 10.1093/jac/dkv273 [DOI] [PubMed] [Google Scholar]
- Johnson T. J., Bielak E. M., Fortini D., Hansen L. H., Hasman H., Debroy C., et al. (2012). Expansion of the IncX plasmid family for improved identification and typing of novel plasmids in drug-resistant Enterobacteriaceae. Plasmid 68, 43–50. 10.1016/j.plasmid.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Koboldt D. C., Zhang Q., Larson D. E., Shen D., McLellan M. D., Lin L., et al. (2012). VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22, 568–576. 10.1101/gr.129684.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaraju M., Kamatchi C., Jha A. K., Devasena N., Vennila R., Sumathi G., et al. (2015). Complete sequencing of an IncX3 plasmid carrying blaNDM−5 allele reveals an early stage in the dissemination of the blaNDM gene. Indian J. Med. Microbiol. 33, 30–38. 10.4103/0255-0857.148373 [DOI] [PubMed] [Google Scholar]
- Lee K., Lim Y. S., Yong D., Yum J. H., Chong Y. (2003). Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-β-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 41, 4623–4629. 10.1128/JCM.41.10.4623-4629.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. (2013). Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. ArXiv. e-Prints 1303, 3997.
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A., Bo Y., Han L., He J., Lanczycki C. J., Lu S., et al. (2017). CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucl. Acids Res. 45, D200–D203. 10.1093/nar/gkw1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A., Bryant S. H. (2004). CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 32, W327–W331. 10.1093/nar/gkh454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheenko A., Valin G., Prjibelski A., Saveliev V., Gurevich A. (2016). Icarus: visualizer for de novo assembly evaluation. Bioinformatics 32, 3321–3323. 10.1093/bioinformatics/btw379 [DOI] [PubMed] [Google Scholar]
- Milne I., Stephen G., Bayer M., Cock P. J. A., Pritchard L., Cardle L., et al. (2013). Using Tablet for visual exploration of second-generation sequencing data. Brief. Bioinformatics 14, 193–202. 10.1093/bib/bbs012 [DOI] [PubMed] [Google Scholar]
- Miyoshi-Akiyama T., Hayakawa K., Ohmagari N., Shimojima M., Kirikae T. (2013). Multilocus sequence typing (MLST) for characterization of Enterobacter cloacae. PLoS ONE 8:e66358. 10.1371/journal.pone.0066358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naas T., Cuzon G., Villegas M. V., Lartigue M. F., Quinn J. P., Nordmann P. (2008). Genetic structure at the origin of acquisition of the beta-lactamase blaKPC gene. Antimicrob. Agents Chemother. 52, 1257–1263. 10.1128/AAC.01451-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Boulanger A. E., Poirel L. (2012). NDM-4 metallo-β-lactamase with increased carbapenemase activity from Escherichia coli. Antimicrob. Agents Chemother. 56, 2184–2186. 10.1128/AAC.05961-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Poirel L., Walsh T. R., Livermore D. M. (2011). The emerging NDM carbapenemases. Trends Microbiol. 19, 588–595. 10.1016/j.tim.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Pál T., Ghazawi A., Darwish D., Villa L., Carattoli A., Hashmey R., et al. (2017). Characterization of NDM-7 carbapenemase-producing Escherichia coli isolates in the Arabian Peninsula. Microb. Drug Resist. 23, 871–878. 10.1089/mdr.2016.0216 [DOI] [PubMed] [Google Scholar]
- Pałucha A., Mikiewicz B., Hryniewicz W., Gniadkowski M. (1999). Concurrent outbreaks of extended-spectrum beta-lactamase-producing organisms of the family Enterobacteriaceae in a Warsaw hospital. J. Antimicrob. Chemother. 44, 489–499. 10.1093/jac/44.4.489 [DOI] [PubMed] [Google Scholar]
- Papagiannitsis C. C., Dolejska M., Izdebski R., Giakkoupi P., Skalova A., Chudejova K., et al. (2016). Characterisation of IncA/C2 plasmids carrying an In416-like integron with the blaVIM−19 gene from Klebsiella pneumoniae ST383 of Greek origin. Int. J. Antimicrob. Agents 47, 158–162. 10.1016/j.ijantimicag.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Papagiannitsis C. C., Miriagou V., Giakkoupi P., Tzouvelekis L. S., Vatopoulos A. C. (2013a). Characterization of pKP1780, a novel IncR plasmid from the emerging Klebsiella pneumoniae ST147, encoding the VIM-1 metallo-β-lactamase. J. Antimicrob. Chemother. 68, 2259–2262. 10.1093/jac/dkt196 [DOI] [PubMed] [Google Scholar]
- Papagiannitsis C. C., Studentova V., Chudackova E., Bergerova T., Hrabak J., Radej J., et al. (2013b). Identification of a New Delhi metallo-β-lactamase-4 (NDM-4)-producing Enterobacter cloacae from a Czech patient previously hospitalized in Sri Lanka. Folia Microbiol. 58, 547–549. 10.1007/s12223-013-0247-5 [DOI] [PubMed] [Google Scholar]
- Papagiannitsis C. C., Studentova V., Jakubu V., Spanelova P., Urbaskova P., Zemlickova H., et al. (2015). High prevalence of ST131 among CTX-M-producing Escherichia coli from community-acquired infections, in the Czech Republic. Microb. Drug Resist. 21, 74–84. 10.1089/mdr.2014.0070 [DOI] [PubMed] [Google Scholar]
- Pavan M. E., Franco R. J., Rodriguez J. M., Gadaleta P., Abbott S. L., Janda J. M., et al. (2005). Phylogenetic relationships of the genus Kluyvera: transfer of Enterobacter intermedius Izard et al. 1980 to the genus Kluyvera as Kluyvera intermedia comb. nov. and reclassification of Kluyvera cochleae as a later synonym of K. intermedia. Int. J. Syst. Evol. Microbiol. 55, 437–442. 10.1099/ijs.0.63071-0 [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez F. J., Hanson N. D. (2002). Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40, 2153–2162. 10.1128/JCM.40.6.2153-2162.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L., Heritier C., Tolun V., Nordmann P. (2004). Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48, 15–22. 10.1128/AAC.48.1.15-22.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotova V., Papagiannitsis C. C., Skalova A., Chudejova K., Hrabak J. (2017). Comparison of imipenem and meropenem antibiotics for the MALDI-TOF MS detection of carbapenemase activity. J. Microbiol. Methods 137, 30–33. 10.1016/j.mimet.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Seemann T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- Studentova V., Dobiasova H., Hedlova D., Dolejska M., Papagiannitsis C. C., Hrabak J. (2015). Complete nucleotide sequences of two NDM-1-encoding plasmids from the same sequence type 11 Klebsiella pneumoniae strain. Antimicrob. Agents Chemother. 59, 1325–1328. 10.1128/AAC.04095-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-González P., Bobadilla-Del Valle M., Tovar-Calderón E., Leal-Vega F., Hernández-Cruz A., Martínez-Gamboa A., et al. (2015). Outbreak caused by Enterobacteriaceae harboring NDM-1 metallo-β-lactamase carried in an IncFII plasmid in a tertiary care hospital in Mexico City. Antimicrob. Agents Chemother. 59, 7080–7083. 10.1128/AAC.00055-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatopoulos A. C., Philippon A., Tzouvelekis L. S., Komninou Z., Legakis N. J. (1990). Prevalence of a transferable SHV-5 type beta-lactamase in clinical isolates of Klebsiella pneumoniae and Escherichia coli in Greece. J. Antimicrob. Chemother. 26, 635–648. 10.1093/jac/26.5.635 [DOI] [PubMed] [Google Scholar]
- Villa L., Guerra B., Schmoger S., Fischer J., Helmuth R., Zong Z., et al. (2015). IncA/C plasmid carrying blaNDM−1, blaCMY−16, and fosA3 in a Salmonella enterica serovar corvallis strain isolated from a migratory wild bird in Germany. Antimicrob. Agents Chemother. 59, 6597–6600. 10.1128/AAC.00944-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth T., Falush D., Lan R., Colles F., Mensa P., Wieler L. H., et al. (2006). Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60, 1136–1151. 10.1111/j.1365-2958.2006.05172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford N., Fagan E. J., Ellington M. J. (2006). Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J. Antimicrob. Chemother. 57, 154–155. 10.1093/jac/dki412 [DOI] [PubMed] [Google Scholar]
- Woodford N., Turton J. F., Livermore D. M. (2011). Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 35, 736–755. 10.1111/j.1574-6976.2011.00268.x [DOI] [PubMed] [Google Scholar]
- Yang P., Xie Y., Feng P., Zong Z. (2014). blaNDM−5 carried by an IncX3 plasmid in Escherichia coli sequence type 167. Antimicrob. Agents Chemother. 58, 7548–7552. 10.1128/AAC.03911-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong D., Toleman M. A., Giske C. G., Cho H. S., Sundman K., Lee K., et al. (2009). Characterization of a new metallo-β-lactamase gene, blaNDM−1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53, 5046–5054. 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankari E., Hasman H., Cosentino S., Vestergaard M., Rasmussen S., Lund O., et al. (2012). Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67, 2640–2644. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. P., Xue W. C., Meng D. Y. (2016). First report of New Delhi metallo-β-lactamase 5 (NDM-5)-producing Escherichia coli from blood cultures of three leukemia patients. Int. J. Infect. Dis. 42, 45–46. 10.1016/j.ijid.2015.10.006 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Liang Y., Lynch K., Dennis J. J., Wishart D. S. (2011). PHAST: a fast phage search tool. Nucleic Acids Res. 39, W347–W352. 10.1093/nar/gkr485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. Q., Zhao J. Y., Xu C., Zhao H., Jia N., Li Y. N. (2016). Identification of an NDM-5-producing Escherichia coli sequence type 167 in a neonatal patient in China. Sci. Rep. 6:29934. 10.1038/srep29934 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.