Abstract
AIM
To investigate the long term (≥5y) efficacy, predictability, and safety of laser in situ keratomileusis (LASIK) in eyes with thin corneas [central corneal thickness (CCT) <500 µm].
METHODS
A total of 339 patients met the criteria of this study. Finally, 175 eyes of 89 patients who had thin corneas and underwent LASIK≥5y ago returned to our clinic and included in this study. Preoperative parameters recorded included uncorrected visual acuity (UCVA), corrected distance visual acuity (CDVA), manifest refraction, CCT and corneal topography. At returning visits, in addition to visual acuity and manifest refraction, ultrasound CCT and corneal topography were performed. Optical coherence tomography was used to measure the CCT, LASIK flap thickness, and residual stromal bed thickness (RSBT). Safety index, efficacy index, percentage of eyes within ±0.5 D and ±1.0 D of refraction, percent tissue altered (PTA), and percentage stromal bed thickness (PSBT) were calculated.
RESULTS
The safety index was 1.09 and efficacy index was 0.99. The percentages of eyes within ±0.5 D and ±1.0 D were 71.2% and 87.7%, respectively. The mean PTA was 40%±6% (range 20% to 55%); 76 eyes (43.4%) had PTA <40% and 99 eyes (56.6%) had PTA≥40%. The mean RSBT was 303±27 µm (range 240 to 390 µm), and 2 eyes had RSBT<250 µm. The mean PSBT was 61%±9% (range 51% to 85%). No eyes developed ectasia.
CONCLUSION
In this cohort with the PSBT of 50% or more, LASIK is safe with follow-up for at least 5y.
Keywords: LASIK, thin cornea, long-term safety, percent tissue altered, percentage stromal bed thickness
INTRODUCTION
Laser in situ keratomileusis (LASIK) has been performed for about 20y, and has been proven to be safe, effective, and predicable[1]–[4]. However, complications following LASIK do occur[5]–[6]. Corneal ectasia after LASIK is a serious complication[7], and its incidence was reported to be 0.25% to 0.6%[8]–[12]. Management for early ectasia includes intrastromal corneal ring implantation[13]–[16], corneal collagen crosslinking[17]–[18], and rigid gas permeable contact lens[19]–[20]. However, for advanced ectasia, corneal transplant is the only option[21]. Central corneal thickness (CCT) <500 µm has been considered by some as a possible independent risk factor for corneal ectasia.
Recently, Santhiago et al[22] reported that percent tissue altered (PTA) ≥40% was the most significant variable associated with the development of ectasia in eyes with normal preoperative corneal topography. Although a few studies investigated the long-term outcomes of LASIK in eyes with thin corneas[23]–[25], to the best of our knowledge, we are unaware of studies evaluating the PTA criterion for the long-term safety of LASIK on thin corneas. In this study, we investigated the efficacy, predictability, and safety of LASIK in a Chinese population who had thin corneas and underwent LASIK at least 5 years ago.
SUBJECTS AND METHODS
Patients
This study followed the tenets of the Declaration of Helsinki. The Ethics Committee of the Shanxi Eye Research Institute approved the study protocol. Informed consent was obtained from each patient prior to enrollment.
To ensure a minimum of 5y after LASIK, we reviewed the charts of all patients who had thin corneas (preoperative CCT <500 µm) and underwent LASIK between September 1998 and September 2010; 339 patients met the criteria. Between February 2015 and July 2015, we tried to contact all patients via telephone calls. We asked if they had any problems with their vision and requested that they return for checkup. Finally, 89 patients returned to our clinic and participated in this study.
Preoperative Evaluation
Before the LASIK surgery, all subjects had detailed eye examination, including uncorrected visual acuity (UCVA), corrected distance visual acuity (CDVA), autorefraction (OPD-Scan ARK-10000, NIDEK Co. Ltd., Japan), manifest refraction, cycloplegic retinoscopy (compound sodium tropicamide 0.5% ophthalmic solution), intraocular pressure (IOP) with non-contact tonometer (NT-2000, NIDEK Co. Ltd., Japan), slit-lamp evaluation, and fundus examination. Corneal topography was performed using the OPD-Scan (ARK-10000, NIDEK Co. Ltd., Japan). Corneal ultrasound pachymetry was also obtained (UP-1000, NIDEK Co. Ltd., Japan).
Patients wearing contact lenses were instructed to stop soft contact lens use 2wk or hard contact lens use 4wk before the preoperative examination.
LASIK Surgical Technique
All LASIK procedures were performed by 3 surgeons using the same protocol and technique. All cases were performed using topical anesthesia (tetracaine sodium 1% ophthalmic solution). The corneal flap was created using a microkeratome (OUP and M2 produced by Moria, France, and MK-2000 produced by NIDEK, Japan). After the flap was lifted, we first measured residual CCT with ultrasound pachymetry so that we could calculate corneal flap thickness later, then ablation was performed using a 193 nm excimer laser (EC-5000 and EC-5000CXII, NIDEK Co. Ltd., Japan). The procedure was designed as a single-zone or multi-zone ablation based on the preoperative refractive error and CCT. The optical zone diameter ranged from 4.3-6.0 mm, and the transition zone diameter, 7.0-8.5 mm. The mean ablation depth was 84.32±22.75 µm (range 32.0 to 144.2 µm).
After the surgery, patients were instructed to use fluorometholone sodium 0.1% ophthalmic solution 4 times a day for 1wk, starting on the day of surgery, and then taper over the following 4wk. Patients were also instructed to use levofloxacin sodium 0.5% ophthalmic solution and recombinant bovine basic fibroblast growth factor sodium ophthalmic solution 4 times a day for 1mo.
Examinations at Last Visit
At returning visit, all subjects had detailed eye examinations, including UCVA, CDVA, autorefraction, manifest refraction, cycloplegic retinoscopy, IOP, slit-lamp evaluation, and fundus examination. Corneal ultrasound pachymetry was obtained using the AX-II (Quantel Medical Corp, France). Corneal topography measurements were performed using the Allegro Oculyzer (Wavelight GmbH, German).
Optical coherence tomography (OCT) imagining system RTVue XR (Optovue Corp, USA) was used to measure the corneal flap thickness, residual stromal bed thickness (RSBT), and CCT. Three scans were taken for each eye, and the one with the clearest image quality was chosen for measurements. Using the built-in caliper, at the center of the cornea, we measured the following distances: 1) from the anterior corneal surface to the interface of LASIK flap (LASIK flap thickness); 2) from the LASIK flap interface to the posterior corneal surface (RSBT); and 3) from the anterior corneal surface to the posterior corneal surface (CCT).
Data Analysis
The following parameters were analyzed: UCVA, CDVA, manifest refraction spherical equivalent (SE), safety index (postoperative CDVA/preoperative CDVA), efficacy index (postoperative UCVA/preoperative CDVA), preoperative CCT, and postoperative CCT at last visit. The PTA was calculated as (corneal flap thickness + ablation depth)/preoperative CCT[22]. The percentage stromal bed thickness (PSBT) was calculated as RSBT/preoperative CCT.
Data that had normal distribution were expressed as mean± standard deviation. The independent or paired t-test was used where applicable. The SPSS 20.0 software was used for data analysis. A P value less than 0.05 was considered statistically significant.
RESULTS
Totally 89 patients (175 eyes) were included. There were 44 male (85 eyes) and 45 female (90 eyes). The mean follow-up time was 97.2±23.9mo (range 60 to 201mo). The mean age, manifest refractive sphere, cylinder, SE, and CCT were listed in Table 1. A total of 175 eyes, 20 eyes had SE between -1.50 and -3.00 D, 76 eyes had SE between -3.25 D and -6.00 D, 56 eyes had SE between -6.25 D and -9.00 D, 19 eyes had SE between -9.25 D and -12.00 D, and 4 eyes had SE<-12.00 D.
Table 1. Summary of preoperative and postoperative data in 175 eyes.
| Parameters | Mean±SD (range) |
| Preoperative | |
| Age (y) | 25.4±5.8 (18 to 43) |
| UCVA (logMAR) | 0.99±0.25 (0.30 to 2.00) |
| CDVA (logMAR) | 0.02±0.05 (-0.10 to 0.20) |
| Sphere (D) | -6.47±2.60 (-2.00 to -15.00) |
| Cylinder (D) | 0.61±0.58 (0 to 2.50) |
| SE (D) | -6.17±2.56 (-1.50 to -15.00) |
| CCT (µm) (ultrasound) | 487.2±10.2 (459 to 499) |
| Postoperative | |
| UCVA (logMAR) | 0.03±0.12 (-0.20 to 0.60) |
| CDVA (logMAR) | -0.02±0.06 (-0.20 to 0.20) |
| Sphere (D) | -0.75±0.90 (-5.00 to 1.75) |
| Cylinder (D) | 0.44±0.41 (0 to 2.00) |
| SE (D) | -0.51±0.80 (-4.50 to 1.50) |
| CCT (µm) (ultrasound) | 419.4±22.4 (360 to 472) |
| CCT (µm) (OCT) | 417.9±23.8 (357 to 479) |
| Corneal flap thickness (µm) (OCT) | 114.0±16.6 (84 to164) |
| RSBT (µm) (OCT) | 302.9±27.0 (240 to 390) |
| PTA | 40%±6% (20% to 55%) |
| PSBT | 61%±9% (51% to 85%) |
UCVA: Uncorrected visual acuity; CDVA: Corrected distance visual acuity; SE: Spherical equivalent; CCT: Central corneal thickness; OCT: Optical coherence tomography; RSBT: Residual stromal bed thickness; PTA: Percent tissue altered; PSBT: Percentage stromal bed thickness.
Visual Acuity
The mean preoperative UCVA was 0.99±0.25 logMAR, and the mean postoperative UCVA was significantly improved to 0.03±0.12 logMAR at the last visit (t=49.659, P=0.000; Table 1). At the last visit, of the 175 eyes, 36 eyes (20.6%) had UCVA of ≥20/16, 85 eyes (48.6%) had UCVA of 20/20, 36 eyes (20.5%) had CDVA of 20/25, and 18 eyes (10.3%) had UCVA of <20/25 (Figure 1).
Figure 1. Preoperative CDVA and postoperative UCVA and CDVA at last visit.
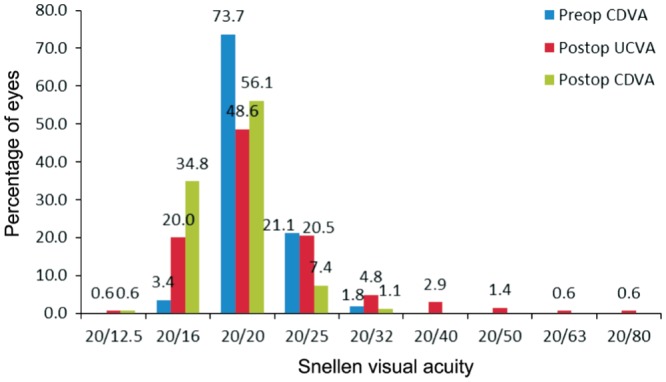
The mean preoperative CDVA was 0.02±0.05 logMAR. A total of 175 eyes, 6 eyes (3.4%) had CDVA of ≥20/16, 129 eyes (73.7%) had CDVA of 20/20, 37 eyes (21.1%) had CDVA of 20/25, and 3 eyes (1.8%) had CDVA of <20/25 (Figure 1). The mean postoperative CDVA at the last visit was -0.02±0.06 logMAR. A total of 175 eyes, 62 eyes (35.4%) had CDVA of ≥20/16, 98 eyes (56.1%) had CDVA of 20/20, 13 eyes (7.4%) had CDVA of 20/25, and 2 eyes (1.1%) had CDVA of <20/25 (Figure 1).
Refraction, Safety, Efficacy, and Accuracy
At the last visit, the mean spherical power was -0.75±0.90 D, and the mean cylinder power was 0.44±0.41 D. The postoperative mean SE was -0.51±0.80 D, which was significantly different from the preoperative mean SE of -6.47±2.60 D (t=-30.683, P=0.000; Table 1).
Compared to preoperative CDVA, postoperative CDVA did not change in 94 eyes (53.7%), gained one line in 73 eyes (41.7%), and gained two lines in 8 eyes (4.6%). No eye lost 1 line or more in CDVA at the last visit (Figure 2). The safety index was 1.09.
Figure 2. Change in CDVA.
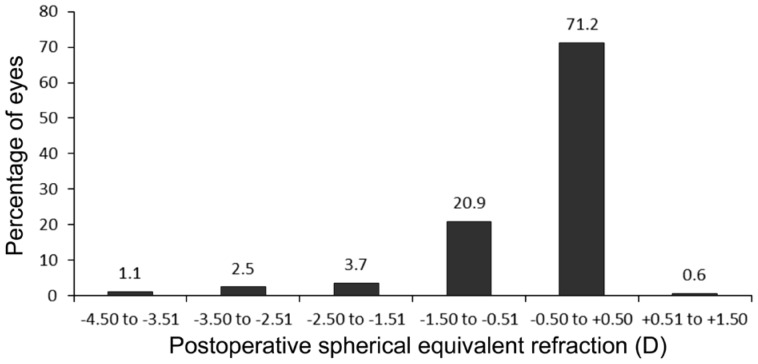
Figure 3 shows the cumulative Snellen visual acuity for preoperative CDVA and postoperative UCVA. The efficacy index was 0.99. Figure 4 displays the postoperative refraction SE; 71.2% and 87.7% of eyes had SE within ±0.50 D and ±1.00 D, respectively.
Figure 3. Preoperative CDVA and postoperative UCVA at last visit.
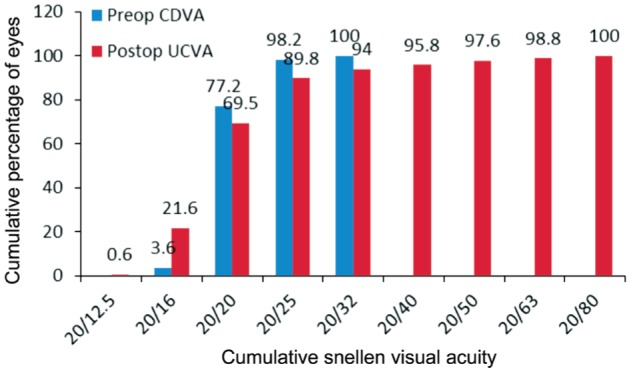
Figure 4. Postoperative spherical equivalent refractive accuracy.
Central Corneal Thickness and Residual Stromal Bed Thickness
The preoperative mean CCT measured by ultrasound pachymeter was 487.2±10.2 µm (range 459 to 499 µm). The postoperative mean CCT values were 419.4±22.4 µm (range 360 to 472 µm) measured by ultrasound pachymeter, and 417.9±23.8 µm (range 357 to 479 µm) measured by OCT (t=0.437, P=0.663).
With OCT, the mean corneal flap thickness was 114.0±16.6 µm (range 84 to 164 µm), and the mean corneal flap thickness calculated through CCT minus residual CCT intraoperatively was 112.3±19.8 µm (range 80 to 164 µm). There were no statistical different between two ways (t=0.253, P=0.422). And the RSBT was 302.9±27.0 µm (range 240 to 390 µm; Figure 5). Two eyes (1.1%) had RSBT<250 µm. The preoperative, intraoperative, and postoperative data for these two eyes were summarized in Table 2. The case 2 generated 2.5 D of myopic regression in this time. Postoperative corneal topography and OCT images for these 2 cases are shown in Figures 6 and 7. We could indicate that both of them didn't develop ectasia based upon these figures.
Figure 5. Postoperative RSBT.
Table 2. Preoperative and postoperative data for 2 eyes with RSBT<250 µm.
| Parameters | Case 1 | Case 2 |
| Preoperative | ||
| Sex | Male | Female |
| Age (y) | 22 | 34 |
| UCVA (logMAR) | 1.40 | 1.10 |
| CDVA (logMAR) | 0.00 | 0.10 |
| Sphere (D) | -13.25 | -9.50 |
| Cylinder (D) | 1.00 | 0.50 |
| SE (D) | -12.75 | -9.25 |
| CCT (µm) (ultrasound) | 470 | 483 |
| Intraoperative | ||
| Ablation pattern | multi-zone | multi-zone |
| Ablation depth (µm) | 117.9 | 101.6 |
| Postoperative | ||
| Follow-up time (mo) | 75 | 104 |
| UCVA (logMAR) | 0.10 | 0.20 |
| CDVA (logMAR) | 0.00 | 0.10 |
| Sphere (D) | -0.75 | -2.50 |
| Cylinder (D) | 0.00 | 0.50 |
| SE (D) | -0.75 | -2.25 |
| CCT (µm) (OCT) | 354 | 383 |
| Corneal flap thickness (µm) (OCT) | 114 | 135 |
| RSBT (µm) (OCT) | 240 | 248 |
| PTA | 49.3% | 49.0% |
| PSBT | 51.1% | 51.3% |
UCVA: Uncorrected visual acuity; CDVA: Corrected distance visual acuity; SE: Spherical equivalent; CCT: Central corneal thickness; OCT: Optical coherence tomography; RSBT: Residual stromal bed thickness: PTA: Percent tissue altered; PSBT: Percentage stromal bed thickness.
Figure 6. Corneal topography (A) and OCT image (B) for the case with RSBT of 240 µm.

Figure 7. Corneal topography (A) and OCT image (B) for the case with RSBT of 248 µm.

Percent Tissue Altered and Percentage Stromal Bed Thickness
The mean PTA was 40%±6% (range 20% to 55%), 76 eyes(43.4%) had PTA<40% and 99 eyes (56.6%) had PTA≥40% (Figure 8).
Figure 8. Postoperative PTA.
The mean PSBT was 61%±9% (range 51% to 85%), and all eyes had PSBT>50% (Figure 9).
Figure 9. Postoperative PSBT.
Postoperative Corneal Topography
At the last visit, corneal curvature values were 38.68±1.72 D (range 33.90 to 42.90 D) for K1, and 39.56±1.77 D (range 35.40 to 44.60 D) for K2. The mean corneal astigmatism magnitude was 0.88±0.50 D (range 0 to 2.1 D). Posterior corneal curvature values were -6.05±0.23 D (range -5.60 to -6.50 D) for K1, and -6.43±0.24 D (range -5.90 to -7.00 D) for K2. The mean posterior corneal astigmatism magnitude was 0.39±0.14 D (range 0 to 0.8 D). None of these eyes had signs of ectasia on corneal topography.
DISCUSSION
LASIK is the most common surgical technique to correct myopia and astigmatism all over the world. Corneal flap creation and stromal ablation during LASIK may affect the corneal biomechanical strength, especially in thin cornea. Giri et al[26] suggested that patients with preoperative CCT of <500 µm should not undergo LASIK. Tabbara and Kotb[27] proposed that preoperative CCT of <500 µm has the highest risk score in the preoperative grading system for the detection of patients who are at risk of corneal ectasia after LASIK. Lazreg et al[28] suggested that thin cornea was one of risk factors of postoperative ectasia. In our study, all patients had preoperative CCT of <500 µm, and, with 5y or more follow-up after the LASIK, none of the patients lost one or more lines of CDVA or had high irregular astigmatism or topographic findings of ectasia.
It was reported that ectasia occurs at an average of 13 to 15.3mo (range 1wk to 62mo)[29]–[31] after LASIK. In this study, the range of follow-up was 84 to 201mo. The fact that no eyes developed ectasia indicates a stable corneal biomechanical strength in these eyes. Moreover, the safety index was 1.09, the effective index was 0.99, and 71.2% and 87.7% of eyes had SE within ±0.50 D and ±1.00 D, respectively. Our data indicates that safety, efficacy, and accuracy were acceptable in eyes with thin corneas (459 to 499 µm).
Some researchers recommend that RSBT should be at least 250 µm[7],[32]. In our study, 2 eyes from 2 patients had RSBT of <250 µm; both have done well. Obviously, much larger numbers are needed to evaluate the potential risk associated with a RSBT under 250 µm, independent of other factors.
Santhiago et al[22] proposed the concept of PTA (percent tissue altered). They included 30 eyes from 16 patients with bilateral normal preoperative Placido-based corneal topography that developed ectasia after LASIK and 174 eyes from 88 consecutive patients with uncomplicated LASIK and at least 3y of postoperative follow-up. They reported that PTA≥40% was significantly associated with the development of ectasia. In our study, 76 eyes (43.4%) had PTA<40% and 99 eyes (56.6%) had PTA≥40%, and there were no signs of ectasia in all these eyes. We noted that, in the study by Santhiago et al[22], ectasia cases had normal corneal topography examined by Placido disk-based device before the surgery. As Placido disk-based corneal topography measures anterior corneal surface only, patients with abnormal posterior corneal surface might not be detected.
In human donor corneas, Randleman et al[33] found that the anterior 40% of the central corneal stroma was the strongest region of the cornea, whereas the posterior 60% of the stroma was at least 50% weaker. The risk for ectasia may therefore be greater with ablations into the posterior stroma. In a previous study, using rabbit eyes, He et al[34] found that it was safe when the PSBT was 50% or more, and ectasia occurred when the PSBT decreased to 30%. In the current study, all eyes had PSBT of >50% (minimum 51%), and no eyes developed ectasia with follow up of ≥5y, demonstrating that it was safe with PSBT of >50%. This ratio of RSBT to preoperative CCT takes into account both RSBT and preoperative CCT, and is thus presumably more appropriate in assessing the safety threshold of LASIK than RSBT alone.
Theoretically, the sum of tissue altered and RSBT should equal the preoperative CCT, and the sum of PTA and PSBT should be 100%. Our results showed that all eyes had PSBT of >50%, whereas 5 eyes (2.9%) had PTA>50%. In this study, preoperative CCT were measured by ultrasound pachymetry, and postoperative corneal flap thickness and RSBT were measured by OCT. For the PTA calculation, we used corneal flap thickness obtained from the OCT at the last visit, tissue ablation depth recorded intraoperatively, and preoperative CCT measured with ultrasound pachymetry. For the PSBT calculation, we used RSBT measured with OCT at the last visit and preoperative CCT measured with ultrasound pachymetry. This may have caused the minor deviation of sum of these two values from the theoretical value of 100%. We believe that OCT measurements at the last visit represent the up-to-date status of the cornea. Additionally, we used both ultrasound pachymetry and OCT to measure the postoperative CCT at the last visit, and we compared the corneal flap thickness measured by OCT and calculated with CCT minus residual CCT intraoperatively. The mean CCT and corneal flap thickness values obtained from these two ways were comparable. Therefore, we believe that PTA and PSBT values calculated in this study were acceptable. Of course, we will use OCT to checkup throughout operational process in future.
In 42 eyes with ectasia after LASIK, Tatar et al[35] showed that ablation depth >75 µm was the most significant risk factor for ectasia. In our study, 108 eyes (61.7%) had ablation depth >75 µm. This demonstrates that ablation depth of >75 µm is probably safe when PSBT was >50%.
Some studies reported the long-term outcomes of thin corneas after refractive surgery. Tomita et al[23] evaluated the long-term (3 to 6y) outcomes of femtosecond laser-assisted LASIK in 291 eyes with thin corneas (<500 µm), and found that LASIK in a thin cornea was as safe and effective and showed similar long-term stability as in eyes with a thicker cornea (≥500 µm). Kymionis et al[24] studied long-term (1 to 3y) outcomes of LASIK in 56 eyes with thin corneas (<500 µm), and suggested that LASIK seems to be a safe and predictable technique for myopic refractive corrections. Djodeyre et al[25] reported refractive and visual outcomes at 3.1 to 8.4y of follow-up in eyes with corneas <470 µm after LASIK with microkeratome. They found that LASIK was effective, safe, and predictable in eyes with corneas thinner than 470 µm, normal preoperative topography, and a RSBT value of greater than 250 µm. In our study, in 175 eyes with follow up of at least 5y, our results were consistent with findings in those studies. Subjects included in these studies were of different ethnicities.
Some limitations of this study were summarized as follows. First, low percentage of patients returned for checkup. A total of 339 patients who had LASIK surgery on thin corneas during the review period, 250 patients could not return due to long distance or were unwilling to return for exams. Over the phone, we asked if they had problems with their vision, and none of those reported problems with their vision. Further study is needed to collect more cases and to evaluate longer term outcomes of LASIK in thin corneas. Second, the accuracy of identifying the interface between the flap and the residual stroma may decrease in eyes that had LASIK many years ago using the OCT. In this study, we did see the high reflective interface in all cases on the OCT images. Santhiago et al[22] also used AC-OCT to measure the flap thickness and RSBT in the control group with at least 3y of postoperative follow-up. Last, our study subjects underwent LASIK procedures between 1998 and 2010. The laser treatment profiles, microkeratome, and screening devices have changed during this period of study. However, this is a common limitation among studies in this area, such as the study by Tomita et al[23] in which different laser treatments and screening devices were used during preoperative and postoperative checkup.
In summary, our results demonstrated that LASIK was safe and effective in Chinese eyes with thin corneas (459 to 499 µm). We recommend that preoperative screening for keratoconus and forme fruste keratoconus should be performed carefully, and then PSBT should be at least 50%. Further studies are warrant to assess the safety of LASIK in eyes with RSBT <250 µm and PTA>40% in larger number of eyes with longer follow ups.
Acknowledgments
Foundations: Supported in part by the National Natural Science Foundation of China (No.11402161); the Programs for Science and Technique Development of Shanxi Province, China (No.20120313025-3); the Science and Technology Research Projects of the Shanxi Provincial Health Department, China (No.201201018); an unrestricted grant from Research to Prevent Blindness, New York, NY, USA.
Conflicts of Interest: Song YW, None; He R, None; Ma JX, None; Koch DD, None; Wang L, None.
REFERENCES
- 1.Khoramnia R, Salgado JP, Wuellner C, Donitzky C, Lohmann CP, Winkler von Mohrenfels C. Safety, efficacy, predictability and stability of laser in situ keratomileusis (LASIK) with a 1000-Hz scanning spot excimer laser. Acta Ophthalmol. 2012;90(6):508–513. doi: 10.1111/j.1755-3768.2010.02052.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosman M, Hall RC, Chan C, Ang A, Koh J, Htoon HM, Tan DT, Mehta JS. Comparison of efficacy and safety of laser in situ keratomileusis using 2 femtosecond laser platforms in contralateral eyes. J Cataract Refract Surg. 2013;39(7):1066–1073. doi: 10.1016/j.jcrs.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 3.Tomita M, Watabe M, Yukawa S, Nakamura N, Nakamura T, Magnago T. Safety, efficacy, and predictability of laser in situ keratomileusis to correct myopia or myopic astigmatism with a 750 Hz scanning-spot laser system. J Cataract Refract Surg. 2014;40(2):251–258. doi: 10.1016/j.jcrs.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 4.Frings A, Intert E, Steinberg J, Druchkiv V, Linke SJ, Katz T. Outcomes of retreatment after hyperopic laser in situ keratomileusis. J Cataract Refract Surg. 2017;43(11):1436–1442. doi: 10.1016/j.jcrs.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Lei Y, Hou J, Zheng X. Redo surgery using IntraLase femtosecond laser for treating a decentered laser in situ keratomileusis flap. J Int Med Res. 2018;46(2):901–907. doi: 10.1177/0300060517718989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilde C, Messina M, Dua HS. Management of recurrent epithelial ingrowth following laser in situ keratomileusis with mechanical debridement, alcohol, mitomycin-C, and fibrin glue. J Cataract Refract Surg. 2017;43(7):980–984. doi: 10.1016/j.jcrs.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Vahdati A, Seven I, Mysore N, Randleman JB, Dupps WJ., Jr Computational biomechanical analysis of asymmetric ectasia risk in unilateral post-LASIK ectasia. J Refract Surg. 2016;32(12):811–820. doi: 10.3928/1081597X-20160929-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bialasiewicz A, Edward DP. Corneal ectasias: study cohorts and epidemiology. Middle East Afr J Ophthalmol. 2013;20(1):3–4. doi: 10.4103/0974-9233.106379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohlhaas M. Iatrogenic keratectasia: a review. Klin Monbl Augenheilkd. 2015;232(6):765–772. doi: 10.1055/s-0035-1545737. [DOI] [PubMed] [Google Scholar]
- 10.Chan C, Ang M, Saad A, Chua D, Mejia M, Lim L, Gatinel D. Validation of an objective scoring system for forme fruste keratoconus detection and post-LASIK ectasia risk assessment in Asian eyes. Cornea. 2015;34(9):996–1004. doi: 10.1097/ICO.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 11.Rapuano CJ. Prevention of iatrogenic keratectasia. Klin Monbl Augenheilkd. 2016;233(6):695–700. doi: 10.1055/s-0042-100734. [DOI] [PubMed] [Google Scholar]
- 12.Thanos S, Oellers P, Meyer Zu Hörste M, Prokosch V, Schlatt S, Seitz B, Gatzioufas Z. Role of thyroxine in the development of keratoconus. Cornea. 2016;35(10):1338–1346. doi: 10.1097/ICO.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 13.Liu XL, Li PH, Fournie P, Malecaze F. Investigation of the efficiency of intrastromal ring segments with cross-linking using different sequence and timing for keratoconus. Int J Ophthalmol. 2015;8(4):703–708. doi: 10.3980/j.issn.2222-3959.2015.04.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arantes JCD, Coscarelli S, Ferrara P, Araújo LPN, Ávila M, Torquetti L. Intrastromal corneal ring segments for astigmatism correction after deep anterior lamellar keratoplasty. J Ophthalmol. 2017;2017:8689017. doi: 10.1155/2017/8689017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicula C, Pop RN, Nicula DV. Comparative results in a combined procedure of intrastromal corneal rings implantation and cross-linking in patients with keratoconus: a retrospective study. Ophthalmol Ther. 2017;6(2):313–321. doi: 10.1007/s40123-017-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torquetti L, Ferrara G, Almeida F, Cunha L, Araujo LP, Machado A, Marcelo Lyra J, Merayo-Lloves J, Ferrara P. Intrastromal corneal ring segments implantation in patients with keratoconus: 10-year follow-up. J Refract Surg. 2014;30(1):22–26. doi: 10.3928/1081597X-20131217-02. [DOI] [PubMed] [Google Scholar]
- 17.Behndig A. Corneal collagen crosslinking for ectasia after refractive surgery. Ophthalmology. 2017;124(10):1440–1441. doi: 10.1016/j.ophtha.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang YM, Chan TC, Yu MCY, Jhanji V. Comparative evaluation of progression rate in keratoconus before and after collagen crosslinking. Br J Ophthalmol. 2017:pii:bjophthalmol-2017-311017. doi: 10.1136/bjophthalmol-2017-311017. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Shimizu E, Yagi-Yaguchi Y, Tomida D, Satake Y, Shimazaki J. A novel entity of corneal diseases with irregular posterior corneal surfaces: concept and clinical relevance. Cornea. 2017;36Suppl1:S53–S59. doi: 10.1097/ICO.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S, Mutalib HA, Sharanjeet-Kaur, Ghoshal R, Retnasabapathy S. Effects of contact lens wearing on keratoconus: a confocal microscopy observation. Int J Ophthalmol. 2017;10(2):228–234. doi: 10.18240/ijo.2017.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang T, Hu Y, Gui M, Hou C, Zhang H. Comparison of refractive outcomes in three corneal transplantation techniques for keratoconus. Graefes Arch Clin Exp Ophthalmol. 2015;253(11):1947–1953. doi: 10.1007/s00417-015-3091-2. [DOI] [PubMed] [Google Scholar]
- 22.Santhiago MR, Smadja D, Gomes BF, Mello GR, Monteiro ML, Wilson SE, Randleman JB. Association between the percent tissue altered and post-laser in situ keratomileusis ectasia in eyes with normal preoperative topography. Am J Ophthalmol. 2014;158(1):87–95.e1. doi: 10.1016/j.ajo.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Tomita M, Watabe M, Mita M, Waring GO., 4th Long-term observation and evaluation of femtosecond laser-assisted thin-flap laser in situ keratomileusis in eyes with thin corneas but normal topography. J Cataract Refract Surg. 2014;40(2):239–250. doi: 10.1016/j.jcrs.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 24.Kymionis GD, Bouzoukis D, Diakonis V, Tsiklis N, Gkenos E, Pallikaris AI, Giaconi JA, Yoo SH. Long-term results of thin corneas after refractive laser surgery. Am J Ophthalmol. 2007;144(2):181–185. doi: 10.1016/j.ajo.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Djodeyre MR, Ortega-Usobiaga J, Beltran J, Baviera J. Long-term comparison of laser in situ keratomileusis versus laser surface ablation in corneas thinner than 470 µm. J Cataract Refract Surg. 2012;38(6):1034–1042. doi: 10.1016/j.jcrs.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 26.Giri P, Azar DT. Risk profiles of ectasia after keratorefractive surgery. Curr Opin Ophthalmol. 2017;28(4):337–342. doi: 10.1097/ICU.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabbara KF, Kotb AA. Risk factors for corneal ectasia after LASIK. Ophthalmology. 2006;113(9):1618–1622. doi: 10.1016/j.ophtha.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 28.Lazreg S, Mesplié N, Praud D, Delcourt C, Kamoun H, Chahbi M, Leoni-Mesplié S, Smadja D, Trattler W, Touboul D, Colin J. Comparison of corneal thickness and biomechanical properties between North African and French patients. J Cataract Refract Surg. 2013;39(3):425–430. doi: 10.1016/j.jcrs.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Khairat YM, Mohamed YH, Moftah IA, Fouad NN. Evaluation of corneal changes after myopic LASIK using the Pentacam®. Clin Ophthalmol. 2013;7:1771–1776. doi: 10.2147/OPTH.S48077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115(1):37–50. doi: 10.1016/j.ophtha.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 31.Roszkowska AM, Sommario MS, Urso M, Aragona P. Post photorefractive keratectomy corneal ectasia. Int J Ophthalmol. 2017;10(2):315–317. doi: 10.18240/ijo.2017.02.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogasawara K, Onodera T. Residual stromal bed thickness correlates with regression of myopia after LASIK. Clin Ophthalmol. 2016;10:1977–1981. doi: 10.2147/OPTH.S116498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randleman JB, Dawson DG, Grossniklaus HE, McCarey BE, Edelhauser HF. Depth-dependent cohesive tensile strength in human donor corneas: implications for refractive surgery. J Refract Surg. 2008;24(1):S85–S89. doi: 10.3928/1081597X-20080101-15. [DOI] [PubMed] [Google Scholar]
- 34.He R, Zhou YX, Chen WY, Wang XJ, Feng Y, Qu M, Liu B, Zhao JW. A study on the effect of different residual stroma thicknesses on the rabbit cornea after laser in situ keratomileusis. Chin J Optom Ophthalmol Vis Sci. 2010;12(2):142–145. [Google Scholar]
- 35.Tatar MG, Aylin Kantarci F, Yildirim A, Uslu H, Colak HN, Goker H, Gurler B. Risk factors in post-LASIK corneal ectasia. J Ophthalmol. 2014;2014:204191. doi: 10.1155/2014/204191. [DOI] [PMC free article] [PubMed] [Google Scholar]