Abstract
AIM
To determine if triptolide influences the contractility and fibronectin production in human Tenon fibroblasts (HTFs).
METHODS
HTFs were cultured in type I collagen gels with or without transforming growth factor beta (TGF-β) and/or triptolide. The diameter of the collagen gel was used to measure contraction. Immunoblot analysis was used to quantify myosin light chain (MLC) phosphorylation and integrin expression. Laser confocal fluorescence microscopy was used to monitor the formation of actin stress fibers. Fibronectin production was measured with an enzyme immunoassay.
RESULTS
Triptolide inhibition of contraction in TGF-β-induced collagen gel mediated by HTFs was dose-dependent and statistically significant at 3 nmol/L (P<0.05) and maximal at 30 nmol/L and significantly time dependent at 2d (P<0.05). Triptolide reduced TGF-β-induced expression of integrins α5 and β1, phosphorylation of MLC, and formation of stress fibers in HTFs. Furthermore, the inhibition of triptolide on the attenuated TGF-β-induced production of fibronectin by HTFs was concentration-dependent and significant at 1 nmol/L (P<0.05) and maximal at 30 nmol/L.
CONCLUSION
Triptolide suppress the contractility of HTFs induced by TGF-β and the production of fibronectin by these cells. It is promising that triptolide treatment may possibly inhibit scar formation after glaucoma filtration surgery.
Keywords: Tenon fibroblast, triptolide, transforming growth factor β, wound healing, fibronectin
INTRODUCTION
One of the complications of glaucoma filtration surgery is the uncontrolled wound-healing at the surgical site[1]. The failure of such surgery is primarily due to scar formation at the filtering bleb site and fibroblasts of Tenon's capsule play a key role in contractile scarring. Contraction of subconjunctival tissue after glaucoma filtration surgery is related to immoderate generation of extracellular matrix (ECM)[2]. Thus, mediation of ECM remodeling has become a major target of antifibrotic therapies[3]–[4].
The multifunctional cytokine, transforming growth factor (TGF)-β, regulates cellular activities including: contraction, differentiation, proliferation, and production of ECM[5]. TGF-β plays a major role in wound healing and fibrosis throughout the body, including conjunctival scarring following glaucoma surgery[6]. Disruption of the TGF-β signaling pathway has been the focus of potential therapies to prevent conjunctival fibrosis[7]. The level of TGF-β type II receptor is higher in scarred blebs of the conjunctiva compared with normal tissue[8]. The success of glaucoma filtration surgery in animal models has been improved by the inhibition of TGF-β activity which reduced conjunctival scarring[9].
Chinese have traditionally used triptolide, a major component of herbal extracts of the Tripterygium wilfordii Hook F, to treatment for rheumatoid arthritis[10]. Triptolide is pharmacologically activite and inhibits of inflammation, cell proliferation, and the immune response. It is also antifibrotic due to its attenuation of TGF-β activity. PG490-88, is a derivative of triptolide that blocks TGF-β activity and inhibited bleomycin-induced lung fibrosis[11] and ECM protein synthesis in TGF-β1-stimulated NRK-49F kidney cells by suppressing Smad2 signaling[12].
Fibroblast-mediated collagen gels contraction has been used as an in vitro model for studies of wound contraction. We have proved that TGF-β stimulates contraction of collagen gel mediated by human Tenon fibroblasts (HTFs) as well as promotes the formation of stress fibers and focal adhesions in these cells[13]. In the current study we examined the effect of triptolide on actin stress fibers formation in HTFs, the contraction of collagen gel mediated by HTFs exposed to TGF-β, the release of fibronectin, integrin expression, and myosin light chain (MLC) phosphorylation.
MATERIALS AND METHODS
Materials
The material and abbreviation is listed as follows: Invitrogen-Gibco (Rockville, MD, USA): 10× Eagle's minimum essential medium (MEM), fetal bovine serum (FBS), and trypsin-EDTA; Corning (Corning, NY, USA): cell culture dishes and 24-well culture plates; Nacalai Tesque (Kyoto, Japan): bovine serum albumin (BSA); Alexis Biochemicals (Carlsbad, CA, USA): triptolide; Nitta Gelatin (Osaka, Japan): reconstitution buffer and native porcine type I collagen; Sigma-Aldrich (St. Louis, MO, USA): anti-β-actin and anti-α-smooth muscle actin (α-SMA) antibodies, collagenase, and protease inhibitor cocktail; Cell Signaling (Beverly, MA, USA): BD Biosciences (San Jose, CA, USA): antibodies to MLC and to phosphorylated MLC; Molecular probes (Eugene, OR, USA): antibodies to integrins α5 and β1, TOTO-3 iodide (642/640) and Alexa Fluor 568-conjugated phalloidin; R&D Systems (Minneapolis, MN, USA): recombinant human TGF-β1; Takara Shuzo (Shiga, Japan): enzyme immunoassay (EIA) kit for fibronectin; Amersham Pharmacia Biotech (Uppsala, Sweden): enhanced chemiluminescence kit and nitrocellulose membranes; Promega (Madison, WI, USA): CytoTox 96 Non-Radioactive Cytotoxicity Assay.
Human Tenon Fibroblasts Isolation and Culture
Human Tenon fibroblasts of subconjunctival tissue from three individuals undergoing strabismus surgery were obtained with informed consent. The present study was performed according to the principles of the Declaration of Helsinki for research involving human subjects[14]. Two Chinese male and one female patients ranging in age from 18 to 30y, had no history of systemic or conjunctival diseases and did not take any topical ocular medications. In brief, a single-cell suspension of HTFs was iolated from the subconjunctival tissue treated with 2 mg/mL of collagenase at 37°C. The cells from each subject were separately maintained in MEM with 10% FBS, and were incubated at 37°C under a humidifying incubator of 5% (vol/vol) CO2. Three to seven passages were used for the present study.
Collagen Gel Contraction Assay
Collagen gel was prepared as described elsewhere[14]. HTFs were suspended in serum-free MEM (SF-MEM) after treating with trypsin-EDTA. Reconstitution buffer, 10×MEM, HTF suspension (1.1×106 cells/mL in SF-MEM) and type I collagen were mixed on ice at the volume ratio of 1:1:2:7 to achieve a final cell density of 2×105/mL and a final type I collagen concentration of 1.9 mg/mL. The mixture was equally added into a 24-well culture plate coated with 1% BSA. A micro spatula was used to free the collagen gels and solidified mixture from the sides of the wells. SF-MEM, triptolide or TGF-β in SF-MEM was added onto the top of each gel. A ruler was used daily to measure the diameter of the gels and gel contraction was calculated at every time point by subtracting the baseline diameter.
Immunoblot Analysis
Immunoblot analysis was performed as described[13]. In brief, ice-cold lysis buffer was used to lyse the collagen gel/HTFs culture[13] and the cell lysates containing 10 µg of protein were subjected to 10% sodium dodecyl sulfate gel electrophoresis. Separated proteins were then transferred onto a nitrocellulose membrane that was blocked for 1h with 5% nonfat milk at room temperature. The specific primary antibodies (1:1000 dilution) were added and incubated for 24h at 4°C. The membrane was washed in Tris-buffered saline with Tween 20, incubated with horseradish peroxidase-conjugated secondary antibodies (1:1000 dilution) for 1h at room temperature, washed again, incubated with enhanced chemiluminescence detection reagents, and exposed to film.
Fluorescence Microscopy
F-actin staining of HTFs cultured in collagen gels was performed as described previously[13]. HTF-collagen mixtures (100 µL) were spread on BSA (1%) coated glass-bottomed dishes. After formation, the collagen gel was collected from the dish and incubated in presence or absence of triptolide for 2h and followed by a culture with or without TGF-β for 3d. The cells were fixed with 1% paraformaldehyde in phosphate-buffered saline for 30min at room temperature, allowed to dry, and then blocked with 1% BSA in phosphate-buffered saline. Cells were incubated for 30min with rhodamine-phalloidin (1:200 dilution) for F-actin staining. The cells were then incubated with TOTO-3 iodide (1:1000 dilution) for 10min to stain nuclei. A laser confocal microscope (LSM5; Carl Zeiss, Hallbergmoos, Germany) was used for imaging.
Enzyme Immunoassay for Fibronectin
Culture medium from collagen gel incubations was centrifuged at 120× g for 10min at 4°C, and the amount of synthesized fibronectin released into the culture supernatant was measured with an EIA kit as specified in the manufactures protocol[15].
Cytotoxicity Assay
Culture medium from collagen gel incubations was collected. To determine the activity of lactate dehydrogenase (LDH) released by HTFs in the culture medium, a colorimetric assay kit (CytoTox 96 non-radioactive cytotoxicity assay, Promega) was employed. A microplate reader was used to measure the absorbance at 490 nm.
Statistical Analysis
Data are expressed as mean±standard deviation (SD). The experiments were performed at least three times (once with the cells from each donor). Significant differences were determined using the Tukey-Kramer test. A significant level of P value of 0.05 was used for all statistical tests.
RESULTS
Effect of Triptolide on Transforming Growth Factor-β-induced Collagen Gel Contraction Mediated by Human Tenon Fibroblasts
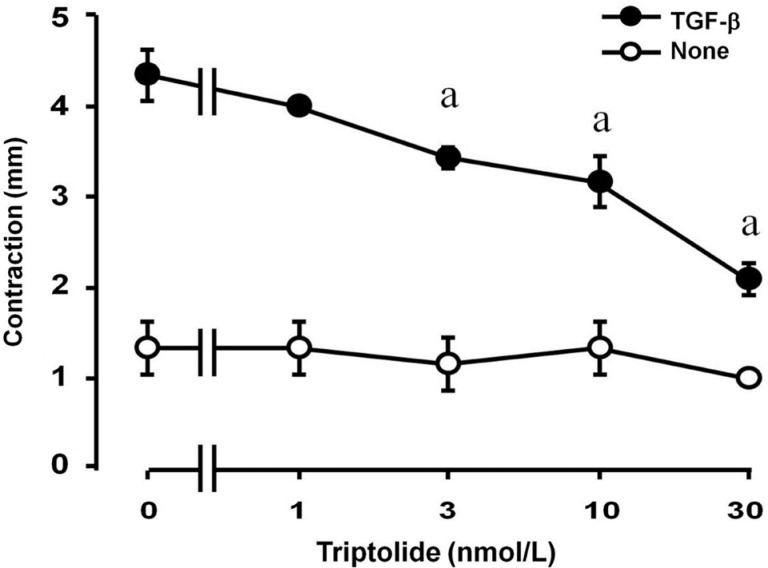
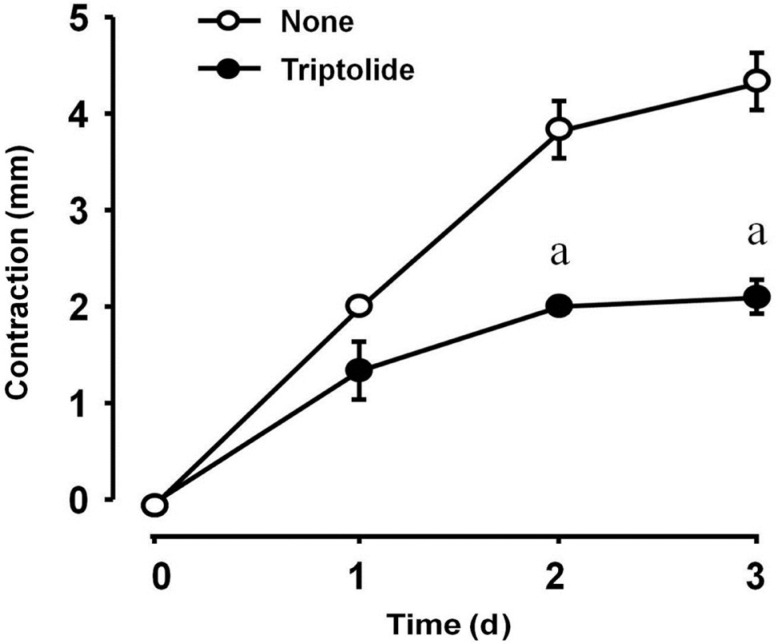
The inhibition of TGF-β-induced gel contraction by triptolide was concentration-dependent and statistically significant at 3 nmol/L and maximal at 30 nmol/L (Figure 1). The effect of triptolide at a concentration of 30 nmol/L was dependent on the incubation time and was statistically significant at 2 and 3d (Figure 2). Collagen contraction was not dependent on triptolide alone (final concentration of 0.1% in dimethyl sulfoxide, data not shown).
Figure 1. Concentration-dependent inhibitory effect of triptolide on TGF-β-induced collagen gel contraction mediated by HTFs.
Cell culture was performed in collagen gels bathed in unsupplemented MEM with or without 1 ng/mL of TGF-β and a range of triptolide doses. After 3d, collagen gel contraction was determined. The experiment was repeated three times with similar results and each data point was measured in triplicate. aA significant difference (P<0.05, Tukey-Kramer test) between points with and without TGF-β. Error bars represent SD.
Figure 2. The longitudinal effect of triptolide on TGF-β-induced collagen gel contraction mediated by HTFs.
Cell incubation was carried out in collagen gels with 1 ng/mL TGF-β over a range of incubation times after which, gel contraction was determined. The experiment was repeated three times with similar results and each data point was measured in triplicate. aA significant difference (P<0.05, Tukey-Kramer test) between points with and without tripolide. Data are presented as mean±SD.
Effect of Triptolide on Transforming Growth Factor-β-induced Formation of Stress Fibers in Human Tenon Fibroblasts
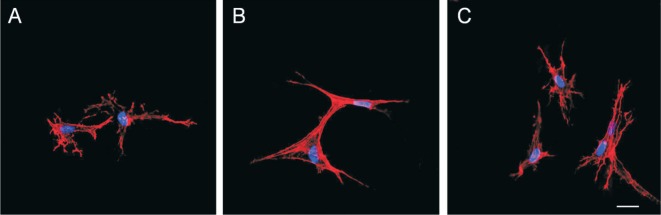
Cell culture in collagen gels for 3d without TGF-β contained no stress fibers and had a dendritic morphology (Figure 3A). Cell culture with TGF-β contained actin stress fibers (Figure 3B) which were attenuated with triptolide (Figure 3C).
Figure 3. Inhibitory effect of triptolide on TGF-β-induced stress fibers formation in HTFs.
Fluoresce micrographs of cells seeded in collagen gel incubated for 2h with or without triptlide, then for 3d with or without TGF-β. A: No TGF-β and triptolide; B: With 1 ng/mL TGF-β; C: With 30 nmol/L triptolide and 1 ng/mL TGF-β. F-actin visualized with rhodamine-phalloidin (red). Cell nuclei visualized with TOTO-3 iodide (blue). Scale bar, 20 µm.
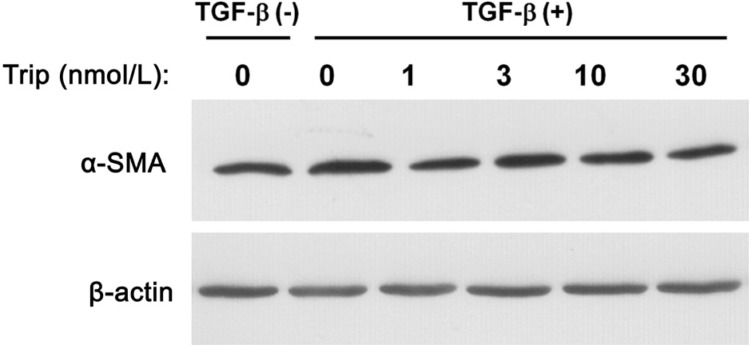
Effects of Triptolide on Myosin Light Chain Phosphorylation and Expression of α-Smooth Muscle Actin in Human Tenon Fibroblasts
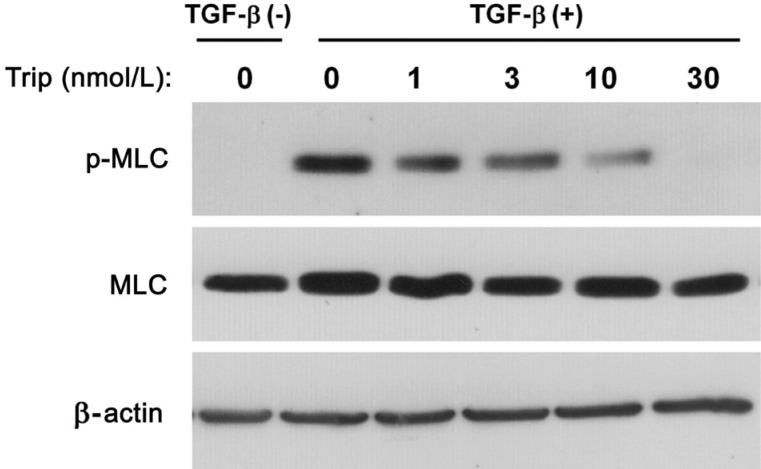
Phosphorylation of MLC was measured in cells cultured with TGF-β. Phosphorylation of MLC was dose-dependently suppressed by triptolide (Figure 4). The expression of α-SMA was not changed in cells cultured under the same conditions (Figure 5).
Figure 4. Triptolide inhibits TGF-β-induced phosphorylation of MLC in HTFs.
Immunoblots from lysates of cells seeded in collagen gel under a 2h incubation in the presence or absence of triptolide, then for 3d with or without TGF-β (1 ng/mL). Antibodies to phosphorylated (p-) or total forms of MLC were used. This result is the representative one from three independent experiments.
Figure 5. Negligible effect of triptolide on expression of α-SMA in HTFs.
Immunoblots from lysates of cells seeded in collagen gel and cultured with or without triptolide for 2h, then for 3d with or without TGF-β (1 ng/mL). Antibodies to α-SMA were used. This result is the representative one from three independent experiments.
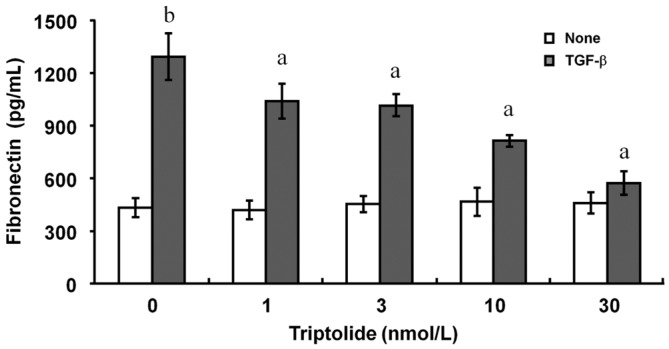
Inhibitory Effect of Triptolide on Transforming Growth Factor-β-induced Fibronectin Release From Human Tenon Fibroblasts
The addition of TGF-β increased the release of fibronectin from these HTFs, and the release was inhibited by triptolide with a dose-dependent characteristic (Figure 6). The statistically significant inhibition by triptolide was started at 1 nmol/L and maximal at 30 nmol/L (Figure 6).
Figure 6. Inhibition by triptolide of TGF-β-induced fibronectin release from HTFs.
Cells were cultured in collagen gel with or without 1 ng/mL of TGF-β and accompanied with various concentrations of triptolide for 3d. The amount of fibronectin in the culture supernatants were determined with the use of an EIA. Data are mean±SD from three independent experiments. aP<0.05 (Tukey-Kramer test) versus the corresponding control (TGF-β alone); bP<0.01 (Tukey-Kramer test) versus control (no TGF-β).
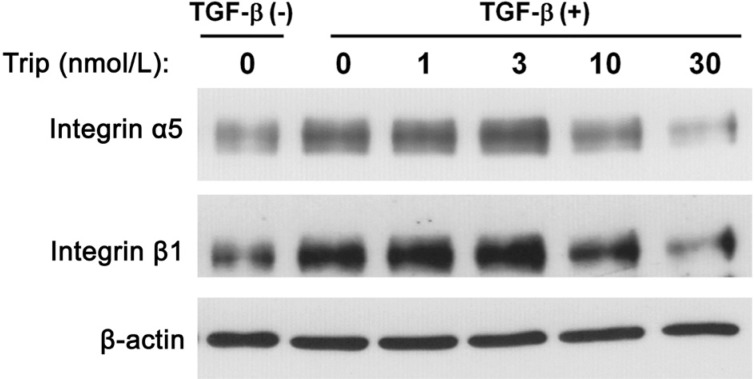
Effect of Triptolide on Integrin Expression in Human Tenon Fibroblasts
Culture of HTFs in the presence of TGF-β increased the amount of integrins α5 and β1 (Figure 7). This up-regulation of integrins was inhibited by triptolide in a concentration-dependent manner, with the maximal effect being apparent at 30 nmol/L (Figure 7).
Figure 7. Triptolide inhibits the expression of integrins α5 and β1 in HTFs.
Immunoblots from lysates of cells seeded in collagen gel cultured for 2h with or without triptlide, then for 3d with or without TGF-β (1 ng/mL). Antibodies to integrins α5 and β1 were used. This result is typical of three independent experiments.
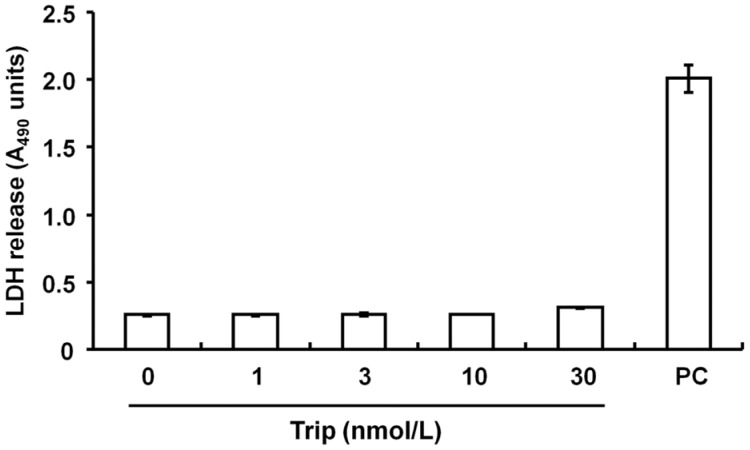
Effect of Triptolide on the Viability of Human Tenon Fibroblasts Co-cultured with Collagen Gels
Exposure of the cells to several doses of triptolide did not affect the release of LDH, indicating it caused no change in cell viability and was not cytotoxic (Figure 8).
Figure 8. Lack of a cytotoxic effect of triptolide on HTFs.
Cells in collagen gels were incubated in the presence or absence of triptolide (Trip) for 3d. Positive control (PC) determined from a cell lysis solution. Experiments were triply repeated and showed consistent results. Data points were measured in triplicate and presented as mean±SD.
DISCUSSION
Scarring after a glaucoma filtering surgery failed the purpose of long-term control of intraocular pressure and arrest of optic nerve damage[16]. The major finding of the current study is that triptolide alleviated TGF-β-induced contraction of collagen gel, MLC phosphorylation, integrin α5 and β1 expression, and the stress fibers formation in HTFs seeded in a collagen gel. Furthermore, triptolide suppressed the production of fibronectin induced by TGF-β in HTFs suggesting that triptolide may inhibit tissue contraction and ECM synthesis at the wound site after glaucoma filtration surgery.
It has been demonstrated that a rapid contractile response by TGF-β in HTFs ensues prior to myofibroblast transdifferentiation[17]. In the current experiment, triptolide suppressed the stress fibers formation induced by TGF-β in HTFs with an associated change in cell morphology. Phosphorylation of MLC facilitates cell contraction and motility by contributing to actin-myosin interactions and the stress fibers formation and contractile rings[18]. In the current study triptolide inhibited TGF-β-induced MLC phosphorylation in HTFs which cultured in collagen gel. α-SMA expression, an indicator for tension changes, was not influenced by TGF-β or triptolide in HTFs in our present study. It could be caused by an insufficient tension change in the floating matrix to achieve a detectable different expression. In another study, the differentiation of fibroblasts into myofibroblasts, which typically express high levels of α-SMA, is thought to play a role in wound contraction and remodeling of fibrotic tissue[19]. To induce a significant change of α-SMA expression in cells cultured in collagen gels, generation of adequate tension within the three-dimensional matrix is crucial[14],[20].
In the present study, triptolide inhibited the TGF-β-induced a dose-dependent up-regulation of fibronectin production and integrin α5β1 expression in HTFs, implying these effects involved in the suppression of TGF-β-induced collagen gel contraction by triptolide. Triptolide also inhibited fibronectin and collagen type III synthesis in TGF-β-stimulated NRK-49F cells[12]. Fibronectin serves as both a structural and regulatory factor and is a central component of the ECM. The hypertrophic scarring is associated with an abundant expression of fibronectin, which could enhance TGF-β-driven scar formation after glaucoma surgery[8]. Integrins α5 and β1 form the major cell surface receptor for fibronectin and mediate adhesion-dependent signaling[21]. Fibronectin accumulation and increased expression of integrin α5β1 contribute to TGF-β-induced myofibroblast differentiation[22]. Our previous studies demonstrated that fibronectin promoted contraction of collagen gel mediated by another cell line, human corneal fibroblasts, in a manner dependent on the formation of stress fibers and focal adhesions and the up-regulation of integrin α5β1[23]. In addition, it protects against myocardial fibrosis associated with inflammatory responses in diabetic cardiomyopathy[24] and against radiation-induced pulmonary fibrosis[25]–[26] in animal models. Thus, triptolide might be expected to attenuate excessive scar formation after glaucoma filtration surgery by inhibiting conjunctival fibrosis at the wound site.
The development of treatments that target TGF-β action has primarily focused on scar formation in systemic or ocular fibrotic disorders. Fibrosis is a major clinical problem that both hinders surgical success and is a principal cause of blindness. In the current study, triptolide inhibited the contractility of HTFs induced by TGF-β cultured in a collagen gel. Fibronectin produced by these cells were also reduced. These results suggest that triptolide may be beneficial as a pharmacological agent for modulation of the conjunctival healing process. Triptolide was safe and clinically beneficial as a treatment for patients with rheumatoid arthritis[27]–[28], in agreement with the present study in which cytotoxicty for HTFs was not detected in vitro. These results are contrary to animal studies in which triptolide damaged several organs including the liver and kidneys, and the drug has been associated with diverse toxic effects including the induction of oxidative stress and apoptosis at the cellular level[29]. Further studies are warranted to determine the toxicity of triptolide and whether triptolide could inhibit scarring along with wound healing in subconjunctiva after glaucoma filtering surgery in vivo.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81770889); the Natural Science Foundation of Guangdong Province (No.2017A030313774); the International Cooperation Item from Research Fund of Jilin Provincial Science and Technology Department (No.20160414055GH).
Conflicts of Interest: Liu Y, None; Liu PP, None; Liu L, None; Zheng XS, None; Zheng H, None; Yang CC, None; Luobu CR, None; Liu Y, None.
REFERENCES
- 1.Fan Gaskin JC, Nguyen DQ, Soon Ang G, O'Connor J, Crowston JG. Wound healing modulation in glaucoma filtration surgery-conventional practices and new perspectives: the role of antifibrotic agents (Part I) J Curr Glaucoma Pract. 2014;8(2):37–45. doi: 10.5005/jp-journals-10008-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahnke T, Kowtharapu BS, Stachs O, Schmitz KP, Wurm J, Wree A, Guthoff RF, Hovakimyan M. Suppression of TGF-b pathway by pirfenidone decreases extracellular matrix deposition in ocular fibroblasts in vitro. PLoS One. 2017;12(2):e0172592. doi: 10.1371/journal.pone.0172592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei D, Dong C, Wu WK, Dong A, Li T, Chan MT, Zhou X, Yuan H. Lentiviral delivery of small hairpin RNA targeting connective tissue growth factor blocks profibrotic signaling in Tenon's capsule fibroblasts. Invest Ophthalmol Vis Sci. 2016;57(13):5171–5180. doi: 10.1167/iovs.16-19480. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Wang J, Wei LJ, Zhu DM, Zhang JS. Biological function and mechanism of lncRNA-MEG3 in Tenon's capsule fibroblasts proliferation: By MEG3-Nrf2 protein interaction. Biomed Pharmacother. 2017;87:548–554. doi: 10.1016/j.biopha.2016.12.040. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Zou H, Zhu XX, Pang J, Xu Q, Jin QY, Ding YH, Zhou B, Huang DS. Transforming growth factor b: a potential biomarker and therapeutic target of ventricular remodeling. Oncotarget. 2017;8(32):53780–53790. doi: 10.18632/oncotarget.17255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordeiro MF. Role of transforming growth factor beta in conjunctival scarring. Clin Sci (Lond) 2003;104(2):181–187. doi: 10.1042/CS20020150. [DOI] [PubMed] [Google Scholar]
- 7.Schlunck G, Meyer-ter-Vehn T, Klink T, Grehn F. Conjunctival fibrosis following filtering glaucoma surgery. Exp Eye Res. 2016;142:76–82. doi: 10.1016/j.exer.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Meyer-Ter-Vehn T, Grehn F, Schlunck G. Localization of TGF-beta type II receptor and ED-A fibronectin in normal conjunctiva and failed filtering blebs. Mol Vis. 2008;14:136–141. [PMC free article] [PubMed] [Google Scholar]
- 9.Mead AL, Wong TT, Cordeiro MF, Anderson IK, Khaw PT. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci. 2003;44(8):3394–3401. doi: 10.1167/iovs.02-0978. [DOI] [PubMed] [Google Scholar]
- 10.Tao XL, Sun Y, Dong Y, Xiao YL, Hu DW, Shi YP, Zhu QL, Dai H, Zhang NZ. A prospective, controlled, double-blind, cross-over study of tripterygium wilfodii hook F in treatment of rheumatoid arthritis. Chin Med J (Engl) 1989;102(5):327–332. [PubMed] [Google Scholar]
- 11.Krishna G, Liu K, Shigemitsu H, Gao M, Raffin TA, Rosen GD. PG490-88, a derivative of triptolide, blocks bleomycin-induced lung fibrosis. Am J Pathol. 2001;158(3):997–1004. doi: 10.1016/S0002-9440(10)64046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu B, Wang YJ, Zhu CF, Lin Y, Zhu XL, Wei S, Lu Y, Cheng XX. Triptolide inhibits extracellular matrix protein synthesis by suppressing the Smad2 but not the MAPK pathway in TGF-beta1-stimulated NRK-49F cells. Nephrol Dial Transplant. 2010;25(10):3180–3191. doi: 10.1093/ndt/gfq239. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Kimura K, Orita T, Teranishi S, Suzuki K, Sonoda KH. Inhibition by all-trans-retinoic acid of transforming growth factor-β-induced collagen gel contraction mediated by human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2014;55(7):4199–4205. doi: 10.1167/iovs.13-13572. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Kimura K, Orita T, Suzuki K, Teranishi S, Mori T, Sonoda KH. Inhibition by a retinoic acid receptor γagonist of extracellular matrix remodeling mediated by human Tenon fibroblasts. Mol Vis. 2015;21:1368–1377. [PMC free article] [PubMed] [Google Scholar]
- 15.Fujitsu Y, Fukuda K, Kumagai N, Nishida T. IL-4-induced cell proliferation and production of extracellular matrix proteins in human conjunctival fibroblasts. Exp Eye Res. 2003;76(1):107–114. doi: 10.1016/s0014-4835(02)00248-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhong H, Sun G, Lin X, Wu K, Yu M. Evaluation of pirfenidone as a new postoperative antiscarring agent in experimental glaucoma surgery. Invest Ophthalmol Vis Sci. 2011;52(6):3136–3142. doi: 10.1167/iovs.10-6240. [DOI] [PubMed] [Google Scholar]
- 17.Meyer-ter-Vehn T, Sieprath S, Katzenberger B, Gebhardt S, Grehn F, Schlunck G. Contractility as a prerequisite for TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2006;47(11):4895–4904. doi: 10.1167/iovs.06-0118. [DOI] [PubMed] [Google Scholar]
- 18.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 19.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63(1):21–29. [PubMed] [Google Scholar]
- 20.Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol. 1999;154(3):871–882. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida T, Inui M, Nomizu M. Peptide therapies for ocular surface disturbances based on fibronectin-integrin interactions. Prog Retin Eye Res. 2015;47:38–63. doi: 10.1016/j.preteyeres.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278(14):12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Yanai R, Lu Y, Kimura K, Nishida T. Promotion by fibronectin of collagen gel contraction mediated by human corneal fibroblasts. Exp Eye Res. 2006;83(5):1196–1204. doi: 10.1016/j.exer.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Xue M, Li CJ, Yang W, Wang SS, Ma ZJ, Zhang XN, Wang XY, Zhao R, Chang BC, Chen LM. Protective effects of triptolide on TLR4 mediated autoimmune and inflammatory response induced myocardial fibrosis in diabetic cardiomyopathy. J Ethnopharmacol. 2016;193:333–344. doi: 10.1016/j.jep.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Chen C, Yang S, Zhang M, Zhang Z, Hong J, Han D, Ma J, Zhang SB, Okunieff P, Zhang L. Triptolide mitigates radiation-induced pulmonary fibrosis via inhibition of axis of alveolar macrophages-NOXes-ROS-myofibroblasts. Cancer Biol Ther. 2016;17(4):381–389. doi: 10.1080/15384047.2016.1139229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Zhang M, Chen C, Cao Y, Tian Y, Guo Y, Zhang B, Wang X, Yin L, Zhang Z, O'Dell W, Okunieff P, Zhang L. Triptolide mitigates radiation-induced pulmonary fibrosis. Radiat Res. 2015;184(5):509–517. doi: 10.1667/RR13831.1. [DOI] [PubMed] [Google Scholar]
- 27.Tao X, Cush JJ, Garret M, Lipsky PE. A phase I study of ethyl acetate extract of the chinese antirheumatic herb Tripterygium wilfordii hook F in rheumatoid arthritis. J Rheumatol. 2001;28(10):2160–2167. [PubMed] [Google Scholar]
- 28.Tao X, Younger J, Fan FZ, Wang B, Lipsky PE. Benefit of an extract of Tripterygium Wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthritis Rheum. 2002;46(7):1735–1743. doi: 10.1002/art.10411. [DOI] [PubMed] [Google Scholar]
- 29.Xi C, Peng S, Wu Z, Zhou Q, Zhou J. Toxicity of triptolide and the molecular mechanisms involved. Biomed Pharmacother. 2017;90:531–541. doi: 10.1016/j.biopha.2017.04.003. [DOI] [PubMed] [Google Scholar]