Abstract
This study investigated the effect of yellow, black, and brown mustard seeds on color, lipid oxidation (thiobarbituric acid-reactive substances [TBARS]), and micro-biological and sensory qualities of meatballs during storage. Heat treatment of mustard seeds affected the TBARS value of meatball samples (p<0.0001). The addition of mustard seeds decreased TBARS value of meatball samples (p<0.0001). Heat treatment of mustard seeds decreased the L*, a* and b* values of meatball samples (p<0.0001). The meatball samples with mustard seeds increased b* value of meatball samples however it decreased a* value of meatball samples (p<0.0001). The addition of mustard seeds decreased aerobic mesophilic bacteria count (p<0.0001), Enterobacteriaceae count (p<0.0001), psychrophilic bacteria count (p<0.0001) and yeast and mold count of meatball samples (p<0.0001). On a given storage day, the yellow mustard added meatballs sample was given higher color, appearance, flavor, acceptability ratings than those added black and brown mustard. Regarding sensory and microbiological properties, mustard seed contributed to microbiological quality and sensorial properties of meatball samples.
Keywords: meatball, mustard seed, Sinapis alba, lipid oxidation, color
Introduction
Microbial growth and lipid oxidation are important factors for the customers to accept meat products (Zhao et al., 1994). These factors induce deterioration, and influence the color, flavor, texture, and nutritional value (Min and Ahn, 2005). For this reason, several studies have focused on the prevention of microbial growth and lipid oxidation during storage (Gök and Bor, 2012). Meat quality can be permanent when lipid oxidation and microbial growth are controlled with the use of antioxidant and antimicrobial compounds (Lee et al., 2010). Currently, producers and consumers mostly prefer antimicrobial and antioxidant compounds that are derived from plant sources (Gök et al., 2011). Several research concluded using mustard seeds to reduce lipid oxidation, improved organoleptic properties in fresh and processed meat products. The inhibition effect of mustard species on spoilage and pathogenic microorganisms in meat products reported by researcher (Olaimat and Holley, 2016; Wójciak and Dolatowski, 2016). Mustard powder as ingredients may affect the physicochemical and organoleptic properties of processed poultry meat, an antimicrobial film or coating as a delivery vehicle appeared to be a more productive approach (Olaimat and Holley, 2015).
The dry seeds of mustard, an annual plant of the Brassicaceae family, are used in food processing. The most common types of mustard include yellow mustard (Sinapis alba), brown mustard (Brassica juncea) and black mustard (Brassica nigra). Mustard contains a rich chemical composition, and its seed flour is commonly used in food processing (Abul-Fadl et al., 2011). Mustard has a spicy flavor, and it is produced with the hydrolysis of glucosinolates using myrosinase enzymes. While mustard seed is generally used as spice, its advantageous chemical composition and relatively low price offer a wide range of possibilities for using it as an additive in human foods and animal feeds (Wanasundara, 2008). Moreover, mustards are functional foods for people since they have beneficial aspects (Abul-Fadl et al., 2011; Lee et al., 2015).
The glucosinolates (thioglucosides) in mustard seeds are pseudo-thioglucosides that include nitrogen and sulphur. When plant tissue is damaged, glucosinolates are hydrolyzed by myrosinase enzymes to produce a variety of compounds like thiocyanates and oxazolidine-2-thiones. Some of these compounds are bioactive, like isothiocyanates and indoles (Bongoni, et al., 2014), or potentially toxic, like nitriles and epithionitriles. The nature of the hydrolysis products depends on the structure of the glucosinolates and the reaction conditions (Fahey et al., 2001; Lambrix et al., 2001). These compounds are essential in food processing (taste, aroma, and flavour attributes) and for human health for their anticarcinogenic and antimicrobial properties (Drewnowski and Gomez-Carneros, 2000).
Glucosinolates are present in broccoli, brussels sprouts, cabbage, and mustard powder. They are also the essence of the pungent flavor of mustard and horseradish. They involve a wide range of antibacterial and antifungal properties, and have a direct or synergistic effect in combination with other compounds (Graumann and Holley, 2008). This antimicrobial activity has been reported to function as a defense mechanism of the crucifers against pathogenic attack (Radulović et al., 2011). The aim of this study is to determine the effect of mustard powders on color of meatballs in addition to their microbial, chemical, and sensory qualities during storage at 4°C.
Materials and Methods
Preparation of mustard seeds
The method of deodorized mustard powder preparation was used cited by Luciano, Belland and Holley (2011). The mustards powders were obtained from Bagdat Food Co. (Turkey). The ground mustard seeds stored them in sealed packaging to prevent their exposure to light. The 200 grams of yellow, brown, and black mustard seeds were used for application. The mustards powder’s packages were removed. The powders were spread out in layers of 2 cm onto stainless steel trays. At this point, the mustard seeds divided into two groups. Group 1 was left untreated (unautoclaved, UNACL), and Group 2 was autoclaved (ACL) at 121°C for 15 min at 1 atm pressure to increase phenolic activity.
Formulation and processing of meatballs
A piece of boneless beef was obtained from a local butcher in Afyonkarahisar (Turkey) and delivered to the laboratory in cold chain. The fat sources of the study were subcutaneous fat and intermuscular fat. Lean beef (max 1.5%) and fat were ground separately using a 3 mm plate meat grinder. Black pepper (0.3%), red pepper (0.3%), cumin (0.15%), and salt (1.8%) were added to the beef along with toasted bread crumbs (5%) and fat before mixing.
The researcher kneaded the mixture for 15 min by hand, and divided the meatball dough into 7 equal batches (2 kg each). The treatments tested had the following composition: control (having no mustard seeds): UNACL×YMM prepared with about 2% unautoclaved yellow mustard, ACL×YMM prepared with about 2% autoclaved yellow mustard; UNACL×BRMM prepared with about 2% unautoclaved brown mustard, ACL×BRMM prepared with about 2% autoclaved brown mustard; UNACL×BLMM prepared with about 2% unautoclaved black mustard, ACL×BLMM prepared with about 2% autoclaved black mustard.
Each batch was kneaded for an additional 30 min to obtain homogeneous dough. The doughs were refrigerated at 4°C for 24 h, and then were shaped (1.0 cm thick and 40 mm diameter) by using a metal shaper. Meatballs weighed approximately 40 g each and packaged with polyethylene bag.
Moisture content and pH measurement
The study determined the moisture and pH value based on the methods of the Association of Official Analytical Chemists (AOAC, 1990).
The determination of 2-thiobarbituric acid-reactive substances (TBARS)
The study used 2-thiobarbituric acid-reactive substances (TBARS) test to determine the extent of oxidative rancidity during storage. The TBARS test (the modified version of Shahidi et al., 1985) was performed according to the methods of Tarladgis et al. (1960). The measurements were carried out at 530 nm (Shimatzu UV-1601, Japan).
Color measurement
Color measurement was performed using a HunterLab model Minolta CR 400 chromometer (Japan). The color of meatballs was measured after every formulation using a Minolta CR-400 (Minolta Co., Japan) spectro-colorimeter equipped with a light source illuminant D65 (10° standard observer) with an 8-mm aperture. Before each measurement, the apparatus was fixed against a white plate. The samples were homogenized and filled into petri dishes before taking the readings. It was ensured that there was no gap between the sample and the lid of the petri dish, and that the lenses of the colorimeter touched the lid of the petri dish. The researcher took six readings, and calculated the mean values for each of the three replications (Gök et al., 2008). The L*, a*, and b* values were determined. Color was described as coordinates: lightness (L*), redness (a*, ±red-green) and yellowness (b*, ±yellow-blue).
Microbiological analysis
The researcher aseptically took 10-g samples from the meatballs, and homogenized them for 3 min with buffered peptone water (BPW, Oxoid Ltd., UK) at dilution 1:9 (w/v) in a Stomacher Lab-Blender 400 (UK). The researcher also made serial decimal dilutions in BPW, and plated them in duplicate for bacterial counts. Total viable counts (mesophilic aerobic bacteria) were determined using plate count agar (PCA Oxoid CM463, Oxoid) after incubation for 24–48 h at 37°C. Psychrotrophic bacteria were determined using plate-count agar after incubation for 10 d at 7°C. The study incubated total coliform counts using Violet Red Bile Dextrose Agar (Merck, 1.10275) at 30°C for 2 d. Yeast and molds were aerobically incubated on Potato Dextrose Agar (Merck, 1.10130) at 30°C for 2 d.
Sensory analysis
Sensory evaluation was carried out by a panel that included 10 trained and members. Panel members were undergraduate and graduate students of the Food Engineering Department of Afyon Kocatepe University. Four training sessions were held to introduce panelists the meatball characteristics and the scale in relation to the study. Each sample was coded with random 3-digit numbers. The researcher schedules only six meatballs per session and two sessions per day not to tire the panelists (total nine sessions per replication). They evaluated the samples for appearance ((visual impression of the products’ surface: shape and color), cut appearance (visual impression at the cross section: particle size and uniformity, glistening of fat, stickiness)); Texture (perceived firmness in the mouth); Color (red color intensity); Flavor (intensity of mustard flavor). The panel members expressed their opinion about the overall acceptability of the meatballs. A continuous scale between 1.0 and 9.0 was used for the evaluation of each attribute. The scores of the hedonic scale was as follows: 1–3 (not acceptable); 4–5 (fairly acceptable); 6–7 (acceptable); and 8–9 (very good) (Gök et al., 2008). The samples were served to panelists in artificial light (incandescent) at room temperature (22°C) in a random order all together. The researcher provided unsalted crackers and deionized rinse water to panelists between the services of individual samples to be used for evaluation. Meatballs samples were internally cooked to 80°C and were served at a temperature of approximately 60°C.
Statistical analysis
The research design was completely randomized having a factorial structure (2×4×4). The factors were heat treatment (non-heated and heated (autoclaved)), meatball type (control (no mustard addition), meatball with 2% yellow mustard, meatball with 2% brown mustard, meatball with 2% black mustard), storage time (0, 4, 7, and 15 d). Three-way ANOVA was applied to data using procedure of the SPSS statistical package program (SPSS Inc., USA) to do this analysis. Lsmeans values were generated and corresponding Tukey’s HSD test. The treatment structure was completely randomized with 3 replications.
Results and Discussion
Moisture, pH and TBARS
A meatball type×storage time (p<0.01) and heat treatment×storage time (p<0.0001) interaction for moisture content existed (Table 1). Storage time influenced the moisture content significantly (p<0.0001) and on the 15 d of storage, moisture content of meat samples increased to 50.13–55.23% (Fig. 1). The increase of moisture content in the core was the result of the moisture permeability of the polyethylene bags in which the samples were placed. The addition of mustard seeds generally increased moisture content of meatball samples exception of BRMM. However, Wu (2013) stated that mustard decreased moisture loss from the mustard added dry fermented sausage. Similarly, Shen et al. (2018) reported that the addition of pickled and dried mustard to pork belly decreased moisture content of samples. This difference can be attributed to food matrix.
Table 1. Probability values (p-values) for all main effects and interactions.
| Source of variation | Moisture | pH | TBARS | L* | a* | b* |
|---|---|---|---|---|---|---|
| Heat treatment | <0.0001 | 0.54 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Meatball type | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Storage time | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Heat treatment×Meatball type | <0.0001 | 0.77 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Heat treatment ×Storage time | 0.99 | 0.01 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Meatball type×Storage time | 0.002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Heat treatment×Meatball type×Storage time | 0.31 | 0.01 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
TBARS, thiobarbituric acid-reactive substances.
Fig. 1. Effect of meatball type and storage time on moisture content of meatball samples.
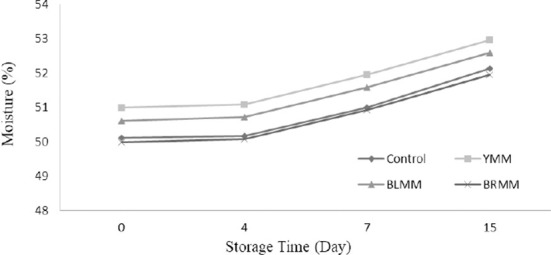
YMM, meatball with 2% yellow mustard; BRMM, meatball with 2% brown mustard; BLMM, meatball with 2% black mustard.
A meatball type×storage time (p<0.0001) and heat treatment×storage time (p<0.01) interaction for pH value content existed (Table 1). Storage time affected the pH values significantly (p<0.0001) and on the day 15 of storage, the pH value of meat samples increased to 5.75–6.01 (Fig. 2). The pH values of meatballs with mustard powders were slightly decrease than control samples. Similarly, Rojas and Brewer (2007) reported that mustard leaf extract reduced the pH values of beef from 5.92 to 5.71. On the other hand, Karwowska and Dolatowski (2013) determined that the addition of 0.2–0.5% of mustard had no effect on pH during storage in sausage samples. However, Lee et al. (2010) reported that the pH of their control group was lower than the pH determined in samples containing 0.1% mustard leaf extract and 0.2% mustard leaf extract. Microbial growth in meat and meat products is a factor that leads to a significant effect on the pH of the product.
Fig. 2. Effect of meatball type and storage time on pH values of meatball samples.
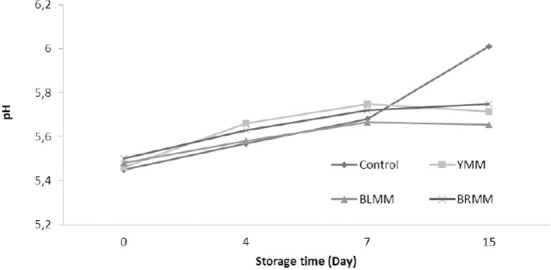
YMM, meatball with 2% yellow mustard; BRMM, meatball with 2% brown mustard; BLMM, meatball with 2% black mustard.
Heat treatment×Meatball type×Storage time interactions were very significant (p<0.0001) (Table 1) on TBARS values. Heat treatment of mustard seeds affected the TBARS value of meatball samples (p<0.0001). The addition of mustard seeds decreased TBARS value of meatball samples (Fig. 3) (p<0.0001). The meatball samples with autoclaved ground mustards had higher antioxidant activity than unautoclaved ground mustards added samples. Similarly, Luciona et al. (2011) reported that the autoclaved powder had the highest level of phenolic content.
Fig. 3. Effect of meatball type and storage time on thiobarbituric acid-reactive substances (TBARS) value of meatball samples.
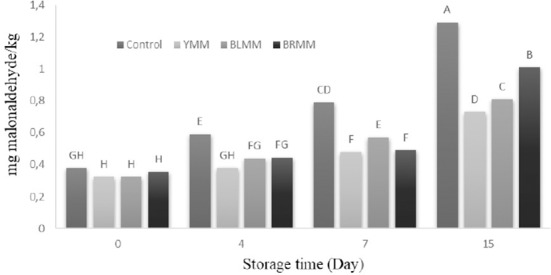
YMM, meatball with 2% yellow mustard; BRMM, meatball with 2% brown mustard; BLMM, meatball with 2% black mustard.
The initial TBARS values of the samples ranged from 0.3 to 0.38 mg malonaldehyde/kg and increased to 0.73 to 1.29 mg malonaldehyde/kg. The TBARS values of the meat samples increased throughout storage period. Control samples had higher TBARS content than any other sample on any day of storage (Fig. 3) (p<0.05). Lipid oxidation was very slow especially in the meatballs that contained mustard seeds can be attributed to the antioxidant compounds of mustard seeds. Lee et al. (2010) also reported that samples containing mustard leaf extract had lower TBA values than the control samples. Shahidi et al. (1993) stated that the addition of up to 2% mustard powder protected cooked chicken meat from lipid oxidation during 20 d of storage. McCarthy et al. (2001) also reported lower lipid oxidation in frozen and ground chicken meat that was supplemented with mustard seeds. It has been proved that the use of autoclaved mustard seeds prevent lipid oxidation, which causes to undesirable taste and aroma in meat products.
Color values (L*, a*, b*)
Heat Treatment×Meatball type×Storage time interactions were very significant for L*, a*, and b* values of meatball samples (p<0.0001) (Table 1). Heat treatment of mustard seeds decreased the L*, a*, and b* values of meatball samples (p<0.0001) (Fig.4). The addition of mustard seeds increased b* value of meatball samples however it decreased a* value of meatball samples (p<0.0001) (Fig. 5). Contrary to this, Wójciak et al. (2014) reported that autoclaved mustard powder decreased yellowness value of sausage but it increased the redness value of sausage. This difference could be explained by the differences in the used mustard species, production method and food matrix. The yellowness values of the samples ranged from 9.73 to 19.31 at the end of the storage (Fig. 6).
Fig. 4. Effect of meatball type and storage time on lightness value of meatball samples.
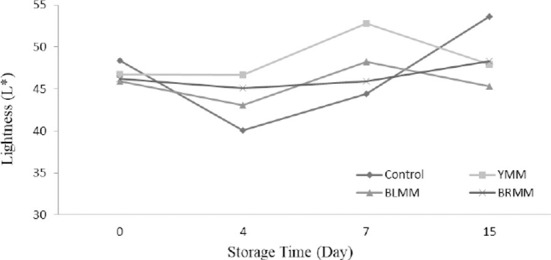
YMM, meatball with 2% yellow mustard; BRMM, meatball with 2% brown mustard; BLMM, meatball with 2% black mustard.
Fig. 5. Effect of meatball type and storage time on redness value of meatball samples.
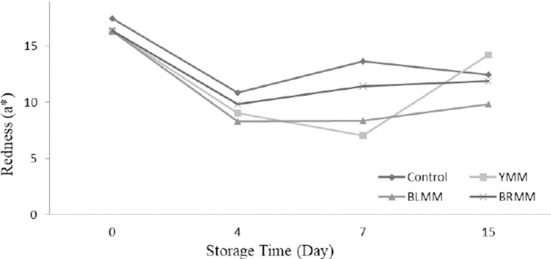
YMM, meatball with 2% yellow mustard; BRMM, meatball with 2% brown mustard; BLMM, meatball with 2% black mustard.
Fig. 6. Effect of meatball type and storage time on yellowness value of meatball samples.
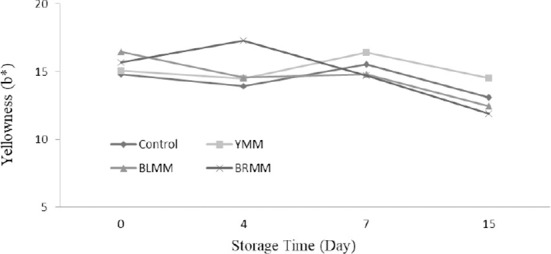
YMM, meatball with 2% yellow mustard; BRMM, meatball with 2% brown mustard; BLMM, meatball with 2% black mustard.
The lightness value of meatball samples increased during the storage time. Many studies showed that the increase in L* during the storage of meatball products is related to methemoglobin formation (MMb) (Fernández-López et al., 2005). Also, MMb formation can be delayed and the L* value can be decreased with the inclusion of antioxidants (Fernández-López et al., 2005). However, the addition of mustard in meatballs increased lightness value. This result can be explained by the mustard seeds color and production methods of meatball. Color formation and stability were important sensory properties of meat products, and affect the acceptability of products by consumers (Zhang et al., 2007).
Microbiological properties
Many studies determined that mustard had antibacterial and antifungal properties (Graumann and Holley, 2008; Lee et al., 2010; Nilson and Holley, 2012; Rhee et al., 2002; Rhee et al., 2003). The antimicrobial and antifungal properties of mustard were attributed to allyl isothiocyanate (AIT) and para-hydroxybenzyl isothiocyanate (p-HBIT) that included mustard (Cordeiro et al., 2013). As shown in Table 2, there was a significant heat treatment×meatball type×storage time interaction for mesophilic aerobic bacteria (p<0.0001), yeast and molds (p<0.0001), psychrophilic bacteria (p<0.0001), and Enterobacteriaceae (p<0.0001). With raised storage time, aerobic mesophilic bacteria count increased (p<0.0001). Similarly, Colle (2015) stated that aerobic mesophilic bacteria count increased longer storage periods for beef patties produced with potato extract and mustard. However, Graumann and Holley (2008) determined that total aerobic mesophilic bacteria counts decreased with storage time for mustard added dry sausage.
Table 2. Probability values (p-values) for all main effects and interactions.
| Source of variation | MAB | EBA | PSB | Yeasts and molds |
|---|---|---|---|---|
| Heat treatment | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Storage time | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Meatball type | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Heat treatment×Storage time | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Heat treatment×Meatball type | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Meatball type×Storage time | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Heat treatment×Meatball type×Storage time | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
MAB, mesophilic aerobic bacteia; EBA, Enterobacteriacea; PSB, psychrophilic bacteria.
The addition of mustard seeds decreased aerobic mesophilic bacteria count (p<0.0001), Enterobacteriaceae count (p<0.0001), psychrophilic bacteria count (p<0.0001), and yeast and mold count of meatball samples (p<0.0001). Similarly, Kumar and Tanwar (2011) determined that the addition of mustard powder to chicken nuggets decreased the total mesophilic aerobic bacteria count at 2.8 Log level at the end of the 15 d. Lee et al. (2010) reported that the addition of mustard leaf ethanolic extract reduced the bacterial count. Enterobacteriaceae count ranged from 2.29 Log CFU/g to 2.49 Log CFU/g on the 1st day and increased to 4.58 Log CFU/g to 5.96 Log CFU/g on the 15th d (Table 3). Enterobacteriaceae count was slightly lower in samples containing mustard than the control sample (p<0.05). Kumar and Tanwar (2011) stated that the addition of mustard powder to chicken nuggets decreased the Enterobacteriaceae counts of samples at 1.0 Log level throughout 15 d. Delaquis and Mazza (1995) reported that the isothiocyanates in mustard cause the inactivation of intracellular enzymes of microorganisms, and negatively affect their development. Kumar and Tanwar (2011) also found that mustard seeds reduced the yeast and mold counts in chicken nuggets.
Table 3. Lsmeans values for heat treatment, meatball type, storage time, heat treatment×meatball type and meatball type×storage time on microbial counts of meatballs*.
| Source variance | MAB | EBA | PSB | Yeasts and molds |
|---|---|---|---|---|
| Heat treatment | ||||
| UNACL | 5.70A | 3.72A | 5.48A | 3.20A |
| ACL | 5.34B | 3.62B | 5.10B | 2.95B |
| Meatball type | ||||
| Control | 6.08 A | 4.03A | 5.82A | 3.52A |
| YMM | 5.06 D | 3.42D | 4.89D | 2.78 D |
| BRMM | 5.38C | 3.57C | 5.11C | 2.91C |
| BLMM | 5.57B | 3.68B | 5.35B | 3.08B |
| Storage time (day) | ||||
| 0 | 3.80D | 2.37D | 3.61D | 2.14D |
| 4 | 4.52C | 3.05C | 4.20C | 2.63C |
| 7 | 5.99B | 4.00B | 5.76B | 3.46B |
| 15 | 7.79A | 5.28A | 7.60A | 4.07A |
| Heat treatment×Meatball type | ||||
| UNACL×YMM | 4.66E | 3.52D | 5.22D | 2.97D |
| UNACL×BRMM | 6.22C | 3.63C | 5.33C | 3.06C |
| UNACL×BLMM | 8.12A | 3.72B | 5.56B | 3.24B |
| ACL×YMM | 4.37F | 3.33E | 4.57G | 2.60F |
| ACL×BRMM | 5.75D | 3.51D | 4.89F | 2.76E |
| ACL×BLMM | 7.47B | 3.64C | 5.14E | 2.93D |
| Meatball type×Storage time | ||||
| Control×0 | 3.82L | 2.29N | 3.65M | 2.16K |
| YMM×0 | 3.81L | 2.38M | 3.60MN | 2.15K |
| BRMM×0 | 3.74M | 2.42M | 3.65M | 2.15K |
| BLMM×0 | 3.81L | 2.39M | 3.53N | 2.11K |
| Control×4 | 4.92H | 3.42I | 4.62I | 2.96G |
| YMM×4 | 4.21K | 2.78L | 3.88L | 2.39J |
| BRMM×4 | 4.43J | 2.96K | 4.03K | 2.48I |
| BLMM×4 | 4.52I | 3.04J | 4.25J | 2.68H |
| Control×7 | 6.92D | 4.43E | 6.61E | 4.07B |
| YMM×7 | 5.33G | 3.74H | 5.05H | 3.04F |
| BRMM×7 | 5.67F | 3.85G | 5.40G | 3.22E |
| BLMM×7 | 6.03E | 4.02F | 5.99F | 3.49D |
| Control×15 | 8.68A | 5.96A | 8.40A | 4.88A |
| YMM×15 | 6.89D | 4.80D | 7.03D | 3.56D |
| BRMM×15 | 7.70C | 5.05C | 7.35C | 3.79C |
| BLMM×15 | 7.90B | 5.30B | 7.64B | 4.06B |
Means within a column with different letters are significantly different (p<0.05).
UNACL, non-heat treatment; ACL, heat treatment; YMM, yellow mustard; BRMM, brown mustard; BLMM, black mustard.
Heat treatment reduced aerobic mesophilic bacteria count (p<0.0001), yeast and molds (p<0.0001), psychrophilic bacteria count (p<0.0001), and Enterobacteriaceae count (p<0.0001) significantly (Table 2). Similarly, Luciano et al. (2011) determined that autoclaved mustard seeds had higher antibacterial effects on E. coli O157:H7 than the other types of mustard seeds. The autoclaving stabilized the level of mustard sinalbin by inactivating the myrosinase enzyme in mustard seed (Luciano et al., 2011; Nilson and Holley, 2012). Mustard sinalbin was hydrolyzed to allyl isothiocyanate (AIT) and para-hydroxybenzyl isothiocyanate by microorganisms. Therefore, ACL meatball types had lower microbial count than UNACL.
Sensory properties
Autoclaving did not affect appearance and texture properties of meatball samples but it was significant for color (p<0.01), flavor (p<0.0001), juiciness (p<0.0001), and acceptability properties of meatball samples (p<0.0001) (Table 4). As shown in Table 4, there was a significant heat treatment×meatball type interaction for flavor (p<0.01), texture (p<0.01), juiciness (p<0.0001), and acceptability (p<0.01) but this interaction did not influence color (p>0.5) and appearance (p>0.49). As storage time increased, color scores, appearance scores, texture scores, flavor scores, juiciness scores, and acceptability scores declined with the lowest scores observed on d 15 (p<0.0001). However, storage time affected all of the sensory properties of meatball (p<0.0001), a meatball type×storage time interaction affected all sensory properties of meatball except appearance scores (p>0.05) (Table 4).
Table 4. Table probability values (p-values) for all main effects and interactions.
| Source of variation | Color | Appearance | Flavor | Texture | Juiciness | Acceptability |
|---|---|---|---|---|---|---|
| Heat treatment | 0.0013 | 0.49 | <.0001 | 0.3 | <.0001 | <.0001 |
| Meatball type | <.0001 | <.0001 | 0.0003 | <.0001 | <.0001 | <.0001 |
| Storage time | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Heat treatment×Meatball type | 0.1 | 0.49 | 0.004 | 0.0008 | <.0001 | 0.0008 |
| Heat treatment ×Storage time | 0.028 | 0.096 | 0.0054 | 0.059 | <.0001 | <.0001 |
| Meatball type×Storage time | 0.021 | 0.046 | 0.016 | 0.0098 | 0.043 | 0.0045 |
| Heat treatment×Meatball type×Storage time | 0.43 | 0.54 | 0.089 | 0.14 | 0.23 | 0.45 |
On a given storage day, the yellow mustard added meatballs sample was given higher color, appearance, flavor, and acceptability ratings than those added black and brown mustard. Li (2012) stated that the addition of the yellow mustard addition in salami decreased acceptability, texture, and flavor scores. However, Shen et al. (2018) stated that the addition of pickle and dried mustard to pork belly contributed to flavor, color and aroma of samples. This difference can be attributed to food matrix and added mustard species. In general, the yellow mustard added meatballs presented better scores than those added black and brown mustard. The autoclaved black mustard added meatballs were given lowest flavor (p<0.01), texture (p<0.01), juiciness (p<0.0001), and acceptability scores (p<0.01) (Table 5). These results can be explained black mustard’s odor and bitter flavor.
Table 5. Lsmeans values for heat treatment, meatball type, storage time, heat treatment×meatball type and meatball type×storage time on sensory properties of meatballs*.
| Source variance | Color | Appearance | Flavor | Texture | Juiciness | Acceptability |
|---|---|---|---|---|---|---|
| Heat treatment | ||||||
| UNACL | 7.15A | 7.10A | 6.58A | 6.60A | 7.25B | 7.03A |
| ACL | 6.90B | 7.05A | 6.20B | 6.53A | 7.33A | 6.65B |
| Meatball type | ||||||
| Control | 7.25B | 7.25A | 6.35B | 6.90A | 7.60A | 7.30A |
| YMM | 7.60A | 7.30A | 6.65A | 6.30C | 7.35B | 7.05A |
| BRMM | 7.10B | 6.65B | 6.40AB | 6.65AB | 7.20C | 6.65B |
| BLMM | 6.15C | 7.10A | 6.15B | 6.40BC | 7.00D | 6.35C |
| Storage time (day) | ||||||
| 0 | 7.38A | 7.43A | 6.74A | 6.86A | 7.59A | 7.14A |
| 4 | 7.18AB | 7.23AB | 6.54AB | 6.66AB | 7.39B | 6.94AB |
| 7 | 6.98B | 7.03B | 6.34B | 6.46BC | 7.19C | 6.74BC |
| 15 | 6.58C | 6.63C | 5.94C | 6.26C | 6.99D | 6.54C |
| Heat treatment×Meatball type | ||||||
| UNACL×YMM | 7.85A | 7.35A | 7.05A | 6.30BC | 7.10C | 7.40A |
| UNACL×BRMM | 7.25A | 6.75A | 6.55B | 6.50ABC | 7.30B | 6.70B |
| UNACL×BLMM | 6.25A | 7.05A | 6.35BC | 6.70AB | 7.00D | 6.70B |
| ACL×YMM | 7.35A | 7.25A | 6.25BC | 6.30BC | 7.60A | 6.70B |
| ACL×BRMM | 6.95A | 6.55A | 6.25BC | 6.80A | 7.10C | 6.60B |
| ACL×BLMM | 6.05A | 7.15A | 5.95C | 6.10C | 7.00D | 6.00C |
| Meatball type×Storage time | ||||||
| Control×0 | 7.60B | 7.60A | 6.70AB | 7.20A | 7.90A | 7.60A |
| YMM×0 | 7.95A | 7.65A | 7.00A | 6.60BC | 7.65B | 7.35AB |
| BRMM×0 | 7.45CB | 7.00ABC | 6.75AB | 6.95AB | 7.50C | 6.95C |
| BLMM×0 | 6.50EF | 7.45AB | 6.50B | 6.70B | 7.30D | 6.65DE |
| Control×4 | 7.40CB | 7.40AB | 6.50B | 7.00AB | 7.70B | 7.40AB |
| YMM×4 | 7.75AB | 7.45AB | 6.80AB | 6.40CD | 7.45C | 7.15BC |
| BRMM×4 | 7.25C | 6.80BCD | 6.55B | 6.75B | 7.30D | 6.75D |
| BLMM×4 | 6.30F | 7.25ABC | 6.30C | 6.50C | 7.10E | 6.45E |
| Control×7 | 7.20C | 7.20ABC | 6.30C | 6.80B | 7.50C | 7.20B |
| YMM×7 | 7.55B | 7.25ABC | 6.60B | 6.20D | 7.25D | 6.95C |
| BRMM×7 | 7.05D | 6.60CD | 6.35C | 6.55C | 7.10E | 6.55DE |
| BLMM×7 | 6.10G | 7.05ABC | 6.10D | 6.30CD | 6.90F | 6.25F |
| Control×15 | 6.80DE | 6.80BCD | 5.90E | 6.60C | 7.30D | 7.00C |
| YMM×15 | 7.15C | 6.85BCD | 6.20CD | 6.00E | 7.05E | 6.75D |
| BRMM×15 | 6.65E | 6.20D | 5.95E | 6.35CD | 6.90F | 6.35E |
| BLMM×15 | 5.70H | 6.65CD | 5.70F | 6.10DE | 6.70G | 6.05G |
Means within a column with different letters are significantly different (p<0.05).
UNACL, non-heat treatment; ACL, heat treatment; YMM, yellow mustad; BRMM, brown mustard; BLMM, black mustard.
Acceptability and flavor scores decreased during the storage periods in all samples. A number of researcher reported that this decline was due to lipid oxidation during storage and to certain compounds (e.g. aldehyde and ketone) that negatively affect the taste of the products (Flores et al., 2004). Thus, the decrease in the flavor and acceptability scores of the samples can be attributed to the undesirable compounds created by lipid oxidation.
Conclusion
This study has proved that adding ground mustard seed to meatballs has a positive effect on their color values, chemical, microbiological, and sensory properties. The use of mustard seed on the meat samples delayed lipid oxidation and extended shelf life. The study also demonstrated that autoclaving and mustard seeds successfully prevented undesirable taste and smell after lipid oxidation. Moreover, the TBA values were lower in meatballs that included autoclaved mustard seeds. Microbiological analysis revealed that the addition of mustard and autoclaving yielded positive results, and were effective in the inhibition of microorganisms. Sensory evaluation showed that the samples containing yellow mustard seeds were favorable, while the samples containing black and brown mustard seeds were less favorable than the control sample. The best overall meatball quality was obtained from the autoclaved ground mustard combination. Therefore, this study proves the efficiency of using ground mustard to improve overall quality of samples and extend shelf life of meatballs. Moreover, adding ground mustard seed decreased lipid oxidation and increased antimicrobial effects in meatballs, which is very important for consumer health.
Acknowledgements
This study was supported by Afyon Kocatepe University Research Fund (Project Number: 13.FEN.BİL.33).
References
- Abul-Fadl MM, El-Badry N, Ammar MS. Nutritional and chemical evaluation for two different varieties of mustard seeds. World Appl Sci J. 2011;15:1225–1233. [Google Scholar]
- AOAC. 15th. Association of Official Analytical Chemist; Washington DC, USA: 1990. Official methods of analysis. [Google Scholar]
- Bongoni R, Verkerk R, Steenbekkers B, Dekker M, Stieger M. Evaluation of different cooking conditions on broccoli (Brassica oleracea var. italica) to improve the nutritional value and consumer acceptance. Plant Foods Hum Nutr. 2014;69:228–234. doi: 10.1007/s11130-014-0420-2. [DOI] [PubMed] [Google Scholar]
- Cordeiro RP, Luciano FB, Holley RA. Evaluation of deodorized yellow mustard concentrations for control of Escherichia coli O157:H7 viability in dry fermented sausage. Food Control. 2013;33:20–24. [Google Scholar]
- Colle MC. University of Idaho; USA: 2015. Effects of dried potato extract on yield, shelf stability, and sensory characteristics of beef patties, chicken frankfurters, and beef top round. [Google Scholar]
- Delaquis PJ, Mazza G. Antimicrobial properties of isothiocyanates in food preservation. Food Tech. 1995;49:73–84. [Google Scholar]
- Drewnowski A, Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: A review. Am J Clin Nutr. 2000;72:1424–1435. doi: 10.1093/ajcn/72.6.1424. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Fernández-López J, Zhi N, Aleson-Carbonell L, Pérez-Alvarez JA, Kuri V. Antioxidant and antibacterial activities of natural extracts: Application in beef meatballs. Meat Sci. 2005;69:371–380. doi: 10.1016/j.meatsci.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Flores M, Durá MA, Marco A, Toldrá F. Effect of Debaryomyces spp. on aroma formation and sensory quality of dry-fermented sausages. Meat Sci. 2004;68:439–446. doi: 10.1016/j.meatsci.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Gök V, Obuz E, Şahin ME, Serteser A. The effects of some natural antioxidants on the color, chemical and microbiological properties of sucuk (Turkish dry-fermented sausage) during ripening and storage periods. J Food Process Preserv. 2011;35:677–690. [Google Scholar]
- Gök V, Bor Y. Effect of olive leaf, blueberry and Zizyphus jujuba extracts on the quality and shelf life of meatball during storage. J Food Agri Environ. 2012;10:190–195. [Google Scholar]
- Gök V, Obuz E, Akkaya L. Effects of packaging method and storage time on the chemical, microbiological, and sensory properties of Turkish pastirma: A dry cured beef product. Meat Sci. 2008;80:335–344. doi: 10.1016/j.meatsci.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Graumann GH, Holley RA. Inhibition of Escherichia coli O157:H7 in ripening dry fermented sausage by ground yellow mustard. J Food Prot. 2008;71:486–493. doi: 10.4315/0362-028x-71.3.486. [DOI] [PubMed] [Google Scholar]
- Karwowska M, Dolatowski ZJ. Antioxidant effects of ground mustard seed in model sausage type product. Food Sci Technol Res. 2013;19:23–28. [Google Scholar]
- Kumar D, Tanwar VK. Effects of incorporation of ground mustard on quality attributes of chicken nuggets. J Food Sci Technol. 2011;48:759–762. doi: 10.1007/s13197-010-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrix V, Reichelt M, Mitchell-Olds T, Kliebenstein DJ, Gershenzon J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell. 2001;13:2793–2807. doi: 10.1105/tpc.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MA, Choi JH, Choi YS, Han DJ, Kim HY, Shim SY, Kim CJ. The antioxidative properties of mustard leaf (Brassica juncea) kimchi extracts on refrigerated raw ground pork meat against lipid oxidation. Meat Sci. 2010;84:498–504. doi: 10.1016/j.meatsci.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Lee YH, Choo C, Waisundara VY. Determination of the total antioxidant capacity and quantification of phenolic compounds of different solvent extracts of black mustard seeds (Brassica nigra) Int J Food Prop. 2015;18:2500–2507. [Google Scholar]
- Li S. Master thesis. University of Manitoba; Canada: 2012. The antimicrobial effects of para-hydroxybenzyl isothiocyanate on Escherichia coli O157:H7 in beaker sausage and the sensory influence of deheated yellow mustard on dry-fermented sausage. [Google Scholar]
- Luciano FB, Belland J, Holley RA. Microbial and chemical origins of the bactericidal activity of thermally treated yellow mustard powder toward Escherichia coli O157:H7 during dry sausage ripening. Int J Food Microbiol. 2011;145:69–76. doi: 10.1016/j.ijfoodmicro.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Manda KR, Adams C, Ercal N. Biologically important thiols in aqueous extracts of spices and evaluation of their in vitro antioxidant properties. Food Chem. 2010;118:589–593. [Google Scholar]
- McCarthy TL, Kerry JP, Kerry JF, Lynch PB, Buckley DJ. Evaluation of the antioxidant potential of natural food/plant extracts as compared with synthetic antioxidants and vitamin E in raw and cooked pork patties. Meat Sci. 2001;58:45–52. doi: 10.1016/s0309-1740(00)00129-7. [DOI] [PubMed] [Google Scholar]
- Min B, Ahn DU. Mechanism of lipid peroxidation in meat and meat products - A review. Food Sci Biotech. 2005;14:152–163. [Google Scholar]
- Nilson AM, Holley RA. Use of deodorized yellow mustard powder to control Escherichia coli O157:H7 in dry cured Westphalian ham. Food Microbiol. 2012;30:400–407. doi: 10.1016/j.fm.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Olaimat AN, Holley RA. Control of Salmonella on fresh chicken breasts by κ-carrageenan/chitosan-based coatings containing allyl isothiocyanate or deodorized oriental mustard extract plus EDTA. Food Microbiol. 2015;48:83–88. doi: 10.1016/j.fm.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Olaimat AN, Holley RA. Inhibition of Listeria monocytogenes on cooked cured chicken breasts by acidified coating containing allyl isothiocyanate or deodorized oriental mustard extract. Food Microbiol. 2016;57:90–95. doi: 10.1016/j.fm.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Prior RL, Cao G, Martin A, Sofic E, McEwen J, O’Brien C, Lischner N, Ehlenfeldt M, WKalt K, Krewer G, Mainland M. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem. 1998;46:2686–2693. [Google Scholar]
- Radulović N, Dekić M, Stojanović-Radić Z. A new antimicrobial glucosinolate autolysis product, 4-isothiocyanatobutanoic acid, from the diffuse wallflower (Erysimum diffusum): Methyl 4-isothiocyanatobutanoate, a long unrecognized artifact of the isolation procedure? Food Chem. 2011;129:125–130. [Google Scholar]
- Rhee KS, Lupton CJ, Ziprin YA, Rhee KC. Effects of sheep production systems on oxidative storage stability of lean lamb patties. Meat Sci. 2003;65:701–706. doi: 10.1016/S0309-1740(02)00317-0. [DOI] [PubMed] [Google Scholar]
- Rhee MS, Dougherty RH, Kang DH. Combined effects of mustard flour, acetic acid, and salt against Escherichia coli O157:H7 stored at 5 and 22°C. J Food Prot. 2002;65:1632–1636. doi: 10.4315/0362-028x-65.10.1632. [DOI] [PubMed] [Google Scholar]
- Rojas MC, Brewer MS. Effect of natural antioxidants on oxidative stability of cooked, refrigerated beef and pork. J Food Sci. 2007;72:282–288. doi: 10.1111/j.1750-3841.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Rubin LJ, Diosady LL, Wood DF. Effect of sulfanilamide on the TBA values of cured meats. J Food Sci. 1985;50:274–275. [Google Scholar]
- Shahidi F, Yang Z, Saleemi ZO, Omar S. Stabilization of mechanically deboned chicken meat lipids with ground mustard seed. J Food Lipids. 1993;1:89–96. [Google Scholar]
- Shen Q, Wang M, Tian J, Hu L, Ren S, Chen J, Ye X, Liu D. Effects of Chinese pickled and dried mustard on nutritional quality, sensory quality, and shelf life of steamed pork belly. Food Sci Nutr. 2018:1–10. doi: 10.1002/fsn3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarladgis BG, Watts BM, Younathan MT, Dugan L. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J Am Oil Chem Soc. 1960;37:44–48. [Google Scholar]
- Wanasundara. Saskatchewan Mustard Development Commission; Canada: 2008. Mustard as an ingredient in food processing: Current uses and the potential; pp. 3–5. [Google Scholar]
- Wu C. M.S. thesis. University of Manitoba; Canada: 2013. Use of completely and partially deodorized yellow and oriental mustards to control Escherichia coli O157:H7 in dry fermented sausage. [Google Scholar]
- Wójciak KM, Karwowska M, Dolatowski ZJ. Use of acid whey and mustard seed to replace nitrites during cooked sausage production. Meat Sci. 2014;96:750–756. doi: 10.1016/j.meatsci.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kong B, Xiong YL. Production of cured meat color in nitrite-free Harbin red sausage by Lactobacillus fermentum fermentation. Meat Sci. 2007;77:593–598. doi: 10.1016/j.meatsci.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wells JH, Mcmillin KW. Applications of dynamic modified atmosphere packaging systems for fresh red meats: Review 3. J Muscle Foods. 1994;5:299–328. [Google Scholar]


