Abstract
The highly refractory nature of cervical cancer to chemotherapeutic drugs and its epithelial-to-mesenchymal transition (EMT) are the key reasons contributing to the poor prognosis of this disease. Golgi Membrane Protein 1 (GOLM1), a protein involved in the trafficking of proteins through the Golgi apparatus, has been shown to be oncogenic in a variety of human cancers. Herein, we found GOLM1 was markedly up-regulated in cervical cancer and GOLM1 down-expression enhanced the anti-tumor effect of methotrexate. By performing mechanistic studies using both in vitro and in vivo models, we found that GOLM1 could target matrix metallopeptidase 13 (MMP13), a member of the MMPs, and regulate the EMT process. Moreover, altered EMT progression compromised the chemotherapy-enhancing effects of GOLM1 knock-down. Finally, we found significantly higher levels of GOLM1 and MMP13 in cervical cancer tissues compared with adjacent noncancerous tissues, and this was also associated with poor cervical cancer patients’ prognosis. Taken together, our results suggest that the GOLM1/MMP13/EMT axis is an important factor involved in regulating methotrexate in cervical cancer, and highlights the potential of novel GOLM1-based clinical modalities as a therapeutic approach in cervical cancer patients.
Keywords: GOLM1, cervical cancer, methotrexate, MMP13/EMT
Introduction
As one of the most common lethal malignancies, cervical cancer represents the main cause of cancer deaths worldwide [1]. Currently, methotrexate-based chemotherapy forms the first-line treatment for patients with cervical cancer, however, drug resistance, either intrinsic or acquired, compromises therapeutic efficacy and represents a significant challenge for the treatment of cervical cancer [2,3]. Although several characteristics such as, epithelial-to-mesenchymal transition (EMT) and the accumulation of cancer stem cells have been suggested as important contributors to cervical cancer chemo-resistance, the precise molecular mechanisms remain largely unknown [4]. EMT is a common feature of various types of tumors. During this process, cancer cells gradually lose expression of epithelial markers and instead, acquire the mesenchymal cell features required for further migration and invasion [5]. Interestingly, recent evidence also suggests that the EMT process is tightly correlated with drug resistance [6]. Nevertheless, exactly how the EMT process is regulated in cervical cancer is still not fully understood and elucidating the mechanisms involved could potentially provide clues for the development of novel cervical cancer therapies.
GOLM1 (Golm 1, NM_016548) is a resident cis-Golgi membrane protein of unknown function. GOLM1 has a single N-terminal transmembrane domain and an extensive C-terminal, coiled-coil domain that faces the luminal surface of the Golgi apparatus [7]. N-terminal cleavage by a furin proprotein convertase resulted in the release of the C-terminal ectomain and its appearance in serum [8]. Golgi has been shown to play an active role in cell migration through posttranslational modification and prominent changes in the Golgi apparatus, as evidenced by the disruption of biochemical composition, structure and functional levels observed in human carcinogenesis and metastasis [9]. Consistently, another Golgi-associated protein, GOLPH3 has recently been shown to act as an oncogene by linking cancer to Golgi and DNA damage signaling. The cleaved form of GOLM1 was detectable in the serum of patients with hepatocellular cancer, a finding that may have diagnostic value [10]. In addition, previous studies demonstrate that GOLM1 mRNA levels can serve as significant predictors of prostate cancer [11]. In prostate cancer, GOLM1 acts as a critical oncogene by promoting prostate cancer cell proliferation, migration and invasion, and inhibiting apoptosis, mainly through activating PI3K-AKT-mTOR signaling pathway [12].
Matrix metalloproteinases (MMPs) are members of zinc-dependent endopeptidases implicated in a variety of physiological and pathological processes [13]. Over the decades, MMPs have been studied for their role in cancer progression, migration, and metastasis. As a result, accumulated evidence of MMPs incriminating role has made them an attractive therapeutic target [14]. Overexpression of MMP13 was observed in esophageal squamous cell carcinoma (ESCC) clinical tissues, and the expression of MMP13 promoted cancer cell aggressiveness [15]. In addition, MMP13 was overexpressed in nasopharyngeal cancer (NPC) cells and exosomes purified from conditioned medium (CM) as well as NPC patients’ plasma and MMP13-containing exosomes in NPC progression which might offer unique insights for potential therapeutic strategies for NPC progressions [16]. High level of MMP13 protein expression is proved to be significant correlation with lymph node metastasis and tumor staging of oral squamous cell carcinoma (OSCC). Multivariate Cox regression model analysis revealed that high level of mRNA and protein expressions of MMP13 were significantly associated with poor prognosis of OSCC [17]. Taken together, these observations indicate that the MMP13 overexpression could be considered as a prognostic marker and potential target of cancer.
Herein, we identified common high-level expression of GOLM1 in cervical cancer clinical tissues and cell lines. Indeed, alteration of GOLM1 expression levels modulates the methotrexate sensitivity of cervical cancer cells. Upon further molecular analysis, we demonstrated that over-expressing of GOLM1 activated the EMT pathway through up-regulation of MMP13, eventually causing drug resistance. Moreover, high GOLM1 and high MMP13 expression was significantly associated with cervical cancer (compared with corresponding non-cancerous tissues), and a tight association with poor prognosis was also identified. Taken together, our data suggest that GOLM1 is a novel factor in the regulation of cervical cancer methotrexate sensitivity. Furthermore, our data provide new direction for the future development of potential molecularly targeted therapies in achieving improved therapeutic outcomes for cervical cancer patients.
Materials and methods
Cell culture and clinical specimens
Human cervical cancer cell lines (C-33A, SiHa, Ca-Ski, C-4-I) and H8, a human normal cervical surface epithelial cell line were obtained from the GuangZhou Jennio Biotech Co., Ltd (GuangZhou, Guangdong, China). All cell lines were cultured in RPMI-1640 (Gibco, Grant Island, NY, USA), which was supplemented with 10% fetal bovine serum in a humidified atmosphere of 5% CO2 at 37°C. Cervical cancer tissue microarray CR1101 (100 cervical cancer tissues and 10 normal cervical tissues) was obtained from Alenabio Company (Alenabio, Xian, China). This study was conducted on a total of 56 archived cervical cancer samples, which were histopathologically and clinically diagnosed at The Third Affiliated Hospital of Zhengzhou University from 2000 to 20010. For the use of these clinical materials for research purposes, prior patient consent and approval from the Institutional Research Ethics Committee were obtained. Clinical information on the samples is summarized in Supplementary Table 1. Cervical cancer tissues and the 23 matched adjacent non-cancerous tissues were frozen and stored in liquid nitrogen until further use. This study was approved by the Ethical Committee of The Third Affiliated Hospital of Zhengzhou University.
Cell transfection
Transfection of vectors, or short hairpin RNA (shRNA) was conducted with Lipofectamine 2000 (Invitrogen). The shRNA against MMP13 or NOB1 were designed and purchased from Genepharma Co., Ltd (Shanghai, China). Lentiviral vector encoding MMP13 or GOLM1 cDNA was constructed by Genepharma Co., Ltd (Shanghai, China) and designated as pLV-MMP13 and pLV-GOLM1. The empty vector was used as control (pLV-vector). SiHa and Ca-Ski cervical cancer cells were transfected with the recombinant vector or empty vector using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) at 60-70% confluence according to the manufacturer’s instructions.
Cloning efficiency, cell proliferation, cell viability and cell apoptosis assays
For evaluation of colony formation capacity, cervical cancer cells were plated in six-well plates at a density of 500 cells per well and then incubated in the plate for 2 weeks until colonies were visible. The cell colonies were fixed for 10 min with 100% methanol and stained with 0.1% crystal violet. For cell proliferation analysis, 5000 cervical cancer cells were plated on 96-well plates. After transfection, absorbance at 490 nm was measured every 24 h for 4 days using MTS reagent (Promega, Madison, WI, USA). For cell viability analysis, the transfected SiHa and Ca-Ski cervical cells were treated with methotrexate at concentrations of 0, 1, 10, 100 or 1000 nM and cultured in a 96-well plate for 72 h. The cell viability was measured by MTS assay as described previously. For cell apoptosis assays, the transfected SiHa and Ca-Ski cervical cancer cells were treated with methotrexate (10 nM) and cultured in six-well plates for 72 h. The cervical cancer cells were then stained with FITC-conjugated Annexin V (BD Biosciences, Heidelberg, Germany) and propidium iodide (5 mg/ml), and analyzed by fluorescence-activated cell sorting analysis.
Transwell and wound-healing assay
Cell migration was measured according to the ability of the cells to migrate across a Transwell filter (8-μm pores, Costar, Cambridge, MA, USA). Indicated cells suspended in serum-free 1640 were added to the upper chamber, and 1640 medium containing 10% fetal bovine serum was added to the lower chamber. After 24 h incubation at 37°C in a 5% CO2 humidified atmosphere, the non-invaded cells were scraped off of the filter using a cotton swab and the cells that migrated to the lower side of the upper chamber, were fixed with 4% paraformaldehyde and stained with crystal violet. The cells per microscopic field were taken pictures and counted in five randomly chosen fields. One day before scratch, cells were trypsinized and seeded equally into six-well tissue culture plates and grew to reach almost total confluence in 24 h. An artificial homogenous wound was created onto the monolayer with a sterile 10-ul tip. After scratching, the cells were washed with serum-free medium. Images of the cells migrating into the wound were captured at time points of 0 h and 24 h, all the experiments were repeated for at least 3 times.
qPCR analysis
Total RNAs were extracted from tissues or cells using TRI reagent (Sigma) or miRNeasy Mini Kit (Qiagen), and the cDNA’s were transcribed through Reverse Transcriptase M-MLV kit (Invitrogen) or Taqman microRNA Reverse Transcription kits (Thermo Fisher Scientific, Dreieich, Germany) according to the manufacturer’s instructions. qPCR was performed using the SYBR Premix Ex Taq (Takara, Shiga, Japan) or Taqman Gene Expression master mix (Thermo Fisher Scientific) in Applied Biosystems ViiATM 7 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Data were calculated with 2-ΔΔCT method and normalized to GAPDH mRNA level. The primers of genes were purchased from Sangon Biotech (Shanghai, China) and the sequences are listed in Supplementary Table 2.
Western blotting
Total proteins were extracted from cervical cancer cells using RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific). Protein concentrations were measured using a BCA Protein Assay Kit (Thermo Fisher Scientific). Standard western blotting techniques, and the Bio-Rad ChemiDoc MP imaging system (Hercules, CA, USA) were used according to the procedure described previously. The primary antibodies used were as follows: MMP13 (1:1000, sc30073, Santa Cruz, CA, USA), TWIST1 (1:1000, sc-6269, Santa Cruz, CA, USA), E-cadherin (1:1000, sc-71009, CST, Santa Cruz, CA, USA), N-cadherin (1:1000, sc-59987, CST, Santa Cruz, CA, USA), Vimentin (1:1000, sc-80975, Santa Cruz, CA, USA) and GAPDH (1:2000, sc-51631, Santa Cruz, CA, USA).
IHC and TUNEL assay
The IHC and TUNEL staining of FFPE tissues were performed as described previously. The primary antibodies used in IHC were as follows: MMP13 (1:200, sc-30073, Santa Cruz, CA, USA), TWIST1 (1:200, sc-6269, Santa Cruz, CA, USA), E-cadherin (1:200, sc-71009, CST, Santa Cruz, CA, USA), Vimentin (1:200, sc-71009, CST, Santa Cruz, CA, USA). Semi-quantitative scoring of IHC was based upon the staining intensity (I: negative, 0; weak, 1; moderate, 2; intense, 3) and the percentage of positive-staining cells (P: 0-5%, scored 0; 6-35%, scored 1; 36-70%, scored 2; and > 70%, scored 3) to obtain a final score (Q) defined as the product of I × P. Low expression was defined as Q < 4 and high expression with Q ≥ 4.
Bioinformatics
In protein-protein interaction (PPI) network construction, STRING 10.0 software (http://string-db.org/) is a web-based database for providing comprehensive interactions information for the already known or predicted proteins. The expression level of GOLM1 or MMP13 genes in the selected cancers was analyzed using Oncomine. For this, we compared clinical specimens of cancer vs. normal patient datasets. In order to reduce our false discovery rate, we selected P < 0.01 as a threshold. We analyzed the results for their p-values and fold change. The functional analysis of GOLM1 was performed using the WEB GESTALT tool (http://bioinfo.vanderbilt.edu/webgestalt) and DAVID tool (http://david.abcc.ncifcrf.gov). The GO option was used, and the significantly (P < 0.05) enriched biological processes and groups of genes possibly contributing to GOLM1-dependent gene expression regulation were identified. The KEGG (http://www.genome.jp/kegg) was used to identify pathways that were most significant to GOLM1. The prognostic value of the GOLM1 genes in cervical cancer was analyzed using SurvExpress (http://kmplot.com/analysis/), a database that integrates gene expression data and clinical data or GEPIA, a web server for cancer and normal gene expression profiling and interactive analyses. The two patient groups (higher and lower expression levels) were compared using a Kaplan-Meier survival plot [18,19].
Animal studies
The Animal Research Committee of The Third Affiliated Hospital of Zhengzhou University approved all experimental protocols and surgical procedures. BALB/c nude mice (SLARC Inc., Shanghai, China; 4-week-old; 15-20 g) were subcutaneously inoculated with 2 × 106 parental SiHa cells, or shCon transfected SiHa cells or shGOLM1 transfected cells, respectively. Seven days after inoculation, mice were subjected to intraperitoneal injection of either methotrexate (15 mg/kg) or saline (100 μl) weekly. Xenograft tumors were measured every week using external calipers and their volumes were calculated based on the equation: V = (length × width2)/2. Five weeks later, mice were killed and tumor weight was measured, followed by IHC and TUNEL staining.
Statistics
Data were presented as Mean ± SD. Group comparisons of normally distributed data were performed with unpaired Student’s t-test. SPSS 17.0 software (IBM, Chicago, IL, USA) was used for all statistical analysis. Values of P < 0.05 were considered statistically significant.
Results
Identification of over-expression GOLM1 levels in cervical cancer
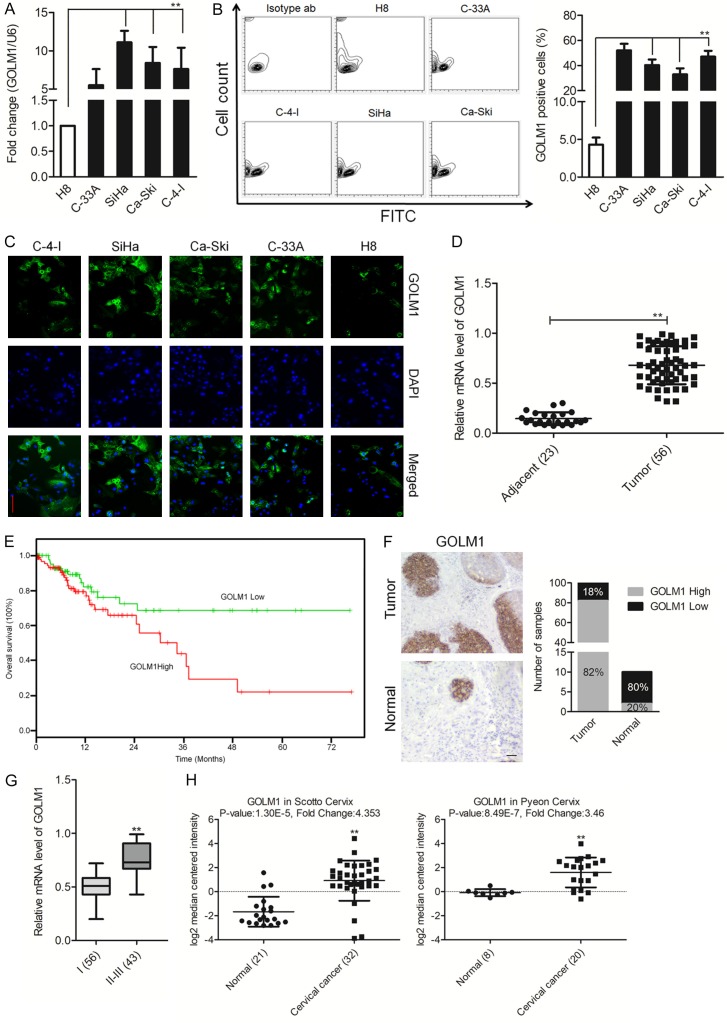
To investigate the roles of GOLM1 in cervical cancer initiation and progression, we firstly detected the expression level of GOLM1 in four human cervical cancer cell lines by qPCR. The result showed that the expression of GOLM1 in all four cervical cancer cell lines were significantly reduced at different degrees compared with H8, a human normal ovarian surface epithelial cell line (Figure 1A). Furthermore, FACS analysis after staining with anti-GOLM1 antibodies revealed the existence of distinct cell subpopulations expressing GOLM1 (Figure 1B), compatible with the existence of a fraction of the cells expressing GOLM1 on H8 cells. Consistently, the increased fluorescence intensity was detected in SiHa and Ca-Ski cervical cancer cells, whereas the H8 cells showed an inconspicuous signal (Figure 1C). Subsequently, the expression of GOLM1 in 56 pairs of clinical cervical cancer tissues was also evaluated by qPCR. As shown in Figure 1D, GOLM1 showed a higher expression in cervical cancer tissues compared with the adjacent non-neoplastic tissues. We further analyzed the clinical outcomes of patients to investigate the correlation between GOLM1 level and clinical characteristics. Statistical analysis revealed that up-regulation of GOLM1 was significantly associated with decreased overall survival of patients (Figure 1E). To further describe GOLM1 expression levels in cervical cancer, immunohistochemical staining of cervical cancer tissues arrays and paired non-neoplastic specimens with GOLM1 antibody was used for further analysis (CR1101). Consistently with our qPCR analysis, strong positive expression of GOLM1 was observed in cervical cancer whereas very weak positive expression of GOLM1 in adjacent normal tissue (Figure 1F). Quantitative analysis pointed out that the average level of GOLM1 in clinical stage II-III primary tumors was greatly higher than in stages of I (Figure 1G). Finally, we examined the expression patterns of GOLM1 in the publicly accessible Oncomine microarray database [20,21]. As shown in Figure 1H, GOLM1 expression was markedly up-regulation in cervical cancer tissues as compared with the matched normal tissues. These results indicated that the increased GOLM1 expression was a frequent event in cervical cancer cells and tissues, which may be involved in cervical cancer progression.
Figure 1.
Over-expression of GOLM1 in cervical cancer tissues and cell lines. A. qRT-PCR analysis of GOLM1 expression in H8 and cervical cancer cell lines. The fold changes of relative expression of GOLM1 versus that of H8 are represented in the vertical axis. Experiments were performed three times. B. Representative FACS dot plots of cells stained with anti-GOLM1 antibody (right) or with an isotype matched antibody (as a background control). Histograms reporting the percentage of GOLM1 cells as assessed by FACS. Mean ± SD of three independent experiments. **P < 0.01, compared with H8 cells. C. Cervical cancer cell lines were fixed and incubated with primary antibodies against GOLM1 and followed immunostained with anti-rabbit FITC-conjugated secondary antibody and then stained with DAPI. The specimens were visualized and photographed using a fluorescence microscope (Scale bar represents 100 μm). Blue depicts the nucleus and green depicts GOLM1. D. Comparison of GOLM1 abundance in 56 paired tumor and adjacent non-tumor tissues. The relative expression of GOLM1 normalized to the internal control GAPDH is shown. **P < 0.01, compared with normal. E. Kaplan-Meier analysis of overall survival of patients with cervical cancer stratified by the expression of GOLM1. F. Immunohistochemical staining of GOLM1 in cervical cancer tissues. G. The level of GOLM1 in different stages was determined by semi-quantitative analyses of IHC staining. **P < 0.01, compared with I. H. Box plots show decreased levels of GOLM1 in cervical cancer tissues compared with normal tissues in two microarray data sets. **P < 0.01, compared with normal tissues.
Reduced GOLM1 expression enhances cervical cancer cell MR through promoting the EMT process
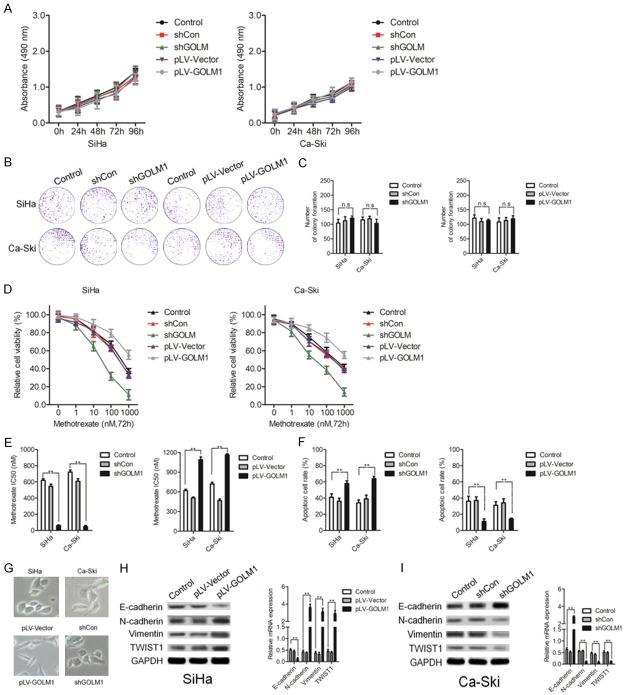
To further explore the biological role of GOLM1, shRNA targeting GOLM1 (shGOLM1) and GOLM1 over-expression vector were used in SiHa and Ca-Ski cervical cancer cells, respectively, for modulating GOLM1 expression. We found that neither increasing GOLM1 expression nor reducing GOLM1 expression influenced the proliferation rate of SiHa and Ca-Ski cervical cancer cells (Figure 2A). Similarly, colony-forming ability was assayed following modulation of GOLM1 levels in both SiHa and Ca-Ski cervical cancer cell lines, and also showed no obvious differences (Figure 2B, 2C). We then examined the effect of modulating GOLM1 levels on the methotrexate potency toward both SiHa and Ca-Ski cervical cancer cell lines. Various concentrations of methotrexate were used and cell viabilities were analyzed 72 h after treatment. Interestingly, down-expression of GOLM1 enhanced the cytotoxicity of methotrexate in SiHa and Ca-Ski cervical cancer cells with remarkably reduced IC50’s observed (Figure 2D, 2E). In contrast, up-regulation of GOLM1 expression levels conferred a higher degree of methotrexate in both SiHa and Ca-Ski cells, with obviously increased IC50 values (Figure 2D, 2E). Flow cytometry analysis of apoptotic cells with modified GOLM1 expression in SiHa and Ca-Ski cells further confirmed the influential role of GOLM1 on the potency of methotrexate treatment (Figure 2F). Intriguingly, SiHa cells with over-expression of GOLM1 levels showed an increase in the number of cells with elongated mesenchymal-like morphology and fewer cell-cell junctions, whereas Ca-Ski cells with epithelial-like morphology were elevated in cervical cancer cell cultures treated with the shGOLM1 (Figure 2G). The phenotypic conversion of epithelial cells to mesenchymal cells, named EMT, has been identified as a key process in the malignant transformation of multiple cancers. Concomitantly, the epithelial-related marker E-cadherin was reduced in SiHa cells transfected with GOLM1, proteins involved in the mesenchymal transition such as, N-cadherin and Vimentin were increased (Figure 2H). qPCR assay confirmed the reduced mRNA expression of E-cadherin and elevation of N-cadherin and Vimentin upon over-expression of GOLM1 levels (Figure 2H). Consistently, down-expression of GOLM1 in cervical cancer cells manifested the opposite effect, with increased epithelial markers and reduced mesenchymal markers (Figure 2I). In conclusion, our results suggest that increased GOLM1 sensitizes cervical cancer cells to methotrexate, and that this effect likely relies on reversing the EMT process.
Figure 2.
Altered GOLM1 expression in cervical cancer cell lines modifies sensitivity to methotrexate. A. MTS analysis of SiHa and Ca-Ski cells transfected with shGOLM1, or negative control (shCon), pLV-GOLM1, negative control (pLV-Vector). B, C. Representative images and statistical analysis of colony formation among SiHa and Ca-Ski cells transfected with shGOLM1 or pLV-GOLM1. D, E. MTS assay and the corresponding IC50 of methotrexate measured in SiHa and Ca-Ski cells transfected with shGOLM1 or pLV-GOLM1. F. Quantification of Annexin V/PI-positive apoptotic cells in SiHa and Ca-Ski cervical cancer cells transfected with shGOLM1 or pLV-GOLM1 after methotrexate treatment (10 nM) via flow cytometric analysis. G. Representative images of the morphological changes of SiHa cells transfected with pLV-GOLM1 or pLV-Vector and Ca-Ski cells with shGOLM1 or shCon. H. EMT-related marker expression in SiHa cells transfected with pLV-hGOLM1 or pLV-Vector were shown by western blotting and qPCR. I. EMT-related marker expression in Ca-Ski cells transfected with shGOLM1 or shCon shown by western blotting and qPCR. **P < 0.01, compared with control.
Identification of MMP13 as a target gene of GOLM1 in NSCLC
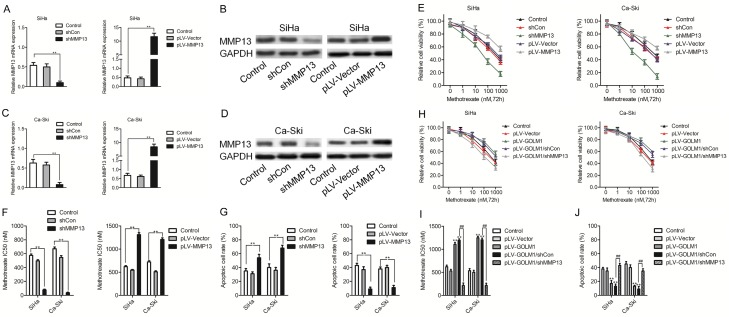
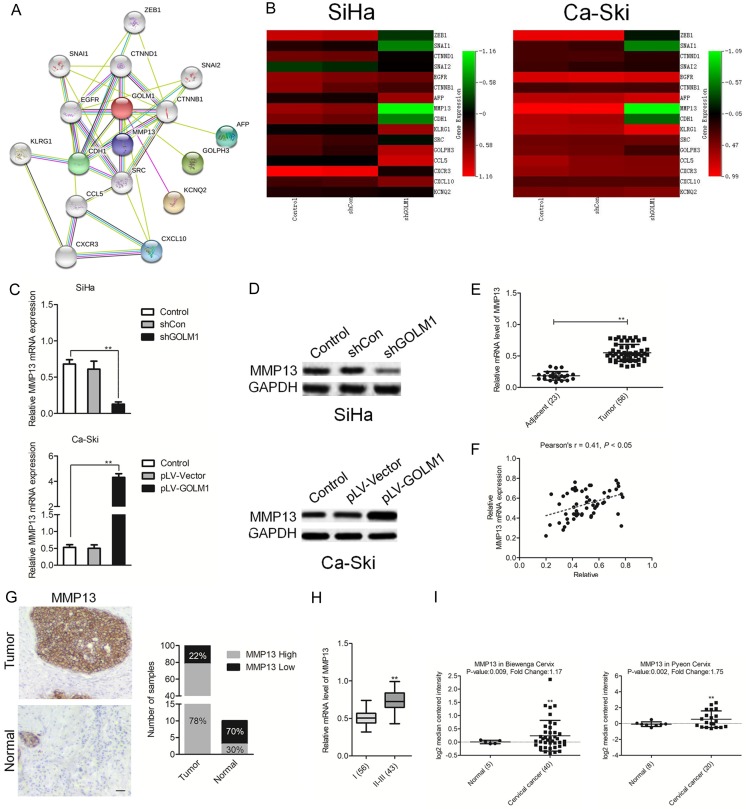
To better understand the molecular mechanism of GOLM1 in cancer metastasis, we mapped GOLM1 onto STRING database to build a PPI network. By using the ‘+ more proteins’ option, additional 16 predicted functional partners were allowed into the network. As shown in Figure 4A, MMP13 acted as a bridge to connect GOLM1. Combining qPCR analysis, we analyzed 16 potential candidate target genes for GOLM1 following decreased expression of GOLM1 in both SiHa and Ca-Ski cervical cancer cell lines. Among these, we found that MMP13 was the only gene showing the remarkably modulation in both SiHa and Ca-Ski cervical cancer cells, namely, reduced MMP13 with shGOLM1 treatment (Figure 3B). Consequently, we then assayed MMP13 protein levels upon modulation of GOLM1 expression. As expected, a positive relationship was identified between MMP13 and GOLM1 in both SiHa and Ca-Ski cervical cancer cell lines (Figure 3C and 3D). qPCR analysis also revealed elevated MMP13 expression in the 56 cervical cancer samples compared with 23 cases of normal tissues (Figure 3E). Importantly, positive relationships between GOLM1 and MMP13 were also detected in our 56 cervical cancer samples (Figure 3F). We future analyzed the expression of MMP13 in cervical cancer and corresponding human tissue samples. A clear increase in MMP13 protein expression was observed in cervical cancer arrays compared with the normal tissues using an immunohistochemistry (IHC) staining assay (Figure 3G). Quantitative analysis pointed out that the average level of MMP13 in clinical stage II-III primary tumors was greatly higher than in stages of I (Figure 1H). Consistent with our results, Oncomine databases also show significantly increased MMP13 in cervical cancer tissue samples (Figure 3I).
Figure 4.
GOLM1 sensitizes cervical cancer cells to methotrexate is MMP13-dependent. A-D. qPCR and western blotting assays confirming the overexpression or knock down of MMP13 in both SiHa and Ca-Ski cells. E, F. MTS assay and the methotrexate IC50 measurements in SiHa and Ca-Ski cells that transfected with the MMP13 shRNA or pLV-MMP13. G. The percentage of apoptotic cells in cervical cancer cells transfected with the MMP13 shRNA or pLV-MMP13 using flow cytometric analysis. H, I. Comparison of cell viability and the IC50 of methotrexate in SiHa and Ca-Ski cells with pLV-GLOM1 alone or pLV-GLOM1 combined with shMMP13 transfection. J. Apoptosis assays in SiHa and Ca-Ski cells with pLV-GLOM1 alone or pLV-GLOM1 combined with shMMP13 transfection. **P < 0.01, compared with control, ##P < 0.01, compared with pLV-GOLM1/shCon.
Figure 3.
GOLM1 directly targets MMP13 and regulates its expression. A. Protein interaction network of MMP13, CDH1, CXCL10, CCL5, CHEK2, CDH1 and TP53. The colored lines between the proteins indicate the various types of evidence demonstrating the interaction. B. A heat map of the expression changes of 15 candidate genes predicted to be regulated by GOLM1 in SiHa and Ca-Ski cells transfected with pLV-GOLM1 or shGOLM1. The scale from 0 to 4 marks the intensity of differential regulation of mRNAs: low expression (green), mid expression (yellow), and high expression (red). C. The expression of MMP13 in SiHa or Ca-Ski cells transfected with pLV-GOLM1 or shGOLM1 as examined by qPCR assays. **P < 0.01, compared with control. D. The expression of MMP13 in SiHa or Ca-Ski cells transfected with pLV-GOLM1 or shGOLM1 as examined by western blotting analysis. E. qPCR examination of MMP13 levels in 56 cervical cancer tissue and control normal tissues. F. The relationship of the expression between GOLM1 and MMP13 in 56 cases of cervical cancer tissues via qPCR assay. G. Representative images and semi-quantitative analyses of IHC staining for MMP13 in cervical cancer tissues and normal tissues. H. The level of MMP13 in different stages was determined by semi-quantitative analyses of IHC staining. **P < 0.01, compared with I-II. I. Box plots show increased levels of MMP13 in cervical cancer compared with normal breast tissues in two microarray data sets. **P < 0.01, compared with normal tissues.
GOLM1 reduces MMP13 expression and results in increased cervical cancer cell methotrexate sensitivity
In order to confirm the possible involvement of MMP13 in regulating chemotherapeutic efficacy, we first modified the expression levels of MMP13 in SiHa and Ca-Ski cervical cancer cell lines using an MMP13 overexpression vector or shRNA targeting MMP13, respectively. qPCR and western blotting analysis confirmed MMP13 overexpression and knock down (Figure 4A-D). Indeed, elevated MMP13 expression in both SiHa and Ca-Ski cervical cancer cell lines increased their viability and decreased the potency of methotrexate, whereas reduced MMP13 manifested the opposite effects (Figure 4E and 4F). Apoptotic cells analyzed by flow cytometry analysis also confirmed the weakened cytotoxic effect of methotrexate against MMP13-overexpressing cells and enhanced cell killing effect in MMP13 low-expressing cells (Figure 4G). To establish whether MMP13 is involved in mediating the chemo-modifying effect of GOLM1, we then overexpressed GOLM1 and shMMP13 simultaneously in both SiHa and Ca-Ski cervical cancer cell lines. Our data above suggested that single overexpression of GOLM1 could decreased the methotrexate sensitivity, as shown by induced IC50’s and decreased numbers of apoptotic cells. This effect, however, when analyzed in cells co-expressing GOLM1 and shMMP13 was largely weakened (Figure 4H-J). Our results therefore suggest that MMP13 may be a strong candidate target gene of GOLM1 with a significant influence on the regulation of chemotherapeutic efficacy.
GOLM1/MMP13 targets the EMT pathway to modulate chemotherapeutic efficacy
Increasing evidence suggests that EMT transformed cells contribute significantly to chemo-resistance through mechanisms such as, reduced cell proliferation, apoptotic resistance and increased numbers of cancer stem cells. We found that samples from 35 cervical cancer tissues with higher levels of GOLM1 were often associated with higher levels of MMP13 and Vimentin (mesenchymal marker) and lower levels of E-cadherin (epithelial marker) expression (Figure 5A). Statistical analysis further confirmed this positive relationship between GOLM1 and mesenchymal markers as well as MMP13 (Figure 5B-E). MMP13, a member of the MMP family, is an important regulator of cell adhesion and migration. Cells undergoing EMT require a series of morphological and molecular changes and MMP members can promote EMT progression through directly increasing the invasive potential of cancer cells. The involvement of MMP13 in cancer development has been reported, but whether it is also implicated in regulating EMT has never been investigated. To address this question, we then examined EMT-related phenotypes in both SiHa and Ca-Ski cervical cancer cell lines with modified MMP13 expression. Enforced MMP13 expression manifested increased SiHa cells with mesenchymal morphology (Figure 5F). Moreover, qPCR assay confirmed reduced epithelial marker (E-cadherin) and increased mesenchymal markers (N-cadherin and Vimentin) accompanied with MMP13 overexpression (Figure 5G). In contrast, an epithelial-like morphology was induced in MMP13-deficient Ca-Ski cervical cancer cells (Figure 5F) and correspondingly, increased epithelial marker (E-cadherin) and reduced mesenchymal markers (N-cadherin and Vimentin) were also detected (Figure 5H). Further western blotting analysis of EMT-related markers confirmed the ability of MMP13 to induce a mesenchymal phenotype and inhibit the epithelial phenotype in both SiHa and Ca-Ski cervical cancer cells (Figure 5I). Finally, we examined whether GOLM1 modulated the EMT process through regulation of MMP13. Indeed, MMP13 down-expression faithfully counteracted the EMT-reversing effect of GOLM1 (Figure 5J). Furthermore, the ability of down-regulation of GOLM1 to regulate the EMT process was abolished in MMP13-over-expxressing cells (Figure 5K). In conclusion, our results show that GOLM1 has a modulatory role on the MMP13/EMT axis, which in turn directly influences the sensitivity of cervical cancer cells to chemotherapy.
Figure 5.
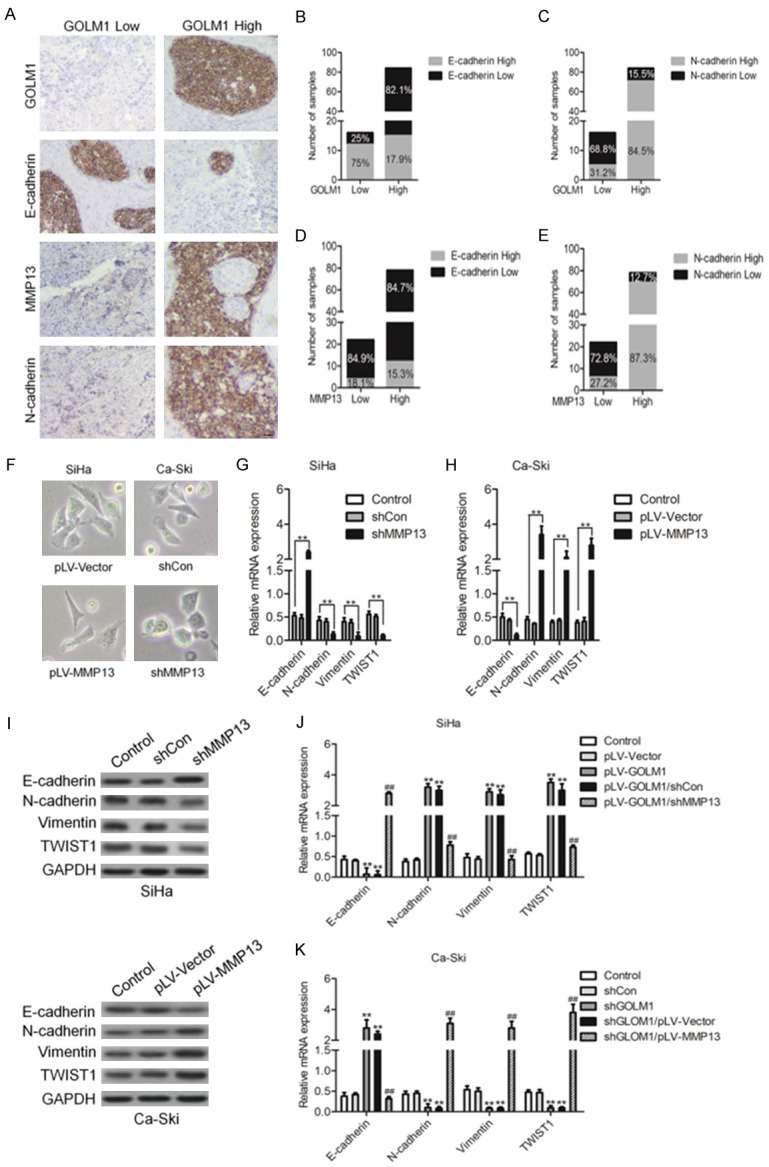
GOLM1 targeting of MMP13 modulates the EMT process in cervical cancer. A. Representative images showing the associations between GOLM1 expression, MMP13 and EMT markers (Vimentin and E-cadherin) in cervical cancer samples. B-E. The percentage scoring of EMT marker (Vimentin and E-cadherin) expression in either GOLM1 low or high expression groups, and in either MMP13 low or high expression groups from cervical cancer samples. F. Morphological changes resulting from MMP13 overexpression in SiHa cells and MMP13-deficient Ca-Ski cells. G-I. Examination of EMT marker (E-cadherin, N-cadherin, Vimentin and TWIST1) expression in MMP13-overexpressing SiHa cells and MMP13 low expressing Ca-Ski cells by qPCR and western blotting assay. J. Comparison of EMT marker (E-cadherin, N-cadherin, Vimentin and TWIST1) expression between GOLM1 single overexpressing and GOLM1/shMMP13 SiHa cells. K. Comparison of EMT marker (E-cadherin, N-cadherin, Vimentin and TWIST1) expression between GOLM1 single down-expressing and shGOLM1/pLV-MMP13 Ca-SKi cells.
Knock down of GOLM1 enhances the chemotherapeutic effect of methotrexate in nude mouse xenograft models
In order to explore the influence of GOLM1 knocked-down on the efficacy of chemotherapy in vivo, we performed studies using mouse xenograft models. In mice, SiHa tumors transfected with shGOLM1 grew at the same rate as those transfected with control plasmids (Figure 6A-C). However, when animal groups were treated with methotrexate, tumors down-expressing GOLM1 grew at a significantly slower rate, with lower tumor volumes and weight, compared with the control group treated with methotrexate (Figure 6A-C). IHC staining of shGOLM1 tumor sections confirmed reduced MMP13 level (Figure 6D, 6E). Moreover, transferase dUTP nick end-labeling (TUNEL) analysis of apoptotic cell numbers further confirmed the improved antitumor effect of methotrexate in tumors with GOLM1 down-expression (Figure 6F). Collectively, our xenograft studies provide further evidence that GOLM1 down-expression enhances the chemotherapeutic effect of methotrexate, which likely relies on its role in counteracting the MMP13-mediated EMT process.
Figure 6.
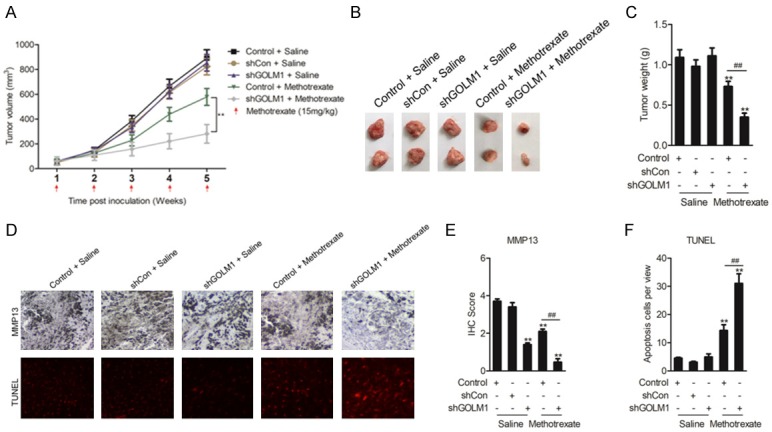
GOLM1 modulates the methotrexate sensitivity of cervical cancer cells in vivo. A. Tumor growth curves of SiHa cells transfected with shCon or shGOLM1 and treated with methotrexate or saline. B. Representative images showing the tumors formed in the five groups, including Control + saline, shCon + saline, shGOLM1 + saline, Control + Methotrexate and shGOLM1 + Methotrexate at the 5th week after subcutaneous transplantation or when mice were killed. C. Comparison of the mean tumor weights of the five groups. D. Representative IHC images for MMP13 or TUNEL staining among the five tumor xenografts groups. E, F. Statistical comparisons of MMP13 expression and apoptotic cell numbers among the five tumor xenografts groups. **P < 0.01, compared with Control + saline, ##P < 0.01, compared with Control + Methotrexate.
GOLM1 associates with prognostic factor in cervical cancer and EMT of cervical cancer cells
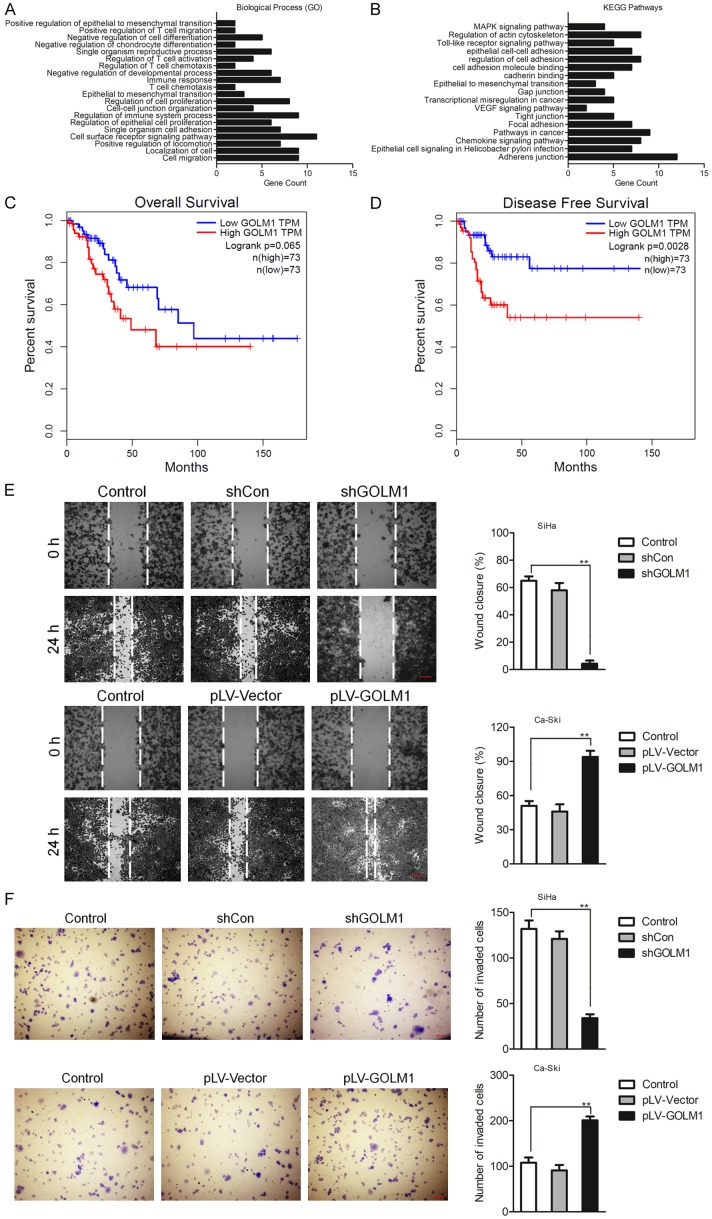
The Gene Ontology (GO) functional annotation showed that GOLM1 were related to different biological processes involved in invasive phenotype in tumor cells (Figure 7A). Furthermore, the analysis in the KEGG pathway database showed that the GOLM1-affected proteins were part of pathways involved in peripheral cell plasticity during migration and invasion processes, such as ‘epithelial cell-cell adhesion’ and ‘Epithelial to mesenchymal transition’ (Figure 7B). We further evaluated the prognostic value of low or high expression of GOLM1 mRNA in a large online clinical microarray database of patients with cervical cancer (GEPIA). Elevated expression of GOLM1 mRNA in tumor was associated with poor disease-free survival (DFS) whereas was not associated with poor overall survival (OS) (Figure 7C, 7D). Mesenchymal cells are associated with malignant properties, such as migration and invasion. To future dissect the roles of GOLM1 in EMT, GOLM1 was either overexpressed in SiHa cells, or depleted in Ca-Ski cells, and the role of GOLM1 on the migration potential of these cells was investigated using wound healing and Transwell assays. As shown in Figure 7E, 7F, the cell migration and invasion potential was significantly elevated in GOLM1-overexpressing SiHa cells, while dramatically decreased in GOLM1-depleted Ca-Ski cells. Taken together, these data demonstrate that GOLM1 promotes cell EMT in cervical cancer cells via up-regulation of MMP13 (Figure 8).
Figure 7.
GOLM1 is a prognostic factor in cervical cancer and GOLM1-regulated pathway analysis. A. Pathway analysis of GOLM1, using Gene Ontology (GO) functional annotation of Web Gestalt bioinformatics database. B. KEGG analysis. Pathways analysis of GOLM1, using KEGG functional annotation of Web Gestalt bioinformatics database. C. Kaplan-Meier analysis of overall survival of cervical cancer patients stratified by the expression of GOLM1. D. Kaplan-Meier analysis of disease-free survival of cervical cancer patients stratified by the expression of GOLM1. E. The migration of cells transfected with shGOLM1 was examined by wound healing assay. F. Transwell assay was performed to detect the invasion of GOLM1 over-expressing Ca-Ski cells. **P < 0.01, compared with control.
Figure 8.
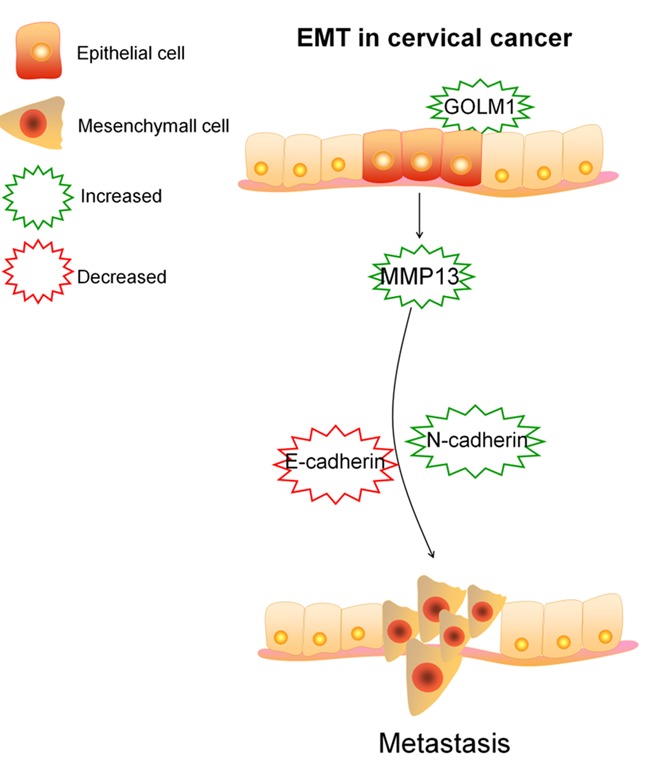
Scheme depicting mechanisms of GOLM1 promotes cell EMT in cervical cancer cells via up-regulating MMP13.
Discussion
The highly malignant nature of cervical cancer, together with its aggressiveness and chemo-resistance all contribute to the poor prognosis of cervical cancer patients [22,23]. Accordingly, a better understanding of the molecular mechanisms underlying these aspects of the disease is urgently needed to enable design of targeted therapies to improve the treatment of cervical cancer. In this study, we identified GOLM1 as being expressed at significantly high levels in cervical cancer cells. Through further mechanistic investigations using both in vitro and in vivo models, we found that GOLM1 targeted the MMP13 gene to regulate EMT progression, which in turn contributed to cervical cancer chemo-resistance. Tight correlations were identified among GOLM1, MMP13 and EMT markers in both cervical cancer patients’ samples and mouse tumor xenograft models. Finally, but importantly, we also found that the expression of GOLM1 and MMP13 correlated poor with cervical cancer patients’ prognosis. Collectively, our study provides new clues for the design of future drugs to enhance the sensitivity of cervical cancer through targeting of the GOLM1/MMP13/EMT axis.
In our study, we found reduced expression of GOLM1 in both cervical cancer cell lines and cervical cancer tissues. Moreover, increased levels of GOLM1 also correlated with poor cervical cancer patient prognosis. However, exactly how GOLM1 regulates the biological behavior of cervical cancer is still not known. Altering the GOLM1 levels within cervical cancer cells in vitro revealed no obvious effects on proliferation, but specifically influenced their sensitivity to methotrexate. Importantly, we observed an interesting phenomenon in that cervical cancer cells with increased GOLM1 expression levels acquired a mesenchymal-like phenotype including, elongated fibroblastoid shape, high expression of Vimentin and N-cadherin, and low expression of E-cadherin. In contrast, cervical cancer cells with decreased GOLM1 expression showed induction of epithelial marker expression. Increasing evidence has confirmed the critical importance of EMT not only in cancer progression, but also for resistance to chemotherapeutic drugs [24]. It has been shown that mesenchymal-type cancer cells with increased expression of genes related to the processes of invasion and metastasis often show resistance to drug treatment [25]. Epithelial cells on the other hand, show less invasive and metastatic potential and are often more sensitive to chemotherapies [26]. The regulatory role of GOLM1 on the EMT process could potentially be performed via several routes, such as direct targeting of EMT transcription factors or components of cell architecture required for EMT progression.
Through our profiling work, we identified the GOLM1 downstream candidate target gene, MMP13, and showed direct regulation by GOLM1. Importantly, we showed significant elevations in MMP13 expression in cervical cancer tissues of our own samples and also of those within the Oncomine databases [27]. Elevated MMP13 expression in cervical cancer cell lines decreased their resistance to methotrexate cytotoxicity and led to EMTs. The MMP13 protein belongs to the subfamily of MMPs, which are important regulators of many fundamental cellular processes such as epithelial adhesion, cell polarity, cell migration and membrane trafficking [28]. The involvement of MMP13 in several types of cancers has also been reported [29]. Our study provides new data indicating that MMP13-modulated EMT is involved in counteracting drug treatment in cervical cancer cells. EMT has long been well known for its role in inducing tumor metastasis. Lately, it has been recognized that malignant tumor properties such as metastasis, immune evasion and chemo-resistance are tightly correlated and can in fact influence each other. Ongoing studies in our lab are focused on exploring whether GOLM1 may also be involved in regulating cervical cancer cells’ invasive and metastatic abilities. In conclusion, our studies identify GOLM1, as a key modulator of cervical cancer chemo-sensitivity. This effect likely relies on its role in repression of the MMP13-mediated EMT process. These results provide new areas of research for developing modalities to enhance chemotherapeutic effects in cervical cancer.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Wang P, Zhang L, Zhang J, Xu G. MicroRNA-124-3p inhibits cell growth and metastasis in cervical cancer by targeting IGF2BP1. Exp Ther Med. 2018;15:1385–1393. doi: 10.3892/etm.2017.5528. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Matsuo K, Shimada M, Saito T, Takehara K, Tokunaga H, Watanabe Y, Todo Y, Morishige KI, Mikami M, Sugiyama T. Risk stratification models for para-aortic lymph node metastasis and recurrence in stage IB-IIB cervical cancer. J Gynecol Oncol. 2018;29:e11. doi: 10.3802/jgo.2018.29.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hata M, Koike I, Miyagi E, Asai-Sato M, Kaizu H, Mukai Y, Takano S, Ito E, Sugiura M, Inoue T. Radiation therapy for patients with bone metastasis from uterine cervical cancer: its role and optimal radiation regimen for palliative care. Anticancer Res. 2018;38:1033–1040. doi: 10.21873/anticanres.12319. [DOI] [PubMed] [Google Scholar]
- 4.Xu D, Liu S, Zhang L, Song L. MiR-211 inhibits invasion and epithelial-to-mesenchymal transition (EMT) of cervical cancer cells via targeting MUC4. Biochem Biophys Res Commun. 2017;485:556–562. doi: 10.1016/j.bbrc.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Y, Sun F, Dong P, Watari H, Yue J, Yu MF, Lan CY, Wang Y, Ma ZB. iASPP induces EMT and cisplatin resistance in human cervical cancer through miR-20a-FBXL5/BTG3 signaling. J Exp Clin Cancer Res. 2017;36:48. doi: 10.1186/s13046-017-0520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha GH, Kim JL, Breuer EK. TACC3 is essential for EGF-mediated EMT in cervical cancer. PLoS One. 2013;8:e70353. doi: 10.1371/journal.pone.0070353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donizy P, Kaczorowski M, Biecek P, Halon A, Szkudlarek T, Matkowski R. Golgi-related proteins GOLPH2 (GP73/GOLM1) and GOLPH3 (GOPP1/MIDAS) in cutaneous melanoma: patterns of expression and prognostic significance. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong Y, Long Q, Xie H, Zhang T, Peng T. Cloning and characterization of human Golgi phosphoprotein 2 gene (GOLPH2/GP73/GOLM1) promoter. Biochem Biophys Res Commun. 2012;421:713–720. doi: 10.1016/j.bbrc.2012.04.067. [DOI] [PubMed] [Google Scholar]
- 9.Hu L, Li L, Xie H, Gu Y, Peng T. The Golgi localization of GOLPH2 (GP73/GOLM1) is determined by the transmembrane and cytoplamic sequences. PLoS One. 2011;6:e28207. doi: 10.1371/journal.pone.0028207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen MH, Jan YH, Chang PM, Chuang YJ, Yeh YC, Lei HJ, Hsiao M, Huang SF, Huang CY, Chau GY. Expression of GOLM1 correlates with prognosis in human hepatocellular carcinoma. Ann Surg Oncol. 2013;20(Suppl 3):S616–624. doi: 10.1245/s10434-013-3101-8. [DOI] [PubMed] [Google Scholar]
- 11.Varambally S, Laxman B, Mehra R, Cao Q, Dhanasekaran SM, Tomlins SA, Granger J, Vellaichamy A, Sreekumar A, Yu J, Gu W, Shen R, Ghosh D, Wright LM, Kladney RD, Kuefer R, Rubin MA, Fimmel CJ, Chinnaiyan AM. Golgi protein GOLM1 is a tissue and urine biomarker of prostate cancer. Neoplasia. 2008;10:1285–1294. doi: 10.1593/neo.08922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan G, Ru Y, Wu K, Yan F, Wang Q, Wang J, Pan T, Zhang M, Han H, Li X, Zou L. GOLM1 promotes prostate cancer progression through activating PI3K-AKT-mTOR signaling. Prostate. 2018;78:166–177. doi: 10.1002/pros.23461. [DOI] [PubMed] [Google Scholar]
- 13.Cao C, Xu N, Zheng X, Zhang W, Lai T, Deng Z, Huang X. Elevated expression of MMP-2 and TIMP-2 cooperatively correlates with risk of lung cancer. Oncotarget. 2017;8:80560–80567. doi: 10.18632/oncotarget.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao XY, Lang XP. Correlation between MMP-7 and bFGF Expressions in non-small cell lung cancer tissue and clinicopathologic features. Cell Biochem Biophys. 2015;73:427–432. doi: 10.1007/s12013-015-0656-y. [DOI] [PubMed] [Google Scholar]
- 15.Zhang DH, Zhang LY, Liu DJ, Yang F, Zhao JZ. Expression and significance of MMP-9 and MDM2 in the oncogenesis of lung cancer in rats. Asian Pac J Trop Med. 2014;7:585–588. doi: 10.1016/S1995-7645(14)60099-7. [DOI] [PubMed] [Google Scholar]
- 16.You Y, Shan Y, Chen J, Yue H, You B, Shi S, Li X, Cao X. Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci. 2015;106:1669–1677. doi: 10.1111/cas.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent-Chong VK, Salahshourifar I, Karen-Ng LP, Siow MY, Kallarakkal TG, Ramanathan A, Yang YH, Khor GH, Rahman ZA, Ismail SM, Prepageran N, Mustafa WM, Abraham MT, Tay KK, Cheong SC, Zain RB. Overexpression of MMP13 is associated with clinical outcomes and poor prognosis in oral squamous cell carcinoma. ScientificWorldJournal. 2014;2014:897523. doi: 10.1155/2014/897523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang F, He Z, Lei H, Chen Y, Lu Z, Zeng G, Wang H. Identification of differentially expressed genes and biological pathways in bladder cancer. Mol Med Rep. 2018;17:6425–6434. doi: 10.3892/mmr.2018.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang B, Li C, Zhao J. Identification of key pathways and genes in colorectal cancer using bioinformatics analysis. Med Oncol. 2016;33:111. doi: 10.1007/s12032-016-0829-6. [DOI] [PubMed] [Google Scholar]
- 20.Pyeon D, Newton MA, Lambert PF, den Boon JA, Sengupta S, Marsit CJ, Woodworth CD, Connor JP, Haugen TH, Smith EM, Kelsey KT, Turek LP, Ahlquist P. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res. 2007;67:4605–4619. doi: 10.1158/0008-5472.CAN-06-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scotto L, Narayan G, Nandula SV, Arias-Pulido H, Subramaniyam S, Schneider A, Kaufmann AM, Wright JD, Pothuri B, Mansukhani M, Murty VV. Identification of copy number gain and overexpressed genes on chromosome arm 20q by an integrative genomic approach in cervical cancer: potential role in progression. Genes Chromosomes Cancer. 2008;47:755–765. doi: 10.1002/gcc.20577. [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Liu Z, Shi F, Wang J. Pin1 modulates chemo-resistance by up-regulating FoxM1 and the involvements of Wnt/betacatenin signaling pathway in cervical cancer. Mol Cell Biochem. 2016;413:179–187. doi: 10.1007/s11010-015-2651-4. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y, Zhou J, Li Y, Ye F, Wan X, Lu W, Xie X, Cheng X. miR-375 mediated acquired chemo-resistance in cervical cancer by facilitating EMT. PLoS One. 2014;9:e109299. doi: 10.1371/journal.pone.0109299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toge M, Yokoyama S, Kato S, Sakurai H, Senda K, Doki Y, Hayakawa Y, Yoshimura N, Saiki I. Critical contribution of MCL-1 in EMT-associated chemo-resistance in A549 non-small cell lung cancer. Int J Oncol. 2015;46:1844–1848. doi: 10.3892/ijo.2015.2861. [DOI] [PubMed] [Google Scholar]
- 25.Guo C, Ma J, Deng G, Qu Y, Yin L, Li Y, Han Y, Cai C, Shen H, Zeng S. ZEB1 promotes oxaliplatin resistance through the induction of epithelial - mesenchymal transition in colon cancer cells. J Cancer. 2017;8:3555–3566. doi: 10.7150/jca.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Wang H, Lian Y, Chen L, Gu L, Wang J, Huang Y, Deng M, Gao Z, Huang Y. GTSE1 promotes cell migration and invasion by regulating EMT in hepatocellular carcinoma and is associated with poor prognosis. Sci Rep. 2017;7:5129. doi: 10.1038/s41598-017-05311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortega CE, Seidner Y, Dominguez I. Mining CK2 in cancer. PLoS One. 2014;9:e115609. doi: 10.1371/journal.pone.0115609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Jin G, Jin H, Wang N, Luo Q, Zhang Y, Gao D, Jiang K, Gu D, Shen Q, Huo X, Hu F, Ge T, Zhao F, Chu W, Shu H, Yao M, Cong W, Qin W. Clusterin facilitates metastasis by EIF3I/Akt/MMP13 signaling in hepatocellular carcinoma. Oncotarget. 2015;6:2903–2916. doi: 10.18632/oncotarget.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou B, Zhao J, Guan S, Feng H, Wangpu X, Zhu C, Zong Y, Ma J, Sun J, Shen X, Zheng M, Lu A. CCR4 promotes metastasis via ERK/NF-kappaB/MMP13 pathway and acts downstream of TNF-alpha in colorectal cancer. Oncotarget. 2016;7:47637–47649. doi: 10.18632/oncotarget.10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.