Abstract
As a Chinese herb medicine (CHM), Bo-Er-Ning capsule (BENC) has been approved in China for adjuvant treatment of cancer, but the particular therapeutic effect of BENC on gastric cancer (GC) has yet to be evaluated. In this study, we implemented an efficacy-driven approach by directly starting the study with a randomized clinical trial to assess the add-on therapeutic effect of BENC on advanced GC patients. Our results showed that the addition of BENC to chemotherapy resulted in higher Karnofsky performance scores and better 3-year overall survival, compared to those treated with the conventional chemotherapy regimen. Subsequently, we explored the mechanism of BENC action on GC cells in the assistance of BATMAN-TCM, the first online bioinformatics analysis tool designed especially for the mechanism study of CHM, by which we identified 263 candidate protein targets of BENC involved in GC treatment. The further enrichment analysis suggested that BENC treatment affected a diversity of biological processes of GC cells, such as cell proliferation, cell cycle and apoptosis, which were further validated in the following in vitro and in vivo assays, indicating such a bioinformatics-assisted approach was feasible and powerful to CHM mechanism study. Thus, as exemplified by BENC, we provided an efficacy-driven and bioinformatics-assisted strategy for CHM research, which may help promote the discovery and application of novel CHM drugs on patients with refractory diseases.
Keywords: Bo-Er-Ning capsule, gastric cancer, Chinese herb medicine, efficacy-driven approach
Introduction
Gastric cancer (GC) is the fifth most common cancer and the second most frequent cancer-related death worldwide. More than 900,000 new cases of GC are diagnosed every year and over 70% of new cases occur in developing countries, accounting for half of the caseloads of the world [1,2]. Unfortunately, approximately two thirds of GC patients are diagnosed at advanced stages, resulting in disappointing outcomes. The median survival time of GC patients is only about one year, with an 5-year overall survival rate ranging 5-20%, primarily due to a high recurrence or metastasis rate [3]. Current standard interventions for GC have not changed for the last decades. Thus, it is an urgent need to develop novel pharmaceuticals to improve the outcomes of GC patients.
So far, over two thirds of chemo-drugs are derived from natural products since launching chemotherapy for cancer treatment over half a century ago [4]. Chinese herb medicine (CHM) is characterized by the application of a blend of several natural sourced ingredients to treat illnesses based on patients’ symptoms. As a key component of traditional Chinese medicine, CHM has a long history and distinguished clinical effect on various diseases, including cancer [5]. Nowadays, CHM research mainly focuses on identifying the active substances and investigating mechanisms of action, under the influence of western biomedical research mode. However, though tremendous efforts have been made, this mechanism-centered approach has led to little progress in developing new CHM formula/drugs in the last decades. Thus, to facilitate the development and clinical application of novel CHM formula/drugs, an efficacy-driven approach for CHM research has recently been proposed [6,7]. Efficacy-driven approach starts the research with a randomized clinical trial to directly evaluate the CHM efficacy in humans, and conducts the mechanism studies in vitro or in animals after the clinical efficacy has been validated. Ethically, efficacy-driven approach is acceptable as some folk CHM recipes have been used in China as well as in other countries for many years, and new CHM drugs will continue to be invented and directly applied to patients in places where traditional Chinese medicine is officially recognized [6,8].
Chinese medicine characterizes cancer as blood stasis, heat toxins, knotted accumulation and formation of swellings and lumps [9]. Bo-Er-Ning capsule (BENC), a patent CHM formula approved by the State Food and Drug Administration (SFDA) of China (Z20054459), is used for the adjuvant treatment of cancer due to its essences of heat-clearing and detoxifying as well as its functions in activating blood flow to dissipate blood stasis. BENC is composed of a series of key pharmaceutical ingredients, including Radix Astragali Seu Hedysari Praeparata, Ligustrum Lucidum, Tulipa Edulis, Portulaca Oleracea, Rhizoma Paridis, Solanum Nigrum, Fructus Perillae, Gallus Gallus Domesticus, Rheum Officinale, Dryobalanops Aromatica and Bombyx Batryticatus (See Supplementary Table 1 for details). Previous study has revealed that BENC exhibited desirable pharmacological effects on preventing recurrence of bladder cancer [10]. Moreover, when applied with HEP regimen (hydroxycamptothecine, etoposide, cisplatin), BENC showed a synergistic anti-tumor effect on advanced non-small cell lung cancer [11]. However, the therapeutic effect of BENC on GC has not been evaluated.
In this study, we started with a randomized clinical trial and confirmed the add-on therapeutic efficacy of BENC on advanced GC patients. Next, in the assistance of bioinformatic analysis tools, we conducted a virtual study to explore the mechanism of BENC action on GC cells. Finally and importantly, we further validated the predicted cellular functional and related signaling impacts of BENC on GC cells using in vitro and in vivo assays. Thus, taking advantage of the efficacy-driven approach and recent advent in bioinformatics, we provided an efficacy-driven and bioinformatics-assisted strategy for CHM research to facilitate the discovery and application of novel CHM formula/drugs on cancer patients.
Material and methods
Patients and clinical data
From June 2008 to June 2012, 112 patients with advanced GC treated by FP regimen (Tegafur + Cisplatin) in Zhangqiu People’s Hospital of Shandong were enrolled for this clinical study by the 3th author and randomly divided into two groups by the 4th author. Among those, 58 patients received BENC accompanying FP regimen (trial group), while 54 patients received FP regimen alone (control group). The general clinical data of two groups were in Table 1. The eligibility criteria were: (1) age from 25 to 75 years; (2) all patients were pathologically confirmed for stage IV or postoperative recurrence and metastasis GC; (3) Karnofsky performance score (KPS) ≥ 80; (4) no previous anticancer therapy; (5) no dysfunction of heart, liver, lung and kidney. All study procedures were approved by the Ethical Committee of Zhangqiu People’s Hospital of Shandong. All patients signed the informed consents, and the study conformed to the ethical principles set forth by the Declaration of Helsinki.
Table 1.
General data of two groups (cases)
| Category | Characteristics | Control group | Trial group |
|---|---|---|---|
| Gender | Male | 24 | 36 |
| Female | 30 | 22 | |
| Histological Type (WHO) | Moderate or poorly differentiated and undifferentiated adenocarcinoma | 26 | 30 |
| Mucinous adenocarcinoma | 6 | 4 | |
| Tubular adenocarcinoma | 6 | 4 | |
| Signet-ring cell carcinoma | 4 | 6 | |
| Well differentiated adenocarcinoma | 12 | 14 | |
| Median Age/Year | ~ | 58.5 | 56.6 |
Treatment regimen for control group: Tegafur 40~60 mg, oral administration, two times a day, from day 1 to day 14 and Cisplatin 40 mg, intravenous infusion administration, from day 1 to day 3. Treatment regimen for trial group: BENC 4 capsules, take orally three times a day (eating with warm water half an hour after a meal) for 14 days (from day 1 to day 14), accompanying Tegafur and Cisplatin treatment (the same drug dosage and usage as the control group). Both groups were evaluated after 3 cycles (both groups take 21 days as one cycle) of chemotherapy (14 days of treatment and 7 days of rest). After 3 cycles of chemotherapy, all patients were observed for appetite, body weight, and Karnofsky performance score (KPS).
Patients were followed up by clinic visit, phone, or mail at least once 6 months starting from day 1 after the first hospitalization. Study endpoints took the overall survival (OS) as the standard. The survival status was obtained from medical records or by direct follow up via telephone or mail. Due to the serious loss of the follow-up visit, the number of control group was 20, and the trial group was 23. Death was defined as GC related death.
Bioinformatics analysis
The potential protein targets for BENC were predicted using the online BATMAN-TCM server [12] and the known GC related targets were obtained from Gene Expression Omnibus (GEO) database (See Supplementary Table 2 for details). We chose four gene expression chips (GSE33429; GSE63089; GSE13195; GSE33335) derived from human GC and adjacent normal tissue.
We first constructed an interaction network for predicted putative drug targets of BENC and the known GC related targets based on the data obtained from the Cytoscape plugin Bisogenet. The network was visualized using Cytoscape (Version 3.2.1) [13]. Next, the topology parameters of each node in the overlap network were calculated using a Cytoscape plugin CytoNCA. The node with a score twice bigger than the median of “Degree centrality” (DC) is considered important and appeared in the network. We finally performed enrichment analysis of the potentially significant targets using ClueGO, a Cytoscape plugin that visualizes non-redundant biological terms for large clusters of genes in a functionally grouped interaction network [14]. These candidates were divided into two types, GO biological process and KEGG signaling pathways.
Cell culture and reagents
Human GC SGC-7901 and BGC-823 cells were obtained from the China Infrastructure of Cell Line Resources (School of Basic Medicine Peking Union Medical College, China) and were maintained in RPMI-1640 medium supplemented with 10% (v/v) FBS and 100 U/ml streptomycin/penicillin in the humidified atmosphere of 5% CO2 at 37°C. RPMI-1640 medium and fetal bovine serum (FBS) were obtained from GIBCO (USA). Annexin-V FITC apoptosis detection kit (#556547) and FITC BrdU Flow kit-PartA (#559619) were purchased from BD Biosciences Pharmingen (USA). Bcl-2 rabbit polyclonal antibody, Bax rabbit polyclonal antibody, Caspase 9/p35/p10 rabbit polyclonal antibody, E-cadherin rabbit polyclonal antibody, Vimentin rabbit polyclonal antibody and Beta-actin mouse monoclonal antibody were bought from Proteintech. BENC were obtained from Shenzhen Jian An Pharmaceutical Limited.
Cellular functional assays
The cell proliferation was assessed by the CCK-8 assay, in which 10 μl of CCK-8 was added to each well, and the cells were incubated for an additional two hours before the absorbance value was measured at a wavelength of 450 nm using an automated microplate reader. Cell invasion and migration assays were performed in Transwell chambers, with or without matrigel on the upper chamber respectively. Cell colony formation assay was carried out and cell colonies were counted after stained with 0.1% crystal violet. Apoptosis detection, cell cycle and Western blot analysis were performed according to the manufacturer’s instructions.
Xenograft mouse work
SGC-7901 cells (2×105/ml) treated with BENC (200 μg mL-1) were inoculated subcutaneously into the left and right flank of 8 NOD/SCID mice using 1-ml needles. Two weeks later, the tumor volumes were measured once every two or three days using the following formula: long diameter × (short diameter)2/2. On day 16, mice were sacrificed and the tumor tissues were weighed. None of mice died during the experiments.
Immunohistochemistry
The slides of tumor tissue sections were disposed of deparaffinization and antigen unmasking, and were then incubated with the antibody against Ki-67 (Abcam, UK) at 4°C overnight. After washing with PBS, the slides were incubated with Polymer Helper and Poly peroxidase-anti-mouse/rabbit IgG (PV-9000, ORIGENE, China), followed by further incubation with diaminobenzidine (DAB). The tissue sections were mounted after counterstained with hematoxylin.
Statistical analysis
The results were analyzed using SPSS17.0.software (USA). Date were represented as the mean ± standard deviation. The differences expressed were using the Student’s t-test.
Results
BENC add-on therapy improved the outcomes of advanced GC patients
We firstly compared the add-on effect of BENC on the quality of life (QOL) between the trial and control groups, as manifested by appetite, body weight as well as Karnofsky Performance Scores (KPS). As showed in Table 2, the appetite was greatly improved in the trial group (77.6%) compared to the control group (11.1%), which was in line with the body weight gaining rate in the trial group (60.3%) verse the control group (9.2%). The differences between two groups were statistically significant (P<0.01).
Table 2.
Changes in appetite and body weight of patients’ in two groups (cases)
| Group | Appetite | Body weight | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Increase | Decrease | Unchange | Effective rate/% | Increase | Decrease | Unchange | Effective rate/% | |
| Trial Group | 45 | 3 | 10 | 77.6 | 35 | 10 | 13 | 60.3 |
| Control Group | 6 | 30 | 18 | 11.1 | 5 | 25 | 24 | 9.2 |
Since the KPS has been widely recognized as a reliable predictor of survival in patients with various cancers [15,16], we next assessed the KPS for both groups. Our result showed a significant increase of KPS in the trial group versus the control group (65.5% vs. 22.2%, Table 3). Finally, we used Kaplan-Meier analysis to evaluate the add-on therapy effect of BENC on advanced GC patients, and the result showed that the 3-year overall survival of the trial group (23.57 months) was greatly improved compared to the control group (18.75 months, Figure 1). The difference between two groups was statistically significant (P<0.05). Together, these results indicated that the adjuvant therapy of BENC significantly improved the QOL and 3-year overall survival of the GC patients in the trial group.
Table 3.
Karnofsky score in two groups (cases)
| Group | Cases | Scores Decrease | Scores Unchange | Add 10 Points | Add 20 Points | Effective rate/% |
|---|---|---|---|---|---|---|
| Trial Group | 58 | 8 | 12 | 26 | 12 | 65.5 |
| Control Group | 54 | 23 | 19 | 11 | 1 | 22.2 |
Figure 1.
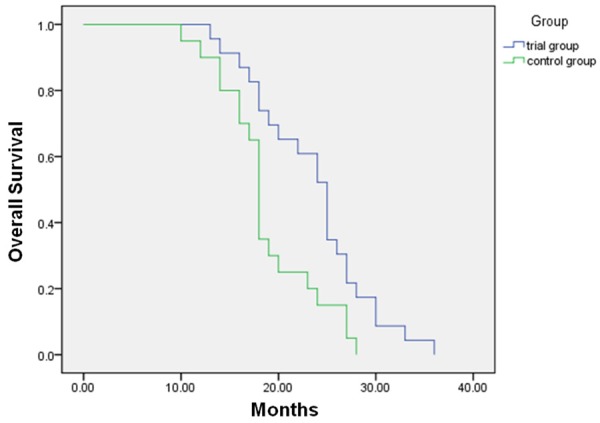
Kaplan-Meier analysis showed significantly improved overall survival of GC patients by add-on therapy with BENC (P=0.01).
BENC inhibited GC cells growth and decreased GC cells viability
It is well-known that CHM exhibits its maximum strength in treating various diseases in a manner of multi-mode of action [15]. Accordingly, the add-on therapeutic efficacy of BENC on GC patients could be attributed to multiple factors, such as modulation on patient’s immune system, synergistic effect with the chemotherapy or direct inhibitory effect on GC cell growth and invasion. Thus, we next determined the role of BENC in GC cell growth and viability.
The CCK-8 assay results showed that the growth of GC cells (SGC-7901 and BGC-823 cell lines) were significantly inhibited following BENC treatment in a time and dose-dependent manner (Figure 2A). Meanwhile, the viability of these cells were determined by the cell colony formation assay, showing the cell clonality of these cells were also decreased in a dose-dependent manner following BENC treatment for 24 hours (Figure 2B). These results demonstrated a direct suppressive role of BENC in GC cell growth and viability.
Figure 2.
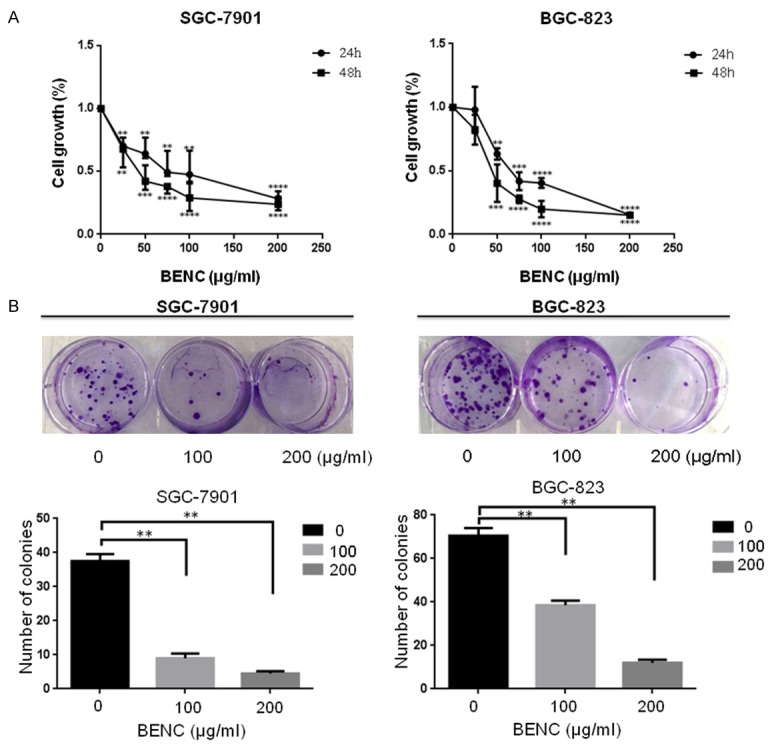
BENC inhibited GC cells growth and decreased GC cells viability. A. CCK-8 assay was performed to measure cell growth rate at 24 h and 48 h after BENC treatment at different dosages. B. Cell colony formation assay was performed to measure cell clonality after BENC treatment for 24 h. **P<0.01 based on the Student t-test.
Identification and enrichment analysis of candidate targets for BENC against GC
Having confirmed the clinical therapeutic efficacy of BENC on GC patients was attributed to its direct inhibitory effects on GC cells, we next conducted an virtual study to explored the underlying mechanism involved in the anti-tumor activity of BENC on GC cells by using the online BATMAN-TCM server [12] and Gene Expression Omnibus (GEO) database.
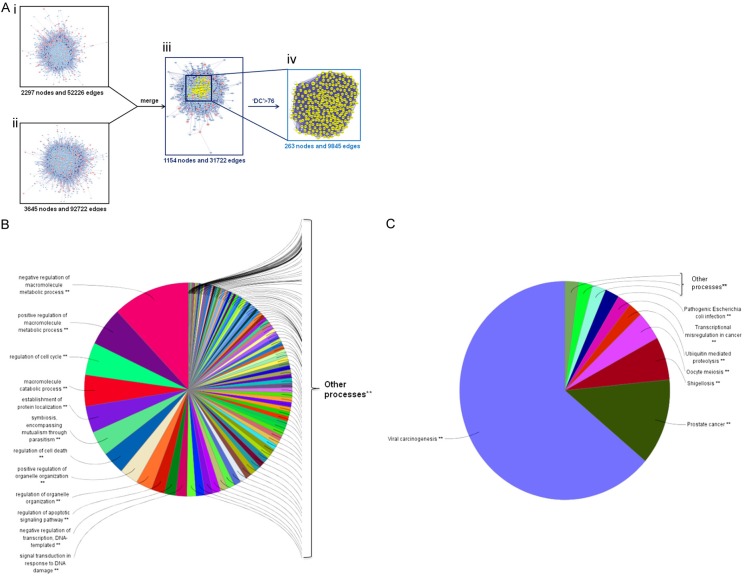
Since the protein-protein interaction (PPI) networks are relevant to visualize the role of various key proteins in cancer, we constructed a putative target network for BENC, containing 2297 nodes and 52226 edges, and a known GC related target network, containing 3645 nodes and 92722 edges, using the Bisogenet, a plugin for Cytoscape (3.2.1). Furthermore, to clarify the pharmacological mechanisms of BENC against GC, we intersected the two networks and thus obtained 1154 nodes and 31722 edges. Then we calculated the topology parameters of each node in the overlap network by using a Cytoscape plugin CytoNCA. Referring to a previous method [16], we identified nodes with degrees that were bigger than twice the median degree of whole nodes as the significant targets. As thus, we constructed a significant target network that contained 263 nodes and 9845 edges (Figure 3A).
Figure 3.
In silico identification and enrichment analysis of candidate targets for BENC against GC. A. (i) The interactive PPI network of BENC putative targets was made of 2297 nodes and 52226 edges. (ii) The interactive PPI network of GC related protein targets was composed of 3645 nodes and 92722 edges. (iii) The interactive PPI network of BENC putative targets and known GC related targets made of 1154 nodes and 31722 edges was shown. (iv) PPI network of significant proteins extracted from iii, in which 263 nodes and 9845 edges were included. B. Candidate significant targets were enriched in the representative biological processes (P<0.05). C. Candidate significant targets were enriched in the representative signalling pathways (P<0.05).
Finally, we performed an enrichment analysis of the identified 263 candidate targets for BENC against GC cells using ClueGO, a Cytoscape plugin [14], and divided these candidates into GO biological process and KEGG signaling pathway, respectively. In this analysis, a wide range of biological processes were disturbed in response to BENC treatment, such as cell growth, cell cycle, cell death and apoptosis (Figure 3B). Meanwhile, the impaired signaling pathways included viral carcinogenesis, transcriptional misregulation in cancer and so on (Figure 3C). Thus, we reasonably speculated that the potential mechanism of BENC acting against GC cells was related to cell proliferation, cell cycle and apoptosis.
BENC promoted apoptosis and arrested GC cells in G2/M phase
To validate the above in silico analysis result, we carried out a serial of cellular functional assays, such as apoptosis assay and cell cycle analysis, by using different GC cell lines. Flow cytometry analysis showed that the apoptotic population that positively stained with annexin V-FITC was significantly increased upon BENC treatment in a dose-dependent manner (Figure 4A and 4B). Consistently, immunoblotting results showed that BENC promoted accumulation of the pro-apoptosis proteins Bax and cleaved Caspase-9, whereas the level of anti-apoptosis protein Bcl-2 was down-regulated by BENC in a dose-dependent manner (Figure 4C). Furthermore, to determine whether BENC induces the disturbance of cell cycle progression, the BrdU-incorporated cell cycle analysis was performed in GC cells. The results showed that BENC treatment could dramatically decrease the proliferation rate of these cells, evidenced by reduced proportion of G0/G1 and S phase, while arresting the cells at G2/M phase (Figure 4D and 4E). Consistently, immunoblotting results showed that BENC promoted accumulation of the G2/M phase-specific marker Cyclin B1, while down-regulating the levels of Cyclin D1 and Cyclin E in both SGC-7901 and BGC-823 cells (Figure 4F). Together, these data indicated that BENC treatment induced apoptosis, inhibition of proliferation and G2/M arrest in GC cells.
Figure 4.
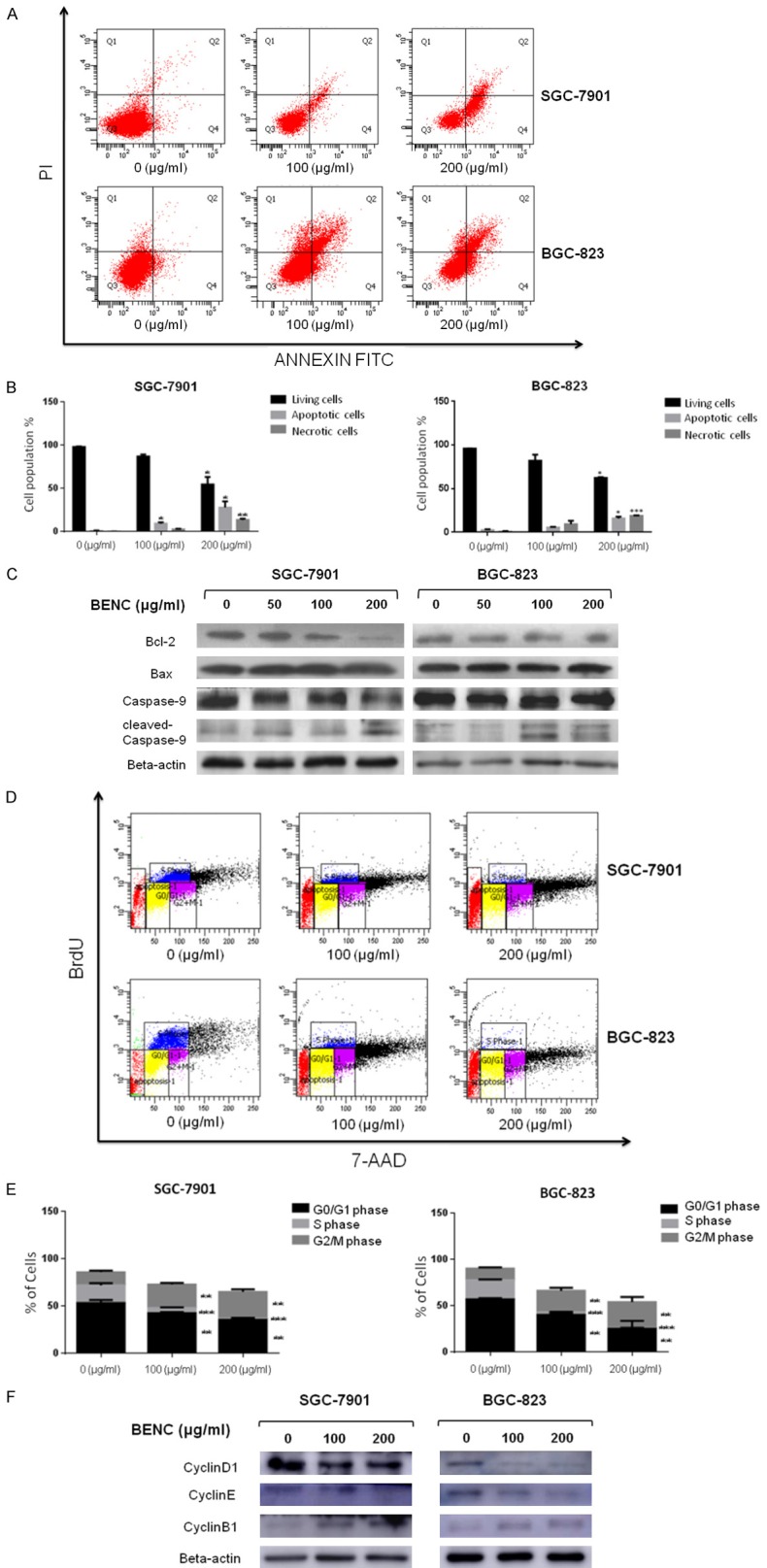
BENC treatment resulted in apoptosis and disturbance of cell cycle progression in GC Cells. A. Induction of apoptosis of SGC-7901 and BGC-823 cells after BENC treatment. Both cell lines were treated with BENC at different concentrations (0, 100, 200 μg/ml) for 24 h when apoptotic events was assessed by flow cytometry. B. Statistical analysis of the percentages of the apoptotic cells. C. SGC-7901 and BGC-823 cells were treated with BENC at different concentrations (0, 50, 100, 200 μg/ml) for 24 h. After proteins were extracted, the levels of Bcl-2, Bax, pro-caspase-9, cleaved-caspase-9 were analyzed by Western Blot. D. Cell cycle analysis of GC cells following BENC treatment (0, 100, 200 μg/ml) for 36 h by flow cytometry. E. Statistical analysis of the proportions of the cells at different phases. **P<0.01 based on the Student t-test. F. SGC-7901 and BGC-823 cells were treated with BENC at different concentrations (0, 100, 200 μg/ml) for 36 h. After proteins were extracted, the levels of Cyclin D1, Cyclin E, Cyclin B1 were analyzed by Western Blot.
BENC suppressed migration and invasion of GC cells
Since the epithelial-mesenchymal transition (EMT) plays an important role in the course of tumor progression, we next determine the effect of BENC on EMT in GC cells. The trans-well assay results revealed that both cell migration and cell invasion were inhibited by BENC in a dose-dependent manner (Figure 5A and 5B). This cellular phenotype might be associated with the inhibitory effect of BENC on EMT process, evidenced by the expression patterns of EMT-related proteins in these cells in response to BENC treatment at different dosages (Figure 5C).
Figure 5.
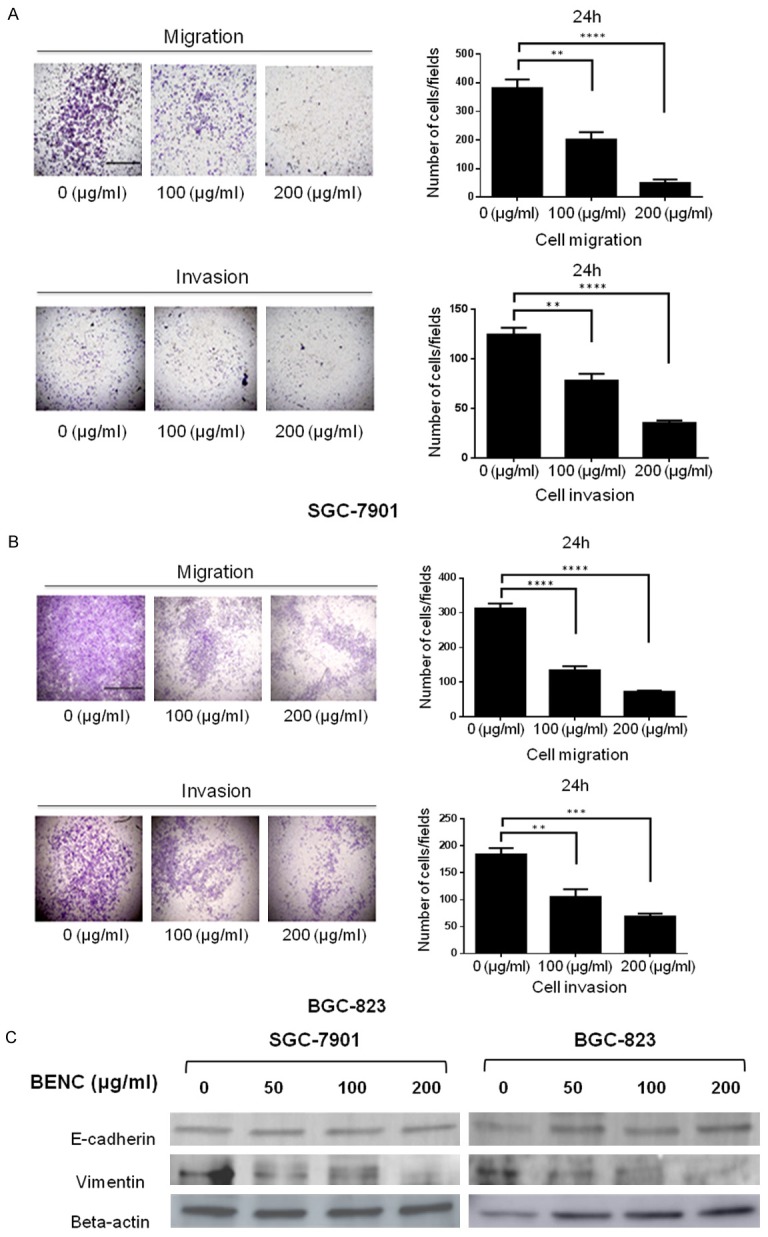
BENC suppressed migration and invasion of SGC-7901 and BGC-823 cells. A, B. Transwell assay was performed to measure cell migration and invasion at 24 h after BENC treatment. C. SGC-7901 and BGC-823 cells were treated with BENC (0, 50, 100, 200 μg/ml) for 24 h. Proteins were extracted, then E-cadherin and Vimentin levels were analyzed by Western Blot. **P<0.01 based on the Student t-test.
Anti-tumor efficacy of BENC in vivo
To investigate the direct tumor suppressive role of BENC in vivo, we further determined the effect of BENC on tumors xenografted onto immunodeficient mice. As shown in Figure 6A, the development of tumors were greatly suppressed by BENC treatment on these mice. By day 16, the tumor volume of BENC-treated group were approximately 7-fold smaller than the control group (P<0.05), in line with the tumor weights, which showed a striking difference between two groups (Figure 6B-D). The subsequent immunohistochemistry analysis revealed that the number of Ki-67 positive cells was significantly decreased in BENC-treated tumors, compared to those in control tumors, indicating an anti-proliferative effect of BENC on these tumors (Figure 6E and 6F). Thus, these data provided convincing evidence showing that BENC possesses direct anti-tumor activity in vivo.
Figure 6.
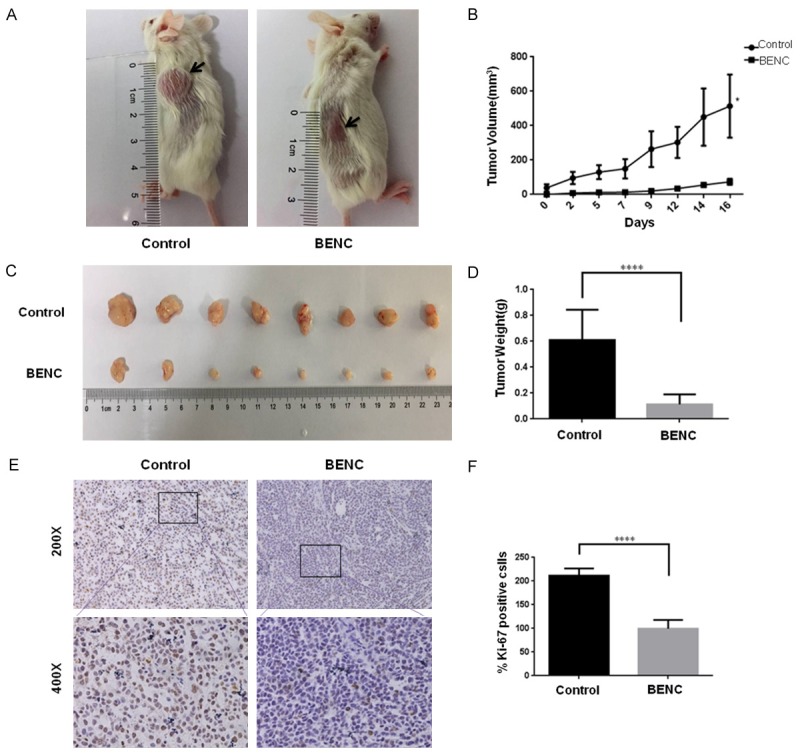
BENC suppressed development of the tumors on xenografted mice. A. The tumor masses inoculated on NOD/SCID mice were carefully checked every another day with naked eyes. B. The tumor volumes were measured and calculated once every two or three days. C, D. The tumors were resected and weighted on day 16. E. Immunohistochemistry staining for Ki-67 was performed by using the tumor slides from control and BENC-treated groups. F. Statistical analysis of the positive ratio of Ki-67 staining. *P<0.05 based on the Student t-test.
Discussion
As an important resource for innovative drug discovery, CHM research has attracted great attention for many years [16,17]. More and more new compounds with various therapeutic activities have been identified from CHM in recent years [18]. In the view of exploring novel drugs, an efficacy study is always beneficial no matter whether the result is positive or not, because if a drug is proved effective, its immediate application in clinical will benefit patients, whereas if the drug is not effective, stopping using it will save resource by avoiding unnecessary basic research. But from a patient’s point of view, it is apparently not the best choice to try a new drug before its efficacy has been fully determined, even if the drug is a CHM that has been applied in clinical practice for a long time. Especially, for the patients who are suffering from serious diseases such as cancer, it is unethical to put them on a new drug to take the risk of failure in efficacy trial, as thus they may miss the optimal treatment window, resulting in serious consequences. Actually, surveys have found that most cancer patients were inclined to take the CHM on the basis of conventional therapy [19]. Therefore, to evaluate the efficacy of BENC on GC, we conducted a clinical trial in advanced GC patients using BENC combined with FP regimen rather than in lieu of FP regimen. Application of this add-on therapy in the efficacy-driven approach took advantage of the efficacy study to promote the clinical use of BENC, but also precluding the ethical problems caused by the failure of the clinical trial. In this study, BENC add-on therapy significantly improved the outcomes of advanced GC patients, evidenced by higher Karnofsky scores and better 3-year overall survival. Importantly, the following cellular functional assays demonstrated that BENC exhibited its anti-tumor effect by directly suppressing the growth and decreasing the viability of GC cells. Thus, having validated the add-on therapeutic efficacy of BENC on GC cells, the clinical trial designed for GC patients treated with BENC combined with lower-dosed chemotherapy or the trial for patients treated with BENC alone warrants further investigation.
In an efficacy-driven approach, mechanism study is still important, though it is undertaken after the efficacy has been confirmed. Identification of the potential targets is a critical step to reveal the underlying mechanisms of CHM actions at the molecular level. However, due to the complexity of the interaction between diverse ingredients of CHM and the networks of various diseases, it remains to be challenging to find the CHM targets by conventional methods [20]. Nowadays, with the advance in computational methods, many informatics methods such as bioinformatics and chemoinformatics have been proposed to apply on the target prediction for CHM [21-23]. Several online databases for herbal ingredients and a number of compound-target interaction prediction methods have been recently developed [24-30]. Among these, BATMAN-TCM is the first online bioinformatics analysis tool designed especially for the mechanism study of TCM, which supports the comprehensive target prediction for all components of CHM and subsequent network pharmacology analyses [12].
Using BATMAN-TCM server and GEO database, we constructed and analyzed BENC and GC-related protein targets network, and finally identified 263 candidate protein targets for BENC involved in GC treatment. The further enrichment analysis using ClueGO plugin predicted that BENC inhibited GC cells growth by affecting the biological processes such as cell cycle and apoptosis. Indeed, the subsequent flow cytometry analysis of BrdU incorporation and Annexin-V staining assays showed a G2/M arrest and significant increased apoptotic events upon BENC treatment. Furthermore, the trans-well assays revealed that BENC could suppress cell migration and invasion, associated with its inhibitory effect on EMT process. The anti-tumor activity of BENC was further determined in immunodeficient mice, in which the growth of xenografted tumors was significantly suppressed by BENC treatment. Thus, using in vitro and in vivo assays, we validated the anti-tumor effects of BENC on GC cells according to the target prediction results by BATMAN-TCM, indicating such a bioinformatics-guided approach was feasible and powerful to CHM mechanism study.
The signaling pathway enrichment was conducted using KEGG PATHWAY. Notably, the viral carcinogenesis related pathways accounted for 2/3 of the total hits. It has been well documented that many signaling pathways are involved in viral carcinogenesis. For instance, the NF-κB signaling pathway plays important roles in HBV-associated hepatocellular carcinoma (HCC) and HPV-associated cervical carcinoma [31,32]. As a key marker for NF-κB signaling pathway, VCAM1 has been identified as a candidate protein target for BENC in this study. Mutant VCAM1 could confer growth and infiltration capacity to HCC cells and down-regulation of VCAM1 inhibits cancer cell adhesion to endothelial cells and EMT process [31,33]. Suppression of VCAM1 activity has been reported as a potential therapeutic strategy for GC [34]. Moreover, a recent study showed that silencing of IKBKG, another candidate protein target for BENC, promoted tumor growth and invasion of breast cancer through the dys-regulation of NF-κB/STAT3 signaling pathways [35]. Thus, the therapeutic effects of BENC on GC cells are attributed to the disturbance of the viral carcinogenesis related pathways, such as NF-κB/STAT3 signaling pathway.
CHM has been recognized for its holistic therapeutic effect on various diseases by combined usage of multiple herbs containing a diversity of active compounds. It shows distinguished medical advantages and efficacy in the treatment of complex diseases such as cancer, owing to its special nature characteristics of multi-components, multi-targets and multi-modes of action [9]. In the present study, our results demonstrated the add-on benefits of TCM in improving outcomes of GC patients, and revealed that BENC suppressed cell growth, invasion and decreased the viability of GC cells through inductions of apoptosis, G2/M arrest and MET process, respectively, displaying a promising potential for its multi-levels, multi-targets and coordinated intervention effects on GC cells. BENC contains a diversity of key active compounds from multiple herbs, such as emodin and rhein from Rheum Officinale, polyphyllin I (PPI) from Rhizoma Paridis, colchicine from Tulipa Edulis and astragaloside IV (AS-IV) from Radix Astragali Seu Hedysari Praeparata. Recent studies have shown that emodin and rhein could induce apoptosis in nasopharyngeal carcinoma cells and glioma cells via targeting the chloride channels and ERK signaling pathway, respectively [36,37], whereas PPI could suppress human osteosarcoma cell growth via inactivation of Wnt/β-catenin pathway to arrest the cells at G2/M phase [38]. In human colon cancer cells, colchicine induced apoptosis via activation of p38 signaling pathway [39], while AS-IV inhibited EMT process by suppressing CREB1 signaling pathway [40]. Therefore, maintaining the diversity of active compounds plays an essential role to exhibit the strength of CHM in treating various cancers, and may help overcome the major challenges encountered in conventional chemotherapy, such as the drug-resistant phenotype.
On the other hand, to bring the BENC into line with modern medicine and better benefit GC patients, it is necessary to further carry out virtual and experimental assays for identification of the bioactive lead components, thereby trimming or withdrawing the redundant and deleterious components/herbs from BENC. At present, many chemoinformatics methods based on molecular docking, pharmacophore and quantitative structure activity relationships have been used for the bioactive leads identification against the drug targets [41,42]. Thus, our next step is to perform a virtual screening of bioactive components from BENC, based on the 263 candidate targets we have identified in this study, and followed by experimental validation.
Conclusion
To facilitate the application of BENC on GC patients, we conducted a randomized clinical trial, in which we demonstrated that the add-on therapy with BENC resulted in better QOL, higher KPS and improved 3-year overall survival in advanced GC patients. The subsequent bioinformatics-assisted mechanism study revealed that BENC disturbed GC cell growth through affecting cell proliferation, cell cycle, apoptosis and EMT process. In perspective, the integration with cell-based biotechnologies and computational technologies will initiate the next revolution of CHM research. Here, we provided a proof-of-concept evidence showing how to use the efficacy-driven and bioinformatics-assisted strategy to benefit the advancement of CHM (Figure 7). In this connection, we speculate that the future development of CHM will be emphasized on accelerating its clinical application so as to benefit the mankind at larger extents.
Figure 7.
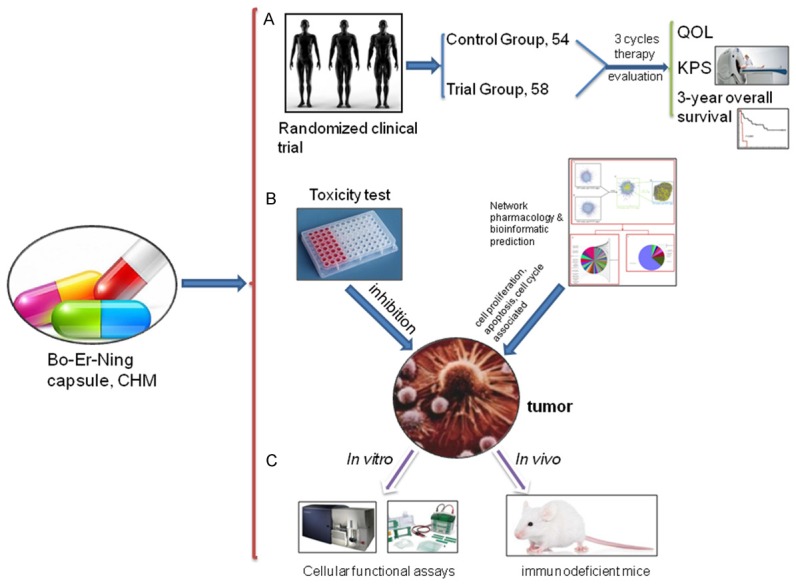
The overall schematic design of the study. A. Starting the study with a randomized clinical trial to assess the add-on therapeutic effect of BENC on GC patients. B. An virtual study was conducted to explore the mechanism of BENC action on GC cells in the assistance of bioinformatic analysis tools. C. Further validation of the predicted cellular functional and related signaling impacts of BENC on GC cells using in vitro and in vivo assays.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 81572416), the Tianjin Natural Science Funds (No. 15JCYBJC54400), the National Key Technologies R&D Program of China (No. 2016YFC1303200), and the Tianjin Medical University Cancer Institute & Hospital Cancer Translational Medicine Seed Funds (No. 1701-1).
Disclosure of conflict of interest
None.
Abbreviations
- CHM
Chinese herb medicine
- BENC
Bo-Er-Ning capsule
- GC
Gastric cancer
- OS
Overall survival
- KPS
Karnofsky performance scores
- QOL
Quality of life
- DC
Degree centrality
- PPI
Protein-protein interaction
- CCK-8
Cell Counting Kit-8
- EMT
Epithelial-Mesenchymal transition
- HCC
Hepatocellular carcinoma
Supplementary Table 1
Supplementary Table 2
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Koehn FE. Cancer drug discovery and development. New York: Humana Press; 2013. Natural products and cancer drug discovery; p. xii.p. 244. [Google Scholar]
- 5.Tang JL, Liu BY, Ma KW. Traditional Chinese medicine. Lancet. 2008;372:1938–40. doi: 10.1016/S0140-6736(08)61354-9. [DOI] [PubMed] [Google Scholar]
- 6.Tang JL. Research priorities in traditional Chinese medicine. BMJ. 2006;333:391–4. doi: 10.1136/bmj.333.7564.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancet T. Complementary medicine: time for critical engagement. Lancet. 2000;356:2023. [PubMed] [Google Scholar]
- 8.Xu J, Yang Y. Traditional Chinese medicine in the Chinese health care system. Health Policy. 2009;90:133–9. doi: 10.1016/j.healthpol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy--from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–31. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 10.Ren ZW MJ, Li BL, et al. Clinical observation of preventing the recurrence for 30 cases of bladder cancer treated with Boerning capsules and HCPT. Chin J TCM WM Crit Care (Chinese) 2005;12 [Google Scholar]
- 11.Liu W FW, Lu YL, et al. Clinical observation of elder advanced NSCLC treated with Boerning capsules plus HEP. Chin J Clin Oncol (Chinese) 2004;31:953–954. [Google Scholar]
- 12.Liu Z, Guo F, Wang Y, Li C, Zhang X, Li H, Diao L, Gu J, Wang W, Li D, He F. BATMAN-TCM: a bioinformatics analysis tool for molecular mechanism of traditional Chinese medicine. Sci Rep. 2016;6:21146. doi: 10.1038/srep21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–3. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling CQ, Wang LN, Wang Y, Zhang YH, Yin ZF, Wang M, Ling C. The roles of traditional Chinese medicine in gene therapy. J Integr Med. 2014;12:67–75. doi: 10.1016/S2095-4964(14)60019-4. [DOI] [PubMed] [Google Scholar]
- 16.Butler MS, Robertson AA, Cooper MA. Natural product and natural product derived drugs in clinical trials. Nat Prod Rep. 2014;31:1612–61. doi: 10.1039/c4np00064a. [DOI] [PubMed] [Google Scholar]
- 17.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–61. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 18.Wong VK, Law BY, Yao XJ, Chen X, Xu SW, Liu L, Leung EL. Advanced research technology for discovery of new effective compounds from Chinese herbal medicine and their molecular targets. Pharmacol Res. 2016;111:546–555. doi: 10.1016/j.phrs.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Ladas EJ, Kelly KM. Milk thistle: is there a role for its use as an adjunct therapy in patients with cancer? J Altern Complement Med. 2003;9:411–416. doi: 10.1089/107555303765551633. [DOI] [PubMed] [Google Scholar]
- 20.Martin C. The plant science decadal vision. Plant Cell. 2013;25:4773–4. doi: 10.1105/tpc.113.251290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XJ, Kong DX, Zhang HY. Chemoinformatics approaches for traditional Chinese medicine research and case application in anticancer drug discovery. Curr Drug Discov Technol. 2010;7:22–31. doi: 10.2174/157016310791162749. [DOI] [PubMed] [Google Scholar]
- 22.Lagunin AA, Goel RK, Gawande DY, Pahwa P, Gloriozova TA, Dmitriev AV, Ivanov SM, Rudik AV, Konova VI, Pogodin PV, Druzhilovsky DS, Poroikov VV. Chemo- and bioinformatics resources for in silico drug discovery from medicinal plants beyond their traditional use: a critical review. Nat Prod Rep. 2014;31:1585–611. doi: 10.1039/c4np00068d. [DOI] [PubMed] [Google Scholar]
- 23.Butte AJ, Ito S. Translational bioinformatics: data-driven drug discovery and development. Clin Pharmacol Ther. 2012;91:949–52. doi: 10.1038/clpt.2012.55. [DOI] [PubMed] [Google Scholar]
- 24.Chen CY. TCM Database@Taiwan: the world’s largest traditional Chinese medicine database for drug screening in silico. PLoS One. 2011;6:e15939. doi: 10.1371/journal.pone.0015939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao X, Hou T, Zhang W, Guo S, Xu X. A 3D structure database of components from Chinese traditional medicinal herbs. J Chem Inf Comput Sci. 2002;42:481–9. doi: 10.1021/ci010113h. [DOI] [PubMed] [Google Scholar]
- 26.Ye H, Ye L, Kang H, Zhang D, Tao L, Tang K, Liu X, Zhu R, Liu Q, Chen YZ, Li Y, Cao Z. HIT: linking herbal active ingredients to targets. Nucleic Acids Res. 2011;39:D1055–9. doi: 10.1093/nar/gkq1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang YC, Huang HC, Chen HH, Juan HF. TCMGeneDIT: a database for associated traditional Chinese medicine, gene and disease information using text mining. BMC Complement Altern Med. 2008;8:58. doi: 10.1186/1472-6882-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue R, Fang Z, Zhang M, Yi Z, Wen C, Shi T. TCMID: traditional Chinese medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Res. 2013;41:D1089–95. doi: 10.1093/nar/gks1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012;40:D1100–7. doi: 10.1093/nar/gkr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin C, Zhang C, Zhu F, Xu F, Chen SY, Zhang P, Li YH, Yang SY, Wei YQ, Tao L, Chen YZ. Therapeutic target database update 2014: a resource for targeted therapeutics. Nucleic Acids Res. 2014;42:D1118–23. doi: 10.1093/nar/gkt1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Deng Q, Wang Q, Li KY, Dai JH, Li N, Zhu ZD, Zhou B, Liu XY, Liu RF, Fei QL, Chen H, Cai B, Zhou B, Xiao HS, Qin LX, Han ZG. Exome sequencing of hepatitis B virus-associated hepatocellular carcinoma. Nat Genet. 2012;44:D1117–21. doi: 10.1038/ng.2391. [DOI] [PubMed] [Google Scholar]
- 32.Tilborghs S, Corthouts J, Verhoeven Y, Arias D, Rolfo C, Trinh XB, van Dam PA. The role of nuclear factor-kappa B signaling in human cervical cancer. Crit Rev Oncol Hematol. 2017;120:D141–50. doi: 10.1016/j.critrevonc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Jin H, Lee WS, Nagappan A, Choi YH, Kim GS, Jung J, Ryu CH, Shin SC, Hong SC, Kim HJ. Morin, a flavonoid from moraceae, inhibits cancer cell adhesion to endothelial cells and EMT by down-regulating VCAM-1 and N-cadherin. Asian Pac J Cancer Prev. 2016;17:D3071–75. [PubMed] [Google Scholar]
- 34.Xia Q, Bai QR, Dong M, Sun X, Zhang H, Cui J, Xi H, Hu XL, Shen Q, Chen L. Interaction between gastric carcinoma cells and neural cells promotes perineural invasion by a pathway involving VCAM1. Dig Dis Sci. 2015;60:D3283–92. doi: 10.1007/s10620-015-3758-x. [DOI] [PubMed] [Google Scholar]
- 35.Elsarraj HS, Valdez KE, Hong Y, Grimm SL, Ricci LR, Fan F, Tawfik O, May L, Cusick T, Inciardi M, Redick M, Gatewood J, Winblad O, Hilsenbeck S, Edwards DP, Hagan CR, Godwin AK, Fabian C, Behbod F. NEMO, a transcriptional target of estrogen and progesterone, is linked to tumor suppressor PML in breast cancer. Cancer Res. 2017;77:D3802–13. doi: 10.1158/0008-5472.CAN-16-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma L, Yang Y, Yin Z, Liu M, Wang L, Chen L, Zhu L, Yang H. Emodin suppresses the nasopharyngeal carcinoma cells by targeting the chloride channels. Biomed Pharmacother. 2017;90:615–625. doi: 10.1016/j.biopha.2017.03.088. [DOI] [PubMed] [Google Scholar]
- 37.Tang N, Chang J, Lu HC, Zhuang Z, Cheng HL, Shi JX, Rao J. Rhein induces apoptosis and autophagy in human and rat glioma cells and mediates cell differentiation by ERK inhibition. Microb Pathog. 2017;113:168–175. doi: 10.1016/j.micpath.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Chang J, Li Y, Wang X, Hu S, Wang H, Shi Q, Wang Y, Yang Y. Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/beta-catenin pathway in vitro and in vivo. Sci Rep. 2017;7:7605. doi: 10.1038/s41598-017-07194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Z, Xu Y, Peng W. Colchicine induces apoptosis in HT29 human colon cancer cells via the AKT and c-Jun N-terminal kinase signaling pathways. Mol Med Rep. 2015;12:5939–44. doi: 10.3892/mmr.2015.4222. [DOI] [PubMed] [Google Scholar]
- 40.Ye Q, Su L, Chen D, Zheng W, Liu Y. Astragaloside IV induced miR-134 expression reduces EMT and increases chemotherapeutic sensitivity by suppressing CREB1 signaling in colorectal cancer cell line SW-480. Cell Physiol Biochem. 2017;43:1617–1626. doi: 10.1159/000482025. [DOI] [PubMed] [Google Scholar]
- 41.Schneider G. Virtual screening: an endless staircase? Nat Rev Drug Discov. 2010;9:273–6. doi: 10.1038/nrd3139. [DOI] [PubMed] [Google Scholar]
- 42.Lauro G, Romano A, Riccio R, Bifulco G. Inverse virtual screening of antitumor targets: pilot study on a small database of natural bioactive compounds. J Nat Prod. 2011;74:1401–7. doi: 10.1021/np100935s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.