Abstract
miRNA, which involves in pathogenesis of thyroid cancer via different targets, has been found aberrantly expressed in thyroid cancer. Modes of actions of miR-26a in papillary thyroid carcinoma (PTC), however, have not been fully understood to this date. In vitro results obtained from this research confirmed miR-26a was down-regulated in PTC cells (i.e. TPC-1 and BCPAP) where the down-regulation of miR-26a was found to be able to promote cell proliferation. In order to explore the mechanisms, potential targets of miR-26a were postulated: cAMP regulated phosphoprotein 19 (ARPP19) turned out to be the target of miR-26a and it was by depleting ARPP19 was the cell proliferation be suppressed. This suggested that miR-26a regulated cell proliferation by targeting ARPP19. In addition, such a depletion of ARPP19 sensitized PTC cells to tamoxifen (TMX) treatment. The above findings indicated miR-26a was a target of interest regarding the treatment of refractory thyroid carcinomas.
Keywords: ARPP19, cell proliferation, miR-26a, tamoxifen, thyroid cancer
Introduction
Papillary thyroid carcinoma (PTC), originating from thyroid follicular cells, is the most common type of thyroid tumor, accounting for 90% of all thyroid cancer occurrences [1]. Although most PTC patients are well prognosticated with the mortality rate less than 10% after surgical resection supplemented with radioiodine and levothyroxine treatment, the remainder whose clinical staging has progressed to metastasis or those whose cancer has recurred usually received poor prognosis [2]. A better understanding of the mechanisms for PTC progression and metastasis is, therefore, necessary for identifying novel diagnostic and therapeutic targets of the malignancy.
miRNAs are short single-strand non-coding RNAs with a length of approximately 19-25 nucleotides. MiRNAs negatively regulate gene expression by inducing mRNA degradation or translational repression through base pair mismatching in the 3’-untranslated regions (UTRs) of their targets [3]. A single miRNA binds generously with different targets and around 40-90% of human genes are regulated by miRNA. It thus plays an important role in several cellular processes, including cell proliferation, differentiation and apoptosis under normal and pathological conditions via regulation of mRNA [4-7]. Previous studies have shown that miRNAs are involved in the pathogenesis of thyroid cancer in which miR-26a aberrantly expressed [8,9]. Modes of actions of miR-26a in PTC, however, have not been fully characterized. In this study, the expression level of miR-26a was measured to assist the investigation of its regulatory roles in PTC cells. It was found that miR-26a could regulate cell proliferation by targeting ARPP19, and that depletion of ARPP19 by miR-26a sensitized PTC cells to tamoxifen.
Materials and methods
Cell culture and transfection
Human PTC cell lines (TPC-1 and BCPAP) and normal human thyroid cell line (Nthy-ori 3-1) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin (100 units/ml) and streptomycin (100 ng/ml) at 37°C in a 5% CO2 atmosphere. Mimics and inhibitor of miR-26a and their corresponding miRNA negative control (miR-NCs) were chemically synthesized and transfected with Lipofectamine® RNAiMAX. Over-expressed or knocked-down plasmids were transfected with Lipofectamine 2000 according to the manufacturer’s protocol.
ARPP19 over-expression and shRNA-mediated knock-down plasmids
The longest transcript human genes of ARPP19 (NCBI Reference Sequence: NM_001306191.1) were cloned into pcDNA 3.1 plasmids using KOD DNA polymerase and then sequenced for validation. The shRNAs were designed and cloned into PLKO.1 vector. The targeted sequences of shRNA were: shRNA1: GGTCTCTGCAACCATCGATTC; shRNA2: GTCTCTGCAACCATCGATTCC; shRNA3: GCAACCATCGATTCCTAACAG.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using TRIzol following the manufacturer’s instructions and was quantified using spectrophotometer (Nanodrop2000). Total RNA (1 μg) was used for reverse transcription and qRT-PCR was carried out with SYBR Premix Ex TaqTM on a real-time PCR system (ABI Prism 7500 fast). GAPDH was used to normalize the expression level of ARPP19. The data were analyzed using the 2-ΔΔCt method. The primers used were as followed: ARPP19-forward: GCCTGGAGGTTCAGATTTCTTA, ARPP19-reverse: CACCAGTGACCTCCGTCTTAT; GAPDH-forward: 5’-GCTGGCGCTGAGTACGTCGTGGAGT-3’, GAPDH-reverse: 5’-CACAGTCTTCTGGGTGGCAGTGATGG-3’.
Western blot
Protein was isolated from cells with RIPA lysis buffer containing 1% protease inhibitor cocktails (Pierce, Rockford, IL, USA). The concentration of total protein was determined by bicinchoninic acid (BCA) assay. 20 µg of protein was loaded and separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% skimmed milk and incubated with primary antibodies at 4°C overnight. Upon rinsing the blot, the membrane was incubated with the corresponding HRP-conjugated secondary antibodies at room temperature for 1 hour. Following the wash of the blot, signals were visualized by electrochemiluminescence (ECL) and analyzed with image processing program (ImageJ). GAPDH was used as an internal control.
Cell counting kit-8 (CCK-8) assay
Cells were seeded into 96-well microtiter plates where transfection was performed. Cell proliferation was examined at 0, 24, 48, and 72 hours after transfection. CCK-8 reagent (10 µl) was added into each well and incubated at 37°C for another 2 hours, after which optical density was measured at a wavelength of 490 nm (OD490). Each sample was assessed in sextuplicate and the data were obtained from three independent experiments and reported as means ± SD.
Bioinformatics analysis
To predict the potential targets of miR-26a, bioinformatics analysis was performed with TargetScan (http//www.targetscan.org) and RNAhybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid).
Luciferase reporter assay
Wild type and mutation of ARPP19 3’-UTR containing the putative binding site of miR-26a were synthesized and sequenced. Cells were seeded in 24-well plates and transfected with reporter vectors together with miR-26a mimics, miR-26a inhibitor or the corresponding miR-NC. After 48 hours of incubation, the activities of firefly and renilla luciferases were determined in transfected cells using the dual-luciferase reporter assay system (Promega, Madison, WI) following the manufacturer’s instructions. Renilla-luciferase activity was used for normalization. Each sample was assessed in sextuplicate and the data were obtained from three independent experiments and reported as means ± SD.
IT click-iT EdU cell proliferation assay
Transfected cells were seeded in 96-well plates and incubated for 48 hours. Upon labelling with 5-ethynyl-2’-deoxyuridine (EdU, Invitrogen), the cells were fixed and permeabilizated, followed by the treatment with Click-iT® reaction cocktails. The cells were allowed to be incubated for 30 minutes and the nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) and visualized under a fluorescence microscope. The EdU positive cells were counted and the ratio of number of EdU positive cells/total number of cells in percentage was defined as the proliferation rate. Each sample was assessed in sextuplicate and the data were obtained from three independent experiments and reported as means ± SD.
Lentivirus production
For lentivirus production, ARPP19 was sub-cloned into lentiviral over-expression vector pLV and sequenced for validation. Over-expressed or shRNA-mediated knocked-down plasmids were transfected into 293T cells together with psPAX2 packaging plasmid and pMD2.G envelope plasmid (Addgene). 64 Hours subsequent to transfection, supernatants were harvested and viral particle was pelleted by centrifugation. The viral titer was then estimated and multiplicity of infection (MOI) of 0.4 was used.
Tumorigenicity assay
The study was approved by Animal Ethic Committee of West China Hospital of Sichuan University, and the experiments with rats were in full compliance with the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013).
4-6 weeks BALB/c nude mice were maintained under specific pathogen free (SPF) conditions. Stably transfected cells were collected and re-suspended in Hank’s buffer and mixed with equal volume of matrigel (BD Biosciences) at a concentration of 5 × 106 cells/mL. The resulting cell suspension (100 μL) was then injected subcutaneously into the flank of nude mice. Tumor diameters were checked every 4 day, and tumor volume was calculated using the equation: (LW2)/2, where L refers to the length of the tumor and W refers to the width of the tumor (L>W). Tumor specimens were prepared for western blot, haematoxylin and eosin (HE) staining and ki67 immunohistochemistry analysis.
Statistical analysis
The statistical analyses were performed using the SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). All data were reported as mean ± SEM; differences between groups were analyzed using two-tailed student’s t-test or one-way ANOVA method. P<0.05 was considered to indicate a statistically significant difference.
Results
MiR-26a is down-regulated in PTC cell lines
Down-regulation of miR-26a has been observed in various cancers, such as bladder cancer, breast cancer, oral squamous cell carcinoma, anaplastic carcinomas as well as thyroid cancer [10-13]. To determine whether miR-26a was down-regulated in PTC cell lines, RT-qPCR was performed both on the PTC cells (TPC-1, BCPAP) as well as on the normal human thyroid cell line Nthy-ori 3-1 for comparison. The PCR results showed that miR-26a was significantly lower in the PTC cell lines compared with the Nthy-ori 3-1 (Figure 1). The results indicated miR-26a was down-regulated in PTC cells.
Figure 1.
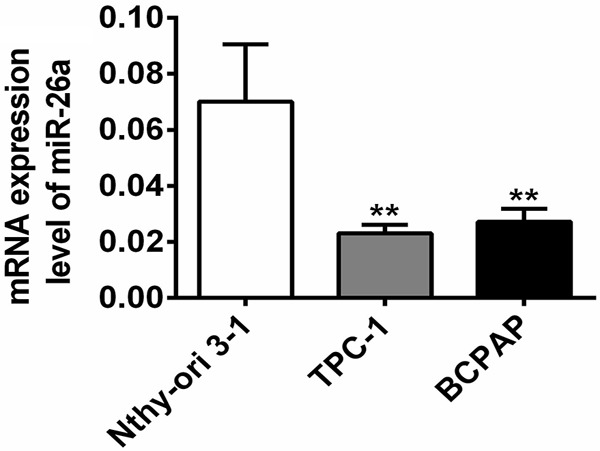
MiR-26a was down-regulated in PTC cell lines. The relative expression of miR-26a in the PTC cell lines of TPC-1, BCPAP and the normal human thyroid cell line Nthy-ori 3-1 was measured by RT-qPCR. **, P<0.01 compared with Nthy-ori 3-1.
MiR-26a inhibits the proliferation of PTC cells
To explore the cellular function of miR-26a in PTC cells, TPC-1 cells were transfected with mimics and inhibitor of miR-26a and the cell growth was measured. The growth curves generated from CCK-8 assay revealed that up-regulation of miR-26a resulted in greater suppression of cell proliferation than the control (mimics NC), whereas down-regulation of miR-26a promoted cell proliferation (Figure 2A). The result was further verified by click-iT EdU cell proliferation assay: the group with up-regulated miR-26a had fewer cells in division with relative to the control (mimics NC), whereas the group with down-regulated of miR-26a had much more dividing cells than the control had (Figure 2B). These results indicated miR-26a inhibited the proliferation of TPC-1 cells and down-regulation of miR-26a promoted the proliferation of cells.
Figure 2.
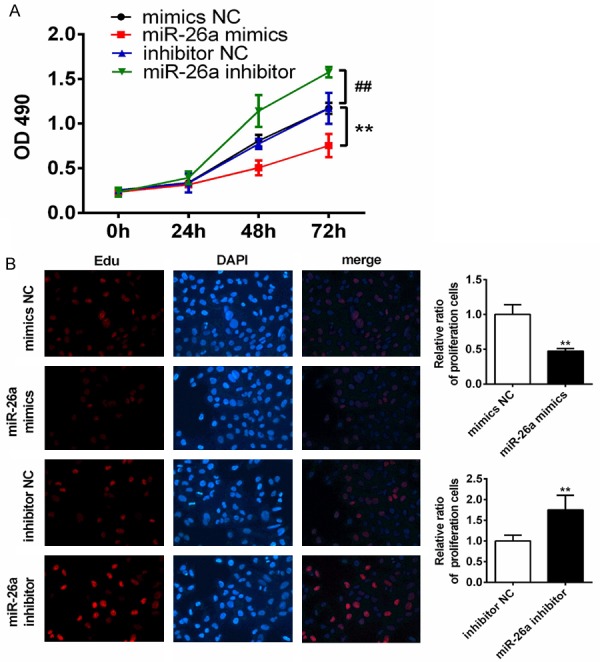
miR-26a inhibited TPC-1 cell proliferation in vitro. A. Growth curves of each group were obtained by CCK-8 assay in TPC-1 cells transfected with mimics of miR-26a or mimics-NC, inhibitor of miR-26a or inhibitor-NC. OD490 was measured at 0, 24, 48 and 72 hours. B. IT click-iT EdU cell proliferation assay was performed to evaluate the number of dividing cells in TPC-1 transfected with mimics of miR-26a or mimics-NC, inhibitor of miR-26a or inhibitor-NC. Representative immunofluorescent images were shown and average number of EdU labeled cells, which indicated dividing cells were counted. (magnification, ×200). **, P<0.01 compared with mimics NC; ##, P<0.01 compared with inhibitor NC.
ARPP19 is a direct target of miR-26a in PTC
In order to find out the mechanism for miR-26a to influence cell proliferation, potential targets of miR-26a were postulated by bioinformatics analysis: cAMP-regulated phosphoprotein 19 (ARPP19) stood out from all the other suppositions. ARPP19 up-regulation associated with hepatocellular carcinoma and promoted the cell growth [14]. Further studies were nevertheless required to confirm the relationship between ARPP19 up-regulation and cell proliferation promoting ability (Figure 3A). Results showed that the expression level of ARPP19 was inversely proportional to the expression of miR-26a. At mRNA and protein levels, overexpression or knock-down of miR-26a resulted in significant decrease or increase in the expression level of ARPP-19, respectively (Figure 3B and 3C). In addition, Luciferase reporter assays were performed to study if miR-26a targeted ARPP19 by binding to its 3’-UTR. It was found that mutations of the putative binding site (i.e. 3’-UTR of ARPP19) of miR-26a eliminate the effect of both mimics and inhibitor of miR-26a on luciferase activity completely (Figure 3D). The results indicated ARPP19 was directly targeted by miR-26a in TPC-1 cells.
Figure 3.
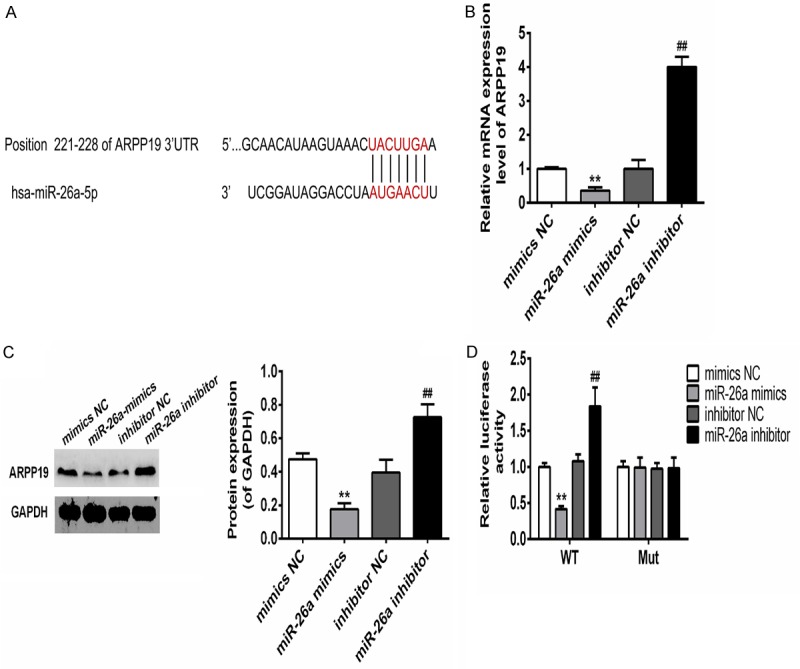
miR-26a directly targeted ARPP-19 in TPC-1 cells. A. Sequences complementary pairings of miR-26a with ARPP19 wild-type and mutant 3’ UTR were shown. B. mRNA levels of ARPP19 in TPC-1 cells transfection with mimics-NC, mimics of miR-26a or inhibitor-NC, inhibitor of miR-26a were determined by qRT-PCR. C. Protein levels of ARPP19 in TPC-1 cells transfection with mimics-NC, mimics of miR-26a or inhibitor-NC, inhibitor of miR-26a were determined by western blot. Representative images of western blot were shown, bands were quantitated by densitometry and normalized against GAPDH. D. Luciferase activities were determined in TPC-1 cells 48 h after co-transfection with the mimics-NC, mimics of miR-26a, inhibitor-NC or inhibitor of miR-26a and Luciferase reporter vector containing WT or mutants of APRR19 3’ UTR. **, P<0.01 compared with mimics-NC; ##, P<0.01 compared with inhibitor NC.
ARPP19 promotes cell proliferation of TPC-1
Although ARPP19 played an important role in regulating mitosis by inhibiting protein phosphatase-2A, the cellular function of ARPP19 had only been occasionally studied [15]. In order to investigate the cellular function of ARPP19, expression level thereof in TPC-1 cells was manipulated by transfecting with over-expressed or shRNA-mediated knocked-down plasmids. The mRNA and protein level of ARPP19 was determined in the transfected cells where the expression level of ARPP19 markedly increased in the presence of over-expressed plasmids and decreased in the absence of plasmids (Figure 4A). The results showed the high efficiency of the constructs. The effect of ARPP19 on cell proliferation was determined by CCK-8 assay and IT click-iT EdU cell proliferation assay. The assay results showed that over-expression of ARPP19 significantly promoted cell growth, while knock-down of ARPP19 hampered it. The observation indicated expression of ARPP19 associated with cell proliferation in vitro (Figure 4B and 4C).
Figure 4.
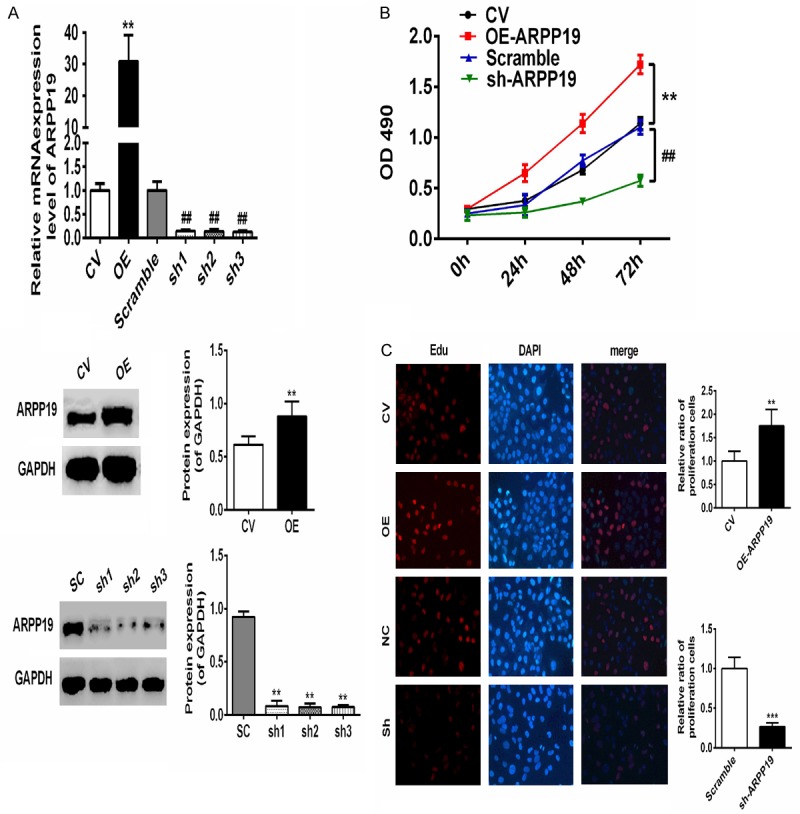
ARPP19 promoted TPC-1 cells proliferation in vitro. A. Top: mRNA levels of ARPP19 in the cells transfected with ARPP19 over-expression plasmids or shRNA-mediated knock-down plasmids were determined by qRT-PCR. Bottom: proteins levels were also confirmed by western blot. Representative images of western blot were shown, bands were quantitated by densitometry and normalized against GAPDH. **, P< 0.01, compared with CV; ##, P< 0.01, compared with scramble. B. Growth curves of each group were obtained by CCK-8 assay in TPC-1 cells transfected with ARPP19 or shRNA of ARPP19 plasmids. OD490 was measured at 0, 24, 48 and 72 hours. C. IT click-iT EdU cell proliferation assay was performed to evaluate the number of dividing cells in TPC-1 transfected with ARPP19 or shRNA of ARPP19 plasmids. Representative immunofluorescent images were shown and average number of Edu labeled cells which indicated dividing cells were counted (magnification, ×200). **, P<0.01 compared with CV; ##, P<0.01; ###, P<0.001 compared with scramble.
Restoration of ARPP19 reverses the effects of miR-26a in vitro and in vivo
With the above results in hand, it was assumed that miR-26a suppressed cell proliferation via up-regulating ARPP19. Considering the low expression level of miR-26a (Figure 1), rescue experiments were performed by co-transfecting the miR-26a mimics with or without ARPP19 followed by determination of the cell proliferation of TPC-1. Growth curves obtained by CCK-8 assay and Edu assay showed expression of miR-26a alone greatly inhibited cell growth whereas expression of ARPP19 alone notably promoted cell growth; co-expression of ARPP19 with miR-26a rescued the inhibitory effects of miR-26a mimics on the cell proliferation (Figure 5A and 5B). Based on the in vitro data, the effect of miR-26a and ARPP19 on tumor growth was further evaluated in vivo. Lentivirus of miR-26a and ARPP19 were either used to infect cells alone or simultaneously. It was found that tumor xenografts derived from cells infected with miR-26a alone were significantly smaller than the negative control while those infected with ARPP19 showed the opposite; co-infection of ARPP19 with miR-26a removed the inhibitory effects of miR-26a (Figure 5C). Expression level of ARPP19 in tumor xenografts was also measured: the expression level of ARPP19 was much lower in miR-26a group and higher in ARPP19 or co-infected groups, compared with their counterparts in the negative control group (Figure 5D). Histologically examining was performed using HE staining and ki67 immunohistochemically staining. Condensed nuclei and weak signal of ki67 indicated necrosis in miR-26a group, whereas large nuclei and strong signal of ki67 indicated active proliferation in ARPP19 and miR-26a+ARPP19 groups (Figure 5E). The in vitro and in vivo observations suggested that miR-26a could target the 3’-UTR of ARPP19 directly and inhibit TPC-1 cell proliferation via ARPP19 up-regulation (Figure 5C).
Figure 5.
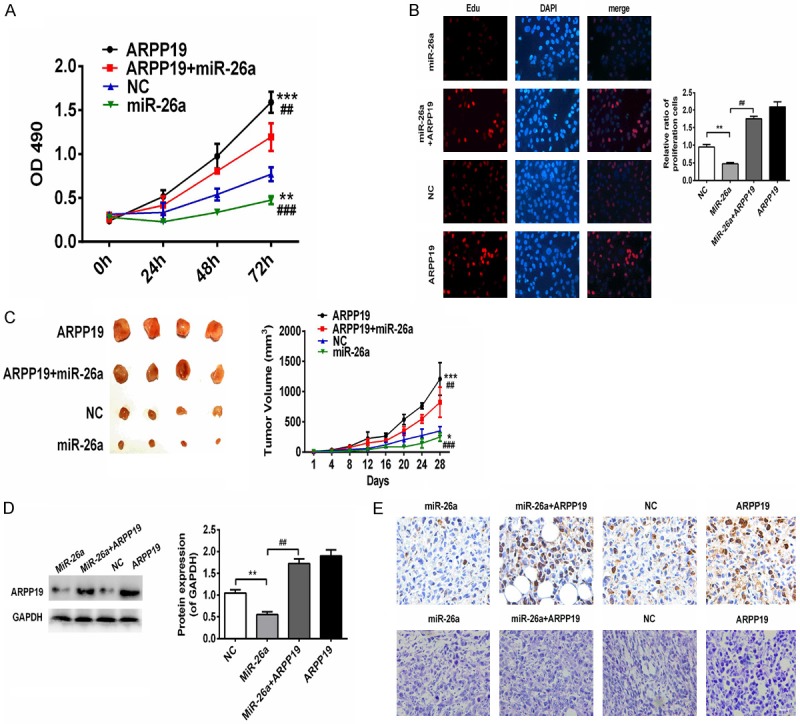
Up-regulation of ARPP1 reversed the inhibitory effects of miR-26a on TPC-1 cells. A. Growth curves were obtained by CCK-8 assay in the cells transfected with miR-26a, ARPP19 alone or co-transfected and NC. OD490 was measured at 0, 24, 48 and 72 hours. **, P<0.01; ***, P<0.001 compared with NC; ##, P<0.01, ###, P<0.001 compared with ARPP19+miR-26a group. B. IT click-iT EdU cell proliferation assay was performed to evaluate the number of dividing cells in TPC-1 transfected with miR-26a, ARPP19 alone or co-transfected and NC. Representative immunofluorescent images were shown and average number of EdU labeled cells which indicated dividing cells were counted (magnification, ×200). **, P<0.01 compared with NC; ##, P<0.01 compared with miR-26a. C. Representative images of tumor xenografts and tumor growth curves were shown. *, P<0.05; ***, P<0.0001, compared with NC; ##, p<0.01, ###, p<0.001 compared with ARPP19+miR-26a group. D. Protein levels of ARPP19 in each group were determined by western blot. **, P<0.01 compared with NC; ##, P<0.01 compared with miR-26a. E. Representative images of HE staining (top) and ki67 immunohistochemically staining (bottom) were shown (magnification, ×200).
Depleting ARPP19 by miR-26a sensitizes PTC to tamoxifen
Tamoxifen (TMX) is the first choice for the patients with estrogen receptor positive breast cancer. Lü and coworkers recently found that depletion of ARPP-19 and ERRγ by miR-320a could sensitize breast cancer cells to TMX [16]. It was, therefore, of interest to investigate if depletion of ARPP19 by miR-26a could also sensitize PTC to TMX. It was initially found out in this study that ARPP19 was involved in the TMX sensitivity of PTC as over-expression of ARPP19 abated the inhibition of TMX, whereas absence of ARPP19 enhanced the inhibitory effect of TMX (Figure 6A). TMX sensitivity of the cells transfected with miR-26a mimics and inhibitor was then determined. Down-regulation of miR-26a reduced the inhibitory effect of TMX, whereas over-expression of miR-26a gave the opposite result (Figure 6B). Considering that ARPP19 was the direct target of miR-26a, further investigation was conducted to examine if the miR-26a-induced TMX sensitivity of cells could be counteracted by ARPP19 over-expression. The results showed the cells co-transfected with miR-26a and ARPP19 were growing notably more rapidly than the cells transfected with miR-26a alone (Figure 6C). The above results indicated up-regulation of miR-26a in TPC-1 could sensitize the cells to TMX via depleting ARPP19.
Figure 6.
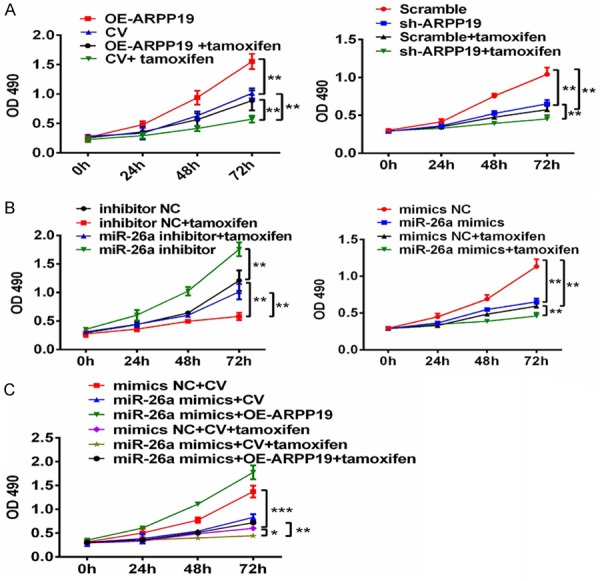
miR-26a and ARPP19 implicated tamoxifen sensitivity of TPC-1 cells. A-C. Growth curves of each group were obtained by CCK-8 assay. OD490 were measured at 0, 24, 48, and 72 hours. *, P<0.05; **, P<0.01; ***, P<0.0001 compared with indicated groups.
Discussion
Almost half of the known miRNAs are located in cancer-associated genomic regions or fragile sites [17]. Dysregulation of miRNAs is involved in tumorigenesis and progression of various cancers by regulating their target genes, and miRNAs are thus regarded as potential targets in cancer treatment. Down-regulation of miR-26a have been found in a number of cancers, such as cholangiocarcinoma, nasopharyngeal carcinoma, ovarian cancer, breast cancer and prostate cancer [18-20]. It has been, therefore, proposed that miR-26a could be used as diagnostic and prognostic biomarker [20,21]. Up-regulation of miR-26, however, also associates with many cancers, with mixed-regulation of miR-26a as well observed in lung cancer, causing difficulty in determining the function of miR-26a in different cancer types. The relationship between miR-26a and cancer should be examined for individual case. In this study, it showed that expression level of miR-26a was significantly down-regulated in PTC cells and the increase in expression level of miR-26 could suppress the proliferation of PTC cells greatly in vitro - suggesting miR-26a could function as an anti-oncogene (Figures 1 and 2).
ARPP19 is highly expressed in embryonic tissues and ARPP19 over-expression may be involved in cell malignancy as ARPP19 regulates mitosis by inhibiting protein phosphatase-2A [22,23]. Song and colleagues found expression level of ARPP19 was proportional to the tumor size of human hepatocellular carcinoma and knock-down of ARPP19 reduced the growth rate of HepG2 and SMMC-7721 cells [14]. Expression of ARPP19 was regulated by miR-320a in breast cancers. Transfecting MCF-7 cells with miR-320 inhibitor to up-regulate ARPP19 led to a notably faster cell growth than the cells transfected with the negative control [16]. It also proved in this study that ARPP19 promoted proliferation of the TPC-1 cells, confirming ARPP19 was a direct target of miR-26a. Furthermore, tumor-suppressive effect of miR-26a was reduced by enforced expression of ARPP19 in vivo and in vitro (Figures 3, 4 and 5). Previous studies reported that apart from ARPP19, miR-26a could as well function as a tumor-suppressor in various cancers through its targeted genes such as TFAP2C, EZH2 and CKS2 [19,24,25]. The results suggested that miR-26a functioned as an anti-oncogene via multiple genes targeting, such as on ARPP19.
Although more than 95% of the thyroid carcinomas are well differentiated types with proper prognosis, the remainder with refractory thyroid carcinomas are left with few treatment options. Developing new chemotherapy and hormonal therapy drugs are hence necessary for refractory thyroid carcinomas treatment [26]. TMX is a selective estrogen-receptor modulator, which was also reported to suppress the growth, migration, and invasion of PTC in vitro and in vivo [27]. Depletion of ARPP19 sensitized TMX-resistant breast cancer cells to TMX. TPC-1 cells with depleted ARPP19 by miR-26a were found more sensitive to TMX than those with normal expression of ARPP19. The findings indicated ARPP19 implicated in the mechanism for TMX-induced apoptosis of cancer cells, however a more precise mechanism would require further investigations to ascertain.
This study availed a better understanding of the cellular function of miR-26a and ARPP19 in thyroid cancer cells. In PTC cells, decreased expression of miR-26a could promote proliferation of PTC cells via de-repression of ARPP-19; miR-26a increased TMX sensitivity of PTC cells by targeting ARPP19. It was hence reasonable to conclude that regulation of miR-26a expression could be useful to refractory thyroid carcinomas treatment.
Acknowledgements
This study was supported by grants from the Department of Sichuan Province, Science and Technology Support Program (grant no. 2017SZ0058).
Disclosure of conflict of interest
None.
References
- 1.Liebner DA, Shah MH. Thyroid cancer: pathogenesis and targeted therapy. Ther Adv Endocrinol Metab. 2011;2:173–195. doi: 10.1177/2042018811419889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mcleod DS, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet. 2013;381:1046–1057. doi: 10.1016/S0140-6736(12)62205-3. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Yu X, Shen J, Chan MT, Wu WK. MicroRNA in intervertebral disc degeneration. Cell Prolif. 2015;48:278–283. doi: 10.1111/cpr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardekani AM, Naeini MM. The role of MicroRNAs in human diseases. Avicenna J Med Biotechnol. 2010;2:161–79. [PMC free article] [PubMed] [Google Scholar]
- 5.Hua K, Jin J, Zhang H, Zhao B, Wu C, Xu H, Fang L. MicroRNA-7 inhibits proliferation, migration and invasion of thyroid papillary cancer cells via targeting CKS2. Int J Oncol. 2016;49:1531–1540. doi: 10.3892/ijo.2016.3660. [DOI] [PubMed] [Google Scholar]
- 6.Ricartefilho J, Fuziwara CS, Yamashita AS, Rezende E, Dasilva MJ, Kimura ET. Effects of let-7 microRNA on cell growth and differentiation of papillary thyroid cancer. Transl Oncol. 2009;2:236–241. doi: 10.1593/tlo.09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wojtas B, Ferraz C, Stokowy T, Hauptmann S, Lange D, Dralle H, Musholt T, Jarzab B, Paschke R, Eszlinger M. Differential miRNA expression defines migration and reduced apoptosis in follicular thyroid carcinomas. Mol Cell Endocrinol. 2014;388:1–9. doi: 10.1016/j.mce.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Pallante P, Visone R, Croce CM, Fusco A. Deregulation of microRNA expression in follicular cell-derived human thyroid carcinomas. Endocr Relat Cancer. 2010;17:F91–104. doi: 10.1677/ERC-09-0217. [DOI] [PubMed] [Google Scholar]
- 9.Pallante P, Battista S, Pierantoni GM, Fusco A. Deregulation of microRNA expression in thyroid neoplasias. Nat Rev Endocrinol. 2013;10:88–101. doi: 10.1038/nrendo.2013.223. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Liu X, He J, Zhou CX, Guo M, He M, Li MF, Chen GQ, Zhao Q. Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis. 2011;32:2–9. doi: 10.1093/carcin/bgq209. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Chen H, Hu Z, Mao Y, Xu X, Zhu Y, Xu X, Wu J, Li S, Mao Q, Zheng X, Xie L. miR-26a inhibits proliferation and motility in bladder cancer by targeting HMGA1. FEBS Lett. 2013;587:2467–2473. doi: 10.1016/j.febslet.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Jia L, Wei S, Gan Y, Guo Y, Gong K, Mitchelson K, Cheng J, Yu GY. Expression, regulation and roles of miR-26a and MEG3 in tongue squamous cell carcinoma. Int J Cancer. 2014;135:2282–2293. doi: 10.1002/ijc.28667. [DOI] [PubMed] [Google Scholar]
- 13.Braun J, Hoangvu C, Dralle H, Huttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 14.Song H, Pan J, Liu Y, Wen H, Wang L, Cui J, Liu Y, Hu B, Yao Z, Ji G. Increased ARPP-19 expression is associated with hepatocellular carcinoma. Int J Mol Sci. 2014;16:178–192. doi: 10.3390/ijms16010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gharbiayachi A, Labbe J, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 16.Lü M, Ding K, Zhang G, Yin M, Yao G, Tian H, Lian J, Liu L, Liang M, Zhu T, Sun F. MicroRNA-320a sensitizes tamoxifen-resistant breast cancer cells to tamoxifen by targeting ARPP-19 and ERRγ*. Sci Rep. 2015;5:8735–8735. doi: 10.1038/srep08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Li L, Wu M, Liu M, Xie X, Guo J, Tang H, Xie X. MiR-26a inhibits proliferation and migration of breast cancer through repression of MCL-1. PLoS One. 2013;8:e65138. doi: 10.1371/journal.pone.0065138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Dong W, Xie J, et al. miR-26a inhibits cell proliferation by regulating TFAP2C expression in ovarian cancer cells. Tumori. 2014;34:908–956. [Google Scholar]
- 20.Wang L, Zhang K, Zhang N, Ma XW, Yan SW, Cao DH, Shi SJ. Serum miR-26a as a diagnostic and prognostic biomarker in cholangiocarcinoma. Oncotarget. 2015;6:18631–18640. doi: 10.18632/oncotarget.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu X, Zhu H, Liu S, Tao G, Jin J, Chu H, Wang M, Tong N, Gong W, Zhao Q, Qiang F, Zhang Z. Expression and prognostic value of microRNA-26a and microRNA-148a in gastric cancer. J Gastroenterol Hepatol. 2017;32:819–827. doi: 10.1111/jgh.13533. [DOI] [PubMed] [Google Scholar]
- 22.Girault JA, Horiuchi A, Gustafson EL, Rosen NL, Greengard P. Differential expression of ARPP-16 and ARPP-19, two highly related cAMP-regulated phosphoproteins, one of which is specifically associated with dopamine-innervated brain regions. J Neurosci. 1990;10:1124–1133. doi: 10.1523/JNEUROSCI.10-04-01124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gharbiayachi A, Labbe J, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, He M, Wang L, Chen Y, Liu X, Dong Q, Chen YC, Peng Y, Yao KT, Kung HF, Li XP. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225–233. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- 25.Lv M, Zhang X, Li M, Chen Q, Ye M, Liang W, Ding L, Cai H, Fu D, Lv Z. miR-26a and its target CKS2 modulate cell growth and tumorigenesis of papillary thyroid carcinoma. PLoS One. 2013;8:e67591. doi: 10.1371/journal.pone.0067591. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Sung T, Choi SH, Lee JM, Jeong JJ, Kang S, Chung WY. Innovative In vitro chemo-hormonal drug therapy for refractory thyroid carcinomas. J Korean Med Sci. 2012;27:729–735. doi: 10.3346/jkms.2012.27.7.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoelting T, Siperstein A, Duh Q, Clark OH. Tamoxifen inhibits growth, migration, and invasion of human follicular and papillary thyroid cancer cells in vitro and in vivo. J Clin Endocrinol Metab. 1995;80:308–313. doi: 10.1210/jcem.80.1.7829632. [DOI] [PubMed] [Google Scholar]


