Abstract
This retrospective cohort study was designed to evaluate the efficacy and safety of nedaplatin plus paclitaxel (NP) compared with carboplatin plus paclitaxel (CP) in platinum-sensitive recurrent ovarian cancer. Patients with histologically proven epithelial ovarian cancer with recurrent interval ≥6 months after finishing platinum-based therapies between January 1, 2009 and December 31, 2014 were investigated. Patients received an intravenous infusion of NP (nedaplatin 80 mg/m2 plus paclitaxel 175 mg/m2) or CP (carboplatin at an area under the curve of 5 plus paclitaxel 175 mg/m2) protocols every 3 weeks for at least 6-8 cycles or until disease progression. Primary end point was progression-free survival (PFS); secondary end points were toxicity and overall survival (OS). 436 patients were included in the study, containing 241 cases receiving CP regimen and 195 cases receiving NP regimen, who were all contained in safety analysis. Because of 61 patients with unbearable toxicity and poor compliance, 375 patients were finally included in the efficacy analysis. With median follow-up of 63.5 months, PFS was 11.0 months with NP regimen versus 9.5 months with CP regimen (P=0.109). Subgroup analysis indicated that PFS of the NP arm was statistically superior to the CP arm when recurrent interval was 6-12 months (P=0.048); median PFS was 10.0 versus 8.0 months, respectively. There was no significant difference in overall survival between two groups. More frequent grade 3-4 neutropenia (13.3% vs 33.6%), thrombocytopenia (5.6% vs 14.5%) and hypersensitivity reactions (5.6% vs 21.9% ) were observed in CP arm (P<0.01). Compared to the CP, NP regimen did not improve 5-year overall survival in platinum-sensitive recurrent ovarian cancer, but it had better tolerance. NP obtained significant benefit in progression-free survival when the recurrent interval was between 6 and 12 months, although the efficacy of two regimens were similar when the recurrent interval ≥12 months.
Keywords: Nedaplatin, carboplatin, platinum-sensitive recurrence, efficacy, toxicity
Introduction
Ovarian cancer is the most mortal gynecologic cancer in women. The incidence of epithelial ovarian cancer in United States in 2015 was approximately 21290 patient cases (14180 deaths) and the data was estimated at 52.1 per 100,000 (22.5 per 100,000 death) in China in 2015 [1,2]. Debulking surgery followed by platinum-based chemotherapy is the standard treatment, and 70% of patients can achieve clinical response, however, the majority of patients will experience recurrence, which is clinically challenging because of the relentless trajectory to eventual drug resistance. Disease-free interval ≥6 months after the last platinum treatment is defined as platinum-sensitive recurrence and platinum-based chemotherapy is still an important part of re-treatment for these recurrent patients [3,4].
In the platinum-sensitive recurrent setting, carboplatin plus paclitaxel (CP) is the most frequently used chemotherapeutic combination [5,6]. Three phase III trials demonstrated significant improvements in progression-free survival in patients with platinum-sensitive ROC treated with platinum-paclitaxel versus conventional platinum-based therapies [7]. However, the incidence of hypersentivity reactions of carboplatin increased with repeated usage and the proportion has been reported to be 23-44%, which limited the rechallenge of CP [8,9].
Nedaplatin (NDP) is a second-generation platinum derivative, which has the same ammine carrier ligands as cisplatin but a different leaving group, with reduced nephrotoxicity, gastrointestinal toxicity and favorable anti-tumor activity. Preclinical studies indicated that the anti-tumor activity of nedaplatin was comparable to cisplatin [10,11]. The studies of Kato T and Noda K showed that the efficacy of nedaplatin against cervical and ovarian cancer was respectively 34-46% and 38% [12,13]. Gao compared the survivorship of patients adopting postoperative chemotherapy by use of carboplatin plus paclitaxel (CP) or nedaplatin plus paclitaxel (NP) against ovarian cancer, indicating similar efficacy between two arms. Nedaplatin was clinically well-tolerated, which was also the most common alternative drugs for carboplatin when incorrigible hematologic toxicity and allergic reaction appeared [14]. Zhang et al reported the responsive rate of nedaplatin in ovarian cancer was 44.8% in a phase II clinical study [15], but the study covered different malignant tumor and no concrete record of progression free survival. We designated the retrospective study to make detailed evaluation about the NP versus the standard CP protocol every 3 weeks in patients with platinum-sensitive recurrent ovarian cancer.
Material and methods
Study design
The aim of the retrospective study was to determine the efficacy and safety of NP protocol for platinum-sensitive recurrent ovarian cancer, which was completed on all patients with ovarian, fallopian tube or primary peritoneal cancer who visited National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College from January 1, 2009 to December 31, 2014. All patients with platinum-sensitive recurrent ovarian cancer receiving CP or NP were retrospectively identified and relevant follow-up information were collected. The human investigations were approved by the institutional review board of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.The written consent were informed to patients and the follow-up ended on May 31, 2017.
Patient population
Eligible patients were ≥18 years old with a histologically confirmed epithelial ovarian, fallopian tube, or primary peritoneal cancer. The included patients should receive standard initial cytoreductive surgery followed by 6-8 cycles of platinum-based chemotherapy. Patients were required to achieve clinical complete response after at least 6 cycles of primary platinum-based chemotherapy, which was defined by negative imaging examination and a normal CA125 level. Disease-free interval was calculated since last infused cycle of platinum, which should be at least 6 months. Inclusion criteria included: Eastern Cooperative Oncology Group (ECOG) performance status ≤2, life-expectancy of at least 12 weeks, ≤2 prior chemotherapy regimens and adequate hepatic, renal, and bone marrow function. Data collected included age, diagnosis date, platinum-free interval(s), neoadjuvant chemotherapy, residual status, International Federation of Gynecology and Obstetrics (FIGO) stages, pathological types, histological grades, prior chemotherapy regimens, lymphatic metastasis, duration of therapy, treatment response, hypersentivity reactions, toxicity, progression-free survival, overall survival.
Treatment protocol and dose modification
CP protocol was the combination of carboplatin (at an area under the curve of 5 based on the Calvert formula intravenously on day 1) and paclitaxel (175 mg/m2 intravenously on day 1). NP regimen was the combination of nedaplatin (80 mg/m2 intravenously on day 1) and paclitaxel (175 mg/m2 intravenously on day 1). Cycles were repeated every 21 days. Without disease progression or intolerable toxicity, patients were treated with a total of 6 cycles of therapy. If complete response (CR), partial response (PR) or stabilization of disease (SD) was achieved after 6 cycles, patients were allowed to remain on therapy with no more than 8 cycles of chemotherapy until progression of disease (PD). In the condition of nedaplatin as an alternative drug after the hypersentivity reaction or ill-tolerated hematologic toxicity of carboplatin, the cumulative cycles of regimen containing nedaplatin should not less than 5 if the response status was considered as CR or PR. The choice of CP or NP protocol was determined by doctor’s experience.
Patient assessment
Treatment response was evaluated by Response Evaluation Criteria in Solid Tumors (RECIST 1.0) for measurable disease and Gynecologic Cancer InterGroup (GCIG) criteria for CA-125 assessable disease [16,17]. Every patient received a complete history inquiry, physical examination, imaging examination and serum CA125 test before the treatment of recurrent ovarian cancer. Clinical, imaging and biochemical assessments were carried out before each cycle and the toxicity was assessed according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) criteria. After treatment, subsequent follow-up was performed every 3 months for 2 years and every 6 months thereafter for 5 years with gynecologic examination, CA-125 and imaging assessment.
Statistical analysis
This was a two-arm parallel non-inferiority study to determine whether NP was noninferior to the standard CP regimen with PFS as main end-point. Secondary outcome measures were overall survival (OS) and safety. A stratified two-sided log-rank test was used to compare the PFS between CP and NP arm. HRs and 95% CIs were calculated from a Cox proportional hazards model to explore the impact of certain prognostic factors on PFS. Stratification factor was treatment-free interval (6 to 12 months vs >12 months). Median follow-up time was calculated by censoring distribution. The safety population contained all patients who received at least one cycle of protocol treatment. Efficacy analysis were performed on population without ill-tolerated and poor medical compliance. SPSS 22.0 software was used for statistical analysis and all hypotheses testing were conducted at the significance level of P=0.05.
Results
Patient population
From January 1, 2009 to December 31, 2014, a total of 436 patients were enrolled in the cohort study with 241 patients receiving carboplatin plus paclitaxel protocol and 195 patients receiving nedaplatin plus paclitaxel regimen. 436 cases were included in safety analysis and 375 cases were included in efficacy analysis after excluding patients with protocol discontinuation ascribing to intolerable toxicity and patient choice. The CP and NP arms were well balanced for baseline disease characteristics (Table 1, Figure 1).
Table 1.
Baseline characteristics
| Characteristics | CP (n=186) | NP (n=189) | P | ||
|---|---|---|---|---|---|
|
|
|
||||
| No. | % | No. | % | ||
| Age (median, years) | 57 | 57 | 0.997 | ||
| ECOG performance status | 0.497 | ||||
| 0-1 | 134 | 72.0 | 142 | 75.1 | |
| 2 | 52 | 28.0 | 47 | 24.9 | |
| Neoadjuvant chemotherapy | 0.243 | ||||
| Yes | 63 | 33.9 | 75 | 39.7 | |
| No | 123 | 66.1 | 114 | 60.3 | |
| Pathological type | 0.455 | ||||
| Serous | 163 | 87.6 | 167 | 88.3 | |
| Mucious | 6 | 3.2 | 2 | 1.1 | |
| Clear cell | 6 | 3.2 | 4 | 2.1 | |
| Endometriod | 11 | 5.9 | 16 | 8.5 | |
| Mixed pathological types above | 37 | 19.9 | 32 | 16.9 | |
| Histologic grade | 0.936 | ||||
| G1 | 4 | 15.1 | 5 | 2.7 | |
| G2 | 64 | 21.5 | 63 | 33.3 | |
| G3 | 118 | 63.4 | 121 | 64.0 | |
| FIGO stages | 0.532 | ||||
| I-II | 16 | 8.6 | 13 | 6.9 | |
| III-IV | 170 | 91.4 | 176 | 93.1 | |
| No. of previous lines of chemotherapy | 0.025 | ||||
| 1 | 154 | 82.8 | 134 | 70.9 | |
| 2 | 23 | 12.4 | 39 | 20.6 | |
| 3 | 9 | 4.8 | 16 | 19.5 | |
| Operation during CP or NP cycles | 0.834 | ||||
| Yes | 27 | 14.5 | 27 | 14.3 | |
| No | 159 | 85.5 | 162 | 85.7 | |
| Measurable disease | 0.404 | ||||
| Yes | 163 | 87.6 | 160 | 84.7 | |
| No | 23 | 12.4 | 29 | 15.3 | |
| Interval since last platinum therapy | 0.566 | ||||
| 6-12 months | 87 | 46.8 | 94 | 49.7 | |
| >12 months | 99 | 53.2 | 95 | 50.3 | |
| Follow-up (Median, years) | 66 | 65 | |||
CP, Carboplatin and Paclitaxel; NP, Nedaplatin and Paclitaxel; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics.
Figure 1.
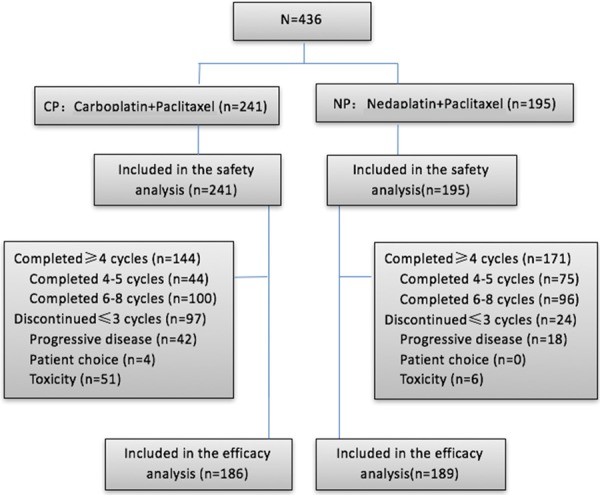
Diagram. CP, carboplatin plus paclitaxel; NP, nedaplatin and paclitaxel; PFS, progression-free survival.
Treatment administration
The median duration of treatment was six cycles (range, 1 to 8 cycles) in the CP arm versus six cycles (range, 1 to 8 cycles) in the NP arm. More cases in the NP arm completed at least 4 cycles of chemotherapy compared to CP arm (86.3% vs 77.8%; P<0.001). The median follow-up duration in the CP arm and NP arm was respectively 66 months and 65 months.
Efficacy
PFS was the main end-point of the analysis. After a median follow-up of 63.5 months and 375 PFS events, NP arm showed a non-significant increase in PFS compared with CP arm, with an HR of 0.847 (two-sided unstratified log-rank test P=0.109, 95% CI, 0.721 to 1.048, Figure 2A). The median PFS was 11.0 months (95% CI, 10.2 to 11.8) and 9.5 months (95% CI, 8.2 to 10.8) for NP arm and CP arm, respectively. Compared with CP arm, there was no OS benefit in NP arm (two-sided unstratified log-rank test P=0.765, HR, 0.847; 95% CI, 0.685 to 1.048). The median OS was 74 months (95% CI, 63.3 to 82.7) in the CP arm versus 73 months (95% CI, 65.3 to 82.7) in the NP group (Figure 2B). According to treatment-free interval (6 to 12 months vs >12 months), we performed subgroup analysis and the results indicated that the PFS of NP protocol (10.0 months) was superior than CP regimen when treatment-free interval was between 6 and 12 months (8.0 months, HR, 0.751; 95% CI, 0.557 to 1.013; P=0.048, Figure 3A), while no significance existed between two group when treatment-free interval was >12 months (PFS, 12 months in both groups, HR, 0.91; 95% CI, 0.672 to 1.233; P=0.543, Figure 3B).
Figure 2.
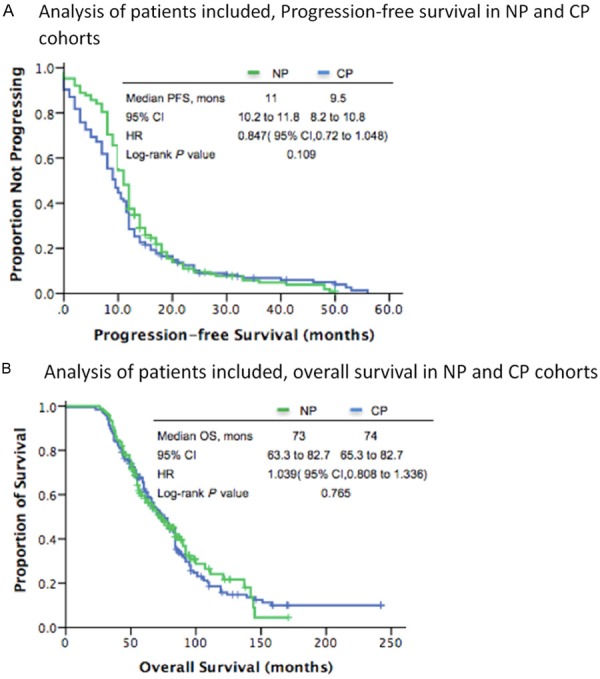
A. NP, nedaplatin plus paclitaxel, CP, carboplatin and paclitaxel, HR, hazard ratio, mons, months, PFS, progression-free survival. B. NP, nedaplatin plus paclitaxel, CP, carboplatin and paclitaxel, HR hazard ratio, mons, months, OS, overall survival.
Figure 3.
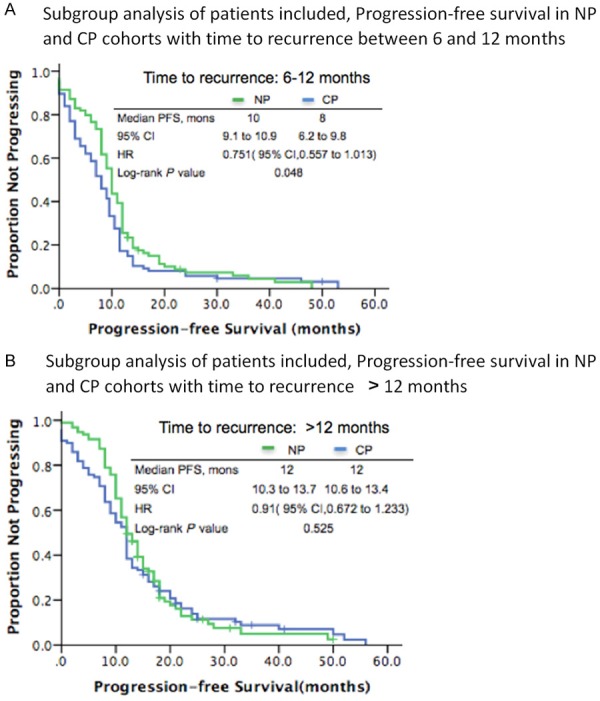
A. NP, nedaplatin plus paclitaxel, CP, carboplatin and paclitaxel, PFS, progression-free survival, HR, hazard ratio, mons, months. B. NP, nedaplatin plus paclitaxel, CP, carboplatin and paclitaxel, PFS, progression-free survival, HR, hazard ratio, mons, months.
An exploratory post-hoc analysis of investigator-assessed objective response (complete or partial response according to RECIST version 1.1 or GCIG) was done for 375 cases to assess response. A significantly higher proportion of patients achieved objective response in NP arm (168 [88.9%] of 189 patients) compared with CP arm (142 [76.3%] of 186 patients P<0.01), including a higher proportion of patients who achieved complete response (117 [61.9%] of 189 patients vs 97 [52.2%] of 186 patients).
Cox regression model evaluated the impact of age, number of previous lines of chemotherapy, recurrent interval, operation at relapse, residual status of debulking, tumor differentiation, pathological classification, ECOG score, therapy protocols and cycles of regimen on PFS. ECOG score, recurrent interval, operation at relapse, therapy protocols and cycles of regimen maintained significance in the multivariate Cox regression model (Figure 4).
Figure 4.
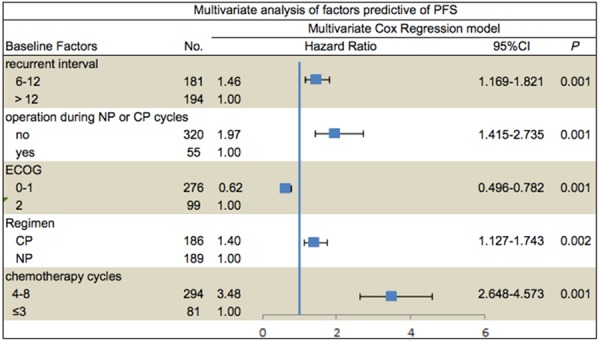
PFS, progression-free survival; HR, hazard ratio; CP, carboplatin and paclitaxel; NP, nedaplatin and paclitaxel, mons, months.
Toxicity
The safety population included 436 patients and adverse events were summarized in Table 2. More patients in the CP arm (33.6%) experienced a grade 3 to 4 neutropenia versus 13.3% with the NP arm (P<0.001). The incidence of grade 3-4 thrombocytopenia was 15% in CP arm versus 5.6% in NP arm (P=0.003). In terms of the incidence of febrile neutropenia and anemia, no statistical significance existed between two arm.
Table 2.
Adverse events according to regimen
| Adverse Events | CP (n=241) | NP (n=195) | P (Grade 3-4) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Grade ≥1 | Grade ≥2 | Grade 3-4 | Grade ≥1 | Grade ≥2 | Grade 3-4 | ||||||||
|
|
|
|
|
|
|
||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||
| Neutropenia | 195 | 80.99 | 81 | 33.6 | 109 | 55.9 | 26 | 13.3 | <0.001 | ||||
| Febrile neutropenia | 9 | 3.73 | 3 | 1.5 | 0.24 | ||||||||
| Thrombocytopenia | 42 | 17.4 | 35 | 14.5 | 31 | 15.9 | 11 | 5.6 | 0.003 | ||||
| Anemia | 139 | 57.7 | 16 | 6.6 | 92 | 47.2 | 8 | 4.1 | 0.261 | ||||
| P (Grade ≥2) | |||||||||||||
| Alopecia | 193 | 80.1 | 165 | 68.55 | 152 | 77.9 | 133 | 68.2 | 0.954 | ||||
| Nausea | 156 | 64.7 | 75 | 31.1 | 107 | 54.9 | 47 | 24.1 | 0.105 | ||||
| Vomiting | 79 | 32.8 | 34 | 14.1 | 68 | 34.9 | 33 | 16.9 | 0.418 | ||||
| Constipation | 118 | 48.9 | 32 | 13.3 | 103 | 52.8 | 27 | 13.8 | 0.863 | ||||
| Diarrhea | 72 | 29.9 | 27 | 11.2 | 63 | 32.3 | 19 | 9.7 | 0.662 | ||||
| Fatigue | 187 | 77.6 | 90 | 37.3 | 136 | 69.7 | 72 | 36.9 | 0.928 | ||||
| Neuropathy | 103 | 42.7 | 43 | 17.8 | 95 | 48.7 | 36 | 18.5 | 0.867 | ||||
| Allergy | 53 | 21.9 | 38 | 15.8 | 11 | 5.8 | 7 | 3.7 | <0.001 | ||||
Abbreviations: CP, carboplatin and paclitaxel; NP, nedaplatin and paclitaxel.
Evaluation of neuropathy, alopecia and gastrointestinal adverse events including nausea, vomiting, constipation and diarrhea were also performed between the CP arm and NP arm, which occurred at a similar incidence in the two treatment arms.
21.9% of cases in CP arm had hypersentivity reaction and the proportion in NP arm was 5.8% (P<0.001). These allergic reactions were all secondary to carboplatin or nedaplatin administration, because in condition of the allergy of paclitaxel, paclitaxel liposome will take place of common paclitaxel, which will not change the whole protocol. These allergic reactions caused by carboplatin led to a significantly higher rate of discontinuation of intented protocols in the CP arm versus NP arm (21.2% vs 3.1%; P<0.001).
Two deaths were reported, which were not considered to be directly caused by progressive disease: one as a result of severe pulmonary infection because of grade 4 neutropenia (in CP arm) and one as a result of intracranial hemorrhage in the context of grade IV thrombocytopenia (in CP arm).
Discussion
For platinum-sensitive recurrent ovarian cancer, chemotherapy is an important part of treatment. Now carboplatin plus paclitaxel and carboplatin plus doxorubicin are regarded as common choices for these population. Nedaplatin is the second-generation platinum derivative, which is proved to be effective in many solid tumors such as head and neck tumor, esophageal cancer, bladder cancer, small cell lung cancer, epithelial ovarian cancer and cervical cancer. Besides these, for partial patients with cisplatin resistance, nedaplatin may still be effective [15]. Takekuma et al carried out a phase II trial in patients with advanced/recurrent uterine cervical cancer and reported that combined chemotherapy of paclitaxel and nedaplatin (paclitaxel 175 mg/m2 and nedaplatin 80 mg/m2 every three weeks) had a response rate of 44.4% (11 complete responses and 8 partial responses), a median PFS of 7.5 months, and a median OS of 15.7 months in 45 patients with advanced or recurrent uterine cervical cancer [18]. However, studies about nedaplatin searched by Pubmed were limited and conclusions usually were set up with a small sample less than 50. In order to systematically evaluate the efficacy and safety of nedaplatin, we designed the retrospective cohort study.
The study contained 436 cases with efficacy and safety analysis of nedaplatin plus paclitaxel, which was the largest-scale study to evaluate the role of nedaplatin against platinum-sensitive recurrent ovarian cancer. The study met its primary objective, indicating statistically significant improvement in NP arm over CP arm in PFS for platinum-sensitive recurrent ovarian cancer when the recurrent interval was between 6 to 12 months (HR, 0.751; 95% CI, 0.557 to 1.013; P=0.048). Equally important was the reduction in severe toxicities associated with NP protocol, including hypersensitivity reactions and bone suppression, which can be dose limiting or protocol discontinuation.
In this cohort study, nedaplatin was proved to be an effective partnering agent for paclitaxel in the setting of platinum-sensitive recurrent ovarian cancer. The NP arm showed no inferiority in PFS to the CP arm (albeit not significant, an increase in median PFS by 1.5 months); median PFS in NP arm and CP arm was 11.0 versus 9.5 months, respectively. Subgroup analysis indicated that median PFS was 10.0 months with NP arm versus 8.0 months with CP arm when treatment-free interval was between 6 and 12 months; NP showed superiority in PFS versus the CP arm with an increase by 2 months (P=0.048); When recurrent interval was >12 months, median PFS in two arms was both 12 months; no statistical difference existed (P=0.543).
The results of our study add the evaluation of new chemotherapy protocol in platinum-sensitive population. About the treatment protocols against platinum-sensitive recurrence, there were several large scale clinical trials. Pujade-Lauraine carried out a randomized, multicenter, phase III trial (the CALYPSO trial) to evaluate the efficacy and safety of the combination of pegylated liposomal doxorubicin (PLD) with carboplatin (CD) compared with carboplatin and paclitaxel (CP) in patients with platinum-sensitive relapsed ovarian cancer. With a median follow-up of 22 months, PFS of the CD arm was statistically superior to the CP arm (HR, 0.821; 95% CI, 0.72 to 0.94; P<0.005); median PFS was respectively 11.3 months versus 9.4 months [4]. Pfisterer J designated the trial (An Intergroup Trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG) by comparing gemcitabine plus carboplatin with single-agent carboplatin in patients with platinum-sensitive recurrent ovarian cancer. With a median follow-up of 17 months, median PFS in group of combined chemotherapy and single carboplatin was respectively 8.6 months and 5.8 months, while myelosuppression was significantly more common in combined chemotherapy [3]. Besides the traditional chemotherapy agents, OCEANS (Ovarian Cancer Study Comparing Efficacy and Safety of Chemotherapy and Antiangiogenic Therapy in Platinum-Sensitive Recurrent Disease) elucidated the efficacy and safety of bevacizumab (BV) in platinum-sensitive recurrent ovarian, primary peritoneal, or fallopian tube cancer. In the OCEANS trial, the study group was GC (gemcitabine combined with carboplatin) plus BV or placebo. The PFS for the BV arm was superior to the placebo arm (HR, 0.484; 95% CI, 0.388 to 0.605; P<0.001); median PFS was respectively 12.4 months vs 8.4 months [19]. Taken together, these data provide robust evidence for combined chemotherapy in platinum-sensitive recurrent ovarian cancer and the range of PFS in above trials was 8.4 to 12.4 months. In our study, the PFS in NP arm was 11.0 months, which was similar to the PFS of other regimens in large clinical studies [17,20].
As the secondary end point, the overall survival in CP and NP arm were respectively 74 months and 73 months without significant difference. OS was not considered as the primary end point because of the possible bias caused by different chemotherapy protocols. Advantages of PFS as the primary end point are that it can objectively reflect tumor shrinkage, stabilization or progression effects, which avoids the confounding impact of subsequent treatment.
Most women with ovarian cancer would experience repeated recurrence, safety was as important as efficacy in assessing the role of a novel combination. Our study showed that the NP protocol was associated with less severe toxicities than CP regimen. CP group had more grade 3-4 neutropenia (34% vs 13%) and thrombocytopenia (15% vs 5.6%) than NP protocol. As to anemia and febrile neutropenia, no significant difference existed between two arms. There were no statistical difference in term of neuropathy and gastrointestinal toxicities, such as, nausea, vomiting, constipation, diarrhea. Grade 3-4 thrombocytopenia and hypersentivity reaction may cause dose limiting, prolonged treatment interval or protocol discontinuation. Grade 3-4 thrombocytopenia commonly needed a longer time to relieve, which might prolong the treatment interval. The proportions of allergy in NP arm and CP arm were respectively 21.9% and 5.8% with significant statistics, which were caused by carboplatin or nedaplatin. Because of the high incidence of hypersensitivity, the proportion of cycle cancellation in CP arm was significantly higher than that in NP arm.
Our study confirmed the efficacy and safety of nedaplatin in platinum-sensitive recurrent ovarian cancer. The study was a retrospective cohort study, so observational bias may exist. The results indicated NP protocol showed superiority in PFS than CP protocol and this may be associated with no cross resistance between carboplatin and nedaplatin which still need further study to verify.
Acknowledgements
Supported by the National Key Research and Development Program of China: 2016YFC1303704.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, Wagner U, Stähle A, Stuart G, Kimmig R, Olbricht S, Le T, Emerich J, Kuhn W, Bentley J, Jackisch C, Lück HJ, Rochon J, Zimmermann AH, Eisenhauer E AGO-OVAR; NCIC CTG; EORTC GCG. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: An intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J. Clin. Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 4.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, Volgger B, Vergote I, Pignata S, Ferrero A, Sehouli J, Lortholary A, Kristensen G, Jackisch C, Joly F, Brown C, Le Fur N, du Bois A. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J. Clin. Oncol. 2010;28:3323–3329. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 5.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, Tropé C ICON and AGO Collaborators. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 6.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R Gynecologic Oncology Group. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J. Clin. Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 7.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, Tropé C ICON and AGO Collaborators. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 8.Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, Belinson J. Clinical features of hypersensitivity reactions to carboplatin. J. Clin. Oncol. 1999;17:1141–1145. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 9.Koshiba H, Hosokawa K, Kubo A, Miyagi Y, Oda T, Miyagi Y, Watanabe A, Honjo H. Incidence of carboplatin-related hypersensitivity reactions in Japanese patients with gynecologic malignancies. Int J Gynecol Cancer. 2009;19:460–465. doi: 10.1111/IGC.0b013e3181a1bf2e. [DOI] [PubMed] [Google Scholar]
- 10.Kawai Y, Taniuchi S, Okahara S, Nakamura M, Gemba M. Relationship between cisplatin or nedaplatin-induced nephrotoxicity and renal accumulation. Biol Pharm Bull. 2005;28:1385–1388. doi: 10.1248/bpb.28.1385. [DOI] [PubMed] [Google Scholar]
- 11.Alberts DS, Fanta PT, Running KL, Adair LP, Garcia DJ, Liu-Stevens R, Salmon SE. In vitro phase II comparison of the cytotoxicity of a novel platinum analog, nedaplatin (254-S), with that of cisplatin and carboplatin against fresh, human ovarian cancers. Cancer Chemother Pharmacol. 1997;39:493–497. doi: 10.1007/s002800050604. [DOI] [PubMed] [Google Scholar]
- 12.Kato T, Nishimura H, Yakushiji M, Noda K, Terashima Y, Takeuchi S, Takamizawa H, Suzuki M, Arai M, Ota M. Phase II study of 254-S (cisdiammine glycolato platinum) for gynecological cancer. Gan to Kagaku Ryoho. 1992;19:695–701. [PubMed] [Google Scholar]
- 13.Noda K, Ikeda M, Yakushiji M, Nishimura H, Terashima Y, Sasaki H, Hata T, Kuramoto H, Tanaka K, Takahashi T, et al. A phase II clinical study of cis-diammine glycolato platinum, 254-S, for cervical cancer of the uterus. Gan To Kagaku Ryoho. 1992;19:885–892. [PubMed] [Google Scholar]
- 14.Gao H, Yuan L, Han Y. Comparisons of the survival time of patients with ovarian cancer adopting post-operative chemotherapy by use of paclitaxel combined with carboplatin or nedaplatin. World J Surg Oncol. 2016;14:168. doi: 10.1186/s12957-016-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P, Feng FY, Wu LY, Hu Y, Liu JW, Gao YJ, Guan XQ, Nan KJ, Suo AL, Wang XW, Zhang MH, Zhang WD, Li CW, Zhang Y, Zhao JB. Phase II multicenter clinical trial of nedaplatin in the treatment of malignant tumors. Chin J Oncol. 2006;28:230–234. [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in tumors: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, du Bois A, Kristensen G, Jakobsen A, Sagae S, Greven K, Parmar M, Friedlander M, Cervantes A, Vermorken J Gynecological Cancer Intergroup. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 18.Takekuma M, Hirashima Y, Ito K, Tsubamoto H, Tabata T, Arakawa A, Itani Y, Furukawa N, Murakoshi H, Takeuchi S. Phase II trial of paclitaxel and nedaplatin in patients with advanced/recurrent uterine cervical cancer: a Kansai Clinical Oncology Group study. Gynecol Oncol. 2012;126:341–345. doi: 10.1016/j.ygyno.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MA, Yi J, Nycum LR. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, Volgger B, Vergote I, Pignata S, Ferrero A, Sehouli J, Lortholary A, Kristensen G, Jackisch C, Joly F, Brown C, Le Fur N, du Bois A. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J. Clin. Oncol. 2010;28:3323–3329. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]


