Abstract
Pregnancy-associated plasma protein A (PAPPA) is a protease that plays important roles in pregnancy, but interestingly acts as an oncogene outside of pregnancy. This review summarizes the oncogenic roles of PAPPA, including its expression levels in multiple malignancies, regulatory and signaling interactions, and pro-tumor functions, which include promoting tumor cell proliferation, invasion, migration and metastasis. These PAPPA activities are linked to IGFBP-4 proteolysis, increased IFG bioavailability, and activation of the NF-κB, PI3K/AKT and ERK signaling pathways. Therefore, PAPPA could be used as a biomarker for monitoring cancer development and progression as well as a potential therapeutic target.
Keywords: PAPPA, cancer, proliferation, regulation, prognosis
Introduction
Pregnancy-associated plasma protein A (PAPPA) was originally found in the plasma of pregnant women [1]. During pregnancy, PAPPA is produced by placental syncytiotrophoblasts and secreted into the serum, and its levels are maintained throughout pregnancy. Aberrant PAPPA levels in circulation are associated with fetal diseases; for instance, low PAPPA levels are associated with fetal Down’s syndrome (trisomy 21), premature birth, low birth weight, and first trimester pre-eclampsia. Conversely, PAPPA levels are increased in diabetic nephropathy patients [1].
Recent studies have clarified the biological functions of PAPPA, which is located on chromosome 9q33.1 and encodes a 1627 amino acid protein of 180 kDa [2]. PAPPA contains structural motifs of the metzincin superfamily, which coordinate the catalytically-essential zinc ion and Met-turn, a methionine-containing 1,4-β-turn [3]. Further structural analysis has revealed that PAPPA is the founding member of a new metzincin subfamily, termed the pappalysins. Thus, PAPPA is a metalloproteinase that cleaves insulin-like growth factor binding proteins (IGFBPs) [4]. The cleavage of IGFBPs leads to the separation of insulin-like growth factors (IGF) from the IGF-IGFBP complex, allowing free IGF to bind IGF receptors and activate the IGF pathway.
Accumulating evidence has demonstrated the proteolytic activity of PAPPA against IGFBP-4 in various tissues and cell types, such as ovarian, smooth muscle, lung and osteoblast cells; for this reason PAPPA is also termed insulin-like growth factor-dependent IGFBP4 protease (IGFBP4 protease) [5]. While PAPPA is ubiquitously expressed in all normal tissues, its levels are very low except in kidney and bone cells [6]. Studies using PAPPA knockout/transgenic mouse models and in vitro cell culture systems have demonstrated that PAPPA is an important regulator of local IGF bioavailability [7]. Additionally, PAPPA plays important roles in bone formation, and inflammatory and injury responses (e.g. wound healing) [8]. Interestingly, recent studies have demonstrated that PAPPA is overexpressed in various tumor types compared with adjacent non-tumor tissues, and that PAPPA overexpression promotes tumor growth and invasion, suggesting PAPPA has oncogenic activity.
Increased PAPPA expression in malignancies
It has been reported that PAPPA is overexpressed in ovarian cancer, along with IGF, IGFBP4 and IGFBP3 [9]. Interestingly, PAPPA and IGF signaling constituents are also present in ascites fluid, and the expression levels of these proteins are associated with the volume of ascites fluid; thus, PAPPA could be an independent prognostic factor for ovarian cancer progression and clinical responses to chemotherapy [10-12]. PAPPA is also strongly expressed in granulosa cell tumors of the ovary, compared with normal ovarian tissues; however, in this tumor type, IGF-I and IGF-II expression did not correspond with PAPPA, but were lower, as was IFGBP4, indicating that PAPPA has tumor-promoting functions independent from IGFBP4 proteolysis in ovarian granulosa cell tumors [9].
Clinical studies of lung cancer have shown that serum PAPPA levels are significantly higher in lung cancer patients compared with healthy subjects [13]. Another study further showed that in advanced lung cancer patients, PAPPA levels were increased 47-fold in the pleural fluid compared with plasma PAPPA levels. Additionally, interleukin 6 (IL-6) was increased more than one hundred folds in the pleural fluid; thus, there was a positive correlation between PAPPA and IL-6 [14].
PAPPA has also been well studied in breast cancer, where it was shown to be overexpressed, particularly in luminal B breast cancer, which has a higher proliferation index than luminal A breast cancer [15], suggesting the tumor-promoting role of PAPPA in breast cancer. However, a recent study reported that PAPPA is epigenetically silenced in human breast cancers. They found that PAPPA silencing was highly prevalent in precursor lesions and invasive breast cancer. Experimental studies manipulating PAPPA expression have shown that increased PAPPA expression suppresses mitosis, but that downregulating PAPPA made breast cancer cells more aggressive [16]. Previous studies have also shown that PAPPA is overexpressed in breast cancer, and that PAPPA expression is significantly correlated with early recurrence. Interestingly, PAPPA positivity is independent of estrogen receptor status and is a clinically significant independent predictor of early recurrence for stage I and II breast cancer [17-19]. These findings suggest a tumor-promoting role for PAPPA in breast cancer.
Ewing sarcoma is one of most malignant bone tumors and is associated with early metastasis. It has been reported that IGF-PAPPA signaling stimulates normal bone growth, and that it is also involved in Ewing sarcoma development, in which PAPPA is frequently overexpressed, and is required for tumor cell proliferation. Therefore, PAPPA is a good target for TCR-based immunotherapy for Ewing sarcoma [20].
In men, PAPPA is also expressed in Leydig, epididymis, rete testis, and seminal vesicle cells. Circulating PAPPA levels are associated testicular and prostate cancer. PAPPA levels can decrease to normal after orchidectomy or prostatectomy [21].
Deeply mining online gene profiling data (www.oncomine.org), we found that PAPPA expression levels were also significantly increased in other malignancies. For example, PAPPA was significantly increased in hepatocellular carcinoma (HCC) compared with normal liver cells [22] (Figure 1A), was increased in focal nodular hyperplasia in livers [22] (Figure 1B). Moreover, PAPPA mRNA levels were significantly increased in prostate cancer [23] (Figure 1C), pancreatic cancer [24] (Figure 1D), thyroid carcinoma (Figure 1E), leukemia [25] (Figure 1F), lymphoma [26] (Figure 1G) and skin basal cell carcinoma [27] (Figure 1H), compared to their matched non-tumor tissues. The clinical significance of PAPPA expression levels in these malignancies are still under investigation.
Figure 1.
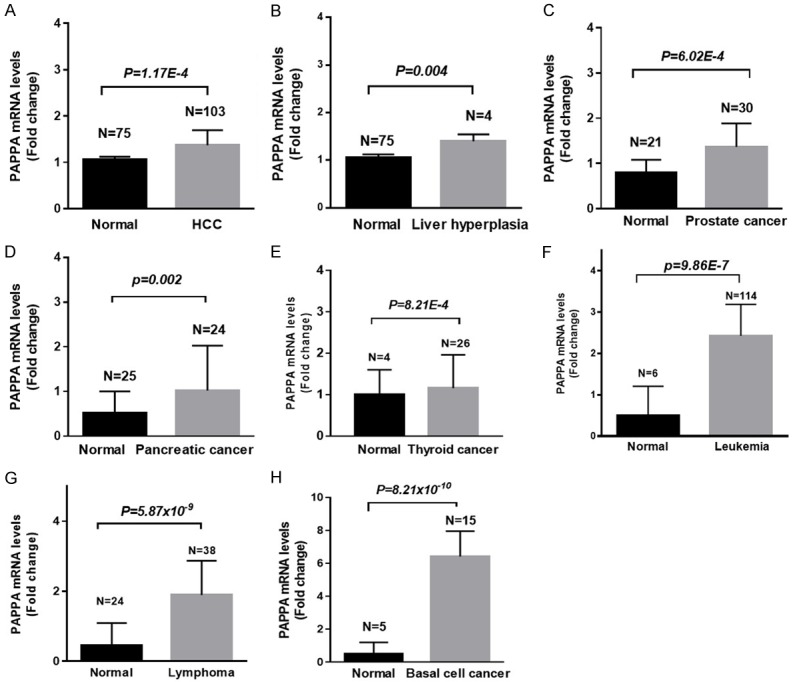
The expression levels of PAPPA in HCC (A), focal nodular liver hyperplasia (B), prostate cancer (C), pancreatic cancers (D), thyroid cancer (E), leukemia (F), lymphoma (G) and skin basal cell carcinoma (H).
Oncogenic roles of PAPPA
As addressed above, PAPPA overexpression is observed in many malignancies, where it is associated with poor outcomes, demonstrating its clinical significance in tumors. Moreover, accumulating evidence has also shown the oncogenic functions of PAPPA, including promoting cancer cell proliferation, migration, invasion and metastasis; thus, PAPPA may be a target for chemotherapy.
The tumor-promoting functions of PAPPA were first observed in the ovarian cancer cell line SKOV3 [28], which has a very low tumorigenic potential. However, increased PAPPA expression in this cell line via PAPPA transfection increased cell proliferation, anchorage-independent growth on soft agar gel, and cell invasion through Matrigel, compared with vector control-transfected cells. Importantly, cells transfected with a mutant PAPPA plasmid also showed tumor-promoting functions, but the degree of colony formation in soft agar gel and invasion through Matrigel was not as high as the cells transfected with wild-type PAPPA, were more similar to vector controls. The tumorigenic capability of PAPPA was also investigated in vivo [28]. These experiments showed that PAPPA overexpression in SKOV3 cells dramatically increased the tumor formation rate in nude mice compared with cells transfection with mutant PAPPA or vector control, despite xenograft tumor cells showing similar PAPPA mRNA and protein levels in the wild-type and mutant PAPPA groups. Given that angiogenesis has critical functions in tumorigenesis and progression, the effects of PAPPA on angiogenesis were also investigated. These results showed that increased PAPPA expression was associated with mature tumor vasculature formation in xenografts and increased vessel area per micrograph in early-stage tumor formation [28].
In contrast, decreased PAPPA expression was shown to inhibit ovarian cancer cell growth, invasion and metastasis [29]. First, both PAPPA mRNA and protein levels were significantly upregulated in intraperitoneal disseminated metastatic tumors after the intraperitoneal inoculation of human ovarian HRA cancer cells into nude mice. However, PAPPA anti-sense-expressing HRA cells showed reduced PAPPA expression levels and markedly inhibited reduced tumor growth in mice. Moreover, administering neutralizing PAPPA antibody could also repress PAPPA expression and tumor growth in vivo in terms of suppressing HRA cell proliferation, invasion and metastasis in nude mice. Furthermore, ovarian cancer cells transfected with PAPPA antisense or treated with PAPPA antibody showed downregulation of IGF-I and AKT/ERK1/2, which are stimulated by IGF-I, as well as a downregulation of uPA and an upregulation of IGFBP-4, a direct PAPPA target.
The tumor-promoting activity of PAPPA is also observed in non-small cell lung cancer, but PAPPA secretion is required for this activity [30]. In vitro studies have shown that PAPPA is secreted by human lung cancer cells but not immortalized normal bronchial epithelial cells. To determine the biological functions of PAPPA in lung cancer cells, PAPPA was knocked down by shRNA in A549 cells. Interestingly, decreasing PAPPA expression did not affect the proliferation of A549 cells in vitro, but did significantly inhibit xenograft growth, in terms of lower tumor weights compared with the vector control group. Interestingly, PAPPA-overexpressing H1299 lung cancer cells caused elevated serum PAPPA levels, which promoted xenograft growth in nude mice, compared with the vector control cells. However, PAPPA-overexpressing H1792 lung cancer cells that did not elevate serum PAPPA levels did not show differences in xenograft growth compared with vector control cells [30]. These findings strongly suggested the essential role of secreted PAPPA for lung cancer growth and progression. Further mechanistic studies indicated that PAPPA-mediated tumor promotion was mainly through IGF pathway activation, which increased AKT phosphorylation.
Clinical studies have shown the increased incidence of melanoma in pregnant women. Moreover, a recent study demonstrated elevated IGF-PAPPA signaling in melanoma tissues and metastatic tumors, suggesting the increased PAPPA levels during pregnancy play critical roles in melanoma formation and metastasis, a hypothesis that has also been supported by in vitro studies [31]. First, silencing PAPPA by siRNA or anti-PAPPA antibody in melanoma cells significantly inhibited tumor cell invasion and migration in vitro and decreased the invasion and migration of melanoma cells in an embryonic chicken model. In contrast, increased PAPPA expression in melanoma cells enhanced cell migration compared with cells with lower PAPPA expression. Interestingly, the migratory capability of melanoma cells was also enhanced after adding PAPPA-rich human pregnant serum in vitro, and this enhancement could be neutralized by an anti-PAPPA antibody [31]. Moreover, another recent study showed lactation attenuated PAPPA-driven pregnancy-associated breast cancer in a PAPPA-transgenic mouse model of breast cancer [32]. This study showed that PAPPA was a pregnancy-dependent oncogene, and that PAPPA overexpression in the transgenic murine mammary led to collagen deposition, which also promoted PAPPA-mediated IGFBP-4 and IGFBP-5 proteolysis, causing proliferative signaling during pregnancy. However, lactation prolonged the time for PAPPA-transgenic mice to not develop mammary tumors, which was through dysregulation of tumor-associated collagen signature (TACS-3) and PAPPA inhibitors STC1 and STC2 [32]. Therefore, extended lactation time is protective against PAPPA-mediated mammary carcinogenesis.
Malignant pleural mesothelioma (MPM) is an extremely deadly disease, the underlying mechanisms of development and progression of which are unclear. However, recent studies have identified that PAPPA is involved in this malignancy [33]. Although PAPPA is differentially expressed in multiple MPM cell lines, its expression is correlated with the migratory ability of MPM cells; cell lines with higher PAPPA expression showed more aggressive features. Additionally, secreted PAPPA protein levels from MPM cells are positively correlated with their migration ability. Similar as seen in other malignancies where higher PAPPA expression was accompanied with elevated IGF-I and IGF-IR and reduced of IGFBP-4. Its migration-promotion activity was also through IGF signaling. For instance, IGF-I recombinant protein could stimulate melanoma cell migration, but this stimulating effect could be blocked by IGFBP-4, while the addition of PAPPA could rescue IGF-I-stimulated migration of MPM cells. Conversely, silencing PAPPA significantly suppressed MPM cell proliferation and migration in vitro and suppressed the metastasis of orthotopic xenografts in nude mice [33]. These findings suggest the oncogenic roles of PAPPA in MPM, such as promoting proliferation, invasion and migration. Thus, PAPPA could be used as a therapeutic target to prevent MPM progression.
Inter-cellular communication between epithelial cells and stromal cells is vital for cancer cell growth and motility. A recent study using computation-based systemic analysis showed that PAPPA is a tumor-promoting stromal factor that maintains hepatocellular cancer cells in vitro via activating NF-κB signaling, promoting hepatocellular carcinoma progression [34].
The oncogenic functions of PAPPA were further supported by the PAPPA-deficient mouse model [35]. In general, there were fewer pathologies in PAPPA knockout mice compared with wild-type mice at different ages. Wild-type mice exhibited more age-related degenerative lesions and tumors, and an earlier onset of these lesions compared with PAPPA knockout mice. Particularly, wild-type mice showed more hyperplasia in the thyroid, pancreatic islets, adrenals and uterus, and had pituitary adenomas and lymphomas at earlier ages than in the PAPPA knockout mice. Additionally, PAPPA knockout mice had extended healthy lifespans, with reduced incidence and delayed occurrence of spontaneous tumor formation [35].
As PAPPA promotes carcinogenesis and malignant progression, it has been used as a therapeutic target for cancers. As addressed above, several antibodies and small molecules against PAPPA could repress it expression, activity and secretion. These treatments reduce IGFBP-4 proteolysis, and subsequently inhibit activation of downstream elements, e.g. decreased IGF-I and IGF-IR expression and reduced AKT and ERK1/2 phosphorylation [11,36-38].
Interactive regulation of PAPPA
IGF signaling includes IGF-I, IGF-II, their receptors IGF-IR and IGF-IIR, and several IGF binding proteins (e.g. IGFBP-4, IFGBP-5), and plays important roles in carcinogenesis and chemotherapy responses. PAPPA is a metalloprotease that is involved in the proteolysis of IGFBP-4 and IGFBP-5. Upon IGF released from IGF-IGFBP complexes, IGF binds to IGF-IR, rapidly increasing IGF bioavailability, and leading to the activation of many signaling pathways. This results in increased cell proliferation, motility and decreased apoptosis, which promote carcinogenesis and malignant transformation. Therefore, PAPPA plays crucial roles in IGF signaling [8].
The regulation of PAPPA has not been well studied. To date, a few reports have investigated the underlying mechanisms of PAPPA regulation. These studies have demonstrated that pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) and IL-1β are the greatest stimulators of PAPPA expression in dermal fibroblasts, arterial endothelial cells and smooth muscle cells in vitro. IL-6 and transforming growth factor-beta (TGF-β) can also stimulate PAPPA expression [1]. Mechanistic studies have revealed that cytokine-induced PAPPA expression is through NF-κB activation, as there are three potential NF-κB binding sites upstream of PAPPA [39].
Recent studies have also revealed that PAPPA can be transcriptionally regulated by p53 [8]. One study identified a p53-binding site in intron 1 of PAPPA; therefore, wild-type p53 can bind to this site and transcriptionally suppress PAPPA, which blocks IFGBP4 proteolysis and reduces IGF bioavailability, leading to decreased IGF signaling and cell cycle arrest and apoptosis, which preserves cellular homeostasis. Interestingly, mutant-p53 can also bind to this site within intron 1, but this binding activates PAPPA expression, leading to an enhancement of IFGBP-4 proteolysis and an induction of IGF bioavailability. Ultimately this leads to increased IGF signaling and increased proliferation and decreased apoptosis [8,40,41].
Previous studies have demonstrated that Bikunin is a Kunitz-type protease inhibitor that inhibits inflammatory responses by suppressing the induction of pro-inflammatory cytokines [42,43], and represses cancer cell invasion and metastasis [44]. High throughput screening by cDNA microarray indicated that PAPPA was downregulated by Bikunin [45]. Genetic knockdown of PAPPA significantly inhibited the invasive properties of ovarian cancer [45]. Therefore, PAPPA could be regulated by Bikunin, and Bikunin-mediated inhibition of cancer cell invasion is PAPPA-dependent.
MicroRNAs (miRNAs) have been reported to be important regulators of gene expression at both the translational and transcriptional levels. Recent studies have shown that PAPPA could be regulated by miRNAs. For instance, a miRNA array and target prediction analysis shows that PAPPA is a target of miR-490-3p, and that a miR-490-3p mimic could inhibit PAPPA upregulation in human coronary artery smooth muscle cells (hCASMCs), resulting in the reduction of PAPPA protease activity on IGFBP-4, which will eventually cause the inhibition of hCASMC proliferation [46]. Moreover, PAPPA is also a direct target of miR-141 [47], showing that miR-141-inhibited vascular smooth muscle cell proliferation goes via targeting PAPPA, while vice versa, PAPPA overexpression attenuated miR-141-induced inhibition of proliferation. In non-small cell lung cancer cells [48], increased miR-214 expression led to decreased invasiveness, while inversely, decreasing miR-214 expression increased cancer cell invasion. Gene profiling and bioinformatic analyses showed that miR-214-mediated inhibition of invasion was through downregulating the metastasis-associated genes PAPPA and α protein kinase 2 (ALPK2); because both PAPPA and ALPK2 are the direct targets of miR-214 [48].
Conclusions
Beyond pregnancy, PAPPA is overexpressed in multiple malignancies and acts as an oncogene. PAPPA could be regulated by pro-inflammatory cytokines, p53 and bikunin, as well as by miRNAs at the transcriptional and translational levels. PAPPA promotes carcinogenesis and malignant progression and exhibits oncogenic functions (such as promoting cancer cell proliferation, invasion, migration and metastasis) in vitro and in vivo. These activities are linked to IGFBP-4 proteolysis and to increased IFG bioavailability and NF-kB, PI3K/AKT and ERK pathways activation (summarized in Figure 2). Therefore, PAPPA could be a biomarker for monitoring cancer formation and progression and could also be used as a target for cancer prevention and therapy.
Figure 2.
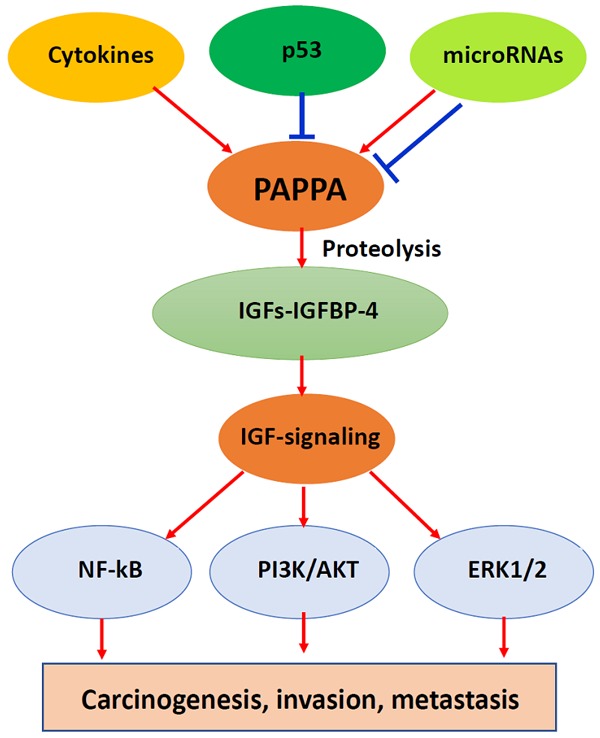
Illustration of the biological functions of PAPPA and signaling pathways in carcinogenesis, invasion and metastasis.
Acknowledgements
This work was supported in part by the grants from the National Nature Science Foundation of China (grant 81672750 to Yang W, grant 81502105 to Bao Y), the grant from the Nature Science Foundation of Shandong Province (grant ZR2016HM36 to Guo Y) and National Nature Science Foundation Cultivation Project of Jining Medical University (grant to Guo Y) and the grant for the Key Laboratory of Higher Education Institutes of Shandong Province, China. We thank James P. Mahaffey, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Disclosure of conflict of interest
None.
Abbreviations
- PAPPA
Pregnancy-associated plasma protein A
- IGF
Insulin-like growth factor
- IGFBP4
IGF-binding protein 4
- TNF-α
tumor necrosis factor α
- TGF-beta
transforming growth factor beta
- IL
interleukin
References
- 1.Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab. 2012;23:242–249. doi: 10.1016/j.tem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kristensen T, Oxvig C, Sand O, Moller NP, Sottrup-Jensen L. Amino acid sequence of human pregnancy-associated plasma protein-A derived from cloned cDNA. Biochemistry. 1994;33:1592–1598. doi: 10.1021/bi00172a040. [DOI] [PubMed] [Google Scholar]
- 3.Overgaard MT, Sorensen ES, Stachowiak D, Boldt HB, Kristensen L, Sottrup-Jensen L, Oxvig C. Complex of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein. Disulfide structure and carbohydrate attachment. J Biol Chem. 2003;278:2106–2117. doi: 10.1074/jbc.M208777200. [DOI] [PubMed] [Google Scholar]
- 4.Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504:36–40. doi: 10.1016/s0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- 5.Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res. 2007;17:10–18. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Fuchtbauer EM, Oxvig C, van Deursen J. Metalloproteinase pregnancyassociated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- 7.Conover CA, Bale LK. Loss of pregnancyassociated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–729. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 8.Conover CA. The IGF-p53 connection in cancer. Growth Horm IGF Res. 2018;39:25–28. doi: 10.1016/j.ghir.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Alexiadis M, Mamers P, Chu S, Fuller PJ. Insulin-like growth factor, insulin-like growth factor-binding protein-4, and pregnancy-associated plasma protein-A gene expression in human granulosa cell tumors. Int J Gynecol Cancer. 2006;16:1973–1979. doi: 10.1111/j.1525-1438.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalli KR, Chen BK, Bale LK, Gernand E, Overgaard MT, Oxvig C, Cliby WA, Conover CA. Pregnancy-associated plasma protein-A (PAPPA) expression and insulin-like growth factor binding protein-4 protease activity in normal and malignant ovarian surface epithelial cells. Int J Cancer. 2004;110:633–640. doi: 10.1002/ijc.20185. [DOI] [PubMed] [Google Scholar]
- 11.Becker MA, Haluska P Jr, Bale LK, Oxvig C, Conover CA. A novel neutralizing antibody targeting pregnancy-associated plasma protein-A inhibits ovarian cancer growth and ascites accumulation in patient mouse tumorgrafts. Mol Cancer Ther. 2015;14:973–981. doi: 10.1158/1535-7163.MCT-14-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomsen J, Hjortebjerg R, Espelund U, Ortoft G, Vestergaard P, Magnusson NE, Conover CA, Tramm T, Hager H, Hogdall C, Hogdall E, Oxvig C, Frystyk J. PAPP-A proteolytic activity enhances IGF bioactivity in ascites from women with ovarian carcinoma. Oncotarget. 2015;6:32266–32278. doi: 10.18632/oncotarget.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulut I, Coskun A, Ciftci A, Cetinkaya E, Altiay G, Caglar T, Gulcan E. Relationship between pregnancy-associated plasma protein-A and lung cancer. Am J Med Sci. 2009;337:241–244. doi: 10.1097/MAJ.0b013e31818967a3. [DOI] [PubMed] [Google Scholar]
- 14.Espelund US, Bjerre M, Hjortebjerg R, Rasmussen TR, Lundby A, Hoeflich A, Folkersen BH, Oxvig C, Frystyk J. Insulin-like growth factor bioactivity, stanniocalcin-2, pregnancy-associated plasma protein-A, and IGF-binding protein-4 in pleural fluid and serum from patients with pulmonary disease. J Clin Endocrinol Metab. 2017;102:3526–3534. doi: 10.1210/jc.2017-00033. [DOI] [PubMed] [Google Scholar]
- 15.Mansfield AS, Visscher DW, Hart SN, Wang C, Goetz MP, Oxvig C, Conover CA. Pregnancyassociated plasma protein-A expression in human breast cancer. Growth Horm IGF Res. 2014;24:264–267. doi: 10.1016/j.ghir.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loddo M, Andryszkiewicz J, Rodriguez-Acebes S, Stoeber K, Jones A, Dafou D, Apostolidou S, Wollenschlaeger A, Widschwendter M, Sainsbury R, Tudzarova S, Williams GH. Pregnancy-associated plasma protein A regulates mitosis and is epigenetically silenced in breast cancer. J Pathol. 2014;233:344–356. doi: 10.1002/path.4393. [DOI] [PubMed] [Google Scholar]
- 17.Kuhajda FP, Abeloff MD, Eggleston JC. Pregnancy-associated plasma protein A: a clinically significant predictor of early recurrence in stage II breast carcinoma. Hum Pathol. 1985;16:228–235. doi: 10.1016/s0046-8177(85)80007-1. [DOI] [PubMed] [Google Scholar]
- 18.Kuhajda FP, Eggleston JC. Pregnancy-associated plasma protein A. A clinically significant predictor of early recurrence in stage I breast carcinoma is independent of estrogen receptor status. Am J Pathol. 1985;121:342–348. [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhajda FP, Eggleston JC. Pregnancy-associated plasma protein A and extensive necrosis. Clinically significant predictors of early recurrence in stage I estrogen receptor-negative breast carcinoma. Lab Invest. 1985;53:101–107. [PubMed] [Google Scholar]
- 20.Kirschner A, Thiede M, Grunewald TG, Alba Rubio R, Richter GH, Kirchner T, Busch DH, Burdach S, Thiel U. Pappalysin-1 T cell receptor transgenic allo-restricted T cells kill Ewing sarcoma in vitro and in vivo. Oncoimmunology. 2017;6:e1273301. doi: 10.1080/2162402X.2016.1273301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bischof P, Megevand M. Pregnancy-associated plasma protein-A concentrations in men with testicular and prostatic tumors. Arch Androl. 1986;16:155–160. doi: 10.3109/01485018608986936. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Cheung ST, So S, Fan ST, Barry C, Higgins J, Lai KM, Ji J, Dudoit S, Ng IO, Van De Rijn M, Botstein D, Brown PO. Gene expression patterns in human liver cancers. Mol Biol Cell. 2002;13:1929–1939. doi: 10.1091/mbc.02-02-0023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, Kalyana-Sundaram S, Wei JT, Rubin MA, Pienta KJ, Shah RB, Chinnaiyan AM. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa M, Yoshida K, Yamashita Y, Ota J, Takada S, Kisanuki H, Koinuma K, Choi YL, Kaneda R, Iwao T, Tamada K, Sugano K, Mano H. Experimental trial for diagnosis of pancreatic ductal carcinoma based on gene expression profiles of pancreatic ductal cells. Cancer Sci. 2005;96:387–393. doi: 10.1111/j.1349-7006.2005.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson A, Ritz C, Lindgren D, Eden P, Lassen C, Heldrup J, Olofsson T, Rade J, Fontes M, Porwit-Macdonald A, Behrendtz M, Hoglund M, Johansson B, Fioretos T. Microarray-based classification of a consecutive series of 121 childhood acute leukemias: prediction of leukemic and genetic subtype as well as of minimal residual disease status. Leukemia. 2007;21:1198–1203. doi: 10.1038/sj.leu.2404688. [DOI] [PubMed] [Google Scholar]
- 26.Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, Bertoni F, Ponzoni M, Scandurra M, Califano A, Bhagat G, Chadburn A, Dalla-Favera R, Pasqualucci L. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riker AI, Enkemann SA, Fodstad O, Liu S, Ren S, Morris C, Xi Y, Howell P, Metge B, Samant RS, Shevde LA, Li W, Eschrich S, Daud A, Ju J, Matta J. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boldt HB, Conover CA. Overexpression of pregnancy-associated plasma protein-A in ovarian cancer cells promotes tumor growth in vivo. Endocrinology. 2011;152:1470–1478. doi: 10.1210/en.2010-1095. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y, Kobayashi H, Suzuki M, Hirashima Y, Kanayama N, Terao T. Genetic downregulation of pregnancy-associated plasma protein-A (PAPP-A) by bikunin reduces IGF-I-dependent Akt and ERK1/2 activation and subsequently reduces ovarian cancer cell growth, invasion and metastasis. Int J Cancer. 2004;109:336–347. doi: 10.1002/ijc.11700. [DOI] [PubMed] [Google Scholar]
- 30.Pan H, Hanada S, Zhao J, Mao L, Ma MZ. Protein secretion is required for pregnancy-associated plasma protein-A to promote lung cancer growth in vivo. PLoS One. 2012;7:e48799. doi: 10.1371/journal.pone.0048799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prithviraj P, Anaka M, McKeown SJ, Permezel M, Walkiewicz M, Cebon J, Behren A, Jayachandran A. Pregnancy associated plasma protein-A links pregnancy and melanoma progression by promoting cellular migration and invasion. Oncotarget. 2015;6:15953–15965. doi: 10.18632/oncotarget.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takabatake Y, Oxvig C, Nagi C, Adelson K, Jaffer S, Schmidt H, Keely PJ, Eliceiri KW, Mandeli J, Germain D. Lactation opposes pappalysin-1-driven pregnancy-associated breast cancer. EMBO Mol Med. 2016;8:388–406. doi: 10.15252/emmm.201606273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Tabata S, Kakiuchi S, The Van T, Goto H, Hanibuchi M, Nishioka Y. Identification of pregnancy-associated plasma protein A as a migration-promoting gene in malignant pleural mesothelioma cells: a potential therapeutic target. Oncotarget. 2013;4:1172–1184. doi: 10.18632/oncotarget.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engelmann JC, Amann T, Ott-Rotzer B, Nutzel M, Reinders Y, Reinders J, Thasler WE, Kristl T, Teufel A, Huber CG, Oefner PJ, Spang R, Hellerbrand C. Causal Modeling of cancer-stromal communication identifies PAPPA as a novel stroma-secreted factor activating NFkappaB signaling in hepatocellular carcinoma. PLoS Comput Biol. 2015;11:e1004293. doi: 10.1371/journal.pcbi.1004293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ. Longevity and agerelated pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerontol A Biol Sci Med Sci. 2010;65:590–599. doi: 10.1093/gerona/glq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhabin SG, Gorin VS, Judin NS. Review: immunomodulatory activity of pregnancy-associated plasma protein-A. J Clin Lab Immunol. 2003;52:41–50. [PubMed] [Google Scholar]
- 37.Yunusova NV, Villert AB, Spirina LV, Frolova AE, Kolomiets LA, Kondakova IV. Insulin-like growth factors and their binding proteins in tumors and ascites of ovarian cancer patients: Association with response to neoadjuvant chemotherapy. Asian Pac J Cancer Prev. 2016;17:5315–5320. doi: 10.22034/APJCP.2016.17.12.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikkelsen JH, Resch ZT, Kalra B, Savjani G, Kumar A, Conover CA, Oxvig C. Indirect targeting of IGF receptor signaling in vivo by substrate-selective inhibition of PAPP-A proteolytic activity. Oncotarget. 2014;5:1014–1025. doi: 10.18632/oncotarget.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 40.Ryan AJ, Napoletano S, Fitzpatrick PA, Currid CA, O’Sullivan NC, Harmey JH. Expression of a protease-resistant insulin-like growth factor-binding protein-4 inhibits tumour growth in a murine model of breast cancer. Br J Cancer. 2009;101:278–286. doi: 10.1038/sj.bjc.6605141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chander H, Halpern M, Resnick-Silverman L, Manfredi JJ, Germain D. Skp2B overexpression alters a prohibitin-p53 axis and the transcription of PAPP-A, the protease of insulin-like growth factor binding protein 4. PLoS One. 2011;6:e22456. doi: 10.1371/journal.pone.0022456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodt P, Fallavollita L, Khatib AM, Samani AA, Zhang D. Cooperative regulation of the invasive and metastatic phenotypes by different domains of the type I insulin-like growth factor receptor beta subunit. J Biol Chem. 2001;276:33608–33615. doi: 10.1074/jbc.M102754200. [DOI] [PubMed] [Google Scholar]
- 43.Fries E, Blom AM. Bikunin--not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000;32:125–137. doi: 10.1016/s1357-2725(99)00125-9. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi H, Suzuki M, Tanaka Y, Kanayama N, Terao T. A Kunitz-type protease inhibitor, bikunin, inhibits ovarian cancer cell invasion by blocking the calcium-dependent transforming growth factor-beta 1 signaling cascade. J Biol Chem. 2003;278:7790–7799. doi: 10.1074/jbc.M210407200. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki M, Kobayashi H, Tanaka Y, Hirashima Y, Kanayama N, Takei Y, Saga Y, Suzuki M, Itoh H, Terao T. Bikunin target genes in ovarian cancer cells identified by microarray analysis. J Biol Chem. 2003;278:14640–14646. doi: 10.1074/jbc.M300239200. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, Chen D, Cao L, Zhang R, Zhou J, Chen H, Li Y, Li M, Cao J, Wang Z. MiR-490-3p modulates the proliferation of vascular smooth muscle cells induced by ox-LDL through targeting PAPP-A. Cardiovasc Res. 2013;100:272–279. doi: 10.1093/cvr/cvt172. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Chen B, Ming L, Qin H, Zheng L, Yue Z, Cheng Z, Wang Y, Zhang D, Liu C, Bin W, Hao Q, Song F, Ji B. MicroRNA-141 inhibits vascular smooth muscle cell proliferation through targeting PAPP-A. Int J Clin Exp Pathol. 2015;8:14401–14408. [PMC free article] [PubMed] [Google Scholar]
- 48.Salim H, Arvanitis A, de Petris L, Kanter L, Haag P, Zovko A, Ozata DM, Lui WO, Lundholm L, Zhivotovsky B, Lewensohn R, Viktorsson K. miRNA-214 is related to invasiveness of human non-small cell lung cancer and directly regulates alpha protein kinase 2 expression. Genes Chromosomes Cancer. 2013;52:895–911. doi: 10.1002/gcc.22085. [DOI] [PubMed] [Google Scholar]


