Abstract
There are limited data on blastomycosis in solid organ transplant recipients with the subsequent development of the immune reconstitution inflammatory syndrome (IRIS). Herein we describe a case of pulmonary blastomycosis in a renal transplant recipient with the development of concomitant IRIS.
Keywords: Transplantation, Immune Reconstitution Inflammatory Syndrome (IRIS), Blastomycosis
1. Introduction
Blastomyces dermatiditis, a dimorphic fungus responsible for Blastomycosis, lives primarily in moist soil and decomposing matter, and inhabits the midwest, south-central, southeastern United States as well as the boreal forests of Ontario and Quebec in Canada [1], [2]. Blastomycosis may produce a spectrum of infections ranging from asymptomatic infections in immunocompetent patients to disseminated disease, including skin, bone, genitourinary tract, and central nervous system (CNS) involvement, in immunocompromised patients. Blastomycosis is usually acquired via inhalation of airborne conidia or traumatic inoculation [3]. Herein, we report an unusual presentation and clinical manifestations of blastomycosis in an immunocompromised renal transplant recipient.
2. Case
A 65 year old man, who had emigrated from Russia 2 years previously, presented 3 months after undergoing a deceased donor renal transplant for end stage renal disease secondary to diabetic nephropathy. His comorbid conditions included: hypertension and coronary artery disease. Shortly after his transplant, he developed urinary retention and underwent a transurethral resection of the prostate 3 weeks after his transplant. Subsequently, he had repeated episodes of urinary tract infection and was also found to have a perinephric fluid collection that was not considered to be clinically significant. His urine cultures were positive for an extended spectrum beta lactamase producing Klebsiella pneumoniae for which he was treated with a prolonged course of ciprofloxacin. His maintenance immunosuppressive regimen consisted of tacrolimus extended release, mycophenolic acid, and prednisone.
While on ciprofloxacin therapy he presented to the Emergency Department on day 0 with fever, non-productive cough, right upper back pain, 7 pound weight loss, vomiting and fatigue. He denied dysuria, frequency, or any other urinary symptoms. He was afebrile, normotensive, and had an oxygen saturation of 96% on room air. His physical examination demonstrated right upper lobe crackles. No laboratory abnormalities were noted but his leukocyte count was reduced (3.9 × 109 cells/L). His trough tacrolimus level was 11.6 µg/L on admission. His urinalysis demonstrated 50 WBCs. A chest X-ray demonstrated a dense consolidation of the right upper lobe. Blood and urine cultures, cytomegalovirus DNA polymerase chain reaction (PCR), Epstein-Barr Virus PCR, urine Legionella antigen, serum cryptococcal antigen, influenza A and B PCR, respiratory syncytial virus PCR, parainfluenza 1,2, and 3 PCR, adenovirus PCR, and human metapneumovirus PCR testing were negative. During his hospital stay, his tacrolimus level continued to increase, with the highest level being 17 µg/L, and therefore this medication was held. Mycophenolic acid was also held given the concern for pulmonary infection. His prednisone dose was increased to 10 mg per day in the setting of his reduction in immunosuppression.
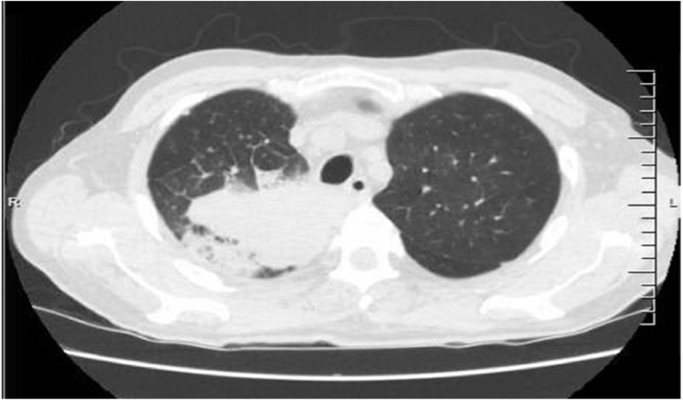
Computed tomography (CT) scan of the chest was performed on day 1 and demonstrated a large area of consolidation in the right upper lobe with a small area of cavitation with numerous lung nodules scattered throughout the lungs bilaterally (Fig. 1).
Fig. 1.
CT of the chest on admission to the hospital.
There was also a small focus of consolidation in the left upper lobe. On day 5, he underwent a diagnostic bronchoscopy with radial probe endoscopic biopsy via ultrasound of the right upper lobe mass. Immediately after the transbronchial needle aspiration of the right upper lobe mass, pus began draining from the area. A washout was performed of the right upper lobe which additionally produced copious amounts of pus. The cytology of the bronchoalveolar lavage (BAL) fluid demonstrated imnumerable large yeasts with a double contoured wall and broad based budding buds consistent with Blastomyces spp. but no apparent inflammatory response was noted. A transbronchial needle aspiration was also consistent with Blastomyces spp. (Fig. 2).
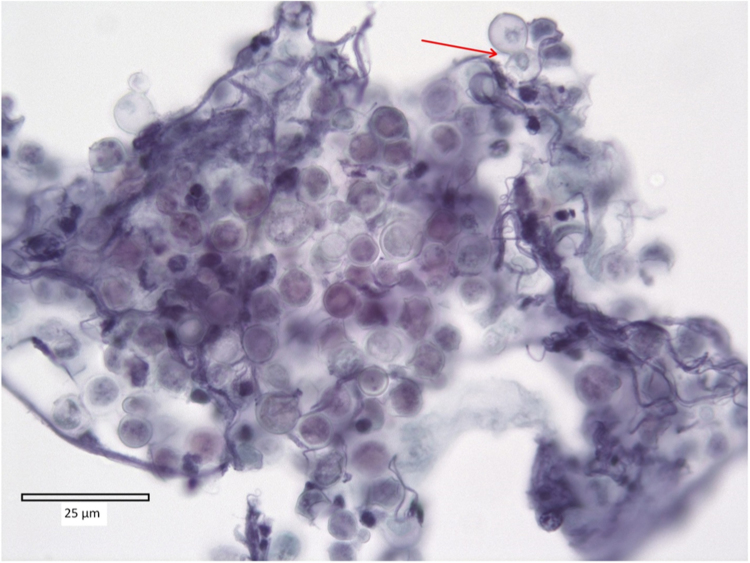
Fig. 2.
BAL fluid sample stained with Papanicolaou stain and demonstrating a cluster of yeast-like fungal organisms. The organism has a diameter of approximately 8 µm and is characterized by a thick “double-contoured” cell wall with sharply defined inner and outer edges. The broad-based budding is marked by the red arrow.
The BAL fluid fungal culture did indeed grow Blastomyces dermatitidis. Bacterial and acid-fast bacilli cultures were negative as were respiratory viral PCR, and Legionella cultures. Of note he did have an elevated BAL fluid galactomannan by the Platelia enzyme immunoassay of > 3.50 optical density. His urine Histoplasma antigen was also positive. Blastomyces serology was also performed by complement fixation and immunodiffusion testing but was nonreactive. He was started on itraconazole capsules 200 mg 3 times a day for 3 days on day 6 and then this was decreased to 200 mg twice a day. He was later switched to the itraconazole solution 200 mg 2 times a day and discharged home. Upon discharge his leukocyte count had risen to 10.3 × 109/L.
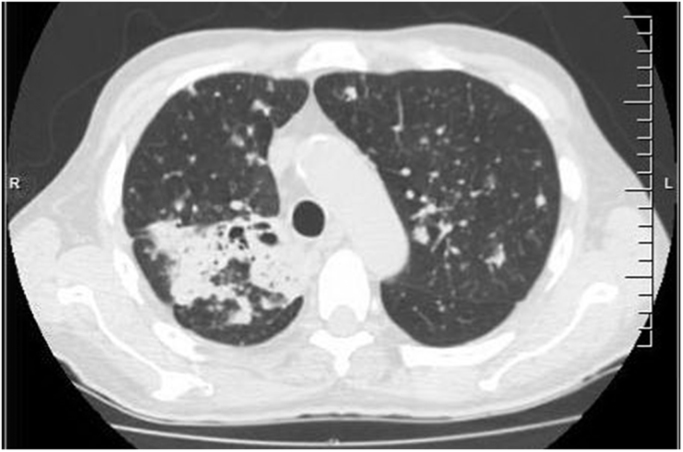
A repeat CT scan of the chest obtained day 26 which was three weeks after the initiation of itraconazole demonstrated cavitation with bronchial dilatation within the previously demonstrated confluent consolidation within the right upper lobe (Fig. 3).
Fig. 3.
Follow up CT of the chest (IRIS).
The left upper lobe lesion was surprisingly slightly larger in size with interval progression in the size and number of innumerable pulmonary nodules, several of which were cavitating. The patient however was afebrile and clinically improved with no respiratory complaints. A repeat bronchoscopy performed day 40 demonstrated mucopurulence in left lung. Bacterial, fungal, acid-fast bacilli, and Legionella cultures of the BAL fluid were negative. The BAL fluid galactomannan, pneumocystis PCR, and the respiratory viral panel PCR were also negative. Additionally, an itraconazole trough serum level was obtained on day 47 and was found to be 1.6 µg/mL, well within the therapeutic range. The patient continued to make an unremarkable recovery, thereafter.
3. Discussion
The primary reservoir of Blastomyces dermatidis is moist soil and decaying matter. Infection occurs through inhalation of conidia, although cutaneous inoculation has been reported. The incubation period is 30–45 days after which symptoms can arise. Infections may range from asymptomatic seroconversion to severe symptomatic disseminated disease. Pulmonary manifestations can range from an acute pneumonia that can mimic community acquired pneumonia to a chronic pneumonia that can mimic carcinoma, tuberculosis, or other fungal infections. In fact, pulmonary manifestations can progress to ARDS, although rare and is associated with high mortality [3]. One would therefore anticipate that large metropolitan areas such as Toronto, Canada where this patient lived would not be a focal point for cases of blastomycosis. However, a survey of cases of blastomycosis in Ontario identified that the highest incidence of cases were in fact noted in the greater Toronto area [1].
Treatment depends on the disease severity and the underlying immune status of the patient. According to the Infectious Diseases Society of America guidelines [4], in mild to moderate disease itraconazole 200 mg orally 3 times per a day for 3 days followed by once or twice daily for 6–12 months is recommended. However in immunosuppressed patients, the initiation of the lipid formulation of amphotericin B at doses of 3–5 mg/kg per day or anmphotericin B deoxycholate dosed at 0.7–1 mg/kg per day for 1–2 weeks or until improvement followed by itraconazole 200 mg 3 times per a day for 3 days followed by twice a day for at least 12 months. Our patient had a history of a renal transplant for which he was on multiple immunosuppressive agents. However, his respiratory status was stable and the infection was confined to his lungs. Therefore it was deemed that therapy with itraconazole would suffice. This view was justified as he did improve clinically after the initiation of therapy.
The unique features of our case includes the positive BAL fluid galactomannan derived from the cross reactivity of the polysaccharide contained on both Blastomyces dermatididis and Histoplasma capsulatum [5] along with the positive urine Histoplasma antigen [6]. In addition, the worsening of his pulmonary findings at the time of his repeat CT scan of the chest three weeks after the initiation of therapy was also not in keeping with his marked clinical improvement. These findings may have been representative of the immune reconstitution inflammatory syndrome (IRIS) although alternative hypotheses could have been entertained for his worsening imaging findings (suboptimal therapeutic drug levels of itraconazole and a new possible superimposed infection) [7]. Regarding the therapeutic drug levels, the itraconazole trough level was assayed and found to be appropriate (1.6 µg/mL, range > 1.0 µg/mL for systemic infection). As for a new possible superimposed infection, the repeat bronchoaveolar lavage was unremarkable. Therefore, it was felt that the worsening CT chest findings were consistent with IRIS.
IRIS has been described in the setting of cryptococcal infection in transplant recipients along with other bacterial and viral infections [8]. It is a diagnosis of exclusion. This clinical syndrome may have occurred in our patient because tacrolimus is known to disrupt T-cell activation proliferation along with the release of cytokines that stimulate macrophages and neutrophil fungicidal activities [9]. Tacrolimus was held during the admission due to elevated levels, as high as 17 µg/L and the medication continued to be held even beyond the timing of the repeat CT Chest. Prednisone sequesters CD4 + cells to the reticular endothelial system, increasing lymphocyte apoptosis, and impedes antigen processing and presentation which may have also enhanced the patient's infiltrates [9]. He remained on prednisone as his only immunosuppressive agent throughout this time. IRIS was considered a plausible explanation for the findings and no adjustment in his therapy was necessary.
This case of pulmonary blastomycosis in a renal transplant recipient illustrates some unique features of this fungal infection. It highlights the interactions between the organism and diagnostic testing as well as the dynamic interactions among the fungal infection, immunosuppressive therapy and the host's immune system.
Conflict of interest
The authors have no conflicts of interest to declare and confirm that each one has made substantial contributions to the information or materials submitted for publication.
References
- 1.Morris S.K., Brophy J., Richardson S.E. Blastomycosis in Ontario, 1994–2003. Emerg. Infect. Dis. 2006;12(2):274–279. doi: 10.3201/eid1202.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St-Germain G., Murray G., Duperval R. Blastomycosis in Quebec (1981–90): report of 23 cases and review of published cases from Quebec. Can. J. Infect. Dis. 1993;4(2):89–94. doi: 10.1155/1993/249823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blastomycosis | Fungal Diseases | CDC. Cdcgov, 2017. Available at: 〈https://www.cdc.gov/fungal/diseases/blastomycosis/index.html〉. (Accessed 8 November 2014).
- 4.Chapman S.W., Dismukes W.E., Proia L.A. Clinical practice guidelines for the management of blastomycosis: 2008 update by the infectious diseases society of America. Clin. Infect. Dis. 2008;46(12):1801–1812. doi: 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 5.Durkin M., Witt J., LeMonte A., Wheat B., Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J. Clin. Microbiol. 2004;42(10):4873–4875. doi: 10.1128/JCM.42.10.4873-4875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheat J., Wheat H., Connolly P. Cross-reactivity in histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin. Infect. Dis. 1997;24:1169–1171. doi: 10.1086/513647. [DOI] [PubMed] [Google Scholar]
- 7.Hage C., Kleiman M., Wheat L. Histoplasmosis in solid organ transplant recipients. Clin. Infect. Dis. 2010;50(1):122–123. doi: 10.1086/649056. [DOI] [PubMed] [Google Scholar]
- 8.Sun Hsin-Yun, Singh Nina. Opportunistic infection-associated immune reconstitution syndrome in transplant recipients. Clin. Infect. Dis. 2011;53:168–176. doi: 10.1093/cid/cir276. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier G., Safdar N., Klein B., Andes D. Blastomycosis in solid organ transplant recipients. Transpl. Infect. Dis. 2007;9(4):310–317. doi: 10.1111/j.1399-3062.2007.00227.x. [DOI] [PubMed] [Google Scholar]