Abstract
AIM
To test the feasibility and performance of a novel upper gastrointestinal (GI) capsule endoscope using a nurse-led protocol.
METHODS
We conducted a prospective cohort analysis of patients who declined gastroscopy (oesophagogastroduodenoscopy, OGD) but who consented to upper GI capsule endoscopy. Patients swallowed the upper GI capsule following ingestion of 1 liter of water (containing simethicone). A series of positional changes were used to exploit the effects of water flow and move the upper GI capsule from one gravity-dependent area to another using a nurse-led protocol. Capsule transit time, video reading time, mucosal visualisation, pathology detection and patient tolerance was evaluated.
RESULTS
Fifty patients were included in the study. The mean capsule transit times in the oesophagus and stomach were 28 s and 68 min respectively. Visualisation of the following major anatomical landmarks was achieved (graded 1-5: Poor to excellent): Oesophagus, 4.8 (± 0.5); gastro-oesophageal junction (GOJ), 4.8 (± 0.8); cardia, 4.8 (± 0.8); fundus, 3.8 (± 1.2); body, 4.5 (± 1); antrum, 4.5 (± 1); pylorus, 4.7 (± 0.8); duodenal bulb, 4.7 (± 0.7); second part of the duodenum (D2), 4.7 (± 1). The upper GI capsule reached D2 in 64% of patients. The mean video reading time was 48 min with standard playback mode and 20 min using Quickview (P = 0.0001). No pathology was missed using Quickview. Procedural tolerance was excellent. No complications were seen with the upper GI capsule.
CONCLUSION
The upper GI capsule achieved excellent views of the upper GI tract. Future studies should compare the diagnostic accuracy between upper GI capsule and OGD.
Keywords: Capsule endoscopy, Upper gastrointestinal, Gastroscopy, Oesophagus, Stomach
Core tip: The demand for diagnostic upper gastrointestinal (GI) endoscopy is high. Capsule endoscopy is well tolerated and is a first line small bowel investigative modality. Capsule endoscopy of the upper GI tract has previously been limited by technology and complexity of use. We demonstrate the feasibility of a nurse-led protocol using simple patient positional changes to move the novel upper GI capsule around a water-filled stomach. This technique provides excellent mucosal views in the oesophagus, stomach and (battery life allowing) duodenum and is well tolerated. The upper GI capsule might be a potential non-invasive, patient-friendly, alternative for diagnostic upper GI endoscopy.
INTRODUCTION
Capsule endoscopy is the method of choice to image the small bowel mucosa and is an accepted alternative to colonoscopy[1,2]. However, the short length of the oesophagus, the volume of the stomach and the convoluted shape of the gastroduodenum present challenges to its role as a non-invasive upper gastrointestinal examination technique: Transit can be rapid through a straight lumen[3] and visualisation may be limited to the dependent part of the stomach.
The PillCam® ESO2 capsule (Given Imaging Ltd., Yoqneam, Israel) has cameras at both ends and is capable of high image acquisition rates (18 frames per second) to maximise oesophageal imaging and a 30-min battery life. Meta-analyses have shown that it is an effective tool to detect Barrett’s oesophagus, oesophageal varices and oesophagitis[4-6]. Three studies have also shown that it can be used to identify patients with suspected upper gastrointestinal bleeding who need gastroscopy[7-9]. In a comparative study in dyspeptic patients, Marelli et al[10] identified all major pathology detected by gastroscopy using an ESO2. These examinations were performed following a fast alone: better visualisation may be achieved after ingestion of simethicone and water to distend the stomach[11-13].
The upper gastrointestinal (UGI) capsule (Medtronic Ltd, Dublin, Ireland) represents the most recent technological advance in this field. Preserving dual-camera image capture, each with a 174° field of view, the UGI capsule captures as many as 35 frames per second for 10 min followed by 18 frames per second for a further 80 min. This study describes the first reported experience of UGI capsule endoscopy using a simple, nurse-led protocol comprising a sequence of patient positional changes following the ingestion of water and simethicone.
MATERIALS AND METHODS
Study population
We performed a prospective observational study at our tertiary hospital. Patients were offered UGI capsule endoscopy if they refused gastroscopy. All indications were considered. Those who had Crohn’s disease were required to undergo a PillCam Patency capsule (Medtronic Ltd.) examination first.
Simple positional interchange technique
The UGI capsule endoscopy system includes an external portable data recorder. The recorder is connected to the patient by an array of leads on the chest and abdominal skin during the examination. This interface supports data export from the capsule to the memory drive of the data recorder. A small monitor in the recorder allows real-time viewing. When the procedure is complete, the data recorder is docked onto a workstation installed with Rapid 9® software (Medtronic Ltd.) and video images are exported for further analysis by the physician.
The simple positional interchange technique (SPIT) was performed by nursing staff on the Clinical Investigation Unit, Royal Hallamshire Hospital. Patients first drank one litre of water containing 80 mg simethicone. Immediately before swallowing the UGI capsule, 20 mg of hyoscine butylbromide was given intramuscularly to reduce gastric peristalsis[14] and optimise gastric views. Patients were asked to swallow the UGI capsule in the right lateral position using an adaptation of the previously described simplified ingestion procedure (SIP)[15]. In brief, this entailed swallowing small sips of water (approximately 15mL) every 30 s until the UGI capsule entered the stomach. If patients were unable to swallow the capsule while lying in the horizontal plane, the head of the bed was incrementally elevated until swallowing was successful. If this failed, then patients swallowed the capsule sitting upright. The real-time views detected when the UGI capsule entered the stomach. Once the capsule entered the stomach, patients were asked to position themselves to face three planes (left/right lateral decubitus and supine/prone) at three angles (30° head down/up and horizontal) for 2 min per position (Figure 1). Additional positional changes and sips of water were used to improve views of the gastric mucosa as necessary. When complete gastric mucosal assessment was achieved patients were asked to sit upright to assist passive capsule movement towards the pylorus. If the capsule had not reached the first part of the duodenum 60 min after ingestion then 10 mg of intramuscular metoclopramide was administered as per our standard protocol[11]. Patient tolerance in the form of procedural pain, discomfort and distress scores were recorded using previously validated visual analogue scales (VAS. 0: No symptom; 10: Intolerable symptom)[16,17].
Figure 1.
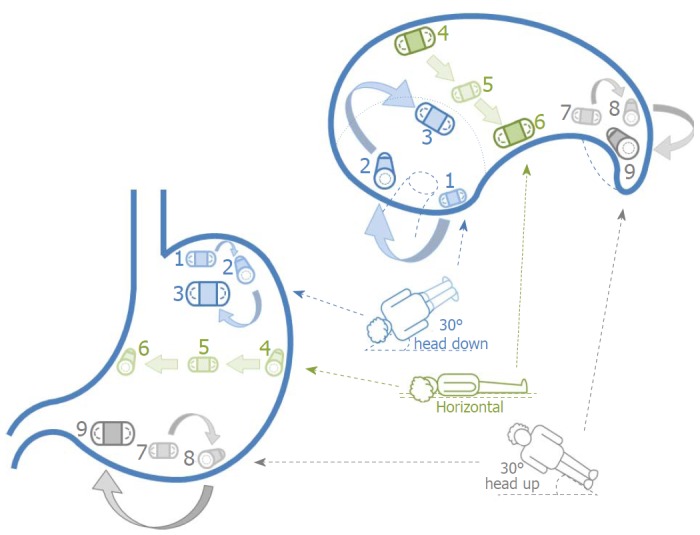
Schematic of the simple positional interchange technique. Coronal views are illustrated on the left and transverse views (with the cranial end closest to the reader) on the right. Capsule movement is achieved by exploiting the effects of water flow from one gravity dependent area to another with patient positional change. Once the UGI capsule enters the stomach, the examination bed is tilted 30° head down (depicted in blue) and patients lie supine (position 1), on their left lateral (position 2) and then prone (position 3). The bed is returned to the horizontal plane (depicted in green) and patients lie on their left lateral (position 4), supine (position 5) and then right lateral (position 6). The bed is finally adjusted to 30° head up (depicted in grey) and patients lie supine (position 7), on their left lateral (position 8) and then prone (position 9). UGI: Upper gastrointestinal.
Video interpretation and analysis
UGI capsule videos were reported by one of two co-authors (Sidhu R and McAlindon ME), each with experience of reading over 1000 small bowel capsule endoscopy videos. Rapid 9® software (Medtronic Ltd.) was used to review videos and has the capacity to playback recordings up to 100 frames per second in an accelerated reading mode. Analysis of videos included grading of mucosal visualisation (Table 1) using an adapted protocol[18]. Capsule transit time, video reading time, completion of examination to the second part of the duodenum (D2), pathology detection and procedural complications were recorded. The service evaluation was registered with the Clinical Effectiveness Unit (registration number 7073), Sheffield Teaching Hospitals NHS Foundation Trust (STH), United Kingdom.
Table 1.
Upper gastrointestinal mucosal visualisation grading
| Grade | Description |
| 1 | Poor view. More than 75% obscured by debris/bubbles/poor image clarity/illumination |
| 2 | Sub-optimal view. More than or equal to 50% obscured by debris/bubbles/poor image clarity/illumination |
| 3 | Reasonable view. Less than 50% obscured by debris/bubbles/poor image clarity/illumination |
| 4 | Good view. Less than 25% obscured by debris/bubbles/poor image clarity/illumination |
| 5 | Excellent. 100% complete view of the landmark |
Views of each major landmark were graded; oesophagus, gastro-oesophageal junction; gastric cardia, fundus, body (anterior, posterior wall, greater and lesser curve), antrum, pylorus, and the first (D1) and second part of the duodenum (D2).
SPSS V.22.0 (IBM) was used for statistical analysis. Continuous data was represented as mean ± SD: The student’s t-test or one-way analysis of variance (ANOVA) was used for comparisons. Categorical data was represented as an absolute number and/or percentage: The χ2 test or Fisher’s exact probability test was used for comparisons. P < 0.05 (two-sided) was considered statistically significant.
RESULTS
Patient demographics
Fifty patients (40% male) with a mean age of 57 (± 15.7) years were included in the study protocol. Indications for investigation included dyspepsia (32%), iron deficiency anemia (14%), variceal screening (42%), suspected upper GI Crohn’s disease (4%) and assessment of oesophageal ulcer healing (8%).
Performance characteristics
SPIT was achieved in 90% of patients: Five had difficulty lying prone. Complete examination to D2 was achieved in 64%. The mean (± SD) time of capsule transit in the oesophagus, stomach and duodenum was 28 (± 95) s, 68 (± 25) min and 11 (± 15) min respectively. Routine administration of hyoscine was abandoned after the first 33 patients because of concern that it might be delaying capsule entry into the duodenum. Analysis, however, failed to demonstrate any delaying effect of the drug on gastric transit: The mean gastric transit time with hyoscine butylbromide was 69 (± 25) min and 66 (± 26) min without (P = 0.67).
Mucosal visualisation and pathology detection
The mean reading time for capsule videos was 48 (± 18) min with standard mode. All 50 studies were subsequently de-identified and re-read by one reader (MEM) in a randomised, blinded fashion using the Quickview (Medtronic Ltd.) option in the pre-set mode (the software selecting 10% of the most relevant lesions for viewing by the reader) to examine the stomach (oesophagus and duodenum being read in standard mode with frame rate selected by the reader according to his usual practice): Reading time was significantly reduced to 20 (± 5) min (P = 0.0001).
Visualisation of the upper GI tract was graded as follows: Oesophagus, 4.8 (± 0.5); gastro-oesophageal junction (GOJ), 4.8 (± 0.8); cardia, 4.8 (± 0.8); fundus, 3.8 (± 1.2); body, 4.5 (± 1); antrum, 4.5 (± 1); pylorus, 4.7 (± 0.8); duodenal bulb (D1), 4.7 (± 0.7); D2, 4.7 (± 1) (Figure 2). Withdrawal of hyoscine administration did not affect any visualisation scores. The visualisation grade at the fundus was significantly lower when compared to all other areas of the upper GI tract (P < 0.05 for comparisons to the oesophagus, GOJ, cardia, body, D1 and D2) (Figure 3). The whole circumference of the Z-line was seen in 92.5% of cases. Inability to achieve prone positions during SPIT did not render lower overall gastric visualisation compared to complete SPIT; combined mean scores of cardia, fundus, body, antrum and pylorus visualisation were 4 (± 1) vs 4.2 (± 1.4), respectively (P = 0.38). Detected pathology included: oesophagitis (n = 12), Barrett’s oesophagus (n = 1), hiatus hernias (n = 7), Cameron’s ulcer (n = 1), gastric inlet patch (n = 1), oesophageal varices (n = 8), gastric varices (n = 2), portal hypertensive gastropathy (n = 5), gastritis (n = 20), benign gastric polyps (n = 10), gastric ulcers (n = 2), duodenitis (n = 4), duodenal polyp (n = 1), villous atrophy (n = 1) and angioectasia (n = 7) (Figure 4). No pathology was missed using the Quickview reading software in the stomach when compared to standard mode.
Figure 2.
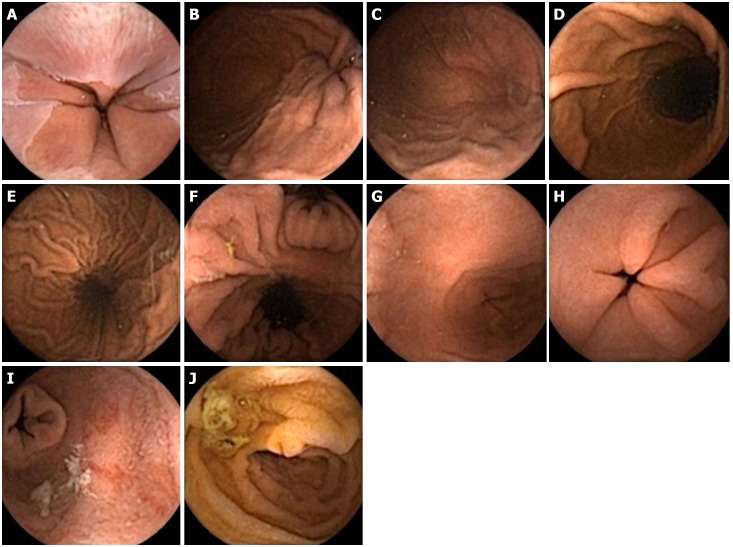
Normal views of the upper gastrointestinal tract seen with the upper gastrointestinal capsule. A: Gastroesophageal junction; B: Cardia; C: Fundus; D: Greater curvature; E: Lesser curvature; F: Incisura angularis; G: Antrum; H: Pylorus; I: First part of duodenum (retrograde view); J: Second part of duodenum (ampulla also seen).
Figure 3.
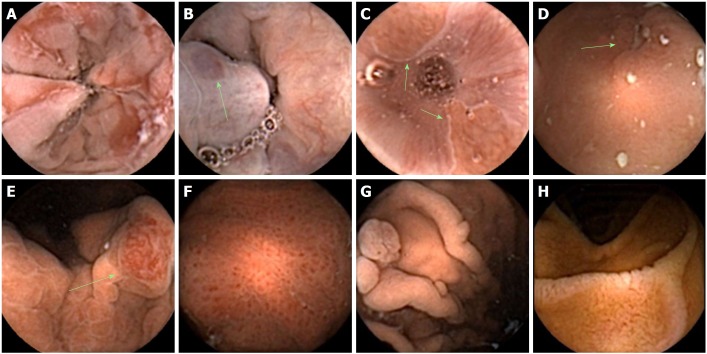
Suboptimal views in the fundus. A: Mucus; B: Bubbles; C: Insufficient distension.
Figure 4.
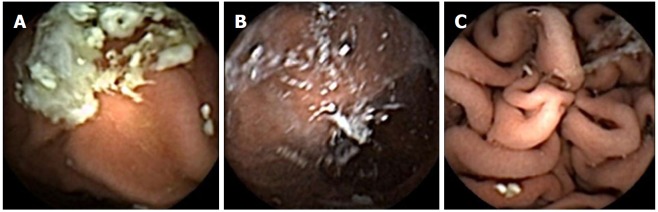
Pathology detected by upper gastrointestinal capsule. A: Erosive esophagitis; B: Oesophageal varices; C: Barrett’s oesophagus; D: Gastric ulcer; E: Gastric angioectasia; F: Portal hypertensive gastropathy; G: Benign cystic fundic gland polyps; H: Coeliac disease.
Patient tolerance and safety
Mean procedural pain, discomfort and distress scores were: 0.4 (± 1), 0.4 (± 1) and 0.3 (± 0.9) respectively. No complications were seen. All patients were willing to undergo a repeat procedure if it was necessary.
DISCUSSION
UGI capsule endoscopy achieved oesophagogastric examination in all patients, although limited battery life precluded duodenal examination in a third. All studies using swallowed water for gastric distension, simethicone and the SPIT were performed by nursing staff according to protocol. Patients were able to comply with the SPIT in 90% of cases although difficulties with lying prone in the remainder did not affect outcome. SPIT provided excellent views of all areas of the oesophagus and stomach, both D1 and D2 were visualised clearly when the capsule traversed the pylorus within the 90-minute time frame and pathology was identified throughout. The procedure was extremely well tolerated and no complications occurred.
Gastroscopy is performed in 1% of the United Kingdom population per annum[19]. In the United States, an increase in 50% of gastroscopy utilisation was estimated within the space of a decade between 2000 and 2010[20]. However, gastroscopy is an uncomfortable procedure[16,21,22] and the majority of findings do not significantly affect management[23]. This would suggest a role for a well-tolerated, non-invasive alternative that could select the minority of patients who need upper gastrointestinal biopsies or endoscopic therapy. Unlike the small and large bowel, which are long, relatively straight with constant lumina, the upper gastrointestinal tract comprises three quite different structures: the short, tubular, small diameter oesophagus and duodenum and the voluminous stomach, the gastroduodenum being convoluted in shape. Technologies to date have tried to address these challenges by developing capsules with cameras at both ends, maximising image capture rate and battery life and controlling capsule movement. Although there is no equivalent data for the oesophagus, there is evidence that a double-ended pill camera is better than a single-ended one in terms of diagnostic yield in the small bowel[24,25]. Intuitively it seems likely that a single-ended capsule leading with the blind end is less likely to get complete views of the GOJ than one with cameras at both ends. Similarly, our experience is that a single ended device may miss proximal lesions in the duodenal bulb if transit through the bulb is rapid[26].
The Pillcam® ESO, capturing a total of fourteen frames (seven from each end) per second[27] was superseded by the ESO2[28], capturing a total of 18 frames per second. The 35 frames per second delivered by the UGI capsule would deliver almost 1000 oesophageal images in the average transit time of 28 s shown in our evaluation. This improvement is likely to have resulted in better oesophageal views: The entire GOJ was seen in only 50% of ESO2 studies[3] compared to 92.5% in this series. Whether or not this translates to better diagnostic yield in the oesophagus and the rest of the upper gastrointestinal tract needs to be confirmed.
We, and others, have demonstrated some degree of control with an external handheld magnet[11,29,30], which has shown promise in comparison with conventional gastroscopy[26,31]. Rey et al[32] visualised between 85%-93% of gastric landmarks in a controlled trial comparing gastroscopy with capsule endoscopy controlled using a large fixed external magnet developed by Olympus and Siemens. Both modalities identified 58% of pathologies and both missed lesions identified by the other. A similar system was found to have a sensitivity of only 62% in comparison to gastroscopy but only 21 of 189 patients recruited had focal pathology[33]. More recently, Liao et al[12] demonstrated that capsule endoscopy controlled by a robot magnet achieved 90% sensitivity (irrespective of size and location) in detecting focal lesions compared to gastroscopy in a large 350 patient multicenter study in Chinese patients with dyspepsia. Such techniques, however, require expertise and cost-effectiveness studies are needed. Therefore, the prospect of a simple, nurse-led, protocol driven UGI examination is attractive: cost and expertise required is mainly limited to the capsule and the interpretation of the videos.
The SPIT protocol is easy to follow in clinical practice. The patient is asked to rotate along their longitudinal axis almost 360° from the right lateral to prone position, a series of manoeuvres which are performed 30° head down, horizontal and 30° head up. This aims to achieve complete gastric imaging as was reported for capsule endoscopy using handheld external[34] and static robot magnets[35]. Qian et al[35] demonstrated the benefits of the left lateral, supine and right lateral positions for imaging the fundus, cardia and antropyloric regions respectively. Rahman et al[34] found that visualising incisura, antrum and pylorus was best achieved by using the handheld magnet to position the capsule opposite the gravity-dependent positions on the greater curve and antrum in the supine patient. We have used the prone position to achieve the same capsule position and viewpoints. The combination of patient positional changes in Rahman’s study achieved good to excellent views of all areas of the upper gastrointestinal tract. These previous studies were performed using single ended camera capsules: it is likely that greater coverage is obtained using a double-ended capsule providing a view of almost 360°. Studies comparing diagnostic yield of the two modalities are warranted. Five patients were unable to achieve the prone position but otherwise completed SPIT without obvious impact on landmark visualisation. Nonetheless, SPIT may not be feasible for all those with mobility restrictions.
Capsule reading was time consuming at 48 min and most of the viewing is repetitive gastric imaging making reading a tedious task. However, image recognition software continues to be developed which can exclude sequentially identical images, or select images which are different or identified as pathological, thereby reducing the size of the video to be viewed. The Quickview system is such a software and in its previous iteration in the Pillcam® SB2 (Given Imaging Ltd.) was shown to have a sensitivity of 92.3% in detecting small bowel pathology[36]. Perhaps such software may prove more useful in the large volume stomach in which the capsule images the same areas repeatedly, compared to the small bowel in which transit distally is more constant and subject to less repetitive imaging of the same region. No pathology was missed when Quickview was used to view the stomach. In this study, videos were re-read with Quickview in a randomised order and anonymised. Even so, they were re-read by MEM, one of the co-authors involved in the initial video interpretation using standard mode. Unbiased Quickview video interpretation by an independent reader, blinded to the findings at standard reading would provide more reliable comparison. Future larger comparative studies are needed to confirm the value Quickview in UGI capsule endoscopy.
The UGI capsule visualised the fundus less well. This is consistent with other studies using capsule endoscopy, even with external actuation techniques such as magnetic steering[18,30]. During gastroscopy, gas insufflation is used to inspect the proximal stomach, which is collapsed in the fasted state. While varying amounts of water have been used to distend the stomach during upper GI capsule endoscopy[10,11], we have previously shown that 1000 mL improves mucosal clarity and distension compared to 200 mL[11]. Some UGI videos were obscured by adherent mucus in the proximal stomach. The use of mucolytics such as N-acetylcysteine or pronase has been shown to be of benefit in improving mucosal visibility during gastroscopy[37-39], although this did not translate to the only capsule endoscopy study to date[40]. Routine use of hyoscine has been advocated to improve visualisation in OGD[14]. This did not appear to make a difference in our experience, although as with water- and gas- distension techniques and mucolytics, the potential benefits of these agents should be investigated further.
A 64% complete examination to D2 was disappointing. Hyoscine may delay gastric emptying[41], but although this was not a study powered to investigate its effects, hyoscine did not appear to have an obvious effect on gastric transit in this small cohort. Meltzer et al[42] found that only one half of their ESO2 (30 min) examinations reached the duodenum. Using a modified version of the ESO2 (with a 90-min battery life) and pre-procedural intravenous erythromycin, Gralnek et al[7] achieved duodenal entry of the capsule in 97.8% of cases. Therefore the use of promotility agents might be considered, unless rendered redundant by further improvements in battery life.
The development of transnasal and single-fibre endoscopy as well as Cytosponge acknowledges the need for less-invasive technologies for upper gastrointestinal screening and surveillance[43]. In this feasibility study, anxiety, discomfort and pain scores associated with the UGI capsule and SPIT were excellent, consistent with previous studies of capsule endoscopy of the oesophagus[44,45], small bowel[16] and colon[46]. Furthermore, Gupta et al[47] found that adult subjects expressed a preference for capsule endoscopy compared to sedated endoscopy for Barrett’s oesophagus screening, raising the possibility that compliance with investigation might be better if less-invasive techniques are offered.
There are limitations to this study and with the technologies. This is an observational cohort study that suggests that UGI capsule endoscopy is feasible, and when technological development allows more reliable duodenal imaging, randomised controlled trials of diagnostic yield compared to gastroscopy are needed. Cost effectiveness studies should consider the costs of the supporting systems and their maintenance (endoscopes, stack systems, monitors, computer software), disinfection, accessories and disposables (which includes the capsule), training requirements and the time taken to perform procedures (including interpreting images). Capsule endoscopy at present remains only diagnostic. The technology to biopsy lesions has been reported but remains in the experimental phase[48]. However, whilst most endoscopists have a low threshold for taking biopsies, the use of non-invasive tests for Helicobacter pylori might reduce this and our experience of investigating patients with dyspepsia is that biopsies only increased diagnostic yield by 2.4%[23].
Within the context of the limitations, this study shows that upper GI capsule endoscopy can be performed by nurses in a protocol-driven manner using the novel UGI capsule (Medtronic Ltd.). The SPIT, combined with gastric insufflation using water and simethicone appears to allow excellent visualisation of the whole stomach, albeit with slightly reduced visibility in the fundus. The oesophagus and gastro-oesophageal junction are well seen although further work is needed to allow more reliable visualisation of the duodenum. The procedure is extremely well tolerated by patients.
ARTICLE HIGHLIGHTS
Research background
Upper gastrointestinal (UGI) endoscopy (gastroscopy) is the method of choice to investigate dyspepsia, but is an uncomfortable test which carries the risk of intubation and sedation. Dyspepsia is a common symptom of which potential malignant lesions are an uncommon cause. Therefore a non-invasive alternative which might appropriately select those patients who require gastroscopy in order to obtain biopsy samples for histological analysis or for endotherapy is desirable. Capsule endoscopy is well tolerated and is a first line small bowel imaging tool, but lack of control of capsule movement limits visualisation to the dependent parts of the stomach only. Control can be achieved using external magnets, but this requires operator skill and magnetic devices which may be expensive. A simpler method would be to use swallowed water as a medium in which to move the capsule in the flow of water to different dependent parts of the stomach using patient positional change.
Research motivation
Several techniques using magnets to control capsule movement have been developed, but movement in water flow induced by patient positional change might offer an effective, simpler and less expensive alternative which has not been studied. An assessment of the areas of the upper gastrointestinal tract a capsule endoscope is capable of visualising is necessary in order to determine if such a technique might be feasible. Were this to be so, comparative trials with gastroscopy in identifying pathology would be warranted.
Research objectives
Our aims were to determine the visualisation quality of different upper gastrointestinal landmarks using a capsule endoscope moved around a water-filled stomach using a novel patient positional change technique, to assess procedural completion and patient tolerance of the procedure and time taken to read and report the videos.
Research methods
This was an observational study of a cohort of patients undergoing capsule endoscopy because they declined to undergo gastroscopy. Visualisation quality of different landmarks (oesophagus, gastro-oesophageal junction, cardia, fundus, body, antrum, pylorus, duodenal bulb and second part of duodenum) was scored (1-5: Poor-excellent) as was patient tolerance in terms of pain, discomfort and distress (0-10: No - intolerable). Video reading times in both standard and Quickview mode were compared.
Research results
Complete oesophagogastric examination was achieved with excellent views in all 50 patients. However, the battery-life for the UGI capsule expired before reaching D2 in 36%. Future adaptations are necessary to either promote earlier exiting of the capsule from the stomach into the duodenum (by positional change or prokinetics) or extend battery life. Reading time was lengthy, at 48 min. Using Quickview reduced this to 20 min and no pathology was missed. Further blinded comparative trials are needed to determine the reliability of Quickview in this setting. For patients, the procedure was extremely well tolerated and no complications were seen with the UGI capsule in this study.
Research conclusions
Our study demonstrates the feasibility of achieving excellent views of the oesophagus, stomach and duodenum (when seen) using a novel nurse-led protocol to move the upper gastrointestinal (GI) capsule through a series of patient positional changes. Future randomised control trials assessing diagnostic yield against gastroscopy will be needed to demonstrate reliability. However, the results we report suggest that this protocol may be a well-tolerated and less invasive alternative means to examining the upper GI tract endoscopically.
Research perspectives
These findings suggest that UGI capsule endoscopy is feasible, allows visualisation of all oesophagogastric landmarks and is extremely well tolerated by patients. Technological improvement, for example in battery life, is likely to ensure more reliable imaging of the duodenum. If so, the simple positional interchange technique using the UGI capsule should be compared to gastroscopy in terms of diagnostic yield. Further studies to improve video reading time are needed.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was registered as service evaluation with the clinical effectiveness unit (CEU number 7073), Sheffield Teaching Hospitals NHS Foundation Trust, United Kingdom.
Informed consent statement: Capsule endoscopy was performed on patients who declined to undergo gastroscopy and all provided written informed consent for the capsule examination which was performed in all cases as part of routine clinical practice. The capsule examinations were not performed as part of a clinical research trial. In these patients who refused to have gastroscopy, the capsule endoscopy protocol was registered as a service evaluation with the department of clinical effectiveness unit (CEU number 7073, Sheffield Teaching Hospitals NHS Foundation Trust) and the evaluation is presented in this paper.
Conflict-of-interest statement: Professor McAlindon ME has acted as a consultant for Medtronic Ltd. All remaining authors have no conflict of interest to report.
Data sharing statement: No additional data are available.
STROBE statement: The authors have read and prepared the manuscript in accordance with the STROBE statement.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 18, 2018
First decision: May 9, 2018
Article in press: June 16, 2018
P- Reviewer: Christodoulou DK, Efthymiou A, Hosoe N, Kato J, Perez-Cuadrado-Robles E S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
Contributor Information
Hey-Long Ching, Academic Department of Gastroenterology and Hepatology, Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Trust, Sheffield S10 2JF, United Kingdom. hey-long.ching@sth.nhs.uk.
Ailish Healy, Academic Department of Gastroenterology and Hepatology, Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Trust, Sheffield S10 2JF, United Kingdom.
Victoria Thurston, Academic Department of Gastroenterology and Hepatology, Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Trust, Sheffield S10 2JF, United Kingdom.
Melissa F Hale, Academic Department of Gastroenterology and Hepatology, Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Trust, Sheffield S10 2JF, United Kingdom.
Reena Sidhu, Academic Department of Gastroenterology and Hepatology, Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Trust, Sheffield S10 2JF, United Kingdom.
Mark E McAlindon, Academic Department of Gastroenterology and Hepatology, Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Trust, Sheffield S10 2JF, United Kingdom.
References
- 1.Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G; ESGE Clinical Guidelines Committee. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220–227. doi: 10.1055/s-0029-1243968. [DOI] [PubMed] [Google Scholar]
- 2.Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, Rondonotti E, Adler SN, Albert J, Baltes P, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352–376. doi: 10.1055/s-0034-1391855. [DOI] [PubMed] [Google Scholar]
- 3.Krok KL, Wagennar RR, Kantsevoy SV, Thuluvath PJ. Esophageal capsule endoscopy is not the optimal technique to determine the need for primary prophylaxis in patients with cirrhosis. Arch Med Sci. 2016;12:365–371. doi: 10.5114/aoms.2016.59263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhardwaj A, Hollenbeak CS, Pooran N, Mathew A. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett’s esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2009;104:1533–1539. doi: 10.1038/ajg.2009.86. [DOI] [PubMed] [Google Scholar]
- 5.Colli A, Gana JC, Turner D, Yap J, Adams-Webber T, Ling SC, Casazza G. Capsule endoscopy for the diagnosis of oesophageal varices in people with chronic liver disease or portal vein thrombosis. Cochrane Database Syst Rev. 2014:CD008760. doi: 10.1002/14651858.CD008760.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y, Gao R, Liao Z, Hu LH, Li ZS. Meta-analysis of capsule endoscopy in patients diagnosed or suspected with esophageal varices. World J Gastroenterol. 2009;15:1254–1258. doi: 10.3748/wjg.15.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gralnek IM, Ching JY, Maza I, Wu JC, Rainer TH, Israelit S, Klein A, Chan FK, Ephrath H, Eliakim R, et al. Capsule endoscopy in acute upper gastrointestinal hemorrhage: a prospective cohort study. Endoscopy. 2013;45:12–19. doi: 10.1055/s-0032-1325933. [DOI] [PubMed] [Google Scholar]
- 8.Meltzer AC, Pinchbeck C, Burnett S, Buhumaid R, Shah P, Ding R, Fleischer DE, Gralnek IM. Emergency physicians accurately interpret video capsule endoscopy findings in suspected upper gastrointestinal hemorrhage: a video survey. Acad Emerg Med. 2013;20:711–715. doi: 10.1111/acem.12165. [DOI] [PubMed] [Google Scholar]
- 9.Sung JJ, Tang RS, Ching JY, Rainer TH, Lau JY. Use of capsule endoscopy in the emergency department as a triage of patients with GI bleeding. Gastrointest Endosc. 2016;84:907–913. doi: 10.1016/j.gie.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 10.Marelli L, Jaboli FM, Jackson L, Palmer H, Erian G, Hamilton M, Epstein O. A pilot study comparing ESO-2 capsule endoscopy with conventional upper endoscopy for the assessment of uncomplicated heartburn and dyspepsia. Frontline Gastroenterol. 2013;4:96–101. doi: 10.1136/flgastro-2012-100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hale MF, Drew K, Sidhu R, McAlindon ME. Does magnetically assisted capsule endoscopy improve small bowel capsule endoscopy completion rate? A randomised controlled trial. Endosc Int Open. 2016;4:E215–E221. doi: 10.1055/s-0035-1569846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao Z, Hou X, Lin-Hu EQ, Sheng JQ, Ge ZZ, Jiang B, Hou XH, Liu JY, Li Z, Huang QY, et al. Accuracy of Magnetically Controlled Capsule Endoscopy, Compared With Conventional Gastroscopy, in Detection of Gastric Diseases. Clin Gastroenterol Hepatol. 2016;14:1266–1273.e1. doi: 10.1016/j.cgh.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Zou WB, Hou XH, Xin L, Liu J, Bo LM, Yu GY, Liao Z, Li ZS. Magnetic-controlled capsule endoscopy vs. gastroscopy for gastric diseases: a two-center self-controlled comparative trial. Endoscopy. 2015;47:525–528. doi: 10.1055/s-0034-1391123. [DOI] [PubMed] [Google Scholar]
- 14.Veitch AM, Uedo N, Yao K, East JE. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat Rev Gastroenterol Hepatol. 2015;12:660–667. doi: 10.1038/nrgastro.2015.128. [DOI] [PubMed] [Google Scholar]
- 15.Gralnek IM, Rabinovitz R, Afik D, Eliakim R. A simplified ingestion procedure for esophageal capsule endoscopy: initial evaluation in healthy volunteers. Endoscopy. 2006;38:913–918. doi: 10.1055/s-2006-944718. [DOI] [PubMed] [Google Scholar]
- 16.Irvine AJ, Sanders DS, Hopper A, Kurien M, Sidhu R. How does tolerability of double balloon enteroscopy compare to other forms of endoscopy? Frontline Gastroenterol. 2016;7:41–46. doi: 10.1136/flgastro-2014-100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elphick DA, Donnelly MT, Smith KS, Riley SA. Factors associated with abdominal discomfort during colonoscopy: a prospective analysis. Eur J Gastroenterol Hepatol. 2009;21:1076–1082. doi: 10.1097/MEG.0b013e32832357b3. [DOI] [PubMed] [Google Scholar]
- 18.Liao Z, Duan XD, Xin L, Bo LM, Wang XH, Xiao GH, Hu LH, Zhuang SL, Li ZS. Feasibility and safety of magnetic-controlled capsule endoscopy system in examination of human stomach: a pilot study in healthy volunteers. J Interv Gastroenterol. 2012;2:155–160. doi: 10.4161/jig.23751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provision of gastrointestinal endoscopy and related services for a district general hospital. Working Party of the Clinical Services Committee of the British Society of Gastroenterology. Gut. 1991;32:95–105. doi: 10.1136/gut.32.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnenberg A, Amorosi SL, Lacey MJ, Lieberman DA. Patterns of endoscopy in the United States: analysis of data from the Centers for Medicare and Medicaid Services and the National Endoscopic Database. Gastrointest Endosc. 2008;67:489–496. doi: 10.1016/j.gie.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 21.Brandt LJ. Patients’ attitudes and apprehensions about endoscopy: how to calm troubled waters. Am J Gastroenterol. 2001;96:280–284. doi: 10.1111/j.1572-0241.2001.03508.x. [DOI] [PubMed] [Google Scholar]
- 22.Campo R, Brullet E, Montserrat A, Calvet X, Moix J, Rué M, Roqué M, Donoso L, Bordas JM. Identification of factors that influence tolerance of upper gastrointestinal endoscopy. Eur J Gastroenterol Hepatol. 1999;11:201–204. doi: 10.1097/00042737-199902000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Ching HL, Hale MF, Sidhu R, McAlindon ME. Reassessing the value of gastroscopy for the investigation of dyspepsia. Frontline Gastroenterol. 2018;9:62–66. doi: 10.1136/flgastro-2017-100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triantafyllou K, Papanikolaou IS, Papaxoinis K, Ladas SD. Two cameras detect more lesions in the small-bowel than one. World J Gastroenterol. 2011;17:1462–1467. doi: 10.3748/wjg.v17.i11.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remes-Troche JM, Jiménez-García VA, García-Montes JM, Hergueta-Delgado P, Roesch-Dietlen F, Herrerías-Gutiérrez JM. Application of colon capsule endoscopy (CCE) to evaluate the whole gastrointestinal tract: a comparative study of single-camera and dual-camera analysis. Clin Exp Gastroenterol. 2013;6:185–192. doi: 10.2147/CEG.S45215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ching HL, Hale MF, Sidhu R, Beg S, Ragunath K, McAlindon ME. Magnetically assisted capsule endoscopy (MACE) of the upper GI tract to select patients for endoscopy and reduce hospital admissions.: Presented at the BSG Annual Meeting 2017. Manchester, UK. BSG2017-942 [Google Scholar]
- 27.Koslowsky B, Jacob H, Eliakim R, Adler SN. PillCam ESO in esophageal studies: improved diagnostic yield of 14 frames per second (fps) compared with 4 fps. Endoscopy. 2006;38:27–30. doi: 10.1055/s-2005-921034. [DOI] [PubMed] [Google Scholar]
- 28.Laurain A, de Leusse A, Gincul R, Vanbiervliet G, Bramli S, Heyries L, Martane G, Amrani N, Serraj I, Saurin JC, et al. Oesophageal capsule endoscopy versus oesophago-gastroduodenoscopy for the diagnosis of recurrent varices: a prospective multicentre study. Dig Liver Dis. 2014;46:535–540. doi: 10.1016/j.dld.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Hale MF, Rahman I, Drew K, Sidhu R, Riley SA, Patel P, McAlindon ME. Magnetically steerable gastric capsule endoscopy is equivalent to flexible endoscopy in the detection of markers in an excised porcine stomach model: results of a randomized trial. Endoscopy. 2015;47:650–653. doi: 10.1055/s-0034-1391329. [DOI] [PubMed] [Google Scholar]
- 30.Rahman I, Pioche M, Shim CS, Lee SP, Sung IK, Saurin JC, Patel P. Magnetic-assisted capsule endoscopy in the upper GI tract by using a novel navigation system (with video) Gastrointest Endosc. 2016;83:889–895.e1. doi: 10.1016/j.gie.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Ching HL, Hale MF, Campbell JA, Healy A, Thurston V, Sidhu R, et al. Magnetically steered capsule endoscopy (MSCE) of the upper and mid gut in recurrent and refractory iron deficiency anaemia. Presented at the BSG Annual Meeting 2017. Manchester, UK. BSG2017-1021 [Google Scholar]
- 32.Rey JF, Ogata H, Hosoe N, Ohtsuka K, Ogata N, Ikeda K, Aihara H, Pangtay I, Hibi T, Kudo SE, et al. Blinded nonrandomized comparative study of gastric examination with a magnetically guided capsule endoscope and standard videoendoscope. Gastrointest Endosc. 2012;75:373–381. doi: 10.1016/j.gie.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Denzer UW, Rösch T, Hoytat B, Abdel-Hamid M, Hebuterne X, Vanbiervielt G, Filippi J, Ogata H, Hosoe N, Ohtsuka K, et al. Magnetically guided capsule versus conventional gastroscopy for upper abdominal complaints: a prospective blinded study. J Clin Gastroenterol. 2015;49:101–107. doi: 10.1097/MCG.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 34.Rahman I, Kay M, Bryant T, Pelitari S, Salter S, Dimitrov B, Patel P. Optimizing the performance of magnetic-assisted capsule endoscopy of the upper GI tract using multiplanar CT modelling. Eur J Gastroenterol Hepatol. 2015;27:460–466. doi: 10.1097/MEG.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 35.Qian Y, Wu S, Wang Q, Wei L, Wu W, Wang L, Chu Y. Combination of Five Body Positions Can Effectively Improve the Rate of Gastric Mucosa’s Complete Visualization by Applying Magnetic-Guided Capsule Endoscopy. Gastroenterol Res Pract. 2016;2016:6471945. doi: 10.1155/2016/6471945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koulaouzidis A, Smirnidis A, Douglas S, Plevris JN. QuickView in small-bowel capsule endoscopy is useful in certain clinical settings, but QuickView with Blue Mode is of no additional benefit. Eur J Gastroenterol Hepatol. 2012;24:1099–1104. doi: 10.1097/MEG.0b013e32835563ab. [DOI] [PubMed] [Google Scholar]
- 37.Basford PJ, Brown J, Gadeke L, Fogg C, Haysom-Newport B, Ogollah R, Bhattacharyya R, Longcroft-Wheaton G, Thursby-Pelham F, Neale JR, et al. A randomized controlled trial of pre-procedure simethicone and N-acetylcysteine to improve mucosal visibility during gastroscopy - NICEVIS. Endosc Int Open. 2016;4:E1197–E1202. doi: 10.1055/s-0042-117631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee GJ, Park SJ, Kim SJ, Kim HH, Park MI, Moon W. Effectiveness of Premedication with Pronase for Visualization of the Mucosa during Endoscopy: A Randomized, Controlled Trial. Clin Endosc. 2012;45:161–164. doi: 10.5946/ce.2012.45.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim GH, Cho YK, Cha JM, Lee SY, Chung IK. Effect of pronase as mucolytic agent on imaging quality of magnifying endoscopy. World J Gastroenterol. 2015;21:2483–2489. doi: 10.3748/wjg.v21.i8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu SG, Qian YY, Tang XY, Zhu QQ, Zhou W, Du H, An W, Su XJ, Zhao AJ, Ching HL, et al. Gastric preparation for magnetically controlled capsule endoscopy: A prospective, randomized single-blinded controlled trial. Dig Liver Dis. 2018;50:42–47. doi: 10.1016/j.dld.2017.09.129. [DOI] [PubMed] [Google Scholar]
- 41.Stacher G, Bergmann H, Havlik E, Schmierer G, Schneider C. Effects of oral cyclotropium bromide, hyoscine N-butylbromide and placebo on gastric emptying and antral motor activity in healthy man. Gut. 1984;25:485–490. doi: 10.1136/gut.25.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meltzer AC, Ali MA, Kresiberg RB, Patel G, Smith JP, Pines JM, Fleischer DE. Video capsule endoscopy in the emergency department: a prospective study of acute upper gastrointestinal hemorrhage. Ann Emerg Med. 2013;61:438–443.e1. doi: 10.1016/j.annemergmed.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 43.di Pietro M, Chan D, Fitzgerald RC, Wang KK. Screening for Barrett’s Esophagus. Gastroenterology. 2015;148:912–923. doi: 10.1053/j.gastro.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eliakim R, Yassin K, Shlomi I, Suissa A, Eisen GM. A novel diagnostic tool for detecting oesophageal pathology: the PillCam oesophageal video capsule. Aliment Pharmacol Ther. 2004;20:1083–1089. doi: 10.1111/j.1365-2036.2004.02206.x. [DOI] [PubMed] [Google Scholar]
- 45.Gralnek IM, Adler SN, Yassin K, Koslowsky B, Metzger Y, Eliakim R. Detecting esophageal disease with second-generation capsule endoscopy: initial evaluation of the PillCam ESO 2. Endoscopy. 2008;40:275–279. doi: 10.1055/s-2007-995645. [DOI] [PubMed] [Google Scholar]
- 46.Ojidu H, Palmer H, Lewandowski J, Hampton J, Blakeborough T, Epstein O, McAlindon ME. Patient tolerance and acceptance of different colonic imaging modalities: an observational cohort study. Eur J Gastroenterol Hepatol. 2018;30:520–525. doi: 10.1097/MEG.0000000000001090. [DOI] [PubMed] [Google Scholar]
- 47.Gupta M, Beebe TJ, Dunagan KT, Schleck CD, Zinsmeister AR, Talley NJ, Locke GR 3rd, Iyer PG. Screening for Barrett’s esophagus: results from a population-based survey. Dig Dis Sci. 2014;59:1831–1850. doi: 10.1007/s10620-014-3092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koulaouzidis A, Iakovidis DK, Karargyris A, Rondonotti E. Wireless endoscopy in 2020: Will it still be a capsule? World J Gastroenterol. 2015;21:5119–5130. doi: 10.3748/wjg.v21.i17.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]