Abstract
Chronic kidney disease (CKD) is an important problem throughout the world, associated with the increase of blood urea nitrogen (BUN) and serum creatinine (sCre) and with renal tubular injuries. It is crucial to elucidate the molecular mechanisms of renal injuries to identify the new therapeutics and early diagnostic methods. We focused on cell adhesion molecule-1 (CADM1) protein. CADM1, its isoform SP4, is expressed in the epithelial cells of various tissues, including renal distal tubules, localized on the lateral cell membrane, mediates cell-cell adhesion via trans-homophilic binding, and interacts with various proteins. We previously reported that its expression was downregulated by post-proteolytic cleavage (α- and β-shedding) in pulmonary diseases. To investigate whether CADM1 α-shedding occurs in human nephropathies, we performed Western blotting and immunohistochemical analysis of specimens with arterionephrosclerosis (AS) and diabetic nephropathy (DN) from autopsied kidneys. CADM1 α-shedding was induced in AS and DN kidneys and derived from the decrease in full-length CADM1 (FL-CADM1) and increase of the COOH-terminal fragment (α-CTF). In particular, the reduced FL-CADM1 level was correlated with tubular and tubulointerstitial injuries and the increases in BUN and sCre levels. Apoptosis of renal tubular epithelial cells (TECs) was promoted in both nephropathies, and it was significantly correlated with the decrease in the FL-CADM1. Furthermore, FL-CADM1 knockdown by small interfering RNA downregulated anti-apoptotic Bcl-2 protein and promoted apoptosis of cultured renal TECs. The present study suggests that the reduction of FL-CADM1 leads to renal TEC apoptosis and could exacerbate renal tubular and tubulointerstitial injuries, which contribute to the development of CKD.
Keywords: apoptosis, arterionephrosclerotic nephropathy, CADM1, DM nephropathy, shedding
INTRODUCTION
The populations of both the US and Japan face aging. The percentage of Americans over 65 years of age is expected to reach 20% by 2050, a rate already reached in Japan, with 24% older than 65 years (5). The prevalence of chronic kidney disease (CKD) in Japan is 13%, and it has the second highest prevalence of end-stage renal disease in the world (5). CKD is also common in the US, at 11%, ranking it third. The affected populations continue to increase in Europe, Australia, India, China, and Southeast Asia (21). Diabetes, hypertension, and vascular disease caused by atherosclerosis account for the majority of cases (21). Furthermore, less than one-half of CKD patients develop end-stage renal disease due to the high risk of mortality associated with cardiovascular events (2). Since tubules are responsible for the reabsorption and secretion of various solutes, damage to the tubules is a key mediator of kidney injury. Therefore, it is indispensable to reveal the mechanisms of renal injury, particularly the apoptosis of renal tubular epithelial cells (TECs), and develop methods for its prevention and therapy. Also, it is crucial to search for early indicators of declining renal injuries before the emergence of clinical dysfunction.
Cell adhesion molecule 1 (CADM1) is an intercellular adhesion molecule that belongs to the immunoglobulin (Ig) superfamily and is also known as tumor suppressor in lung cancer 1 (TSLC1), Necl-2, IgSF4A, and SynCAM1 (24, 33). CADM1 is a cell adhesion molecule with many functions, including tumor suppression, mast cell survival, synapse formation, and spermatogenesis (34). The cytoplasmic domain of CADM1 contains a protein 4.1-binding motif and a class II (PDZ)-binding motif (17), and it is associated with members of a group of scaffolding proteins, membrane-associated guanylate kinase homologs, including membrane palmitoylated protein 1–3, Ca2+/calmodulin-dependent serine kinase, and Pals-2 (33). In epithelial cells, CADM1 is localized on the lateral cell membrane and mediates neighboring cell-cell adhesion via the trans-homophilic binding (15, 32) and is implicated in transmitting cell attachment signals to actin reorganization in the cytoplasm through activating the phosphatidylinositol 3-kinase (PI3K) pathway for the formation and maintenance of the adhesion-based epithelial structure (32). Various types of epithelial cells express CADM1, including renal tubular epithelial cells, pulmonary cells, biliary cells, neurons, mast cells, and pancreatic endocrine cells (50), but its expression could be regulated by postproteolytic cleavage, referred to as shedding (7, 34). The ectodomain of CADM1 is cleaved at one of two sites, yielding two fragments, the α-COOH-terminal fragment (α-CTF) and β-CTF (34). Our laboratory recently identified CADM1 shedding as a key event in the development of pulmonary diseases (30, 50). CADM1 ectodomain shedding rates increase in emphysematous lungs, and the mitochondrial localization of α-CTF contributes to lung epithelial apoptosis (30). On the other hand, CADM1 α-shedding was increased in idiopathic interstitial pneumonia (IIP) and consequently contributed to alveolar epithelial cell apoptosis by decreasing the full-length CADM1 (FL-CADM1) level (51).
CADM1 expression was noted in the distal tubules of the kidneys (35), but it was not clearly elucidated whether the shedding of CADM1 is correlated with the renal pathology. We hypothesized that this shedding was induced in the kidneys with nephropathies and evaluated whether further α-CTF or FL-CADM1 reduction induces apoptosis. In the present study, we examined specimen histologically diagnosed with injured kidneys from the autopsy records of Kindai University Hospital, and focused on the specimens with diabetes mellitus nephropathy (DN) and arterionephrosclerosis (AS). According to a previously described method (14, 30), protein extracts were prepared from the autopsied kidney paraffin sections and examined the CADM1 expression by Western blotting. We also examined whether these alterations in expression might be involved in tubular and tubulointerstitial injuries, renal tubular epithelial cell apoptosis, and renal dysfunction. Furthermore, we investigated CADM1 shedding (increased α-CTF or reduced FL-CADM1) effects on the apoptosis of cultured renal TECs.
MATERIALS AND METHODS
Antibodies and reagents.
A rabbit anti-CADM1 polyclonal antibody directed against the COOH-terminal 15-amino acid peptide was generated in our laboratory, as described previously (10). Single-strand DNA (ssDNA) (rabbit IgG; Immuno-Biological Laboratories, Gunma, Japan), caspase-3 (no. 9662, Cell Signaling Technology), Bax (sc-493, Santa Cruz Biotechnology), Bcl-2 (sc-7382, Santa Cruz Biotechnology), and β-actin (Sigma) were purchased. Peroxidase-conjugated secondary antibodies were obtained from Amersham (Buckinghamshire, UK) and Jackson ImmunoResearch (West Grove, PA), respectively.
Human samples.
We reviewed the clinical records, autopsy records, and pathological specimens archived in Kindai University Hospital (Osaka, Japan) to identify autopsied patients. The control group comprised patients who had died of diseases without clinical or histological evidence of kidney disease. Kidneys were removed at autopsy and fixed with 10% buffered formalin for several days and embedded with paraffin [formalin fixed paraffin embedded (FFPE)]. The specimens were routinely prepared for pathological diagnoses and selected randomly from the newly autopsied to avoid sampling bias. The Ethics Committee of Kindai University approved the experimental protocol (26–279).
Protein extraction and Western blot analysis.
Protein was extracted from 20-μm-thick sections of paraffin-embedded kidneys according to the method described by Rodriguez-Rigueiro et al. (38) with minor modification (17, 34). The procedures for protein extraction from cultured cells and Western blot analyses were performed as described previously (22). Immunoreactive band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD). We excluded the specimens with less or no band of β-actin due to excessive autolysis.
RNA extraction and RT-PCR.
RNA was extracted from 20-μm-thick sections of paraffin-embedded kidneys using an RNeasy FFPE Kit (Invitrogen), according to the manufacturer’s instruction. cDNA was synthesized with superscript IV reverse transcriptase (Invitrogen). The primer used for DNA amplification are as follows: CADM1, forward, 5′-GCTCACTCGGATTATATGCTGT-3′ and reverse, 5′-TATAGCTGTGTCTGCGTCTGCT-3′; β-actin, forward, 5′-CACCAACTGGGACGACAT-3′ and reverse, 5′-ACAGCCTGGATAGCAACG-3′. PCR was performed with the KOD FX Neo (TOYOBO, Osaka, Japan), according to the manufacturer’s instructions.
Histological and pathological examination.
Kidney sections (4 μm thick) adjacent to those used for protein extraction were immunostained as described previously (22) using primary antibodies against CADM1 and ssDNA. Color reaction and counterstaining were performed using the peroxidase substrate 3-amino-9-ethylcarbazole (Vector Laboratories, Burlingame, CA) and hematoxylin, respectively.
Grading of renal tubular and tubulointerstitial injuries.
A pathologist (A.I.) quantified the renal tubular and interstitial injuries of hematoxylin- and eosin (HE)-stained sections, as previously reported (17). Briefly, injury levels in each section were scored based on the extent of inflammation, interstitial fibrosis, and tubular degeneration. Three kinds of indexes were graded as follows: 0, none; 1, mild; 2, moderate; and 3, severe. When the extent was regarded to be between two grades, 0.5 was added to the lower score. Each of these indexes was summed, and total indexes (0~9) were expressed as the individual index.
Cell culture.
CNT cells, a rabbit renal distal tubular cell line, were a gift from Riken BioResource Center (RCB1912; Riken, Ibaraki, Japan) (45). CNT cells were grown and maintained according to the manufacturer’s instructions.
α-CTF and RNAi transfection.
The plasmid vector expressing CADM1-α-CTF was used previously (14, 30). For exogenous expression of α-CTF, CNT cells were transfected using Lipofectamine 3000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. To silence the expression of endogenous CADM1, we constructed small interfering RNA (siRNA) sequences according to Masuda’s report (28). The targeted sequence was as follows: 5′-AACGAAAGACGTGACAGTGAT-3′ (CADM1 siRNA).
Apoptotic assay.
For terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) and ssDNA immunofluorescence assay, CNT cells were grown to 60–70% confluence in μ-Dishes (ibidi) and transfected with indicated vectors or left untransfected. After 48 h, cells were fixed with 4% paraformaldehyde at 4°C for 10 min and permeabilized with 0.25% Triton X-100 in PBS at 4°C for 5 min. TUNEL assays were conducted on cultured cells using an In Situ Cell Death Detection Kit (Roche Applied Science, Upper Bavaria, Germany), as described in the previous report (30). After permeabilization, the cells were incubated with the TUNEL reaction mixture containing terminal deoxynucleotidyl transferase and FITC-labeled dUTP for 1 h at 37°C, followed by nuclear counterstaining with 4,6-diamidino-2-phenylindole. For immunofluorescence assays of ssDNA, the permeabilized cells were incubated with 3% BSA in PBS at room temperature for 1 h, and then with anti-ssDNA antibody for 4°C overnight. After being washed with PBS, the cells were treated with the Alexa 488-conjugated secondary antibody (Invitrogen) and with 4,6-diamidino-2-phenylindole for nuclear counterstaining at 4°C for 2 h.
Statistical analysis.
Differences among experimental groups were analyzed using Student’s t-test for quantification. Correlations were analyzed using Spearman’s rank test. A P value < 0.05 was considered significant.
RESULTS
Renal features of specimens histopathologically diagnosed as nephropathies.
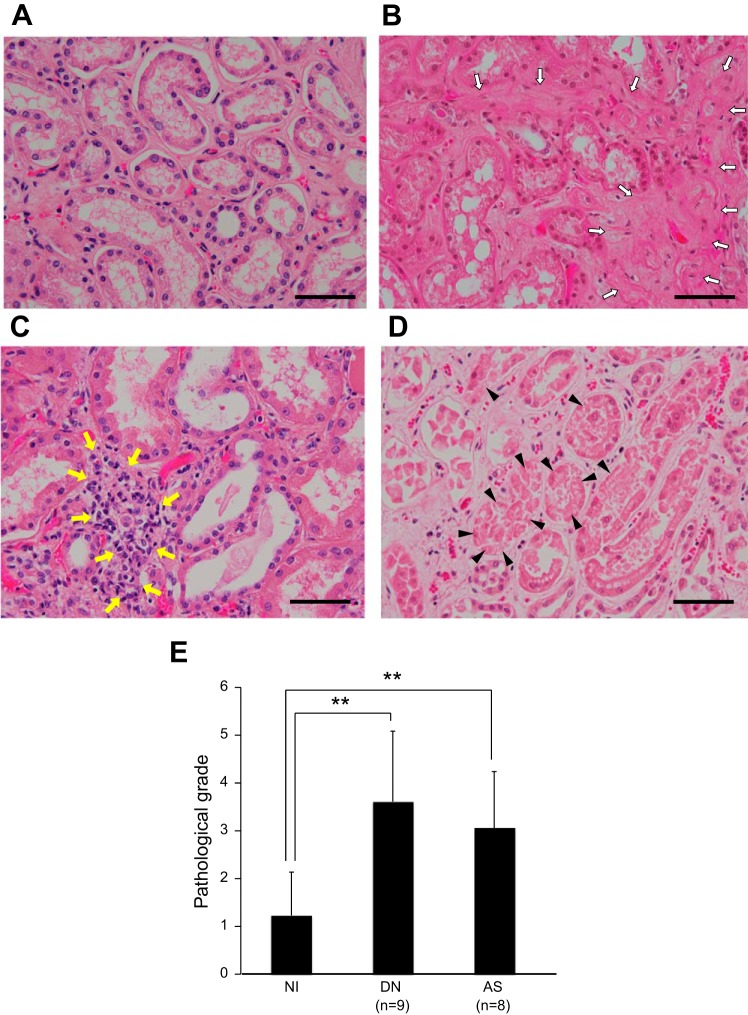
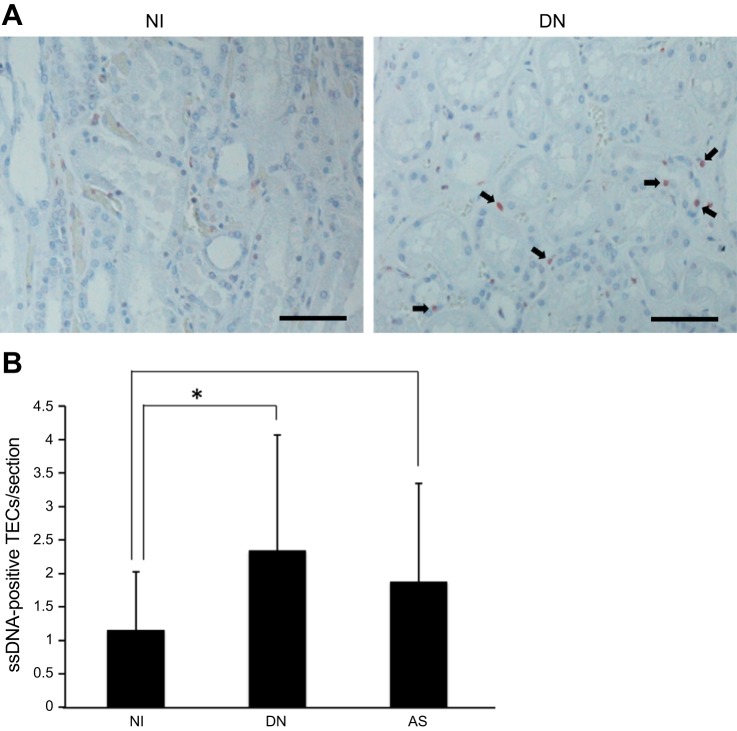
Thirty autopsied kidneys were histologically assessed by pathologists independent of this study, and 13 noninjured (NI) and 17 injured subjects were enrolled with AS or DN. The clinical parameters of specimens used in this study are shown in Table 1. The serum creatinine (sCre) level was markedly increased in the specimens with AS. Furthermore, the BUN and sCre levels were more increased in DN. To investigate the histological characterization and pathological levels, we stained the renal sections with HE stain (Fig. 1). By comparing NI sections (Fig. 1A), AS and DN had histopathological features, such as tubular degeneration, fibrosis, and inflammation (Fig. 1, B–D). Moreover, tubular and tubulointerstitial injury grades (pathological grades) were quantified using blind scoring methods. We measured the injury scores in terms of inflammation, degeneration, and interstitial fibrosis and summed the scores as the pathological grade. Although the pathological grade was 1.23 ± 0.90 in the NI kidneys, it was 2.19 ± 1.39 in AS (Fig. 1E). Furthermore, this grade was 2.34 ± 1.73 in the DN kidneys, and significantly higher than in NI sections (Fig. 1E). Next, the renal sections were immunostained with an antibody targeting ssDNA to detect apoptotic cells (8, 16, 47) and counterstained with hematoxylin to count total tubular cells in the kidney (Fig. 2A). The proportions of ssDNA-positive tubular cells were 2.0- and 1.6-fold higher in the DN and AS kidneys, respectively (Fig. 2B).
Table 1.
Clinical characteristics of normal patients and those with nephropathies
| NI | DN | AS | |
|---|---|---|---|
| n | 13 | 8 | 7 |
| Age, yr | 68.5 ± 10.8 (48–81) | 67.4 ± 12.9 (45–83) | 73.9 ± 7.9 (62–83) |
| Sex (male/female) | 9/4 | 5/3 | 6/1 |
| GFR, ml·min−1·1.73 m−2 | 58.1 ± 8.7 (27–129) | 25.4 ± 7.2* (5–52) | 27.1 ± 4.7* (5–43) |
| BUN, mg/dl | 40.8 ± 5.7 (14–79) | 66.4 ± 12.5* (21–128) | 59.4 ± 9.5* (21–93) |
| sCre, mg/dl | 1.14 ± 0.16 (0.49–2.48) | 3.81 ± 1.07* (0.59–8.59) | 2.37 ± 0.84 (0.59–8.59) |
Values are means ± SE (with range in parentheses); n, no. of subjects. NI, noninjured; DN, diabetic nephropathy; AS, arterionephrosclerosis; eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen; sCre, serum creatinine.
P < 0.05 vs. NI.
Fig. 1.
Representative images of renal tissues stained with HE stain. A: noninjured (NI). B: diabetes mellitus nephropathy (DN); C and D: nephropathy with arterionephrosclerosis (AS). White arrows, interstitial fibrosis; yellow arrows, inflammation; black arrowheads, tubular degeneration. Scale bar = 100 μm. E: pathological grade in NI and each nephropathy (DN and AS). **P < 0.01.
Fig. 2.
Apoptotic cells in nephropathies. A: representative images of immunohistochemical staining of ssDNA against NI and DN kidney. The sections were counterstained with hematoxylin. The arrows indicate ssDNA-positive TECs. Scale bar = 100 μm. B: the numbers of apoptotic cells among human renal tubular cells per section (×400). Values are means ± SD. NI, n = 13; DN, n = 9; AS, n = 8. *P < 0.05.
Increased ectodomain shedding of CADM1 in nephropathies.
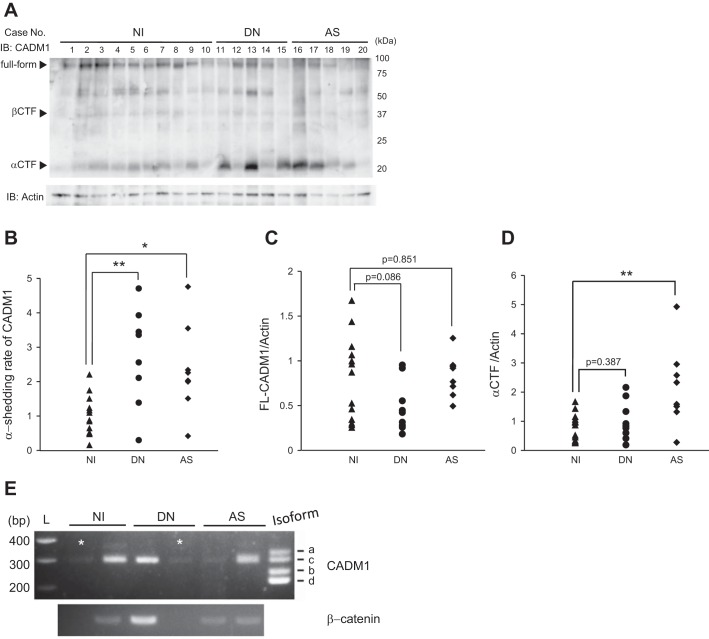
Next, we extracted proteins from renal sections (FFPE) and analyzed the extracts by Western blot using a CADM1 antibody targeting the COOH-terminal domain (Fig. 3A). Consistent with our laboratory’s previous report (30), the CADM1 antibody detected three forms of CADM1 of ~100, 35, and 18 kDa, corresponding to FL-CADM1, β-CTF, and α-CTF, respectively (Fig. 3A). The β-CTF band was thin, and there were no significant differences among the groups (data not shown), but the α-CTF band was significantly increased in the kidneys with nephropathies (Fig. 3A). The CADM1 α-shedding rates were calculated as the band intensities of α-CTF relative to those of FL-CADM1. The two nephropathy groups had higher α-shedding rates than those of the control group (Fig. 3B). By comparing the band intensities of FL-CADM1 and β-actin bands, there was a tendency whereby FL-CADM1 was downregulated in nephropathies, especially DN kidneys (Fig. 3C). We assessed the acceleration of α-CTF and could observe α-CTF induction to some extent, but there was no significance (Fig. 3D). Therefore, we found increased α-shedding in the kidneys with nephropathies. To examine whether decreased FL-CADM1 expression was derived from the downregulated transcript, we tried to analyze the CADM1 mRNA from NI, AS, and DN specimens. But we could not sufficiently isolate RNA from renal FFPE sections and investigate the CADM1 transcription levels (Fig. 3E). On the other hand, we found the major isotype of CADM1 in NI, AS, and DN kidneys via RT-PCR assay in the present study. CADM1 has several isoforms from alternative mRNA splicing, which occurs in the juxtamembranous extracellular region (3). Especially, four membrane-spanning isoforms, CADM1 SP1–SP4, are found in humans (3). By comparing the control band size, we found that the major is isoform SP4 in NI, AS, and DN kidneys (Fig. 3E).
Fig. 3.
Western blot analysis of CADM1 in nephropathies. A: Western blot analysis using anti-COOH-terminal-CADM1 antibody. Arrowheads indicate bands corresponding to the FL, β-CTF, and α-CTF forms of CADM1. B: CADM1 ectodomain shedding rates (amount of α-CTFs relative to FL-CADM1). *P < 0.05. **P < 0.01. C and D: FL- and α-CTF-CADM1 levels, respectively, relative to β-actin are plotted as dots in each patient sample. Significance between two groups was analyzed using the t-test. Values are means ± SD. NI, n = 13; DN, n = 9; AS, n = 8. P values ≤ 0.05 are shown. E: total RNAs from renal sections of human NI, AS, and DN were analyzed by RT-PCR using a primer set encompassing the CADM1 extracellular juxtamembrane region. The PCR products were electrophoresed together with CADM1 isoform (SP1–4) size markers. L, 100-base pair (bp) ladder. RNAs were also PCR-amplified using a primer set for β-actin to indicate RNA loading per lane. *FFPE samples that did not have the sufficient RNA quantity.
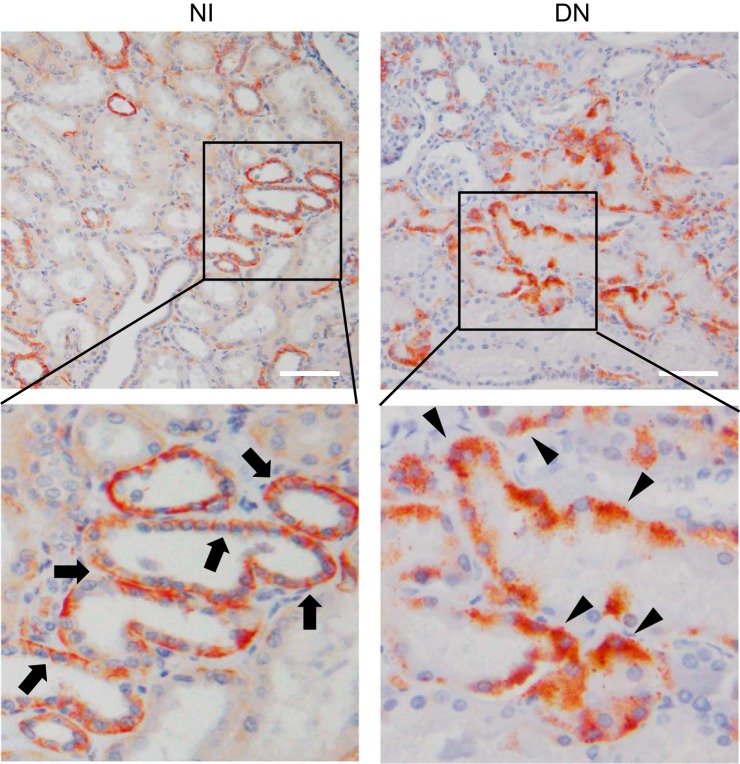
We analyzed the immunohistochemistry with anti-COOH-terminus CADM1 antibody to examine the localization (Fig. 4). FL-CADM1 was localized at the cell membrane, particularly the lateral side in NI kidneys (Fig. 4, top left and bottom left), which suggests that CADM1 exists as a full form. However, in the diabetic nephropathies, CADM1, mainly α-CTF, was localized in the cytoplasm (Fig. 4, top right and bottom right), which revealed that the transmembranous regions had been lost, and α-CTF existed in the cytoplasm of DN kidneys.
Fig. 4.
Representative localizations of CADM1 in tubular epithelial cells. NI (left) and DN kidneys (right). Immunohistochemical stain was performed with a COOH-terminal CADM1 antibody (top row: ×200). The arrows and arrowheads indicate the CADM1 stain at the lateral and/or basal membrane and at the intracytoplasm, respectively. Scale bar = 100 μm.
Association between CADM1 shedding and renal pathological or clinical parameters.
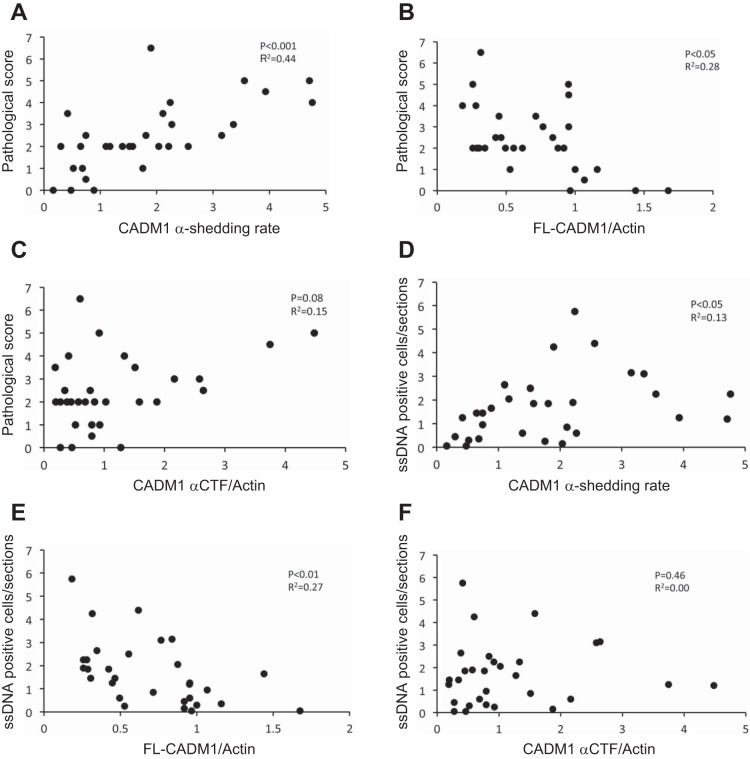
To assess the association between the pathological grade and α-shedding rate, we summed the specimens of each group for this analysis. A scatter plot of all cases and correlation assays revealed that the degree of tubular and tubulointerstitial injuries (pathological grade) was correlated with the α-shedding rate (Fig. 5A). A negative correlation between the FL-CADM1 level and pathological grade was observed (Fig. 5B). On the other hand, we analyzed the association between α-CTF (normalized by β-actin) and the pathological grade, but there was no significant correlation (Fig. 5C). Next, we focused on CADM1 shedding and TEC apoptosis. Of note, the promotion of CADM1 shedding was correlated with renal ssDNA-positive TECs (Fig. 5D). Furthermore, the reduction of FL-CADM1 was correlated with renal ssDNA-positive TECs (Fig. 5E), but α-CTF induction and TEC apoptosis showed no significant correlation (Fig. 5F). These results suggest that reduced FL-CADM1 affects renal TEC survival. Furthermore, we examined the association between the CADM1 expression parameters (α-shedding rate, FL-CADM1, and α-CTF level) and clinical parameters, such as estimated glomerular filtration rate, blood urea nitrogen (BUN), and sCre (Table 2). The CADM1 shedding rate and expression levels were not significantly correlated with clinical parameters. However, the FL-CADM1 level was comparably correlated with BUN and sCre (Table 2). On the other hand, the α-CTF level was not associated with the clinical parameters.
Fig. 5.
Correlation between CADM1 ectodomain shedding and tubular injuries or TEC apoptosis. A–C: correlation of CADM1 shedding rate (A), FL-CADM1 (B), or α-CTF (C) with tubular injuries. D–F: correlation of CADM1 shedding rate (D), FL-CADM1 (E), or α-CTF (F) with tubular epithelial cell apoptosis. n = 28. P values ≤ 0.05 are shown.
Table 2.
Correlation between CADM1 shedding rate, FL-CADM1, α-CTF, and renal cinical parameters
| α-Shedding Rate |
FL-CADM1/Actin |
α-CTF/Actin |
||||
|---|---|---|---|---|---|---|
| Clinical Parameter | R2 | P value | R2 | P value | R2 | P value |
| eGFR, ml·min−1·1.73 m−2 | 0.04 | 0.269 | 0.01 | 0.556 | 0.01 | 0.557 |
| BUN, mg/dl | 0.02 | 0.452 | 0.06 | 0.209 | 0 | 0.917 |
| sCre, mg/dl | 0.05 | 0.223 | 0.09 | 0.131 | 0 | 0.804 |
Correlations were analyzed by Spearman’s test.
FL-CADM1 reduction induces apoptosis in cultured renal tubular cells.
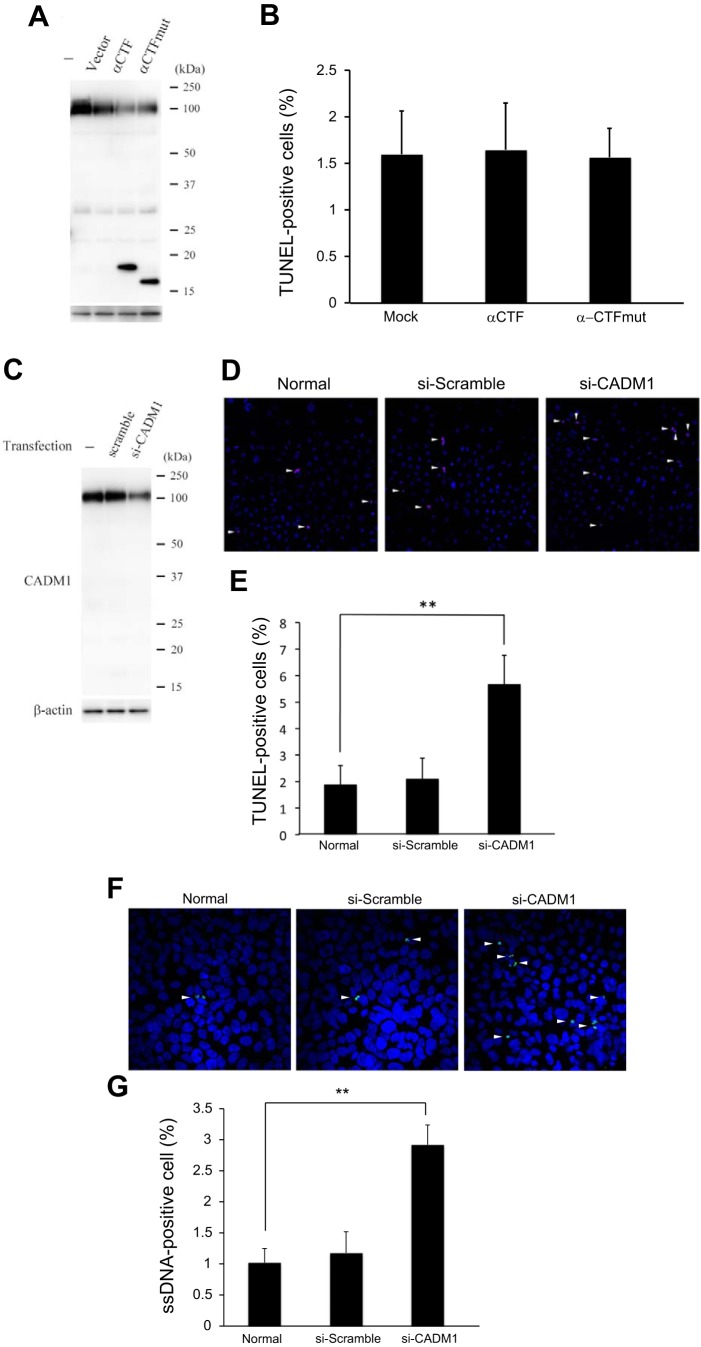
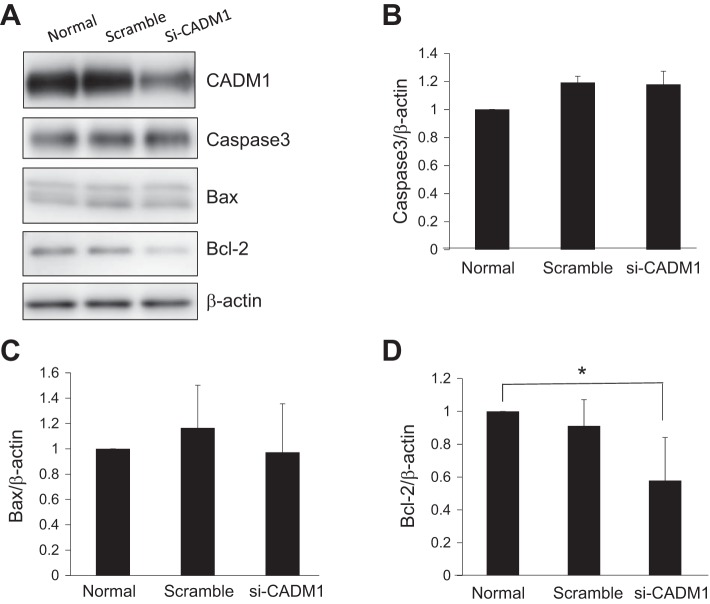
It is important to validate whether increased α-CTF or decreased FL-CADM1 induces apoptosis in the TEC cells. At first, to test whether α-CTF induces apoptosis, we exogenously transfected cultured CNT cells with α-CTF vector and confirmed α-CTF expression by Western blot (Fig. 6A). We verified the apoptotic cells transfected with α-CTF using TUNEL methods after 48 h, but found no significant differences in apoptotic cells in mock and α-CTF-transfected CNT cells (Fig. 6B). Next, to investigate the effects of FL-CADM1 reduction on cell survival, CNT cells were transfected with a vector encoding specific siRNA targeting CADM1. After 48 h of transfection, the CNT cells were subjected to Western blot analyses (Fig. 6C). Based on triplicate assays, control siRNA had no effects on the level (101.7 ± 10.1%), and CADM1-targeting siRNA reduced the level of FL-CADM1 to ~60.1 ± 11.1%. The observed rates of TUNEL-positive cells were 1.88 ± 0.71 and 2.09 ± 0.78% in untreated and control siRNA-transfected cells, respectively, but the rate was 5.68 ± 1.08% with CADM1-targeting siRNA knockdown (Fig. 6, D and E). So as to investigate the effects of downregulated FL-CADM1 on apoptotic cells by another method, we, furthermore, performed the ssDNA immunofluorescence assay (Fig. 6F). Compared with non- and control siRNA treated (1.02 ± 0.23 and 1.18 ± 0.34, respectively), significantly higher apoptotic cells were found in the cells transfected with CADM1 siRNA (2.92 ± 0.32) (Fig. 6G). Therefore, the reduction of FL-CADM1 significantly affected the apoptosis of cultured CNT cells. Furthermore, we examined the expression of apoptosis-related proteins, caspase-3, Bax, and Bcl-2, by Western blot assay (Fig. 7A). Although we could not find the significant changes of caspase-3 and proapoptotic Bax expression (Fig. 7, B and C), anti-apoptotic Bcl-2 protein was significantly decreased in the CADM1 siRNA-treated CNT cells (Fig. 7D). We suggest FL-CADM1 reduction leads into the Bcl-2 downregulation and further induction of apoptosis in the renal tubular cells.
Fig. 6.
A decreased FL-CADM1 level, not increased α-CTF, induces the apoptosis of CNT cells. A: CNT cells were left untreated or transfected with mock or α-CTF or α-CTFmut. After 48 h, CADM1 expression was examined by Western blot analyses. B: the numbers of apoptotic cells in CNT cells/section. C: CNT cells were left untreated or transfected with control or CADM1-targeting siRNA. After 48 h, CADM1 expression was examined by Western blot analyses, compared with β-actin levels. The CADM1 levels were further normalized by the value in untreated cells. FL-CADM1 levels were calculated from triplicate experiments for each cell type. D: after 48 h, CNT cells untreated and transfected with either control or the siRNA vector were analyzed by TUNEL assays. TUNEL (green) and DAPI (blue) fluorescent images were merged. White arrowheads indicate TUNEL-positive cells. E: the numbers of apoptotic cells in CNT cells/section (n = 3). The rates of TUNEL-positive cells are presented as means ± SD. **P values ≤ 0.05. F: after 48 h, CNT cells untreated and transfected with either control or the siRNA vector were analyzed by ssDNA immunofluorescence assays. ssDNA (green) and DAPI (blue) fluorescent images were merged. White arrowheads indicate ssDNA-positive cells. G: the numbers of apoptotic cells in CNT cells/section (n = 3). The rates of ssDNA-positive cells are presented as means ± SD. **P values ≤ 0.05.
Fig. 7.
Decreased FL-CADM1 level effects on the decrease of Bcl-2, but not caspase-3 and Bax. A: after 48 h, CNT cells untreated and transfected with either control or the siRNA vector were analyzed by Western blot assays. Capase-3 (B), Bax (C), and Bcl-2 (D) levels relative to β-actin are shown. The rates of each are presented as means ± SD. Significance between two groups was analyzed using the t-test. *P values ≤ 0.05.
DISCUSSION
CADM1 is downregulated by the hypermethylation of the CADM1 gene promoter and/or the loss of heterozygosity in many types of cancers (33). Also, miR-214, frequently upregulated in a variety of cancers, targeted the 3′-UTR of CADM1 mRNA directly and suppressed its translation (31). On the other hand, our laboratory previously showed the mechanism of FL-CADM1 reduction by protein shedding. The α-shedding of CADM1 is dependent on a disintegrin and metalloproteinase 10 (ADAM10) that is also a membrane-bound protein expressed in various organs (34, 40). Some substrates of ADAM10 were found in distal tubules, such as meprin A (13) and klotho (9, 46). Meprin A is shed during renal ischemia reperfusion injury, a well-known acute kidney injury model (16). This protease has been implicated in several inflammatory pathologies, such as edema formation, leukocyte infiltration, and thrombosis. Moreover, a substrate of ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death (41). Targeted inhibition of active ADAM10 is expected to become a potential therapy for ADAM10-dependent tumor development and drug resistance (1), and its inhibition by chemicals or gene knockout attenuates the inflammatory response in animal models of vascular damage, including hypertension and atherosclerosis (6). For example, the exposure of cells to tunicamycin-induced apoptosis was accompanied by the cleavage of caspase-3 and poly (ADP-ribose) polymerase 1, and ADAM10 induction (19), which was significantly inhibited by treatment with GI254023X (a specific ADAM10 inhibitor). Therefore, ADAM10 may be a promising therapeutic target for nephropathies.
In this study, we found a strong correlation of FL-CADM1 reduction with apoptosis, the renal pathological grade, and dysfunctions. Because the loss of cell polarity can generally trigger apoptosis to eliminate damaged epithelial cells (29, 39), FL-CADM1 decrease could cause renal TECs through the loss of epithelial polarity. Of note, we observed that TECs expressed CADM1 SP4, which might strengthen cell-cell binding and epithelial polarity via the trans-homophilic CADM1 interaction, resulting in anti-apoptotic function of CADM1. Moreover, FL-CADM1 was connected to PI3K by forming a protein complex with membrane palmitoylated protein 3 and disk-large at the cell-cell contact sites (32). The PI3K pathway is crucial for the signals mediated by trans-homophilic CADM1 interaction to the cytoplasm, leading to cytoskeletal remodeling and the formation and maintenance of the epithelial structure (32). CADM1 functions as the machinery for TEC survival in the distal tubules. Some reports demonstrated that intrinsic and extrinsic apoptotic factors are involved in the apoptosis of proximal tubular cells (12, 44, 48, 49). In the present study, we found Bcl-2 expression was significantly decreased in the cultured CADM1-downregulated renal tubular cells and might lead into apoptosis. CADM1 shedding remains intriguing as the mechanism of apoptosis in the distal tubules.
It remains unclear why the renal α-CTF level was not significantly correlated with the pathological grade, despite increased α-shedding, but α-CTF could be degraded into a smaller peptide. Our laboratory previously found a CADM1-intracellular domain (C-ICD), which has a theoretical molecular weight of 5.3 kDa (51 amino acids). C-ICD generated by γ-secretase induces the apoptosis of lung epithelial cells (11, 34). C-ICD has band 4.1- and PDZ-binding domains (50), which are indispensable for the induction of apoptosis and the suppression of epithelial-mesenchymal transition (25–27). The quantity of α-CTF relative to FL-CADM1 increased in emphysematous lungs, but that of C-ICD was below the detection limit of usual Western blot (11). In addition to the technical problem due to the molecular size, we think that the amount of the C-ICD fragment is very small compared with α-CTF. This is probably because of the rapid degradation of C-ICD, as many other ICD fragments are generated by γ-secretase, including Notch ICD, in a proteasome-dependent manner (18). Moreover, it remains controversial where C-ICD is localized in the intracellular region. C-ICD may be transported into the nucleus together with Ca2+/calmodulin-dependent serine kinase and modulate target genes. ICDs of γ-secretase substrates, such as CD44 (36) and β-amyloid precursor protein (20), often act as nuclear signaling molecules that regulate gene expression. Otherwise, C-ICD could accumulate in the mitochondria and accelerate apoptosis. In particular, C-ICD is likely to accumulate in the mitochondrial intermembrane space, because it is small enough to pass through the porin pores (4, 23). Further experiments are needed to clarify the effects of C-ICD on renal TECs, especially apoptosis.
Although renal pathological examination by renal biopsy is the standard for evaluating nephropathies, it is impractical for physicians to closely monitor patients due to the invasive methodology. Thus various biomarkers for renal injuries in urine have already been reported, such as urinary nephrin, urinary vascular endothelial growth factor in podocytes, and kidney injury molecule-1, α1-microglobulin, β2-microglobulin, and L-type fatty acid binding protein in tubular cells. Podocyte damage and proximal tubule dysfunction biomarkers could be validated as a practical approach for the diagnosis of early DN by further studies with larger cohorts (37). The patients who display increased levels of urinary α1-microglobulin and kidney injury molecule-1 show that tubular functional defects precede the onset of albuminuria in early diabetes mellitus patients (43). We have actually detected the CADM1 NH2-terminal fragment (C-NTF) in urine from some patients with nephropathies. This urinary CADM1 NH2-terminal fragment detection provides a noninvasive and inexpensive diagnostic alternative for the diagnosis and monitoring of urinary tract disorders, i.e., early detection of tubular disorders of DN and AS nephropathies, especially in the collecting duct system.
The ratios of β-CTF to FL-CADM1 were significantly higher in emphysematous lungs (30), but there was no significant difference in the β-shedding rate in IIP lungs (51). In this study, β-shedding was low level or not increased in human nephropathies. Our laboratory reported that phorbol-12-myristate-13-acetate treatment led to an α-CTF increase in COS-7-CADM1 cells, but that in β-CTF it was not apparent (34). Moreover, α-CTF production was prevented by ADAM10 inhibitor, but that of β-CTF was not (34). Therefore, β-shedding was an independent mechanism of ADAM10 and perhaps not involved in the renal pathology. Finally, we discuss whether CADM1 SP4 is shed by ADAM10 in the TECs. Shirakabe et al. (42) recently reported that the main sheddase of CADM1 is ADAM17 and further many O-glycosylatable threonine residues in exon 8 prevent CADM1 shedding, and exon 9 strengthens the CADM1 shedding rate through non-glycosylatable amino acids. Although CADM1 SP4 includes exon 8, but not exon 9, CADM1 SP4 shedding could occur in the injured TECs. We suppose some explanations for this discrepancy. The first is the difference between epithelial and mesenchymal cells. The second is the in vitro and in vivo systems for examining CADM1 shedding. In other tissues, for example in lung, alveolar type I and II cells express CADM1 SP4, and its shedding also occurs in the emphysema and IIP (11, 30, 51). We expect that CADM1 SP4 could be cleaved in various diseases, perhaps through the activation of ADAM10.
In conclusion, we show that CADM1 shedding occurred in human DN and AS nephropathies. Especially, the decrease of FL-CADM1 was correlated with renal TEC apoptosis, an aggravated renal pathology, and renal dysfunction. In fact, the decrease of FL-CADM1 induced apoptosis in cultured renal epithelial cells. Therefore, the reduction of FL-CADM1 deteriorates nephropathies due to the induction of TEC apoptosis. It is valuable to focus on the urinary NH2-terminal CADM1 fragment for diagnosis before CKD.
GRANTS
This work was supported by a grant from the Osaka Kidney Foundation (OKF16–0006 to T. Kato) and grants from the Japan Society for the Promotion of Science Kakenhi (no. 14454911 to T. Kato, no. 25860302 to M. Hagiyama, no. 24590492 to A. Ito), Ministry of Education, Culture, Sports, Science and Technology-Supported Program for the Strategic Research Foundation at Private Universities 2015–17 (to A. Ito), and 21st Century Joint Research Enhancement Grant of Kindai University (to A. Ito).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.K. and A.I. conceived and designed research; T.K., M.H., Y.T., and A.Y. performed experiments; T.K., M.H., Y.T., and A.I. analyzed data; T.K., M.H., Y.T., A.Y., and A.I. interpreted results of experiments; T.K., M.H., and A.I. prepared figures; T.K. drafted manuscript; T.K., Y.T., and A.I. edited and revised manuscript; T.K., M.H., Y.T., A.Y., and A.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Makoto Suzuki for establishing CNT cells.
REFERENCES
- 1.Atapattu L, Saha N, Chheang C, Eissman MF, Xu K, Vail ME, Hii L, Llerena C, Liu Z, Horvay K, Abud HE, Kusebauch U, Moritz RL, Ding BS, Cao Z, Rafii S, Ernst M, Scott AM, Nikolov DB, Lackmann M, Janes PW. An activated form of ADAM10 is tumor selective and regulates cancer stem-like cells and tumor growth. J Exp Med 213: 1741–1757, 2016. doi: 10.1084/jem.20151095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athyros VG, Tziomalos K, Katsiki N, Doumas M, Karagiannis A, Mikhailidis DP. Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: An update. World J Gastroenterol 21: 6820–6834, 2015. doi: 10.3748/wjg.v21.i22.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biederer T. Bioinformatic characterization of the SynCAM family of immunoglobulin-like domain-containing adhesion molecules. Genomics 87: 139–150, 2006. doi: 10.1016/j.ygeno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Colombini M. Pore size and properties of channels from mitochondria isolated from Neurospora crass. J Membr Biol 53: 79–84, 1980. doi: 10.1007/BF01870576. [DOI] [Google Scholar]
- 5.Costello-White R, Ryff CD, Coe CL. Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Age (Dordr) 37: 9808, 2015. doi: 10.1007/s11357-015-9808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreymueller D, Pruessmeyer J, Groth E, Ludwig A. The role of ADAM-mediated shedding in vascular biology. Eur J Cell Biol 91: 472–485, 2012. doi: 10.1016/j.ejcb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Fogel AI, Li Y, Giza J, Wang Q, Lam TT, Modis Y, Biederer T. N-glycosylation at the SynCAM (synaptic cell adhesion molecule) immunoglobulin interface modulates synaptic adhesion. J Biol Chem 285: 34864–34874, 2010. doi: 10.1074/jbc.M110.120865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankfurt OS, Robb JA, Sugarbaker EV, Villa L. Apoptosis in breast carcinomas detected with monoclonal antibody to single-stranded DNA: relation to bcl-2 expression, hormone receptors, and lymph node metastases. Clin Cancer Res 3: 465–471, 1997. [PubMed] [Google Scholar]
- 9.Gołembiewska E, Stępniewska J, Kabat-Koperska J, Kędzierska K, Domański M, Ciechanowski K. The role of Klotho protein in chronic kidney disease: studies in animals and humans. Curr Protein Pept Sci 17: 821–826, 2016. doi: 10.2174/1389203717666160526123646. [DOI] [PubMed] [Google Scholar]
- 10.Hagiyama M, Ichiyanagi N, Kimura KB, Murakami Y, Ito A. Expression of a soluble isoform of cell adhesion molecule 1 in the brain and its involvement in directional neurite outgrowth. Am J Pathol 174: 2278–2289, 2009. doi: 10.2353/ajpath.2009.080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagiyama M, Yoneshige A, Inoue T, Sato Y, Mimae T, Okada M, Ito A. The intracellular domain of cell adhesion molecule 1 is present in emphysematous lungs and induces lung epithelial cell apoptosis. J Biomed Sci 22: 67, 2015. doi: 10.1186/s12929-015-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He P, Zhou G, Qu D, Zhang B, Wang Y, Li D. HBx inhibits proliferation and induces apoptosis via Fas/FasL upregulation in rat renal tubular epithelial cells. J Nephrol 26: 1033–1041, 2013. doi: 10.5301/jn.5000304. [DOI] [PubMed] [Google Scholar]
- 13.Herzog C, Haun RS, Ludwig A, Shah SV, Kaushal GP. ADAM10 is the major sheddase responsible for the release of membrane-associated meprin A. J Biol Chem 289: 13308–13322, 2014. doi: 10.1074/jbc.M114.559088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue T, Hagiyama M, Yoneshige A, Kato T, Enoki E, Maenishi O, Chikugo T, Kimura M, Satou T, Ito A. Increased ectodomain shedding of cell adhesion molecule 1 from pancreatic islets in type 2 diabetic pancreata: correlation with hemoglobin A1c levels. PLoS One 9: e100988, 2014. doi: 10.1371/journal.pone.0100988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito A, Ichiyanagi N, Ikeda Y, Hagiyama M, Inoue T, Kimura KB, Sakurai MA, Hamaguchi K, Murakami Y. Adhesion molecule CADM1 contributes to gap junctional communication among pancreatic islet α-cells and prevents their excessive secretion of glucagon. Islets 4: 49–55, 2012. doi: 10.4161/isl.18675. [DOI] [PubMed] [Google Scholar]
- 16.Katagiri D, Hamasaki Y, Doi K, Okamoto K, Negishi K, Nangaku M, Noiri E. Protection of glucagon-like peptide-1 in cisplatin-induced renal injury elucidates gut-kidney connection. J Am Soc Nephrol 24: 2034–2043, 2013. doi: 10.1681/ASN.2013020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato T, Kurosawa TM, Taketo MM. Proteinuria-induced chronic kidney disease in the ICGN/Oa mice with a mutation of Tensin2 gene. Ren Fail 31: 229–238, 2009. doi: 10.1080/08860220802669834. [DOI] [PubMed] [Google Scholar]
- 18.Kim DY, Ingano LA, Kovacs DM. Nectin-1 alpha, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/gamma-secretase-like cleavage. J Biol Chem 277: 49976–49981, 2002. doi: 10.1074/jbc.M210179200. [DOI] [PubMed] [Google Scholar]
- 19.Kim YH, Jung JC. Suppression of tunicamycin-induced CD44v6 ectodomain shedding and apoptosis is correlated with temporal expression patterns of active ADAM10, MMP-9 and MMP-13 proteins in Caki-2 renal carcinoma cells. Oncol Rep 28: 1869–1874, 2012. doi: 10.3892/or.2012.1986. [DOI] [PubMed] [Google Scholar]
- 20.Kimberly WT, Zheng JB, Guénette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem 276: 40288–40292, 2001. doi: 10.1074/jbc.C100447200. [DOI] [PubMed] [Google Scholar]
- 21.Kohan DE, Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 86: 896–904, 2014. doi: 10.1038/ki.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koma Y, Furuno T, Hagiyama M, Hamaguchi K, Nakanishi M, Masuda M, Hirota S, Yokozaki H, Ito A. Cell adhesion molecule 1 is a novel pancreatic-islet cell adhesion molecule that mediates nerve-islet cell interactions. Gastroenterology 134: 1544–1554, 2008. doi: 10.1053/j.gastro.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 23.Krasilnikov OV, Sabirov RZ, Ternovsky VI, Merzliak PG, Muratkhodjaev JN. A simple method for the determination of the pore radius of ion channels in planar lipid bilayer membranes. FEMS Microbiol Immunol 5: 93–100, 1992. doi: 10.1111/j.1574-6968.1992.tb05891.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuramochi M, Fukuhara H, Nobukuni T, Kanbe T, Maruyama T, Ghosh HP, Pletcher M, Isomura M, Onizuka M, Kitamura T, Sekiya T, Reeves RH, Murakami Y. TSLC1 is a tumor-suppressor gene in human non-small-cell lung cancer. Nat Genet 27: 427–430, 2001. doi: 10.1038/86934. [DOI] [PubMed] [Google Scholar]
- 25.Mao X, Seidlitz E, Ghosh K, Murakami Y, Ghosh HP. The cytoplasmic domain is critical to the tumor suppressor activity of TSLC1 in non-small cell lung cancer. Cancer Res 63: 7979–7985, 2003. [PubMed] [Google Scholar]
- 26.Mao X, Seidlitz E, Truant R, Hitt M, Ghosh HP. Re-expression of TSLC1 in a non-small-cell lung cancer cell line induces apoptosis and inhibits tumor growth. Oncogene 23: 5632–5642, 2004. doi: 10.1038/sj.onc.1207756. [DOI] [PubMed] [Google Scholar]
- 27.Masuda M, Kikuchi S, Maruyama T, Sakurai-Yageta M, Williams YN, Ghosh HP, Murakami Y. Tumor suppressor in lung cancer (TSLC)1 suppresses epithelial cell scattering and tubulogenesis. J Biol Chem 280: 42164–42171, 2005. doi: 10.1074/jbc.M507136200. [DOI] [PubMed] [Google Scholar]
- 28.Masuda M, Maruyama T, Ohta T, Ito A, Hayashi T, Tsukasaki K, Kamihira S, Yamaoka S, Hoshino H, Yoshida T, Watanabe T, Stanbridge EJ, Murakami Y. CADM1 interacts with Tiam1 and promotes invasive phenotype of human T-cell leukemia virus type I-transformed cells and adult T-cell leukemia cells. J Biol Chem 285: 15511–15522, 2010. doi: 10.1074/jbc.M109.076653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCaffrey LM, Macara IG. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol 21: 727–735, 2011. doi: 10.1016/j.tcb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Mimae T, Hagiyama M, Inoue T, Yoneshige A, Kato T, Okada M, Murakami Y, Ito A. Increased ectodomain shedding of lung epithelial cell adhesion molecule 1 as a cause of increased alveolar cell apoptosis in emphysema. Thorax 69: 223–231, 2014. doi: 10.1136/thoraxjnl-2013-203867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Momose K, Minami A, Shimono Y, Mizutani K, Nobutani K, Azuma T, Takai Y. miR-214 and hypoxia down-regulate Necl-2/CADM1 and enhance ErbB2/ErbB3 signaling. Genes Cells 18: 195–202, 2013. doi: 10.1111/gtc.12027. [DOI] [PubMed] [Google Scholar]
- 32.Murakami S, Sakurai-Yageta M, Maruyama T, Murakami Y. Trans-homophilic interaction of CADM1 activates PI3K by forming a complex with MAGuK-family proteins MPP3 and Dlg. PLoS One 9: e110062, 2014. doi: 10.1371/journal.pone.0082894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami Y. Involvement of a cell adhesion molecule, TSLC1/IGSF4, in human oncogenesis. Cancer Sci 96: 543–552, 2005. doi: 10.1111/j.1349-7006.2005.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagara Y, Hagiyama M, Hatano N, Futai E, Suo S, Takaoka Y, Murakami Y, Ito A, Ishiura S. Tumor suppressor cell adhesion molecule 1 (CADM1) is cleaved by a disintegrin and metalloprotease 10 (ADAM10) and subsequently cleaved by γ-secretase complex. Biochem Biophys Res Commun 417: 462–467, 2012. doi: 10.1016/j.bbrc.2011.11.140. [DOI] [PubMed] [Google Scholar]
- 35.Nagata M, Sakurai-Yageta M, Yamada D, Goto A, Ito A, Fukuhara H, Kume H, Morikawa T, Fukayama M, Homma Y, Murakami Y. Aberrations of a cell adhesion molecule CADM4 in renal clear cell carcinoma. Int J Cancer 130: 1329–1337, 2012. doi: 10.1002/ijc.26160. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T, Wong AJ, Saya H. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol 155: 755–762, 2001. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrica L, Vlad A, Gluhovschi G, Gadalean F, Dumitrascu V, Gluhovschi C, Velciov S, Bob F, Vlad D, Popescu R, Milas O, Ursoniu S. Proximal tubule dysfunction is associated with podocyte damage biomarkers nephrin and vascular endothelial growth factor in type 2 diabetes mellitus patients: a cross-sectional study. PLoS One 9: e112538, 2014. doi: 10.1371/journal.pone.0112538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez-Rigueiro T, Valladares-Ayerbes M, Haz-Conde M, Blanco M, Aparicio G, Fernández-Puente P, Blanco FJ, Lorenzo MJ, Aparicio LA, Figueroa A, Figueroa A. A novel procedure for protein extraction from formalin-fixed paraffin-embedded tissues. Proteomics 11: 2555–2559, 2011. doi: 10.1002/pmic.201000809. [DOI] [PubMed] [Google Scholar]
- 39.Royer C, Lu X. Epithelial cell polarity: a major gatekeeper against cancer? Cell Death Differ 18: 1470–1477, 2011. doi: 10.1038/cdd.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol 164: 769–779, 2004. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulte M, Reiss K, Lettau M, Maretzky T, Ludwig A, Hartmann D, de Strooper B, Janssen O, Saftig P. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death Differ 14: 1040–1049, 2007. doi: 10.1038/sj.cdd.4402101. [DOI] [PubMed] [Google Scholar]
- 42.Shirakabe K, Omura T, Shibagaki Y, Mihara E, Homma K, Kato Y, Yoshimura A, Murakami Y, Takagi J, Hattori S, Ogawa Y. Mechanistic insights into ectodomain shedding: susceptibility of CADM1 adhesion molecule is determined by alternative splicing and O-glycosylation. Sci Rep 7: 46174, 2017. doi: 10.1038/srep46174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shore N, Khurshid R, Saleem M. Alpha-1 microglobulin: a marker for early detection of tubular disorders in diabetic nephropathy. J Ayub Med Coll Abbottabad 22: 53–55, 2010. [PubMed] [Google Scholar]
- 44.Song XF, Ren H, Andreasen A, Thomsen JS, Zhai XY. Expression of Bcl-2 and Bax in mouse renal tubules during kidney development. PLoS One 7: e32771, 2012. doi: 10.1371/journal.pone.0032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi K, Suzuki M. Glucagon increases intracellular free calcium in a distal tubular cell line. Nihon Jinzo Gakkai Shi 36: 289–297, 1994. [PubMed] [Google Scholar]
- 46.van Loon EP, Pulskens WP, van der Hagen EA, Lavrijsen M, Vervloet MG, van Goor H, Bindels RJ, Hoenderop JG. Shedding of klotho by ADAMs in the kidney. Am J Physiol Renal Physiol 309: F359–F368, 2015. doi: 10.1152/ajprenal.00240.2014. [DOI] [PubMed] [Google Scholar]
- 47.Verzola D, Procopio V, Sofia A, Villaggio B, Tarroni A, Bonanni A, Mannucci I, De Cian F, Gianetta E, Saffioti S, Garibotto G. Apoptosis and myostatin mRNA are upregulated in the skeletal muscle of patients with chronic kidney disease. Kidney Int 79: 773–782, 2011. doi: 10.1038/ki.2010.494. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Parrish AR. Loss of α(E)-catenin promotes Fas mediated apoptosis in tubular epithelial cells. Apoptosis 20: 921–929, 2015. doi: 10.1007/s10495-015-1129-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu TK, Wei CW, Pan YR, Cherng SH, Chang WJ, Wang HF, Yu YL. Vitamin C attenuates the toxic effect of aristolochic acid on renal tubular cells via decreasing oxidative stress–mediated cell death pathways. Mol Med Rep 12: 6086–6092, 2015. doi: 10.3892/mmr.2015.4167. [DOI] [PubMed] [Google Scholar]
- 50.Yoneshige A, Hagiyama M, Fujita M, Ito A. Pathogenic Actions of Cell Adhesion Molecule 1 in Pulmonary Emphysema and Atopic Dermatitis. Front Cell Dev Biol 3: 75, 2015. doi: 10.3389/fcell.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoneshige A, Hagiyama M, Inoue T, Mimae T, Kato T, Okada M, Enoki E, Ito A. Increased ectodomain shedding of cell adhesion molecule 1 as a cause of type II alveolar epithelial cell apoptosis in patients with idiopathic interstitial pneumonia. Respir Res 16: 90, 2015. doi: 10.1186/s12931-015-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]