Abstract
The IL-17 family of cytokines has emerged over the last two decades as a pleiotropic group of molecules that function in a wide variety of both beneficial and detrimental (pathological) processes, mainly in mucosal barrier tissue. The beneficial effects of IL-17 expression are especially important in the lung, where exposure to foreign agents is abundant. IL-17A plays an important role in protection from both extracellular bacteria and fungi, as well as viruses that infect cells of the mucosal tracts. IL-17 coregulated cytokines, such as IL-22, are involved in maintaining epithelial cell homeostasis and participate in epithelial cell repair/regeneration following inflammatory insults. Thus, the IL-17/IL-22 axis is important in both responding to, and recovering from, pathogens. However, aberrant expression or overexpression of IL-17 cytokines contributes to a number of pathological outcomes, including asthma, pneumonitis, and generation or exacerbation of pulmonary fibrosis. This review covers the good, bad, and ugly aspects of IL-17 in the lung.
Keywords: asthma, fibrosis, IL-17, IL-22, neutrophils
INTRODUCTION
First identified in 1995, IL-17 (IL-17A) was recognized for its similarity to a cytokine produced by the herpesvirus, Herpesvirus saimiri (129). The discovery of IL-17 was quickly followed by its identification as a cytokine secreted by a unique subset of CD4+ T cells, termed Th17 cells (42, 115, 130). The IL-17 family of cytokines consists of six related proteins, IL-17A–IL-17F (49, 62, 105, 130). The two most well-known IL-17 family members, IL-17A and IL-17F, signal through the IL-17 receptor, which is a multimeric receptor consisting of at least two subunits, IL-17RA and IL-17RC (16). In this review, IL-17 will be taken to mean IL-17A. Other variants of the IL-17 receptor complex also exist, consisting of multimeric IL-17RA and IL-17RB subunits, which preferentially bind IL-17B and IL-17E, and IL-17RE, which binds IL-17C (25, 48). Ligation of the IL-17RA/RC receptor complex results in initial recruitment of ACT1 and TRAF6 adaptor proteins, activating NF-κB and MAPK pathways, and leads to production of proinflammatory cytokine/chemokines, as well as cell proliferation and differentiation (43, 50, 87, 129, 134, 135).
The various IL-17 receptor complexes are present on the surface of a variety of cells, including epithelial cells, fibroblasts, keratinocytes, and granulocytes, such as neutrophils and eosinophils (50, 110, 121, 129, 135). The wide cellular distribution of IL-17R contributes to the pleiotropic nature of the IL-17 family, allowing it to partake in a variety of physiological processes both beneficial and detrimental. Originally thought to be mainly produced by Th17 lymphocytes, IL-17 is now appreciated to be secreted by many innate-like lymphocytes including, γδ T cells, invariant natural killer cells (iNKT), and type three innate lymphoid cells (ILC3) (60, 66, 76, 88, 102, 133). Many of these cell types are present in relatively high numbers at mucosal surfaces making IL-17 an important mediator of both host defense and homeostatic physiological processes at mucosal barrier sites, such as the lung, gut, and oropharynx. This review will focus on the beneficial and detrimental roles of IL-17 in mediating host defense and chronic diseases of the lung. We categorize these effects as good (host defense), bad (autoimmune/inflammatory), and ugly (fibrotic).
GOOD: ROLE OF IL-17 IN DEFENSE AGAINST BACTERIAL PATHOGENS IN THE LUNG
The IL-17 family and IL-17-related cytokines have important endogenous functions at mucosal surfaces, and expression of these cytokines confers protection from a variety of extracellular pathogens. IL-17-deficient mice are more susceptible to a variety of respiratory pathogens, including Klebsiella pneumoniae, Streptococcus pneumoniae, and Pseudomonas aeruginosa (7, 76). Lung-associated bacterial burdens were higher in IL-17−/− or IL-17R−/− mice, and knockout mice developed increased inflammation and disease severity. IL-23 is a regulator of IL-17 production and IL-23-deficient mice infected with S. pneumoniae failed to produce IL-17 and IL-6 and had higher bacterial burden in the both the lungs and blood (56). Neutrophil recruitment was also impaired in IL-23-deficient mice, and the authors surmised that this deficiency was the cause of the increased susceptibility to S. pneumoniae (56). Similarly, IL-17R−/− mice are extremely sensitive to K. pneumoniae infection and fail to recruit innate immune cells, such as neutrophils and macrophages due to decreased production of G-CSF and MIP-2 (131, 132). Interestingly, infection of mice with P. aeruginosa drives expression of another IL-17 family member, IL-17C, in an IL-17A-dependent manner (118). IL-17C is known to be secreted by epithelial cells in response to Toll-like receptor stimulation by bacterial pathogens, signaling in an autocrine manner that further boosts expression of the neutrophil-recruiting CXC chemokines CXCL1 and CXCL2 (KC and MIP-2), which facilitates bacterial clearance (59, 90, 118).
More recently, the cellular source of IL-17 production needed for protection and clearance of bacteria has been shown to vary from pathogen to pathogen. IL-23-driven expression of IL-17 from γδ T cells has been shown to be important in the clearance of K. pneumoniae (41, 76). Likewise, IL-17 produced from ILC3 cells has also been shown to be important for K. pneumoniae clearance in a TNF-α-dependent fashion (124). For clearance of Pseudomonas, ILC3, Th17 T cells, and γδ T cells are all thought to be predominant sources of IL-17 (7). It is still unclear how different cell types may vary in their responsiveness to differing pathogens to produce IL-17. However, it is believed that IL-17 from any of these sources may stimulate epithelial cells to generate chemokine gradients to recruit immune cells, such as neutrophils that are important in bacterial clearance (21).
IL-17-deficient mice are also more susceptible to developing postinfluenza bacterial pneumonia from Staphylococcus aureus and S. pneumoniae (58). Influenza A infection was shown to significantly inhibit IL-1β signaling, which decreased expression of IL-17 critically needed for the clearance of S. aureas (93). Similarly, IL-1 receptor-deficient mice had significantly higher bacterial titers and subsequently developed a more severe pneumonia following influenza challenge (93). Interestingly, a specific micro-RNA, MIR-155, was shown to be preferentially induced by influenza infection, decreasing expression of IL-23 and IL-17, thus resulting in decreased bacterial clearance and increased severity of S. aureus postinfluenza bacterial pneumonia (86). Additionally, mice deficient for the IL-17 coregulated cytokine, IL-22, displayed increased epithelial cell damage and fibrosis following influenza challenge (85). IL-22−/− mice were also more susceptible to postinfluenza bacterial pneumonia challenge with S. pneumoniae developing a more severe IFNγ response and increased mortality (52).
GOOD: ROLE OF IL-17 IN DEFENSE AGAINST FUNGAL PATHOGENS IN THE LUNG
IL-17 is also critically important in host defense against fungal pathogens at mucosal barrier sites, especially in the lung. IL-23−/− mice infected with Pneumocystis carinii failed to generate an IL-17 response and developed increased fungal burdens in the lung and impaired fungal clearance (95). IL-17RB−/− mice infected with Pneumocystis failed to generate a protective Th2 response and did not produce inducible bronchus-associated lymphoid tissue (33). IL-17−/− mice infected with the opportunistic fungal pathogen, Cryptococcus neoformans, displayed increased dissemination to the brain and blood following fungal challenge; however, clearance of the fungus from the lungs was not impaired (107, 119). But, more recently, IL-17−/− mice infected with a less virulent strain of C. neoformans, 52D, did display defects in fungal clearance (77). These mice also displayed defects in IFNγ production and recruitment of CD11c+, CD11b+ myeloid cells following infection (77). Interestingly, IL-17RA−/− mice infected with C. neoformans displayed reduced expression of IL-17 by neutrophils but increased IL-17 expression from γδ T cells, indicating that the cellular source of IL-17 during fungal infection is also important (120).
GOOD AND BAD: ROLE OF IL-17 AND IL-17-RELATED CYTOKINES IN EPITHELIAL CELL HOMEOSTASIS
Outside of host defense at mucosal surfaces, IL-17 and IL-17-related cytokines, such as IL-22, are especially important in homeostatic processes in mucosal tissues, especially in mucosal epithelial cells. IL-22 has long been known to regulate epithelial barrier function in the gut; however, epithelial cells in the lung express both the IL-17 and IL-22 receptors, indicating that these cytokines play important homeostatic roles in the lung as well (1, 21, 67, 85). However, data are conflicting about whether these roles are always beneficial, suggesting the effects of IL-17 and IL-22 are highly context and/or temporally dependent. Mice deficient for IL-22 developed a decreased eosinophilic response to OVA-induced allergic lung inflammation, indicating a proinflammatory role for the cytokine (10). However, when IL-22 was depleted following challenge, by way of neutralizing antibody therapy, the opposite effect was observed, and IL-22-depleted mice had less allergic inflammation and proinflammatory cytokine expression, indicating a tissue-protective and/or -restorative effect of IL-22 expression at later time points (10). Furthermore, transgenic mice that produced lung-specific IL-22 had decreased levels of IL-13, eosinophilic infiltration, mucus production, and decreased airway hyperreactivity in an OVA-induced model of acute asthma exacerbation, indicating that expression of IL-22 may be beneficial for epithelial repair following allergic challenge (35). Interestingly, IL-22 has also been shown to induce autophagy, while IL-17 seems to inhibit autophagy in alveolar epithelial cells (47, 68). Autophagy is an important mechanism to maintain epithelial cell homeostasis in the face of cellular stress. For example, epithelial cells from autophagy-deficient mice produced large quantities of IL-1β in response to respiratory syncytial virus infection and developed increased IL-17-driven lung pathology (91). Together, these data indicate that the IL-17/IL-22 axis play complex roles in regulating homeostatic processes, such as autophagy, and are important in dictating pathological outcomes to both infectious and noninfectious inflammatory stimuli. Given the pleiotropic nature of IL-17, it is not surprising that along with these beneficial actions, IL-17 can also contribute to a number of inflammatory and fibrotic lung diseases as well.
BAD: ROLE OF IL-17 IN ACUTE LUNG INJURY
As important as IL-17 is to driving proper leukocyte infiltration to facilitate bacterial or fungal clearance, a delicate balance must be struck. Aberrant IL-17 signaling can lead to excess inflammation, which can have damaging results. One such pathology, acute respiratory distress syndrome (ARDS), can develop following traumatic injury and/or a major inflammatory episode, such as sepsis, and is characterized by severe lung dysfunction, fluid accumulation (edema), hypoxia, and excessive neutrophil infiltration/activation (24). Elevated levels of both IL-17 and IL-22 were detected in ARDS patients (63, 127). Furthermore, IL-17−/− mice were protected from developing LPS-induced lung injury (a model for ARDS) and did not recruit neutrophils following challenge (63, 127). Recently, a population of lung-specific ILC3-like cells was identified as a major source of IL-17 during LPS-induced lung injury. These cells drove excessive neutrophil recruitment, which suggests an innate response rather than an adaptive one is important in driving pathogenesis (74). Moreover, depletion of circulating inflammatory monocytes decreased IL-17 levels following LPS challenge and decreased the severity of lung injury (53). The exact mechanism of cross-talk between circulating monocytes and ILC3 cells in this model remains unclear, however, IL-1β was decreased following monocyte depletion, which may have led to decreased IL-17 secretion (53).
BAD: INFLAMMATION AND CYSTIC FIBROSIS
Cystic fibrosis or CF is a progressive genetic disease that mostly affects the lung. CF is inherited as an autosomal recessive gene, and there are over 1,700 mutations known to affect the cystic fibrosis transmembrane regulator (CFTR) gene, which functions as a chloride ion channel. Dysfunctional CFTR leads to accumulation of thickened mucus, which traps particles in the airways and creates a niche for chronic bacterial infection, especially with P. aeruginosa. It is now appreciated that IL-17 signaling plays an important role in CF pathogenesis.
Several studies have demonstrated IL-17A and IL-17F were significantly elevated in the sputum of CF patients experiencing an exacerbation of their disease with evidence of colonization by P. aeruginosa (29, 71). Immunoreactive IL-17 is found in neutrophils and mononuclear cells of CF patients (15). In addition, T cells have been shown to contribute to IL-17 levels in CF lungs (20), and higher percentages of Th17 cells in the blood were strongly associated with poor lung function in CF patients (75). In addition to classic Th17 cells, γδ T cells and NK T cells are also known sources of IL-17 in CF lungs (8, 108) and ILC3s produce IL-17 in response to P. aeruginosa infections (7). IL-17 receptors are localized to basal airway cells, and signaling of IL-17 in bronchial epithelial cells leads to the upregulation of proinflammatory cytokines and chemokines, which as mentioned above help to recruit even more neutrophils to the airways (71). This proinflammatory milieu is believed to promote pathogenesis of CF. Interestingly, airway epithelial cells harboring the common CFTRΔ508 mutation are especially sensitive to IL-17 stimulation, resulting in higher levels of CXCL8 secretion, increased expression of NOD1, NOD2, TLR4, and both the IL-17RA and IL-17RC receptors (94). Other studies have suggested that IL-17 promotes CXCL8 expression in bacteria-infected CF airways via regulation of p38 phosphorylation (73). Taken together, mutant CFTR and IL-17 prime the airway epithelium to be overly sensitized to bacterial stimulation, resulting in heightened inflammation. Not only does IL-17 signaling promote inflammation in CF, but It has also been suggested to induce changes in bronchial epithelial cell bicarbonate secretion, which may explain why airway surface liquid pH is abnormal (alkaline) in CF (57).
In animal models of chronic CF-like bacterial colonization, infection of mice with P. aeruginosa-loaded beads leads to upregulation of Th1 and Th17 cytokines along with an influx of neutrophils and M2 macrophages (12). Using CFTR-deficient mouse models, neutralization of IL-17 before bacterial infection significantly improved the outcomes in the CFTR mutant mice (46). When considering both human and murine studies together, it is clear that IL-17 induction can be beneficial by helping to recruit inflammatory cells to help clear the infection; however, in the setting of CF, where the bacteria are not easily cleared due to biofilm formation and mucus trapping, the prolonged induction of IL-17 leads to persistent inflammation, lung damage, and alterations to epithelial cell homeostasis.
BAD: HYPERSENSITIVITY PNEUMONITIS
Hypersensitivity pneumonitis (HP), which is also known as extrinsic allergic alveolitis, is an acute or chronic interstitial lung disease associated with inflammation of the alveoli, thought to be caused by a hypersensitivity to inhaled dusts or environmental substances. Although limiting exposure to environmental triggers can improve disease symptoms, causative agents are not always easy to identify, and some patients will progress to more fibrotic presentations of HP (92). IL-17 is known to be elevated in the bronchoalveolar lavage fluid of HP patients, even to a greater extent than in patients with idiopathic pulmonary fibrosis (IPF) to be discussed below (9). To better understand the pathogenesis of HP and its association with IL-17, several murine models have been developed. One such model involves repeated exposure of mice to Saccharopolyspora rectivirgula, which is correlated with a dose-dependent increase in IL-17 (3, 44, 100). The IL-17 was found in Th17 cells (100), but also in neutrophils and monocyte/macrophages (44). Exposure of IL-17RA−/− mice to S. rectivirgula resulted in less inflammation and fibrosis than in wild-type mice (100), and the protection of these mice from fibrotic HP development correlates with expression of IL-17RA on structural rather than hematopoietic cells (44). Similarly, in a model of Bacillus subtilis-induced HP in mice, both Th17 cells and γδ T cells expressing IL-17 were found to be important in eliminating the microorganism and, thus, preventing inflammation and fibrosis (99, 101). One of these studies also showed that the γδ T cells expressing IL-17 expressed the canonical Vγ6/Vδ1 T-cell receptor (99). Taken together, most data support a pathogenic role for IL-17 in driving inflammation and fibrosis in HP, although some forms caused by bacterial allergens may benefit from IL-17-mediated clearance of the organism. Thus, clinical applications of anti-IL-17 therapies will need to be specifically targeted.
BAD AND UGLY: ASTHMA AND CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Asthma is another chronic inflammatory disease of the airways that is characterized by narrowed airways, reversible airflow obstruction, and excess mucin production. The narrowing is the result of smooth muscle cell activation, and this can be complicated by deposition of extracellular matrix proteins which remodel the airways, making it difficult to breathe. While allergic asthma is classically associated with Th2 cytokines, there is mounting evidence that neutrophilic forms of severe asthma are associated with IL-17 and that these endotypes are generally difficult to treat (28). Previous studies have shown that IL-17 levels are increased in patients with moderate to severe asthma when compared with patients with mild asthma or normal volunteers (19). Importantly, consistent with higher levels of IL-17 in these patients, expression of the extracellular matrix components, type I and type III collagens were also higher and treatment with corticosteroids had no ability to diminish expression of these matrix components (19). Some of the mechanisms that may account for this correlation between IL-17 and airway remodeling include the ability of IL-17 to promote release of profibrotic transforming growth factor (TGF)-β from eosinophils of asthmatic patients in a p38 MAPK-dependent manner (2).
Additionally, IL-17 is known to enhance the expression of α-smooth muscle actin in fibrocytes (45) [an inflammatory cell type increased in asthmatic tissue (98)], which could promote tissue contraction and airway narrowing. Furthermore, IL-17 can promote production of profibrotic and proangiogenic factors from fibrocytes as well (45). Finally, IL-17 has been shown to have direct profibrotic effects on lung fibroblasts to promote production of TGF-β and collagen proteins (84, 134). In addition to stimulation of profibrotic mediators, IL-17A acts directly on epithelial cells increasing expression of mucus-producing genes, such as MUC5A and promotes goblet cell hyperplasia (a hallmark of asthma induced airway remodeling) (123). Interestingly, inhibition of TNF-α reduced MUC5A expression in epithelial cells; however, antibody neutralization of the TNF-α receptor 2 protein (TNFr2) had the opposite effect and increased IL-17 expression and airway remodeling in an OVA-induced allergic asthma model, suggesting a complex regulatory network (61, 64). Thus, it is clear that IL-17 responses in asthma portend worse outcomes because they are associated with significant inflammation and airway remodeling.
Similarly to severe asthma, chronic inflammation and small airway fibrosis are also pathological features of chronic obstructive pulmonary disease (COPD), and there are currently few effective treatments for this condition, which is highly correlated with cigarette smoke exposure. IL-17 has also been suggested to play a key role in airway remodeling in animal models of COPD. Using cigarette smoke exposure or overexpression of IL-1β, two stimuli that are relevant to COPD pathogenesis, mice deficient in the IL-17RA receptor were protected from airway inflammation and fibrosis (128). Sources of IL-17 noted in both models included CD4+ Th17 cells, γδ T cells, ILC3s, neutrophils, and CD8 T cells (128). Similar cell types were also shown to accumulate in an inducible IL-18 transgenic mouse model of COPD, where IL-18 overexpression upregulates Th1, Th2, and Th17 responses. In this model, IL-17 was also associated with mucus metaplasia, airway fibrosis, and vascular remodeling (55). Thus, IL-17 likely promotes pathological development of fibrosis in both asthma and COPD.
Another consequence of cigarette smoke exposure is the potential to develop lung cancer. Although a review of how IL-17 regulates cancer as a whole is beyond the scope of this review, it is interesting that two recent publications in this journal have addressed this issue. First, Xu et al. (125) demonstrated that delivery of an adenovirus overexpressing IL-17 to a Kras mutant mouse promoted the growth of the lung tumor and induced expression of matrix metalloproteinase-9. Not surprisingly, IL-17 expression increased the motility and invasiveness of this cancer as well (125). Shortly after this report, a study by Jungnickel et al. that explored the impact of cigarette smoke and Hemophilus influenzae on the growth of Lewis lung carcinoma cells in vivo showed that metastatic growth of this tumor was reduced in IL-17−/− mice compared with controls (54). In this study, IL-17 did not directly affect cancer cell proliferation, but rather is believed to modulate tumor-associated inflammation to promote pathogenesis. Thus, a current model suggests that cigarette smoke may alter barrier function and allow bacterial factors to translocate to the lung, where, in turn, the microorganisms can stimulate IL-17-dependent inflammation to promote tumorigenesis (54).
UGLY: LUNG FIBROSIS
Given the considerable evidence of lung remodeling induced by IL-17 in asthma and COPD, it is no surprise that lung fibrosis is also associated with expression of IL-17. IPF is a progressive scarring disease of the lung that eventually results in death from respiratory insufficiency. The disease is believed to be related to repetitive epithelial cell injury, chronic inflammation, and activation of mesenchymal cells to produce extracellular matrix. In IPF tissues, IL-17 can be localized to areas of active disease where IL-17 was noted in regenerating epithelium, CD3+ T cells, and macrophages (79). It is also elevated in the bronchoalveolar lavage of IPF patients (117).
In addition to these clinical data, numerous animal modeling studies have explored the role of IL-17 in driving lung fibrosis, with some conflicting results. The most commonly used model involves administration of the chemotherapeutic agent, bleomycin, into the mice to induce a fibrotic remodeling response (73a). In studies using bleomycin, IL-17 has been shown to be produced by γδ T cells (14, 38, 117). However, the consequences of this are unclear. One study showed that mice deficient in this innate T-cell subtype display reduced inflammation and delayed epithelial repair, suggesting a protective role for IL-17-producing γδ T cells (14). In a similar study, histological and biochemical analyses of fibrosis showed that bleomycin-induced pathology and fibrosis was more severe in T-cell receptor (TCR) δ-deficient mice when compared with WT mice (96). However, in this study, the authors concluded that IFNγ-producing γδ T cells suppressed IL-17 production (96). Other studies showed bleomycin-induced IL-17 led to significant neutrophilia (117) and promoted pulmonary fibrosis (38, 117). Neutralization of IL-17 in vivo resulted in improved resolution of the acute inflammation and attenuated the development of fibrosis (72), even in models that involved repetitive bleomycin challenge (116). While IL-17−/− mice were protected from bleomycin-induced fibrosis (117) in one study, this has not been seen uniformly by others (134). Interestingly, genetic ablation of the IL-17RA receptor did not seem to show reduced levels of collagen deposition, although it was associated with a shift to production of collagen V, increased IL-17, and reduced myofibroblast apoptosis (34). It is not immediately clear why IL-17 has not shown a uniform phenotype in all bleomycin models. Clearly the source of IL-17 (innate vs. adaptive T cells) may influence outcomes and because IL-17 is known to be regulated by gut microbiota, some discrepancies may relate to alterations in the lung or gut microbiota between studies that are not fully understood (81).
For studies in which IL-17 may promote fibrosis, potential mechanisms include the ability of IL-17 to increase synthesis and secretion of collagen from epithelial cells and the promotion of epithelial-mesenchymal transition (72). Conversely the IL-17 coregulated cytokine IL-22 was shown to inhibit EMT in lung epithelial cells in response to bleomycin challenge (65). As with the asthma studies above, IL-17R is increased on fibroblasts following bleomycin treatment, and exogenous IL-17 can promote proliferation, α-smooth muscle actin expression, and collagen production (31). This study also showed that IL-17 stimulation of fibroblasts resulted in NF-κB activation and that the profibrotic effects could be limited via inhibition of the adaptor protein NF-κB activator 1 (Act 1) associated with IL-17R in fibroblasts (31). IL-17 has been shown to suppress autophagy in epithelial cells (68), and autophagy is known to regulate lung fibrosis (80). Consistent with this, in one study, IL-17 neutralization was associated with increased autophagy, which presumably facilitated collagen degradation in the lung (72).
There have also been a number of studies that have explored the upstream regulators that may influence production of IL-17 in the lung during fibrogenesis. Studies have suggested that B cell-activating factor (BAFF) is elevated in IPF patients and that BAFF is required for CD3+ T cells to produce IL-17 (36). IL-27 has also been shown to regulate Th17 cell differentiation and to attenuate bleomycin-induced fibrosis (30). Osteopontin, a matricellular protein elevated during bleomycin-induced fibrosis has also been shown to regulate the ratio of pathogenic Th17 cells vs. protective Th1 cell phenotypes (82). Finally, increased levels of adenosine are believed to promote IL-17 and IL-6 levels during fibrosis (70).
UGLY: SILICA-INDUCED LUNG FIBROSIS
Silica instillation into the lung is another commonly used method to study lung fibrosis developing in response to a particulate matter, and this model is associated with macrophage activation and secretion of IL-1β (73a). The pathogenesis is also characterized by an early alveolitis, including neutrophil accumulation, thought to promote tissue damage. Recently, it has become appreciated that silica-induced pathogenesis is also highly dependent on IL-17 signaling. IL-17 is upregulated during silicosis (69, 103), and production has been localized to γδ T cells and Th17 cells, but not macrophages, neutrophils, NK, or CD8 T cells (69). T-regulatory cells (Tregs) have also been suggested to promote Th17 differentiation during silicosis (104) but as noted below, Tregs can also be regulatory. Neutralization of IL-17 can delay neutrophil accumulation and blunt inflammatory and fibrotic responses (22, 72) and promote Treg accumulation (22). Ex vivo cocultures between macrophages and lymphocytes suggest that neutralizing IL-17 increased the function of Treg cells to suppress Th1 and promote Th2 responses (109). Interestingly, the mineral dust-induced gene known as mdig or Mina53 that is expressed in alveolar macrophages exposed to silica has also been suggested to regulate the balance of Th17 and Tregs (111).
Inhibition of IL-1 signaling is also known to blunt IL-17 responses to silica (103), which is not surprising given the known role of IL-1β in promoting Th17 differentiation (78). Not surprisingly, depletion of alveolar macrophages limits IL-17 responses to silica, suggesting these cells are the first to encounter silica and to respond via production of IL-1β and IL-23 to regulate the early alveolitis that develops (69). However, one report does suggest that IL-17 may be dispensable for the chronic inflammatory response and ultimate fibroproliferation in this model (69).
UGLY: LUNG AND STEM CELL TRANSPLANTATION
Lung transplantation represents a therapeutic modality for many end-stage lung diseases, but its effectiveness is limited by the development of serious complications that lead to graft dysfunction. Among these complications are acute and chronic rejection, which can eventually lead to the development of bronchiolitis obliterans (OB), an obstructive and fibrotic disease of the small airways (113). Human studies have shown that patients with end-stage lung disease often develop Th17-specific memory T-cell responses specific for collagen type V, a neo auto-antigen associated with lung transplantation (13). Animal models of acute rejection stimulated by instillation of anti-MHC class I antibodies endobronchially show diminished autoimmune responses in mice deficient in IL-17 that also correlate with reduced collagen type V (6). In animal models of orthotopic lung transplantation, CD4+ T cells are the major source of IL-17, and depletion of CD4+ cells can reduce acute rejection and development of OB. Interestingly, however, in two different studies, production of IL-17 was found to promote acute inflammation, but to have little impact on the ultimate level of fibrosis seen in OB (122, 126). This is somewhat in contrast to another study that showed that IL-17 blockade could reduce both severity of acute rejection and development of OB. Interestingly, in this study, IL-17 blockade actually led to an increased frequency of both Th17 cells, but also γδ T cells, while still showing decreased OB development (39). More work in this area is needed to fully understand the role of IL-17 during lung transplantation, but most evidence suggests IL-17 can promote inflammatory and autoimmune responses that are likely to drive rejection and the eventual development of fibroproliferation and obstructive lung disease.
Pulmonary complications are also hallmarks of stem cell transplantation, and these can include development of idiopathic pneumonia syndrome (IPS) or OB (89). Interestingly, patients who develop IPS have elevated IL-6 levels, which can promote Th17 responses (112). Furthermore, patients who develop IPS have also been shown to harbor occult infections, often herpesvirus infections (97), and early viral infection can predispose the patient toward the development of IPS (114). This led our group to develop a murine model of bone marrow transplantation (BMT) followed by infection with a γherpesvirus (γHV-68). Infection of syngeneic BMT mice with γHV-68 leads to the development of severe pneumonitis and fibrosis (26). This pathology is associated with a skewing of T-cell responses to favor Th17 accumulation (27, 40, 134). This has been linked to BMT-induced alterations in antigen-presenting cells to favor Th17 skewing (134), and recently, it was shown that blocking recruitment of myeloid cells to the lung via the creation of CCR2−/− into WT BMT chimeras resulted in even more Th17 cells and accumulation of granulocytes correlating with worse pathology (40). Thus, similar to other fibrotic lung diseases described above, IL-17 appears to play an important role in regulating both the recruitment of granulocytes and the stimulation of extracellular matrix production from lung fibroblasts (40, 134). Interestingly, neutralization of IL-17 during the fibroproliferative phase was more effective at reducing pathology than was neutralization during the inflammatory phase (134).
ANTI-IL-17 THERAPY AND LUNG DISEASE
Recently, three IL-17-related monoclonal antibodies have been developed, brodalumab, which inhibits the human IL-17A receptor, and secukinumab, as well as ixekizumab, which both target and neutralize IL-17A itself (18, 83). Although, not covered specifically in this review, anti-IL-17 therapy has proven efficacious for the treatment of psoriasis and psoriatic arthritis (4). Given the pathogenic role of IL-17 in various mouse models of lung disease there is much excitement about the possibility of using IL-17-neutralizing therapy for the treatment of various lung diseases such as asthma, COPD, and possibly fibrosis [the current state of the use of monoclonal antibody therapy in the treatment of asthma is reviewed in detail in Ref. 32].
Unfortunately, an early clinical trial using brodalumab to treat patients with uncontrolled asthma did not show a significant effect (17). However, more recent evidence suggests that asthma in humans may be able to be separated into at least three distinct subtypes: Th2-dominated, Th17-dominated, and Th2/Th17 low, which suggests that αIL-17 therapy may yet prove to be efficacious in treating at least some forms of asthma (23). Furthermore, preclinical modeling has suggested that neutralization of both IL-13 (Th2) and IL-17 (Th17) cytokines as a combination therapy has the best efficacy for limiting eosinophilia, mucus secretion, neutrophilic inflammation, and airway hyperreactivity (23). Similar to asthma, both COPD and IPF appear to be multifaceted diseases in humans with some studies, suggesting that increased levels of IL-17 correlate with a more severe disease progression (11, 117), while other studies suggest that Th17 differentiation is actually decreased (37, 106). Although no clinical trials have specifically looked at the efficacy of IL-17 neutralization for COPD or IPF, preclinical data suggest that αIL-17 therapy may be efficacious in at least some forms of these diseases.
SUMMARY
IL-17 is an important cytokine for regulating mucosal defense. As summarized above, IL-17 can be good in the sense that IL-17 helps activate epithelial cells to recruit neutrophils for host defense. Efficient clearance of pathogens by neutrophils can limit development of pneumonia caused by bacteria and fungi. However, in the case of persistent infections or allergens such as what can occur in CF, chronic viral infections and asthma, prolonged release of IL-17 can have the bad effects of promoting persistent neutrophil and eosinophil recruitment, degranulation, and tissue destruction. In the case of persistent or repetitive lung injuries, IL-17 expression can disturb epithelial cell homeostasis and support the development of extracellular matrix with ugly consequences (see Fig. 1). Given the context, cell-type and temporal differences in IL-17 regulation and effects, more research is needed to better understand the long-term consequences of anti-IL-17 therapies. Given that there are now drugs on the market to target IL-17 signaling, it may be time to consider their usefulness in some forms of chronic lung disease.
Fig. 1.
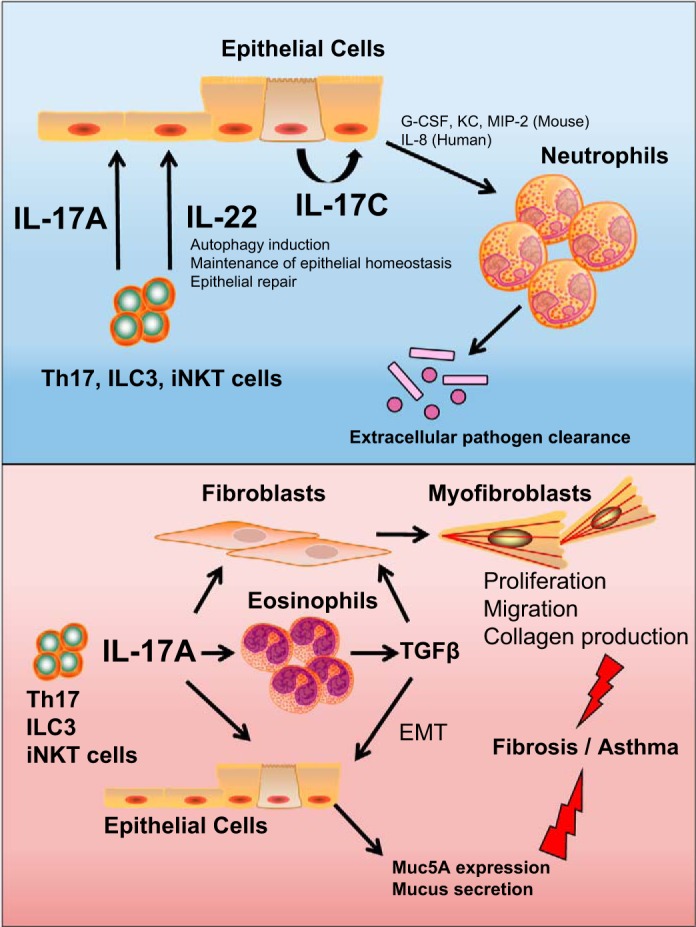
The IL-17 family and IL-17-related cytokines have a variety of beneficial and detrimental effects in the lung. Top, blue panel: IL-17A and IL-17C serve beneficial effects in the lung and aid in the recruitment of neutrophils to clear extracellular pathogens (21, 51, 118). IL-22 also serves a protective role in the lung and induces autophagy in epithelial cells, reducing inflammation and promoting epithelial repair following pathogen damage (47, 65, 85). Of course, prolonged neutrophil accumulation can also lead to tissue damage. Bottom, red panel: IL-17A has detrimental effects during exacerbations of asthma, COPD, and pulmonary fibrosis by promoting eosinophil recruitment/activation leading to the secretion of profibrotic mediators such as TGF-β (2). IL-17A can act directly on both epithelial cells and fibroblasts, promoting epithelial to mesenchymal transition, as well as myofibroblast differentiation and extracellular matrix production, resulting in fibrosis (84, 134). Furthermore, IL-17A can stimulate epithelial cells to upregulate mucus-producing gene products, which can exacerbate asthma (123).
GRANTS
This work was supported by National Institutes of Health Grants HL-127805 and AI-117229 (to B. B. Moore) and T32-HL-007749 (to S. J. Gurczynski).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.G. prepared figures; S.J.G. and B.B.M. drafted manuscript; S.J.G. and B.B.M. edited and revised manuscript; S.J.G. and B.B.M. approved final version of manuscript.
REFERENCES
- 1.Aden K, Rehman A, Falk-Paulsen M, Secher T, Kuiper J, Tran F, Pfeuffer S, Sheibani-Tezerji R, Breuer A, Luzius A, Jentzsch M, Häsler R, Billmann-Born S, Will O, Lipinski S, Bharti R, Adolph T, Iovanna JL, Kempster SL, Blumberg RS, Schreiber S, Becher B, Chamaillard M, Kaser A, Rosenstiel P. Epithelial IL-23R signaling licenses protective IL-22 responses in intestinal inflammation. Cell Reports 16: 2208–2218, 2016. doi: 10.1016/j.celrep.2016.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Muhsen S, Letuve S, Vazquez-Tello A, Pureza MA, Al-Jahdali H, Bahammam AS, Hamid Q, Halwani R. Th17 cytokines induce pro-fibrotic cytokines release from human eosinophils. Respir Res 14: 34, 2013. doi: 10.1186/1465-9921-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews K, Ghosh MC, Schwingshackl A, Rapalo G, Luellen C, Waters CM, Fitzpatrick EA. Chronic hypersensitivity pneumonitis caused by Saccharopolyspora rectivirgula is not associated with a switch to a Th2 response. Am J Physiol Lung Cell Mol Physiol 310: L393–L402, 2016. doi: 10.1152/ajplung.00305.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attia A, Abushouk AI, Ahmed H, Gadelkarim M, Elgebaly A, Hassan Z, Abdel-Daim MM, Negida A. Safety and efficacy of brodalumab for moderate-to-severe plaque psoriasis: a systematic review and meta-analysis. Clin Drug Investig 37: 439–451, 2017. doi: 10.1007/s40261-017-0500-9. [DOI] [PubMed] [Google Scholar]
- 6.Basha HI, Ramachandran S, Tiriveedhi V, Takenaka M, Subramanian V, Nath DS, Benshoff N, Patterson GA, Mohanakumar T. Critical role for IL-17A/F in the immunopathogenesis of obliterative airway disease induced by anti-MHC I antibodies. Transplantation 95: 293–300, 2013. doi: 10.1097/TP.0b013e3182772244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayes HK, Ritchie ND, Evans TJ. Interleukin-17 is required for control of chronic lung infection caused by Pseudomonas aeruginosa. Infect Immun 84: 3507–3516, 2016. doi: 10.1128/IAI.00717-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazett M, Bergeron ME, Haston CK. Streptomycin treatment alters the intestinal microbiome, pulmonary T-cell profile and airway hyperresponsiveness in a cystic fibrosis mouse model. Sci Rep 6: 19189, 2016. doi: 10.1038/srep19189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellanger AP, Gbaguidi-Haore H, Gondoin A, Pallandre JR, Vacheyrou M, Valot B, Soumagne T, Reboux G, Dalphin JC, Millon L. Positive fungal quantitative PCR and Th17 cytokine detection in bronchoalveolar lavage fluids: Complementary biomarkers of hypersensitivity pneumonitis? J Immunol Methods 434: 61–65, 2016. doi: 10.1016/j.jim.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, Teixeira MM, Charron S, Fick L, Erard F, Warszawska K, Wolk K, Quesniaux V, Ryffel B, Togbe D. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med 183: 1153–1163, 2011. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
- 11.Bhavani S, Tsai CL, Perusich S, Hesselbacher S, Coxson H, Pandit L, Corry DB, Kheradmand F; Five-Year Prospective Longitudinal Exacerbation Study of Chronic Obstructive Pulmonary Disease (LES-COPD) . Clinical and immunological factors in emphysema progression. Am J Respir Crit Care Med 192: 1171–1178, 2015. doi: 10.1164/rccm.201504-0736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bielen K, 's Jongers B, Boddaert J, Raju TK, Lammens C, Malhotra-Kumar S, Jorens PG, Goossens H, Kumar-Singh S. Biofilm-induced Type 2 innate immunity in a cystic fibrosis model of Pseudomonas aeruginosa. Front Cell Infect Microbiol 7: 274, 2017. doi: 10.3389/fcimb.2017.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobadilla JL, Love RB, Jankowska-Gan E, Xu Q, Haynes LD, Braun RK, Hayney MS, Munoz del Rio A, Meyer K, Greenspan DS, Torrealba J, Heidler KM, Cummings OW, Iwata T, Brand D, Presson R, Burlingham WJ, Wilkes DS. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am J Respir Crit Care Med 177: 660–668, 2008. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, Putney L, Ferrick DA, Hyde DM, Love RB. IL-17 producing γδ T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation 31: 167–179, 2008. doi: 10.1007/s10753-008-9062-6. [DOI] [PubMed] [Google Scholar]
- 15.Brodlie M, McKean MC, Johnson GE, Anderson AE, Hilkens CM, Fisher AJ, Corris PA, Lordan JL, Ward C. Raised interleukin-17 is immunolocalised to neutrophils in cystic fibrosis lung disease. Eur Respir J 37: 1378–1385, 2011. doi: 10.1183/09031936.00067110. [DOI] [PubMed] [Google Scholar]
- 16.Bugeon L, Gardner LM, Rose A, Gentle M, Dallman MJ. Cutting edge: Notch signaling induces a distinct cytokine profile in dendritic cells that supports T cell-mediated regulation and IL-2-dependent IL-17 production. J Immunol 181: 8189–8193, 2008. doi: 10.4049/jimmunol.181.12.8189. [DOI] [PubMed] [Google Scholar]
- 17.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, Lin SL. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 188: 1294–1302, 2013. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 18.Campa M, Mansouri B, Warren R, Menter A. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis. Dermatol Ther (Heidelb) 6: 1–12, 2016. [Erratum in Dermatol Ther 6: 305–305, 2016.] doi: 10.1007/s13555-015-0092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 111: 1293–1298, 2003. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 20.Chan YR, Chen K, Duncan SR, Lathrop KL, Latoche JD, Logar AJ, Pociask DA, Wahlberg BJ, Ray P, Ray A, Pilewski JM, Kolls JK. Patients with cystic fibrosis have inducible IL-17+IL-22+ memory cells in lung draining lymph nodes. J Allergy Clin Immunol 131: 1117–1129, 2013. doi: 10.1016/j.jaci.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen K, Eddens T, Trevejo-Nunez G, Way EE, Elsegeiny W, Ricks DM, Garg AV, Erb CJ, Bo M, Wang T, Chen W, Lee JS, Gaffen SL, Kolls JK. IL-17 receptor signaling in the lung epithelium is required for mucosal chemokine gradients and pulmonary host defense against K. pneumoniae. Cell Host Microbe 20: 596–605, 2016. doi: 10.1016/j.chom.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Li C, Weng D, Song L, Tang W, Dai W, Yu Y, Liu F, Zhao M, Lu C, Chen J. Neutralization of interleukin-17A delays progression of silica-induced lung inflammation and fibrosis in C57BL/6 mice. Toxicol Appl Pharmacol 275: 62–72, 2014. doi: 10.1016/j.taap.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM, Butler CA, Hargadon B, Sciurba JC, Gieseck RL, Thompson RW, White S, Abbas AR, Jackman J, Wu LC, Egen JG, Heaney LG, Ramalingam TR, Arron JR, Wynn TA, Bradding P. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med 7: 301ra129, 2015. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 24.Confalonieri M, Salton F, Fabiano F. Acute respiratory distress syndrome. Eur Respir Rev 26: 160116, 2017. doi: 10.1183/16000617.0116-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conti HR, Peterson AC, Brane L, Huppler AR, Hernández-Santos N, Whibley N, Garg AV, Simpson-Abelson MR, Gibson GA, Mamo AJ, Osborne LC, Bishu S, Ghilardi N, Siebenlist U, Watkins SC, Artis D, McGeachy MJ, Gaffen SL. Oral-resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. J Exp Med 211: 2075–2084, 2014. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coomes SM, Farmen S, Wilke CA, Laouar Y, Moore BB. Severe γherpesvirus-induced pneumonitis and fibrosis in syngeneic bone marrow transplant mice is related to effects of transforming growth factor-β. Am J Pathol 179: 2382–2396, 2011. doi: 10.1016/j.ajpath.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coomes SM, Wilke CA, Moore TA, Moore BB. Induction of TGF-β1, not regulatory T cells, impairs antiviral immunity in the lung following bone marrow transplant. J Immunol 184: 5130–5140, 2010. doi: 10.4049/jimmunol.0901871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosmi L, Liotta F, Annunziato F. Th17 regulating lower airway disease. Curr Opin Allergy Clin Immunol 16: 1–6, 2016. doi: 10.1097/ACI.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 29.Decraene A, Willems-Widyastuti A, Kasran A, De Boeck K, Bullens DM, Dupont LJ. Elevated expression of both mRNA and protein levels of IL-17A in sputum of stable cystic fibrosis patients. Respir Res 11: 177, 2010. doi: 10.1186/1465-9921-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong Z, Lu X, Yang Y, Zhang T, Li Y, Chai Y, Lei W, Li C, Ai L, Tai W. IL-27 alleviates the bleomycin-induced pulmonary fibrosis by regulating the Th17 cell differentiation. BMC Pulm Med 15: 13, 2015. doi: 10.1186/s12890-015-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong Z, Yang Y, Zhang T, Li Y, Kang Q, Lei W, Cao Y, Niu X, Wang D, Tai W. siRNA-Act1 inhibits the function of IL-17 on lung fibroblasts via the NF-κB pathway. Respiration 86: 332–340, 2013. doi: 10.1159/000348403. [DOI] [PubMed] [Google Scholar]
- 32.Durham AL, Caramori G, Chung KF, Adcock IM. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl Res 167: 192–203, 2016. doi: 10.1016/j.trsl.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eddens T, Elsegeiny W, Garcia-Hernadez ML, Castillo P, Trevejo-Nunez G, Serody K, Campfield BT, Khader SA, Chen K, Rangel-Moreno J, Kolls JK. Pneumocystis-driven inducible bronchus-associated lymphoid tissue formation requires Th2 and Th17 immunity. Cell Reports 18: 3078–3090, 2017. doi: 10.1016/j.celrep.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabro AT, da Silva PH, Zocolaro WS, de Almeida MS, Rangel MP, de Oliveira CC, Minatel IO, Prando ED, Rainho CA, Teodoro WR, Velosa AP, Saber AM, Parra-Cuentas ER, Popper HH, Capelozzi VL. The Th17 pathway in the peripheral lung microenvironment interacts with expression of collagen V in the late state of experimental pulmonary fibrosis. Immunobiology 220: 124–135, 2015. doi: 10.1016/j.imbio.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Fang P, Zhou L, Zhou Y, Kolls JK, Zheng T, Zhu Z. Immune modulatory effects of IL-22 on allergen-induced pulmonary inflammation. PLoS One 9: e107454, 2014. doi: 10.1371/journal.pone.0107454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.François A, Gombault A, Villeret B, Alsaleh G, Fanny M, Gasse P, Adam SM, Crestani B, Sibilia J, Schneider P, Bahram S, Quesniaux V, Ryffel B, Wachsmann D, Gottenberg JE, Couillin I. B cell activating factor is central to bleomycin- and IL-17-mediated experimental pulmonary fibrosis. J Autoimmun 56: 1–11, 2015. doi: 10.1016/j.jaut.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Galati D, De Martino M, Trotta A, Rea G, Bruzzese D, Cicchitto G, Stanziola AA, Napolitano M, Sanduzzi A, Bocchino M. Peripheral depletion of NK cells and imbalance of the Treg/Th17 axis in idiopathic pulmonary fibrosis patients. Cytokine 66: 119–126, 2014. doi: 10.1016/j.cyto.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, Fick L, Charron S, Lagente V, Eberl G, Le Bert M, Quesniaux VF, Huaux F, Leite-de-Moraes M, Ryffel B, Couillin I. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One 6: e23185, 2011. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta PK, Wagner SR, Wu Q, Shilling RA. IL-17A blockade attenuates obliterative bronchiolitis and IFN-γ cellular immune response in lung allografts. Am J Respir Cell Mol Biol 56: 708–715, 2017. doi: 10.1165/rcmb.2016-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurczynski SJ, Procario MC, O’Dwyer DN, Wilke CA, Moore BB. Loss of CCR2 signaling alters leukocyte recruitment and exacerbates γ-herpesvirus-induced pneumonitis and fibrosis following bone marrow transplantation. Am J Physiol Lung Cell Mol Physiol 311: L611–L627, 2016. doi: 10.1152/ajplung.00193.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med 202: 761–769, 2005. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol 18: 349–356, 2006. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Hartupee J, Liu C, Novotny M, Sun D, Li X, Hamilton TA. IL-17 signaling for mRNA stabilization does not require TNF receptor-associated factor 6. J Immunol 182: 1660–1666, 2009. doi: 10.4049/jimmunol.182.3.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasan SA, Eksteen B, Reid D, Paine HV, Alansary A, Johannson K, Gwozd C, Goring KA, Vo T, Proud D, Kelly MM. Role of IL-17A and neutrophils in fibrosis in experimental hypersensitivity pneumonitis. J Allergy Clin Immunol 131: 1663–1673, 2013. doi: 10.1016/j.jaci.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi H, Kawakita A, Okazaki S, Yasutomi M, Murai H, Ohshima Y. IL-17A/F modulates fibrocyte functions in cooperation with CD40-mediated signaling. Inflammation 36: 830–838, 2013. doi: 10.1007/s10753-013-9609-z. [DOI] [PubMed] [Google Scholar]
- 46.Hsu D, Taylor P, Fletcher D, van Heeckeren R, Eastman J, van Heeckeren A, Davis P, Chmiel JF, Pearlman E, Bonfield TL. Interleukin-17 pathophysiology and therapeutic intervention in cystic fibrosis lung infection and inflammation. Infect Immun 84: 2410–2421, 2016. doi: 10.1128/IAI.00284-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu M, Yang S, Yang L, Cheng Y, Zhang H. Interleukin-22 alleviated palmitate-induced endoplasmic reticulum stress in INS-1 cells through activation of autophagy. PLoS One 11: e0146818, 2016. doi: 10.1371/journal.pone.0146818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang J, Yuan Q, Zhu H, Yin L, Hong S, Dong Z, Jin W, Dong C. IL-17C/IL-17RE augments T cell function in autoimmune hepatitis. J Immunol 198: 669–680, 2017. doi: 10.4049/jimmunol.1600977. [DOI] [PubMed] [Google Scholar]
- 49.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol 169: 443–453, 2002. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 50.Hwang SY, Kim JY, Kim KW, Park MK, Moon Y, Kim WU, Kim HY. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-κB- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther 6: R120–R128, 2004. doi: 10.1186/ar1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivanov S, Bozinovski S, Bossios A, Valadi H, Vlahos R, Malmhäll C, Sjöstrand M, Kolls JK, Anderson GP, Lindén A. Functional relevance of the IL-23-IL-17 axis in lungs in vivo. Am J Respir Cell Mol Biol 36: 442–451, 2007. doi: 10.1165/rcmb.2006-0020OC. [DOI] [PubMed] [Google Scholar]
- 52.Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, Blanc F, De Trez C, Van Maele L, Dumoutier L, Huerre MR, Eberl G, Si-Tahar M, Gosset P, Renauld JC, Sirard JC, Faveeuw C, Trottein F. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J Virol 87: 6911–6924, 2013. doi: 10.1128/JVI.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Z, Zhou Q, Gu C, Li D, Zhu L. Depletion of circulating monocytes suppresses IL-17 and HMGB1 expression in mice with LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 312: L231–L242, 2017. doi: 10.1152/ajplung.00389.2016. [DOI] [PubMed] [Google Scholar]
- 54.Jungnickel C, Wonnenberg B, Karabiber O, Wolf A, Voss M, Wolf L, Honecker A, Kamyschnikow A, Herr C, Bals R, Beisswenger C. Cigarette smoke-induced disruption of pulmonary barrier and bacterial translocation drive tumor-associated inflammation and growth. Am J Physiol Lung Cell Mol Physiol 309: L605–L613, 2015. doi: 10.1152/ajplung.00116.2015. [DOI] [PubMed] [Google Scholar]
- 55.Kang MJ, Choi JM, Kim BH, Lee CM, Cho WK, Choe G, Kim DH, Lee CG, Elias JA. IL-18 induces emphysema and airway and vascular remodeling via IFN-γ, IL-17A, and IL-13. Am J Respir Crit Care Med 185: 1205–1217, 2012. doi: 10.1164/rccm.201108-1545OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim B-J, Lee S, Berg RE, Simecka JW, Jones HP. Interleukin-23 (IL-23) deficiency disrupts Th17 and Th1-related defenses against Streptococcus pneumoniae infection. Cytokine 64: 375–381, 2013. doi: 10.1016/j.cyto.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 57.Kreindler JL, Bertrand CA, Lee RJ, Karasic T, Aujla S, Pilewski JM, Frizzell RA, Kolls JK. Interleukin-17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 296: L257–L266, 2009. doi: 10.1152/ajplung.00344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR. Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 186: 1666–1674, 2011. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kusagaya H, Fujisawa T, Yamanaka K, Mori K, Hashimoto D, Enomoto N, Inui N, Nakamura Y, Wu R, Maekawa M, Suda T, Chida K. Toll-like receptor-mediated airway IL-17C enhances epithelial host defense in an autocrine/paracrine manner. Am J Respir Cell Mol Biol 50: 30–39, 2014. doi: 10.1165/rcmb.2013-0130OC. [DOI] [PubMed] [Google Scholar]
- 60.Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol 13: 144–151, 2011. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SU, Sung MH, Ryu HW, Lee J, Kim HS, In HJ, Ahn KS, Lee HJ, Lee HK, Shin DH, Lee Y, Hong ST, Oh SR. Verproside inhibits TNF-α-induced MUC5AC expression through suppression of the TNF-α/NF-κB pathway in human airway epithelial cells. Cytokine 77: 168–175, 2016. doi: 10.1016/j.cyto.2015.08.262. [DOI] [PubMed] [Google Scholar]
- 62.Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, Dowd P, Gurney AL, Wood WI. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci USA 97: 773–778, 2000. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Q, Gu Y, Tu Q, Wang K, Gu X, Ren T. Blockade of interleukin-17 restrains the development of acute lung injury. Scand J Immunol 83: 203–211, 2016. doi: 10.1111/sji.12408. [DOI] [PubMed] [Google Scholar]
- 64.Li XM, Chen X, Gu W, Guo YJ, Cheng Y, Peng J, Guo XJ. Impaired TNF/TNFR2 signaling enhances Th2 and Th17 polarization and aggravates allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol 313: L592–L601, 2017. doi: 10.1152/ajplung.00409.2016. [DOI] [PubMed] [Google Scholar]
- 65.Liang M, Wang J, Chu H, Zhu X, He H, Liu Q, Qiu J, Zhou X, Guan M, Xue Y, Chen X, Zou H. Interleukin-22 inhibits bleomycin-induced pulmonary fibrosis. Mediators Inflamm 2013: 209179, 2013. doi: 10.1155/2013/209179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao CM, Zimmer MI, Wang CR. The functions of type I and type II natural killer T cells in inflammatory bowel diseases. Inflamm Bowel Dis 19: 1330–1338, 2013. doi: 10.1097/MIB.0b013e318280b1e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, Ivanov JA, Fu YY, Takashima S, Hua G, Martin ML, O’Rourke KP, Lo YH, Mokry M, Romera-Hernandez M, Cupedo T, Dow L, Nieuwenhuis EE, Shroyer NF, Liu C, Kolesnick R, van den Brink MRM, Hanash AM. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528: 560–564, 2015. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu H, Mi S, Li Z, Hua F, Hu ZW. Interleukin 17A inhibits autophagy through activation of PIK3CA to interrupt the GSK3B-mediated degradation of BCL2 in lung epithelial cells. Autophagy 9: 730–742, 2013. doi: 10.4161/auto.24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lo Re S, Dumoutier L, Couillin I, Van Vyve C, Yakoub Y, Uwambayinema F, Marien B, van den Brûle S, Van Snick J, Uyttenhove C, Ryffel B, Renauld JC, Lison D, Huaux F. IL-17A-producing γδ T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol 184: 6367–6377, 2010. doi: 10.4049/jimmunol.0900459. [DOI] [PubMed] [Google Scholar]
- 70.Luo F, Le NB, Mills T, Chen NY, Karmouty-Quintana H, Molina JG, Davies J, Philip K, Volcik KA, Liu H, Xia Y, Eltzschig HK, Blackburn MR. Extracellular adenosine levels are associated with the progression and exacerbation of pulmonary fibrosis. FASEB J 30: 874–883, 2016. doi: 10.1096/fj.15-274845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, Pirhonen J, Kolls JK. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-α and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol 175: 404–412, 2005. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, Wang XX, Liu HZ, Sun W, Hu ZW. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-β1-dependent and -independent mechanisms. J Immunol 187: 3003–3014, 2011. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- 73.Mizunoe S, Shuto T, Suzuki S, Matsumoto C, Watanabe K, Ueno-Shuto K, Suico MA, Onuki K, Gruenert DC, Kai H. Synergism between interleukin (IL)-17 and Toll-like receptor 2 and 4 signals to induce IL-8 expression in cystic fibrosis airway epithelial cells. J Pharmacol Sci 118: 512–520, 2012. doi: 10.1254/jphs.11240FP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73a.Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol 49: 167–179, 2013. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muir R, Osbourn M, Dubois AV, Doran E, Small DM, Monahan A, O’Kane CM, McAllister K, Fitzgerald DC, Kissenpfennig A, McAuley DF, Ingram RJ. Innate lymphoid cells are the predominant source of IL-17A during the early pathogenesis of acute respiratory distress syndrome. Am J Respir Crit Care Med 193: 407–416, 2016. doi: 10.1164/rccm.201410-1782OC. [DOI] [PubMed] [Google Scholar]
- 75.Mulcahy EM, Hudson JB, Beggs SA, Reid DW, Roddam LF, Cooley MA. High peripheral blood Th17 percent associated with poor lung function in cystic fibrosis. PLoS One 10: e0120912, 2015. doi: 10.1371/journal.pone.0120912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murakami T, Hatano S, Yamada H, Iwakura Y, Yoshikai Y. Two types of interleukin 17A-producing γδ T cells in protection against pulmonary infection with Klebsiella pneumoniae. J Infect Dis 214: 1752–1761, 2016. doi: 10.1093/infdis/jiw443. [DOI] [PubMed] [Google Scholar]
- 77.Murdock BJ, Huffnagle GB, Olszewski MA, Osterholzer JJ. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and γ interferon production. Infect Immun 82: 937–948, 2014. doi: 10.1128/IAI.01477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nalbant A, Eskier D. Genes associated with T helper 17 cell differentiation and function. Front Biosci (Elite Ed) 8: 427–435, 2016. doi: 10.2741/e777. [DOI] [PubMed] [Google Scholar]
- 79.Nuovo GJ, Hagood JS, Magro CM, Chin N, Kapil R, Davis L, Marsh CB, Folcik VA. The distribution of immunomodulatory cells in the lungs of patients with idiopathic pulmonary fibrosis. Mod Pathol 25: 416–433, 2012. doi: 10.1038/modpathol.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Dwyer DN, Ashley SL, Moore BB. Influences of innate immunity, autophagy, and fibroblast activation in the pathogenesis of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 311: L590–L601, 2016. doi: 10.1152/ajplung.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Dwyer DN, Dickson RP, Moore BB. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol 196: 4839–4847, 2016. doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh K, Seo MW, Kim YW, Lee DS. Osteopontin potentiates pulmonary inflammation and fibrosis by modulating IL-17/IFN-γ-secreting T-cell ratios in bleomycin-treated mice. Immune Netw 15: 142–149, 2015. doi: 10.4110/in.2015.15.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, Baumgartner S. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 366: 1181–1189, 2012. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 84.Peters M, Köhler-Bachmann S, Lenz-Habijan T, Bufe A. Influence of an allergen-specific Th17 response on remodeling of the airways. Am J Respir Cell Mol Biol 54: 350–358, 2016. doi: 10.1165/rcmb.2014-0429OC. [DOI] [PubMed] [Google Scholar]
- 85.Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn JF. IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol 182: 1286–1296, 2013. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Podsiad A, Standiford TJ, Ballinger MN, Eakin R, Park P, Kunkel SL, Moore BB, Bhan U. MicroRNA-155 regulates host immune response to postviral bacterial pneumonia via IL-23/IL-17 pathway. Am J Physiol Lung Cell Mol Physiol 310: L465–L475, 2016. doi: 10.1152/ajplung.00224.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 8: 247–256, 2007. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 88.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453: 65–71, 2008. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 89.Radhakrishnan SV, Hildebrandt GC. A call to arms: a critical need for interventions to limit pulmonary toxicity in the stem cell transplantation patient population. Curr Hematol Malig Rep 10: 8–17, 2015. doi: 10.1007/s11899-014-0244-z. [DOI] [PubMed] [Google Scholar]
- 90.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, Modrusan Z, Sai T, Lee W, Xu M, Caplazi P, Diehl L, de Voss J, Balazs M, Gonzalez L Jr, Singh H, Ouyang W, Pappu R. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 12: 1159–1166, 2011. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 91.Reed M, Morris SH, Owczarczyk AB, Lukacs NW. Deficiency of autophagy protein Map1-LC3b mediates IL-17-dependent lung pathology during respiratory viral infection via ER stress-associated IL-1. Mucosal Immunol 8: 1118–1130, 2015. doi: 10.1038/mi.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riario Sforza GG, Marinou A. Hypersensitivity pneumonitis: a complex lung disease. Clin Mol Allergy 15: 6, 2017. doi: 10.1186/s12948-017-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robinson KM, Choi SM, McHugh KJ, Mandalapu S, Enelow RI, Kolls JK, Alcorn JF. Influenza A exacerbates Staphylococcus aureus pneumonia by attenuating IL-1β production in mice. J Immunol 191: 5153–5159, 2013. doi: 10.4049/jimmunol.1301237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roussel L, Rousseau S. IL-17 primes airway epithelial cells lacking functional cystic fibrosis transmembrane conductance Regulator (CFTR) to increase NOD1 responses. Biochem Biophys Res Commun 391: 505–509, 2010. doi: 10.1016/j.bbrc.2009.11.088. [DOI] [PubMed] [Google Scholar]
- 95.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun 75: 3055–3061, 2007. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Segawa S, Goto D, Iizuka A, Kaneko S, Yokosawa M, Kondo Y, Matsumoto I, Sumida T. The regulatory role of interferon-γ producing γδ T cells via the suppression of T helper 17 cell activity in bleomycin-induced pulmonary fibrosis. Clin Exp Immunol 185: 348–360, 2016. doi: 10.1111/cei.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seo S, Renaud C, Kuypers JM, Chiu CY, Huang ML, Samayoa E, Xie H, Yu G, Fisher CE, Gooley TA, Miller S, Hackman RC, Myerson D, Sedlak RH, Kim YJ, Fukuda T, Fredricks DN, Madtes DK, Jerome KR, Boeckh M. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 125: 3789–3797, 2015. doi: 10.1182/blood-2014-12-617035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shipe R, Burdick MD, Strieter BA, Liu L, Shim YM, Sung SS, Teague WG, Mehrad B, Strieter RM, Rose CE Jr. Number, activation, and differentiation of circulating fibrocytes correlate with asthma severity. J Allergy Clin Immunol 137: 750–757, 2016. doi: 10.1016/j.jaci.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simonian PL, Roark CL, Born WK, O’Brien RL, Fontenot AP. γδ T cells and Th17 cytokines in hypersensitivity pneumonitis and lung fibrosis. Transl Res 154: 222–227, 2009. doi: 10.1016/j.trsl.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F, Born WK, O’Brien RL, Fontenot AP. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol 182: 657–665, 2009. doi: 10.4049/jimmunol.182.1.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simonian PL, Roark CL, Wehrmann F, Lanham AM, Born WK, O’Brien RL, Fontenot AP. IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis. J Immunol 182: 6540–6549, 2009. doi: 10.4049/jimmunol.0900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One 6: e23522, 2011. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song L, Weng D, Dai W, Tang W, Chen S, Li C, Chen Y, Liu F, Chen J. Th17 can regulate silica-induced lung inflammation through an IL-1β-dependent mechanism. J Cell Mol Med 18: 1773–1784, 2014. doi: 10.1111/jcmm.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Song L, Weng D, Liu F, Chen Y, Li C, Dong L, Tang W, Chen J. Tregs promote the differentiation of Th17 cells in silica-induced lung fibrosis in mice. PLoS One 7: e37286, 2012. doi: 10.1371/journal.pone.0037286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol 167: 4137–4140, 2001. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 106.Sun N, Wei X, Wang J, Cheng Z, Sun W. Caveolin-1 promotes the imbalance of Th17/Treg in patients with chronic obstructive pulmonary disease. Inflammation 39: 2008–2015, 2016. doi: 10.1007/s10753-016-0436-x. [DOI] [PubMed] [Google Scholar]
- 107.Szymczak WA, Sellers RS, Pirofski LA. IL-23 dampens the allergic response to Cryptococcus neoformans through IL-17-independent and -dependent mechanisms. Am J Pathol 180: 1547–1559, 2012. doi: 10.1016/j.ajpath.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med 184: 252–258, 2011. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang W, Liu F, Chen Y, Song L, Dai W, Li C, Weng D, Chen J. Reduction of IL-17A might suppress the Th1 response and promote the Th2 response by boosting the function of Treg cells during silica-induced inflammatory response in vitro. Mediators Inflamm 2014: 570894, 2014. doi: 10.1155/2014/570894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang W, Smith SG, Beaudin S, Dua B, Howie K, Gauvreau G, O’Byrne PM. IL-25 and IL-25 receptor expression on eosinophils from subjects with allergic asthma. Int Arch Allergy Immunol 163: 5–10, 2014. doi: 10.1159/000355331. [DOI] [PubMed] [Google Scholar]
- 111.Thakur C, Wolfarth M, Sun J, Zhang Y, Lu Y, Battelli L, Porter DW, Chen F. Oncoprotein mdig contributes to silica-induced pulmonary fibrosis by altering balance between Th17 and Treg T cells. Oncotarget 6: 3722–3736, 2015. doi: 10.18632/oncotarget.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Varelias A, Gartlan KH, Kreijveld E, Olver SD, Lor M, Kuns RD, Lineburg KE, Teal BE, Raffelt NC, Cheong M, Alexander KA, Koyama M, Markey KA, Sturgeon E, Leach J, Reddy P, Kennedy GA, Yanik GA, Blazar BR, Tey SK, Clouston AD, MacDonald KP, Cooke KR, Hill GR. Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation. Blood 125: 2435–2444, 2015. doi: 10.1182/blood-2014-07-590232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verleden SE, Sacreas A, Vos R, Vanaudenaerde BM, Verleden GM. Advances in Understanding bronchiolitis obliterans after lung transplantation. Chest 150: 219–225, 2016. doi: 10.1016/j.chest.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 114.Versluys AB, Rossen JW, van Ewijk B, Schuurman R, Bierings MB, Boelens JJ. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant 16: 782–791, 2010. doi: 10.1016/j.bbmt.2009.12.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24: 677–688, 2006. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 116.Wei YR, Qiu H, Wu Q, Du YK, Yin ZF, Chen SS, Jin YP, Zhao MM, Wang C, Weng D, Li HP. Establishment of the mouse model of acute exacerbation of idiopathic pulmonary fibrosis. Exp Lung Res 42: 75–86, 2016. doi: 10.3109/01902148.2016.1144835. [DOI] [PubMed] [Google Scholar]
- 117.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 207: 535–552, 2010. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wolf L, Sapich S, Honecker A, Jungnickel C, Seiler F, Bischoff M, Wonnenberg B, Herr C, Schneider-Daum N, Lehr CM, Bals R, Beisswenger C. IL-17A-mediated expression of epithelial IL-17C promotes inflammation during acute Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol 311: L1015–L1022, 2016. doi: 10.1152/ajplung.00158.2016. [DOI] [PubMed] [Google Scholar]
- 119.Wozniak KL, Hardison SE, Kolls JK, Wormley FL. Role of IL-17A on resolution of pulmonary C. neoformans infection. PLoS One 6: e17204, 2011. doi: 10.1371/journal.pone.0017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wozniak KL, Kolls JK, Wormley FL Jr. Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL-17A production by γδ T cells. BMC Immunol 13: 65, 2012. doi: 10.1186/1471-2172-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu L, Chen X, Zhao J, Martin B, Zepp JA, Ko JS, Gu C, Cai G, Ouyang W, Sen G, Stark GR, Su B, Vines CM, Tournier C, Hamilton TA, Vidimos A, Gastman B, Liu C, Li X. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J Exp Med 212: 1571–1587, 2015. doi: 10.1084/jem.20150204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu Q, Gupta PK, Suzuki H, Wagner SR, Zhang C, W Cummings O, Fan L, Kaplan MH, Wilkes DS, Shilling RA. CD4 T cells but not Th17 cells are required for mouse lung transplant obliterative bronchiolitis. Am J Transplant 15: 1793–1804, 2015. doi: 10.1111/ajt.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xia W, Bai J, Wu X, Wei Y, Feng S, Li L, Zhang J, Xiong G, Fan Y, Shi J, Li H. Interleukin-17A promotes MUC5AC expression and goblet cell hyperplasia in nasal polyps via the Act1-mediated pathway. PLoS One 9: e98915, 2014. doi: 10.1371/journal.pone.0098915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiong H, Keith JW, Samilo DW, Carter RA, Leiner IM, Pamer EG. Innate lymphocyte/Ly6C(hi) monocyte crosstalk promotes Klebsiella pneumoniae clearance. Cell 165: 679–689, 2016. doi: 10.1016/j.cell.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu B, Guenther JF, Pociask DA, Wang Y, Kolls JK, You Z, Chandrasekar B, Shan B, Sullivan DE, Morris GF. Promotion of lung tumor growth by interleukin-17. Am J Physiol Lung Cell Mol Physiol 307: L497–L508, 2014. doi: 10.1152/ajplung.00125.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamada Y, Vandermeulen E, Heigl T, Somers J, Vaneylen A, Verleden SE, Bellon H, De Vleeschauwer S, Verbeken EK, Van Raemdonck DE, Vos R, Verleden GM, Jungraithmayr W, Vanaudenaerde BM. The role of recipient derived interleukin-17A in a murine orthotopic lung transplant model of restrictive chronic lung allograft dysfunction. Transpl Immunol 39: 10–17, 2016. doi: 10.1016/j.trim.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 127.Yan Z, Xiaoyu Z, Zhixin S, Di Q, Xinyu D, Jing X, Jing H, Wang D, Xi Z, Chunrong Z, Daoxin W. Rapamycin attenuates acute lung injury induced by LPS through inhibition of Th17 cell proliferation in mice. Sci Rep 6: 20156, 2016. doi: 10.1038/srep20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yanagisawa H, Hashimoto M, Minagawa S, Takasaka N, Ma R, Moermans C, Ito S, Araya J, Budelsky A, Goodsell A, Baron JL, Nishimura SL. Role of IL-17A in murine models of COPD airway disease. Am J Physiol Lung Cell Mol Physiol 312: L122–L130, 2017. doi: 10.1152/ajplung.00301.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3: 811–821, 1995. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 130.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol 155: 5483–5486, 1995. [PubMed] [Google Scholar]
- 131.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol 25: 335–340, 2001. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 132.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194: 519–527, 2001. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yeste A, Mascanfroni ID, Nadeau M, Burns EJ, Tukpah AM, Santiago A, Wu C, Patel B, Kumar D, Quintana FJ. IL-21 induces IL-22 production in CD4+ T cells. Nat Commun 5: 3753, 2014. doi: 10.1038/ncomms4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou X, Loomis-King H, Gurczynski SJ, Wilke CA, Konopka KE, Ptaschinski C, Coomes SM, Iwakura Y, van Dyk LF, Lukacs NW, Moore BB. Bone marrow transplantation alters lung antigen-presenting cells to promote TH17 response and the development of pneumonitis and fibrosis following γherpesvirus infection. Mucosal Immunol 9: 610–620, 2016. doi: 10.1038/mi.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu L, Wu Y, Wei H, Xing X, Zhan N, Xiong H, Peng B. IL-17R activation of human periodontal ligament fibroblasts induces IL-23 p19 production: Differential involvement of NF-κB versus JNK/AP-1 pathways. Mol Immunol 48: 647–656, 2011. doi: 10.1016/j.molimm.2010.11.008. [DOI] [PubMed] [Google Scholar]


