Abstract
Elevated active plasminogen activator inhibitor-1 (PAI-1) has an adverse effect on the outcomes of intrapleural fibrinolytic therapy (IPFT) in tetracycline-induced pleural injury in rabbits. To enhance IPFT with prourokinase (scuPA), two mechanistically distinct approaches to targeting PAI-1 were tested: slowing its reaction with urokinase (uPA) and monoclonal antibody (mAb)-mediated PAI-1 inactivation. Removing positively charged residues at the “PAI-1 docking site” (179RHRGGS184→179AAAAAA184) of uPA results in a 60-fold decrease in the rate of inhibition by PAI-1. Mutant prourokinase (0.0625–0.5 mg/kg; n = 12) showed efficacy comparable to wild-type scuPA and did not change IPFT outcomes (P > 0.05). Notably, the rate of PAI-1-independent intrapleural inactivation of mutant uPA was 2 times higher (P < 0.05) than that of the wild-type enzyme. Trapping PAI-1 in a “molecular sandwich”-type complex with catalytically inactive two-chain urokinase with Ser195Ala substitution (S195A-tcuPA; 0.1 and 0.5 mg/kg) did not improve the efficacy of IPFT with scuPA (0.0625–0.5 mg/kg; n = 11). IPFT failed in the presence of MA-56A7C10 (0.5 mg/kg; n = 2), which forms a stable intrapleural molecular sandwich complex, allowing active PAI-1 to accumulate by blocking its transition to a latent form. In contrast, inactivation of PAI-1 by accelerating the active-to-latent transition mediated by mAb MA-33B8 (0.5 mg/kg; n = 2) improved the efficacy of IPFT with scuPA (0.25 mg/kg). Thus, under conditions of slow (4–8 h) fibrinolysis in tetracycline-induced pleural injury in rabbits, only the inactivation of PAI-1, but not a decrease in the rate of its reaction with uPA, enhances IPFT. Therefore the rate of fibrinolysis, which varies in different pathologic states, could affect the selection of PAI-1 inhibitors to enhance fibrinolytic therapy.
Keywords: fibrinolytic therapy, active plasminogen activator inhibitor-1, molecular target, prourokinase, animal model
INTRODUCTION
The incidence (12–14, 30, 32, 64, 70) and hospitalization rate (30) for empyema, an infectious pleural injury, is increasing in all age groups, as is mortality in adults (8, 58). Intrapleural fibrinolytic therapy (IPFT) with tissue (tPA) and urokinase (uPA) plasminogen activators is generally successful in treating organizing pleural injury in pediatric patients (6, 7, 9, 19, 72, 73, 76). However, in adults, IPFT has been variably effective with an unclear safety profile and thus remains controversial (16, 18, 35, 53, 58, 68, 69, 79). Understanding the molecular interactions in fibrinolysis is key to developing successful IPFT. Plasminogen activator inhibitor-1 (PAI-1), the major endogenous inhibitor of tPA and uPA, interacts with target proteinases with diffusion-limited rate constants at a 1:1 stoichiometry (31, 77). PAI-1, which can be elevated by orders of magnitude in various disease states, has been suggested as a therapeutic target in a number of thrombotic disorders (87). Levels of PAI-1 antigen in blood and pleural fluids of patients with complicated pleural effusions and empyema are reported to increase from 5–20 ng/ml (1, 87) to hundreds and thousands of nanograms per milliliter (1, 37, 40, 54, 55, 61), respectively. Thus, in pleural injury, overexpressed PAI-1 is potentially capable of inactivating significant amounts of fibrinolysin during IPFT. Although murine models of parapneumonic effusion associated with the formation of pleural adhesions have been reported (80, 85), there are fundamental differences between the human and mouse fibrinolytic systems that limit the use of mouse models. The structure of mouse fibrin differs from that in humans (67). Mouse PAI-1 also notably differs from the human serpin (23), and human uPA possesses poor affinity to murine uPA receptor (41). However, rabbit models of chemical (36, 46) and infectious (47) pleural injury closely recapitulate human empyema, allow for testing of therapeutics because of the close similarity between rabbit and human fibrinolytic systems, and are thus reasonable alternatives to the use of mouse models.
Intrapleural levels of PAI-1 in rabbit models of Streptococcus pneumoniae and Pasteurella multocida empyema (47) and in a tetracycline (TCN)-induced pleural injury (25) approach those observed in humans. TCN-induced pleural injury in rabbits, which recapitulates a number of features of empyema (36, 38, 39, 46, 74), was recently used to study the mechanisms of intrapleural fibrinolysis (46) and the contribution of active PAI-1 to IPFT outcomes (25, 42) and for evaluating the short-term effects of chest computed tomography on IPFT outcomes (48). Active PAI-1 was identified as a biomarker that indicates the severity of fibrosis in TCN-induced pleural injury (42) and as a molecular target for IPFT (25). An increase in the intrapleural level of active PAI-1 coincided with less efficacious IPFT of TCN-induced pleural injury after repeated computed tomography scans of rabbits (48). Intrapleural fibrinolysis in TCN-induced pleural injury is relatively slow, with the minimal time necessary for effective fibrinolysis being 4–8 h (48). Thus overexpression of intrapleural PAI-1 in combination with fast inhibition of exogenous plasminogen activator results in ineffective IPFT (25, 46, 48). Higher levels of active PAI-1 contribute to faster inhibition of intrapleural plasminogen-activating activity and termination of fibrinolysis and adversely affect IPFT outcomes (48). PAI-1 inactivates tPA and uPA with a stoichiometry close to unity (Fig. 1A, ki; 15). Intrapleural neutralization of PAI-1 by redirecting the reaction from the inhibitory (Fig. 1, ki) to the substrate (Fig. 1, ks) branch resulted in an eightfold decrease in the effective dose of prourokinase (scuPA) in TCN-induced pleural injury (25). Although targeting active PAI-1 represents a promising strategy to enhance IPFT in TCN-induced pleural injury (25, 48) and, likely, in empyema, where intrapleural PAI-1 levels were elevated up to 3 orders of magnitude (47), there are other intermolecular mechanisms that could be used for in vivo PAI-1 neutralization (22, 28, 81).
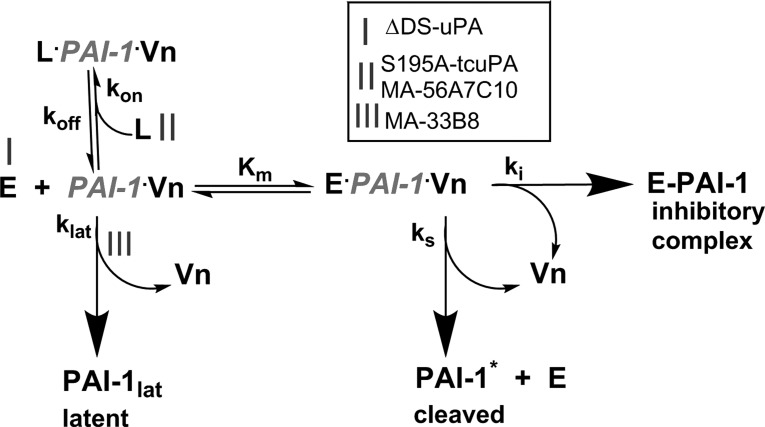
Fig. 1.
Plasminogen activator inhibitor-1 (PAI-1) mechanism and approaches to modulating PAI-1 activity in vivo. The PAI-1 reaction includes inhibitory (ki), substrate (ks), and latent (klat) branches, which result in a loss of activity and formation of a stable inhibitory complex (E-PAI-1), cleaved serpin (PAI-1*), and latent serpin (PAI-1lat), respectively. PAI-1 in an active conformation (PAI-1) complexed with endogenous vitronectin (Vn) in the pleural space interacts with the plasminogen activator [wild-type urokinase (wt-uPA) or urokinase with 179RHRGGS184→179AAAAAA184 substitutions (ΔDS-uPA); enzyme, E] and forms a transient Michaelis complex (E·PAI-1·Vn). Once the enzyme cleaves PAI-1 and forms an acyl-enzyme, conformational changes in the serpin result in its stabilization in an inhibitory complex (E-PAI-1). Under physiological conditions, >90% of the PAI-1 reaction follows the inhibitory branch (ki), resulting in mutual, stoichiometric inhibition of the enzyme and PAI-1. Active PAI-1 can also slowly, spontaneously transform (klat) into an inactive, latent conformation (PAI-1lat), losing the ability to bind Vn. The substrate branch (ks) yields an inactive, cleaved serpin (PAI-1*) and an active enzyme. The PAI-1 reaction was modulated by three distinct mechanisms (mechanisms I–III). In mechanism I, alanine mutations of positively charged residues in the 37-loop of uPA result in ΔDS-uPA, which interacts with PAI-1 via the inhibitory branch (ki) considerably more slowly than the wild-type enzyme. In mechanism II, ligands (L), such as inactive uPA with Ser195Ala substitution (S195A-uPA) or monoclonal antibody MA-56A7C10, compete with uPA and bind PAI-1/Vn with nanomolar affinity [Kd = koff/kon, where Kd is the dissociation constant and koff and kon are the first-order rate constants of dissociation and association, respectively, of PAI-1/S195A-two-chain uPA (tcuPA) or MA-56A7C10] forming nonproductive “molecular sandwich”-type complexes (L·PAI-1·Vn), which compete with the Michaelis complex for uPA (Kd << Km). L·PAI-1·Vn also stabilizes the active conformation of PAI-1, inhibiting the latent branch (klat). The rate of enzyme inactivation by L·PAI-1·Vn becomes limited by a low koff. In mechanism III, monoclonal antibody MA-33B8 accelerates the transition of active PAI-1 or its complex with Vn to inactive PAI-1 (PAI-1lat).
Despite the fact that a number of low-molecular weight (LMW) inhibitors of PAI-1 are available, monoclonal antibodies (mAbs) remain the most effective and specific modulators for PAI-1 targeting (22). mAb-mediated redirection of the PAI-1 reaction to the substrate branch was effective both in vitro (43–45, 51) and in vivo in TCN-induced pleural injury (25). However, the effects of other well-established mechanisms of targeting PAI-1 (Fig. 1, mechanisms I–III) on IPFT with scuPA in this model remain unknown. Thus the focus of the present study was to compare the effects of two major approaches (Fig. 1) to affect the interactions between PAI-1 bound to endogenous vitronectin (Vn; 66, 78, 86) and uPA on the outcomes of IPFT with scuPA in TCN-induced pleural injury. These include 1) decreasing the rate of formation of the stabilized acyl-enzyme without affecting the stoichiometry of the reaction (Fig. 1, mechanisms I and II) and 2) mAb-mediated PAI-1 inactivation (Fig. 1, mechanism III). The rate of the reaction between PAI-1 and target proteinase was decreased by mutating the “PAI-1 docking site” (DS; 4, 75, 89), positively charged residues of 37-loop of uPA, which forms exosite interactions (17, 57) with PAI-1 (Fig. 1, mechanism I), and by using ligands that compete for active PAI-1 with uPA (Fig. 1, mechanism II; 10, 26). The latter bind active PAI-1 with high affinity, forming nonproductive “molecular sandwich”-type complexes with low first-order rate constant of dissociation of PAI-1/two-chain urokinase with Ser195Ala substitution (S195A-tcuPA) or MA-56A7C10 (koff; Fig. 1, mechanism II; 26). Finally, mAb-mediated acceleration of the active-to-latent transition (Fig. 1, mechanism III; 21, 84) was used to test the effect of PAI-1 inactivation on IPFT outcomes. Intrapleural PAI-1 inactivation, but not a decrease in the rate of the reaction between PAI-1 and uPA, improved the efficacy of IPFT in a manner similar to that observed for the redirection of the reaction to the substrate branch (25). These results clarify the contribution of active PAI-1 to the success or failure of IPFT in TCN-induced pleural injury in rabbits and identify the role of rate of fibrinolysis as one of the key factors in the selection of an appropriate mechanism to target PAI-1 in vivo in diverse settings.
EXPERIMENTAL PROCEDURES
Proteins and reagents.
Recombinant scuPA was a gift from Abbott Laboratories (Chicago, IL). Monomeric human Vn and wild-type (wt) recombinant PAI-1 were from Molecular Innovations (Novi, MI). Mouse mAbs MA-56A7C10 and MA-33B8 were selected from a panel raised against the human PAI-1/tPA complex (21). A mutant variant of scuPA (179RHRGGS184→179AAAAAA184; ΔDS-scuPA) was produced as described elsewhere (89). Active standard tcuPA (100,000 IU/mg) was from American Diagnostica (Stamford, CT). Fluorogenic uPA substrate was from Centerchem (Norwalk, CT); plasmin substrate and Glu-plasminogen were from Haematologic Technologies (Essex Junction, VT). A 0.05 M phosphate (pH 7.4) buffer with or without bovine serum albumin (BSA; 1 mg/ml) and/or NaCl (20 mM) were used to perform all in vitro and ex vivo experiments. uPA amidolytic and plasminogen-activating activity was determined using Synergy HT Hybrid Reader (BioTek, Winooski, VT) or Varian Cary Eclipse fluorescence spectrophotometer (Varian, Lincolnshire, IL) as previously described (46, 49). Enzymes wt-uPA, ΔDS-uPA, and plasmin with known concentration were used as standards.
The quality of wild-type and ΔDS-prourokinase preparations used for both in vitro and in vivo experiments was independently confirmed by measuring the fraction of the active enzyme based on its ability to form an inhibitory complex with PAI-1. First, wt- and ΔDS-scuPA were activated by immobilized plasmin (Molecular Innovations) to a two-chain mature form, as previously described (52). Samples of two-chain enzymes (10–25 µg) were mixed with 2–2.5-fold molar excess of human recombinant PAI-1 (Molecular Innovations) in Dulbecco’s phosphate-buffered saline (DPBS) and incubated for 1–2 min at room temperature. SDS-PAGE loading buffer was added to the reaction mixtures, and samples were incubated at 100°C for 2 min. Products of the reaction between an enzyme and PAI-1 were subjected to 4–12% gradient SDS-PAGE (NuPage; Invitrogen by Thermo Fisher Scientific) under nonreducing conditions. Molecular weight markers (Precision Plus Protein Standards, All Blue; Bio-Rad, Hercules, CA) were used to identify the position of the proteins and their complexes. Gels were stained with SimplyBlue SafeStain (Novex by Life Technologies, Carlsbad, CA), per the manufacturer’s protocol. The relative amounts of active (complexed with PAI-1) and inactive wt- and ΔDS-uPA were estimated from the intensity of corresponding bands on gel scans using Bio-Rad GelDoc (data not shown). The molar fraction of active enzymes in all preparations used for this study was determined to be >0.95.
Animal model.
The animal protocol was approved by the Institutional Animal Care and Use Committee at The University of Texas Health Science Center at Tyler. New Zealand, white, pathogen-free, female rabbits (weight, 3.0–3.6 kg) acquired from Charles River Laboratories (Wilmington, MA) were used in this study. The TCN-induced pleural injury model was implemented as previously described, and injury development was monitored via ultrasonography (36, 37, 48). After 48 h, rabbits with pleural injury were treated intrapleurally with scuPA (with or without adjunct), ΔDS-scuPA, or a vehicle/adjunct control (36, 46, 48) using an 18-gauge, 1.25-in.-long Excel Safelet catheter, which was cleared with 0.5 ml of PBS. Aliquots (0.5 ml) of pleural fluid were collected at 10, 20, and 40 min after IPFT. Anesthesia, postoperative pain medication, and animal care were provided as previously reported (42, 48). Rabbits were carefully monitored for signs of pain, discomfort, or distress to ensure animal stability and well-being. Euthanasia was accomplished by intravenous injection of 0.5 ml/kg of pentobarbital sodium and phenytoin solution followed by exsanguination.
Doses of fibrinolysins and adjuncts used in IPFT.
The doses of the fibrinolysin (ΔDS-scuPA) and adjuncts (MA-56A7C10 and MA-33B8) were chosen on the basis of previous studies of IPFT with wt-scuPA alone and in combination with PAI-1-neutralizing mAbs (25, 46). Doses selected for ΔDS-scuPA, 0.5 and 0.25 mg/kg, are equal to the minimal effective and maximal ineffective doses of scuPA alone (36, 46). IPFT with these doses of ΔDS-scuPA allows for the comparison of efficacy between wt- and ΔDS-scuPA. The 0.0625 mg/kg dose corresponds to an ineffective dose of wt-scuPA, which is rendered effective in the presence of two anti-PAI-1 mAbs (0.5 mg/kg; 25). IPFT with this dose of ΔDS-scuPA allowed us to answer the question of whether or not the efficacy of mutant scuPA alone is comparable to that of the combination of wt-scuPA-mAbs. We selected an identical dose (0.5 mg/kg) for MA-56A7C10 and MA-33B8 combined with the maximal ineffective dose of scuPA (0.25 mg/kg) to test whether or not these PAI-1 modulators affect the outcome of IPFT. The initial dose (0.1 mg/kg) of S195A-tcuPA was selected to determine how the adjunct affects IPFT outcomes with different doses of scuPA. Then, a 0.5 mg/kg dose was selected to verify our findings and compare the observed effects with those of other modulators of PAI-1 activity.
Metrics of pleural injury.
Gross lung injury score (GLIS; 25, 48) was determined for each animal during necropsy by counting the number of fibrin strands or webs linking the visceral and parietal pleura and fibrin aggregates. The GLIS was defined as follows: 1 = for each discrete individual fibrin strand, 2 = for each discrete aggregate of <5-mm size; 5 = for each web or large aggregate (>5 mm), 50 = strands too numerous to count, associated with multiple interconnected intrapleural webs including visceral-parietal sheets of fibrin (25, 42, 46, 48). IPFT was considered successful at a GLIS ≤ 10. Pleural injury was also documented by photographing rabbit hemithoraces using a Nikon D300 camera (Nikkor 18–200-mm VR lens) after pleural fluids collection (48).
PAI-1 activity assay.
Levels of active rabbit PAI-1 in the pleural fluids were determined either by titrating active inhibitor with solutions of uPA of a known concentration, as previously described (50), or by using a commercially available ELISA (Molecular Innovations) following the manufacturer’s protocol.
Measurement of uPA amidolytic and plasminogen-activating activity in pleural fluids of animals treated with IPFT.
Amidolytic uPA activity in samples of pleural fluid was measured from time traces of the change in fluorescence emission at 440 nm (excitation 344 nm) of fluorogenic uPA substrate Pefafluor uPA Bz-α-Ala-Gly-Arg-AMC AcOH (where AMC and AcOH are 7-amino-4-methylcoumarin and acetate, respectively) from Centerchem. The substrate concentration was 0.1 mM in DPBS with BSA (1 mg/ml) as previously described (50). The plasminogen-activating activity was measured by determining the accumulation of the product (plasmin) with time, by incubating pleural fluids containing uPA with human Glu-plasminogen (100–200 nM; Haematologic Technologies) as previously described (42, 49, 50). Plasmin activity was determined from the parabolic dependence of an increase in the fluorescence emission at 470 nm (F470; excitation at 352 nm) on time t. The slopes of the linear dependence of the initial increase in the fluorescence emission on t2 (24, 33, 34, 60, 88) were used to calculate rates of plasmin generation. Fluorogenic [6-amino-1-naphthalenesulfonamide (ANS)-based] substrate d-Ala-Phe-Lys-ANS-NH-iC4H9·2HBr [Haematologic Technologies; 0.2 mM in DPBS buffer with BSA (1 mg/ml)] was used. Volumes of 5–0.625 or 1–0.03 µl of pleural fluid were diluted in 100 µl of the reaction mixture to measure the amidolytic and plasminogen-activating activities of uPA. Enzymatic activities were expressed as nanomoles per liter of pleural fluid based on comparison with corresponding activities of solutions of tcuPA (Secusui Diagnostics, Stamford, CT), a standard of known activity. Plasmin and uPA amidolytic activities were measured in either black or white 96-well flat-bottom plates from Costar (Corning, NY) using a Synergy HT Hybrid Reader (BioTek) or Varian Cary Eclipse fluorescence spectrophotometer, respectively.
Immunoprecipitation of active PAI-1 complexed with S195A-tcuPA or with MA-56A7C10 using magnetic beads.
Magnetic beads (Dynabeads M-280 sheep anti-mouse IgG; Invitrogen by Thermo Fisher Scientific), were used for immunoprecipitation (IP) of complexes of active PAI-1 with MA-56A7C10 or with S195A-tcuPA from pleural fluids (n = 2 each) of animals treated with either MA-56A7C10 and scuPA (0.5 and 0.25 mg/kg, respectively) or with S195A-tcuPA (0.5 mg/kg). The pleural fluids of animals treated with mouse IgG (0.5 mg/kg) and vehicle (DPBS) were used as controls in these experiments. Volumes of 100–200 µl of pleural fluids collected at the end of the experiment (24 h after IPFT) were incubated with washed magnetic beads for 2 h on ice. Anti-human uPA mAb (4–8 µg; Molecular Innovations) was added to the pleural fluid of animals treated with S195A-tcuPA and the vehicle control 10 min before the addition of the magnetic beads. After incubation, beads were washed three times with 600 µl of cold DPBS and subsequently resuspended in 20–40 µl of cold DPBS. PAI-1 activity was measured by incubating an aliquot (5–10 µl) of the bead slurry with 0.2 nM uPA and fluorogenic substrate in DPBS with BSA (1 mg/ml). Amidolytic activity of uPA was measured with the fluorogenic substrate, as described in experimental procedures and elsewhere (42). Relative levels of active PAI-1 bound to the resin were estimated from a decrease of the uPA activity. The levels of active PAI-1 complexed with MA-56A7C10 or S195A-tcuPA (an average of 2 independent experiments) in the pleural fluid were expressed in arbitrary units.
Visualization of endogenous PAI-1 coprecipitated with MA-56A7C10 and S195A-tcuPA using Western blot analysis.
Following incubation with pleural fluids, SDS-PAGE loading buffer was added to an aliquot of magnetic bead slurry. The slurry was incubated at 100°C for 2 min and subjected to 4–12% gradient SDS-PAGE (NuPage; Invitrogen by Thermo Fisher Scientific) under nonreducing conditions. Positions of molecular weight markers (Precision Plus Protein Standards, All Blue; Bio-Rad) are shown to the right of the gel. Proteins were transferred to a polyvinylidene difluoride membrane (Trans-Blot Turbo RTA Transfer Kit, Bio-Rad). Membranes were blocked with 5% nonfat milk and incubated overnight (4°C) with goat anti-rabbit polyclonal antibody (Molecular Innovations). Goat anti-rabbit PAI-1 was detected using donkey anti-goat IgG conjugated with horseradish peroxidase (HRP; Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:5,000 dilution. Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA) was used to develop membranes, as per the manufacturer’s protocol. A ChemiDoc XRS+ Molecular Imager and Image Lab software (Bio-Rad) were used to develop and process images.
Measurements of fibrinolytic activity in the pleural fluids.
A fluorescein isothiocyanate (FITC)-labeled fibrin film in a fluorescence quench and dequench assay (25, 46, 49) was used to evaluate the fibrinolytic activity in pleural fluids. Briefly, FITC-fibrinogen (Molecular Innovations) was mixed in a 1:1 molar ratio with unlabeled fibrinogen (Molecular Innovations) at a final concentration of 0.4 mg/ml in 0.05 M HEPES/NaOH (pH 7.4) buffer with addition of 20 mM NaCl and 5 mM CaCl2. To obtain FITC-fibrin film at the bottom of 96-well plates, 50 µl of the solution were incubated with 10 nM of thrombin (Haematologic Technologies), at room temperature for 60–90 min. Polymerization of fibrinogen was monitored by a decrease in the FITC fluorescence emission with time using a Varian Cary Eclipse fluorescence spectrophotometer. The 96-well plates were dried overnight, protected from direct light, washed 3 times with 0.3 ml of HEPES/NaOH buffer, and then either used immediately or stored at −20°C. Fibrinolytic activity in pleural fluids (2–5 µl/100 µl) was determined by monitoring the time-dependent increase in the fluorescence emission of fluorescein due to dequenching, as described previously (25, 46, 49).
Kinetics of intrapleural ΔDS-uPA inactivation and α-macroglobulin/ΔDS-uPA complex formation.
The observed first-order rate constants for intrapleural inactivation of ΔDS-uPA (kobs) and accumulation of α-macroglobulin (αM)/ΔDS-uPA complex (kapp) were determined from measurements of amidolytic activity, as previously described (25, 46). The amidolytic uPA activity in samples of pleural fluids was measured before and after supplementation with a 20–50-fold excess of exogenous wild-type human recombinant PAI-1, corresponding to the activities of total ΔDS-uPA and αM/ΔDS-uPA, respectively. Both uPA and ΔDS-uPA, when complexed with αM, retain amidolytic activity toward LMW substrates (46, 50). Thus, to determine kobs, a single exponential equation was fit to the time dependence of concentration of active free ΔDS-uPA ([free ΔDS-uPA] = [total ΔDS-uPA] − [αM/ΔDS-uPA]). To determine kapp, a single exponential equation was fit to the time dependence of [αM/ΔDS-uPA]. SigmaPlot 12.0 for Windows (SPSS) was used to determine kobs and kapp as described previously (25, 46). The values of kobs for intrapleural inactivation of ΔDS-uPA were also determined from a decrease in intrapleural PA activity with time as previously described (46, 49).
Data analysis and statistics.
The levels of statistical significance were estimated by Kruskal-Wallis one-way analysis of variance on ranks and pairwise multiple-comparison procedures (Holm-Sidak method and Tukey’s test) as previously described (25, 48). A correlation coefficient (r) and visual analysis of the plots of the residuals were used to estimate the quality of the fit in kinetic measurements. To calculate the second-order association rate constant for the reaction of PAI-1 with proteinase (kass) and kapp, plots were fit by linear or single exponential equations, respectively, using nonlinear least squares fitting with the Levenberg-Marquardt algorithm (25, 26, 46, 48).
RESULTS
ΔDS- and wt-scuPA are equally effective in fibrinolytic therapy of tetracycline-induced pleural injury.
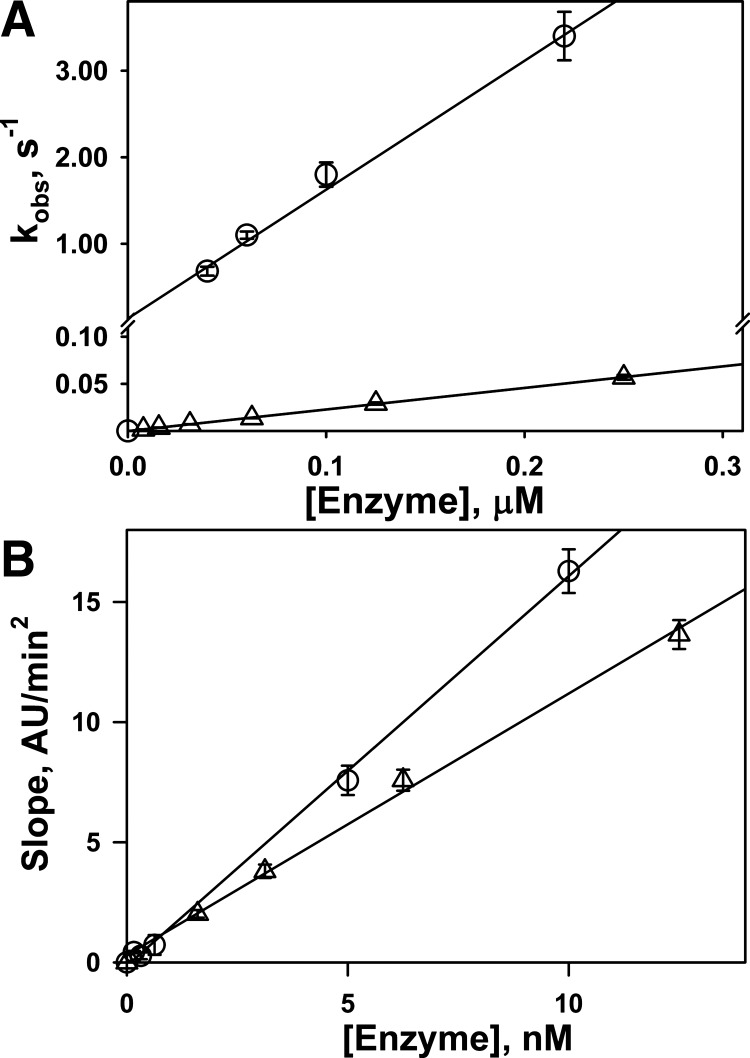
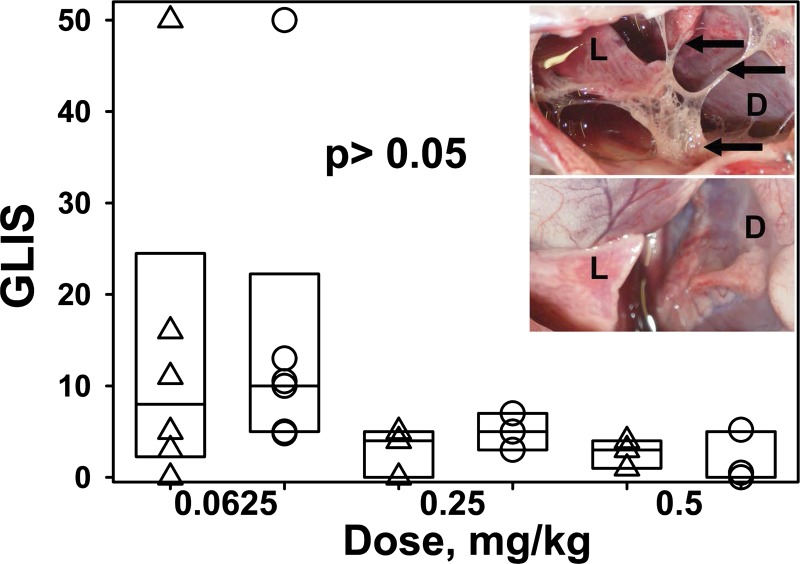
Although there are two mechanisms (Fig. 1, mechanisms I and II) that decrease the rate of the reaction between fibrinolysin and PAI-1, rational engineering of plasminogen activator (Fig. 1, mechanism I) is preferable, since it eliminates the necessity of adjuncts. A mutant variant of scuPA (ΔDS-scuPA) with an alanine substitution of the positively charged residues of the 37-loop (VR1 region) 179RHRGGS184→179AAAAAA184 (89) was used to determine the effect of decreasing the rate of the reaction with serpin on IPFT outcomes. The second-order association rate constant (kass) for the reaction between ΔDS-tcuPA and PAI-1 was decreased by >60-fold (Fig. 2A) compared with wt-tcuPA. In contrast, the plasminogen-activating (Fig. 2B) and amidolytic (not shown) activities of ΔDS-tcuPA were only 1.5–2.5-fold less than those of wild-type enzyme. Similar to wt-uPA, the reaction between ΔDS-tcuPA and PAI-1 follows the inhibitory branch of the mechanism (Fig. 1, ki) and results in inactivation of the enzyme due to the formation of a stabilized acyl-enzyme with a stoichiometry close to unity (not shown). To determine the effect of decreasing the rate of the reaction on IPFT outcomes, three doses of ΔDS-scuPA that differed by nearly an order of magnitude (0.5, n = 3; 0.25, n = 3; and 0.0625 mg/kg, n = 6) were used to treat animals with TCN-induced pleural injury. In control experiments, rabbits with TCN-induced pleural injury were treated with the same doses of wt-scuPA (n = 3, 3, and 6, respectively). The doses selected for studies of ΔDS-scuPA were equal to the minimal effective, maximal ineffective, and a low ineffective dose, respectively, identified for wt-scuPA in previous studies (25, 36, 46). Animals from mixed (experiments and controls) groups (n = 6–8) were treated with a bolus intrapleural injection of the ΔDS- or wt-scuPA at 72 h after induction of pleural injury, as previously described (25, 42, 46, 48). Samples of pleural fluid (0.1–0.5 ml) were withdrawn for further analysis just before IPFT and 10, 20, and 40 min as well as at 24 h after IPFT. IPFT outcomes were assessed at 24 h using GLIS (42, 46), and data obtained for ΔDS- and wt-scuPA groups were compared (Fig. 3). Despite a trend toward decreased GLIS with ΔDS-scuPA (Fig. 3), there was no statistically significant difference (P > 0.05) between animals treated with wt- or ΔDS-scuPA; both proenzymes were equally effective for treatment of TCN-induced pleural injury in rabbits. Moreover, combining the results for all three doses, shown in Fig. 3 (n = 12 for each ΔDS- and wt-scuPA), did not reveal a statistically significant difference between the efficacy of ΔDS-scuPA and wt-scuPA (P > 0.05; not shown). Thus a 60-fold decrease in kass for the reaction with PAI-1 due to 179RHRGGS184→179AAAAAA184 mutation (Fig. 1, mechanism I) did not result in a statistically significant increase in the efficacy of IPFT.
Fig. 2.
Effects of a 179RHRGGS184→179AAAAAA184 urokinase mutant (ΔDS-uPA) on the rate of interaction with plasminogen activator inhibitor-1 (PAI-1) and activation of Glu-plasminogen. A: dependence of the observed first-order rate constants (kobs) for the interaction of PAI-1 with wild-type urokinase (wt-uPA; ○) and ΔDS-uPA (△) on enzyme concentration. The data for wt- and ΔDS-uPA are shown in different scales. The values of kobs were determined as previously described (45, 49, 51). Linear equations were fit (solid lines; r2 > 0.95) to the data, and values of the second-order association rate constant (kass) were determined from the slopes. The ratio of kass for wt-uPA over ΔDS-uPA was 63.0 for inhibition by PAI-1. B: dependence of the rates of accumulation of plasmin due to activation of Glu-plasminogen by wt-uPA (○) and ΔDS-uPA (△) on enzyme concentration. The rates of plasmin accumulation were determined from the slopes of linear equations, which were fit (solid lines; r2 > 0.99) to the data as described previously (49). The ratio of slopes for wt-uPA over ΔDS-uPA for activation of Glu-plasminogen was 1.5. AU, arbitrary units.
Fig. 3.
Prourokinase with 179RHRGGS184→179AAAAAA184 substitutions (ΔDS-scuPA) and wild-type prourokinase (wt-scuPA) are equally effective in intrapleural fibrinolytic therapy (IPFT) of tetracycline (TCN)-induced pleural injury in rabbits. The efficacy of IPFT was determined by gross lung injury score (GLIS; 25, 48), in the treatment of TCN-induced pleural injury with (from left to right) 0.0625 (n = 6), 0.25 (n = 3), and 0.5 (n = 3) mg/kg ΔDS-scuPA (△) and wt-scuPA (○). IPFT was considered successful with a GLIS ≤ 10. Animals were euthanized 24 h after initiating IPFT (72 h after TCN-induced pleural injury). Inset: photographic images of the pleural space taken at 24 h after unsuccessful (top; GLIS = 16) and successful (bottom; GLIS = 0) IPFT with the same dose of ΔDS-scuPA (0.0625 mg/kg). L, lung; D, diaphragm; arrows in the image at top indicate fibrin net and strands. The data are presented as box plots (showing interquartile ranges). There was no statistically significant difference (P > 0.05) in treatment outcomes (GLIS) between ΔDS-scuPA and wt-scuPA at the same dose.
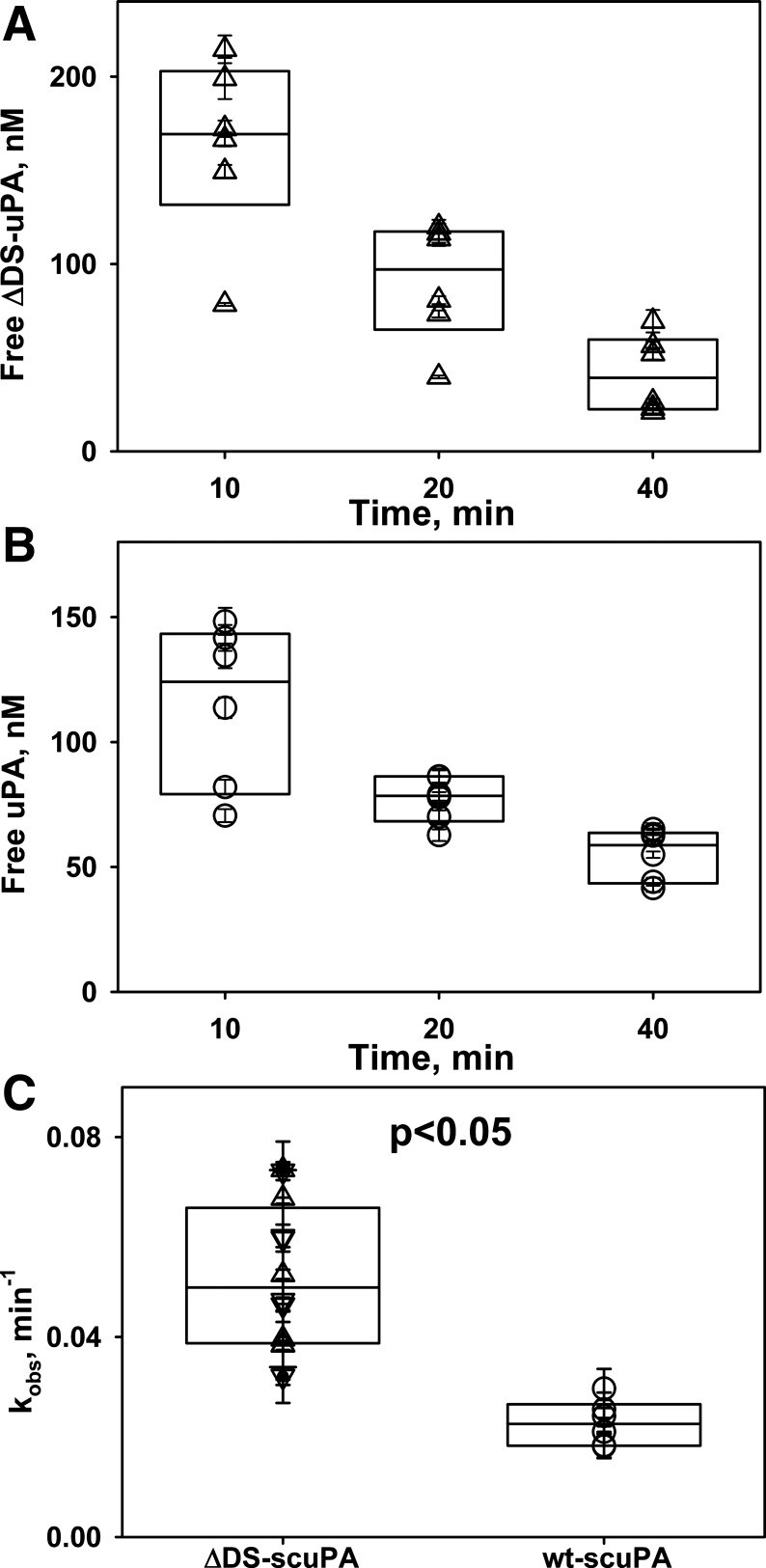
Intrapleural ΔDS-uPA forms more αM complexes than wild-type enzyme but loses activity twice as fast as wild-type enzyme.
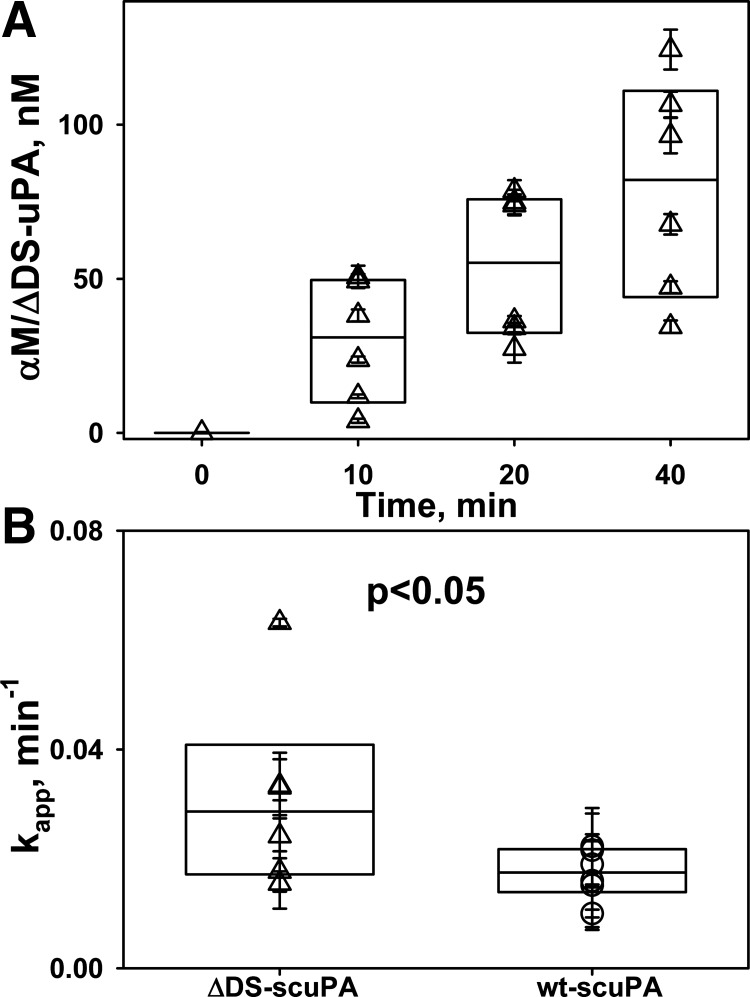
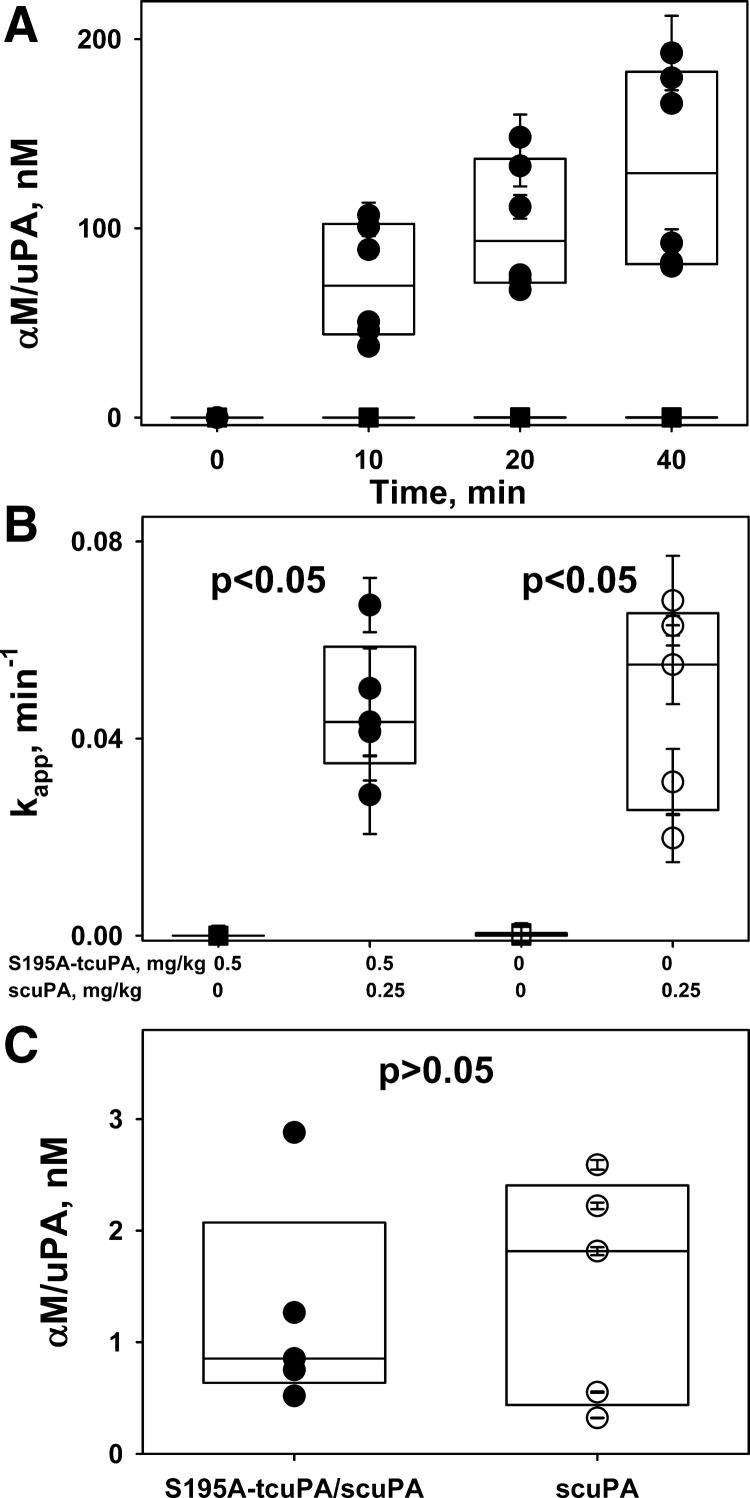
Intrapleural processing of a fibrinolysin and its in vivo half-life critically affect the results of IPFT (46, 48). Although the mechanisms of intrapleural inactivation of ΔDS-uPA were unknown, we posit that the overall processing of ΔDS-uPA is similar to that of the wild-type enzyme. To determine possible differences in the intrapleural processing of ΔDS- and wt-scuPA, which could affect their efficacy in IPFT, aliquots of pleural fluid were analyzed. Similar to the wild-type enzyme, ΔDS-uPA forms intrapleural “molecular cage”-type complexes with αM, where the enzyme is active toward LMW amidolytic substrates but is protected from interaction with high-molecular weight ligands such as PAI-1 (Fig. 5A). Thus, similar to wt-uPA (25, 46), total ΔDS-uPA activity during IPFT can be represented as the sum of αM/ΔDS-uPA complexes (resistant to inhibition by PAI-1; Fig. 5A) and free ΔDS-uPA, which possess plasminogen-activating activity and are prone to inhibition by PAI-1. The latter contributes the most to intrapleural plasminogen activation. Changes in the amidolytic and plasminogen-activating activities and intrapleural levels of molecular cage-type αM/ΔDS-uPA and αM/wt-uPA complexes with time were determined and analyzed as previously described (46, 48). These data were used to evaluate rates of PAI-1-independent intrapleural inactivation of ΔDS-uPA (Fig. 4A) and the kinetics of in vivo formation of αM/ΔDS-uPA (Fig. 5A) and to compare the intrapleural processing of ΔDS-uPA with that of the wild-type enzyme (Figs. 4, B and C, and 5B). Although ΔDS-tcuPA interacts with PAI-1 more slowly than wild-type enzyme (Fig. 2A), the rate constant of PAI-1-independent inactivation of ΔDS-uPA (kobs) was almost twofold higher (P < 0.05) than that for wt-scuPA alone (Fig. 4, B and C). Intrapleural αM/ΔDS-uPA complexes are easily detected starting at 10 min after IPFT (Fig. 5A). The first-order rate constant of αM/ΔDS-uPA complex formation (Fig. 5B, kapp) and maximal level of intrapleural accumulation of αM/ΔDS-uPA (not shown) observed during treatment with 0.0625 mg/kg of ΔDS-scuPA were 1.5–2-fold higher than those for wt-scuPA at the same dose. Thus a decrease in the rate of the reaction between uPA and PAI-1 due to the mutation at the 37-loop (Fig. 1, mechanism I) increases the rate of PAI-1-independent intrapleural inactivation of ΔDS-uPA, and the fraction of the active enzyme in complexes with αM, without a statistically significant increase in the efficacy of IPFT compared with wt-scuPA.
Fig. 5.
Accumulation of intrapleural α-macroglobulin (αM)/urokinase with 179RHRGGS184→179AAAAAA184 substitutions (ΔDS-uPA) “molecular cage” complexes during intrapleural fibrinolytic therapy (IPFT). A: time dependence of the formation of intrapleural αM/ΔDS-uPA during IPFT with ΔDS-scuPA (0.0625 mg/kg). ΔDS-uPA amidolytic activity was measured after samples of pleural fluid withdrawn at 0–40 min were supplemented with an excess (100–200 nM) of exogenous recombinant human plasminogen activator inhibitor-1 (PAI-1) to inhibit free enzyme. PAI-1-resistant ΔDS-uPA amidolytic activity, which represents intrapleural ΔDS-uPA in “molecular cage” complexes with αM (46, 50), was converted to concentrations (nM) and plotted against time. A single exponential equation was fit to the dependence of the concentration of αM/ΔDS-uPA (A) or αM/uPA (not shown) on time to obtain the values of the apparent first-order rate constants (kapp; B) as previously described (46).
Fig. 4.
Intrapleural plasminogen activator inhibitor-1 (PAI-1)-independent inactivation of free urokinase with 179RHRGGS184→179AAAAAA184 substitutions (ΔDS-uPA) is twofold faster than wild-type urokinase (wt-uPA). A: time dependence of the amidolytic activity of intrapleural free uPA (△) during intrapleural fibrinolytic therapy (IPFT) with ΔDS-prourokinase (ΔDS-scuPA; 0.0625 mg/kg). Briefly, the total ΔDS-uPA amidolytic activity was measured in samples of pleural fluid withdrawn at 10–40 min after IPFT. ΔDS-uPA activity that is resistant to an excess of exogenous human recombinant PAI-1 [represents α-macroglobulin (αM)/ΔDS-uPA complexes] was subtracted from total amidolytic activity to estimate the level of free ΔDS-uPA in the sample ([free ΔDS-uPA] = [total ΔDS-uPA] − [αM/ΔDS-uPA]). B: time dependence of free (not complexed) intrapleural uPA amidolytic activity (○) during IPFT with wt-scuPA (0.0625 mg/kg). Amidolytic activity of free uPA was the difference between total uPA activity and activity of αM/uPA complexes. C: observed first-order rate constants (kobs) for the intrapleural inactivation of free ΔDS- and wt-uPA. Values of kobs are given for loss of intrapleural amidolytic (▽) and plasminogen-activating (△) activities of ΔDS-uPA, as well as the plasminogen-activating activity of wt-uPA (○) during IPFT with 0.0625 mg/kg (n = 6). Values of kobs were estimated from the changes in activity with respect to time, as described previously (42, 49). A single exponential equation was fit to the dependence of [free enzyme] on time to obtain kobs of PAI-1-independent inactivation of ΔDS- and wt-uPA as previously described (46). The rate of intrapleural inactivation of ΔDS-uPA was statistically (P < 0.05) higher than that for wt-uPA. There was no statistically significant difference (P > 0.05) between the kobs of ΔDS-uPA inactivation estimated from measurements of amidolytic and Glu-plasminogen-activating activities.
Effect of S195A-tcuPA on outcomes of IPFT with wt-scuPA.
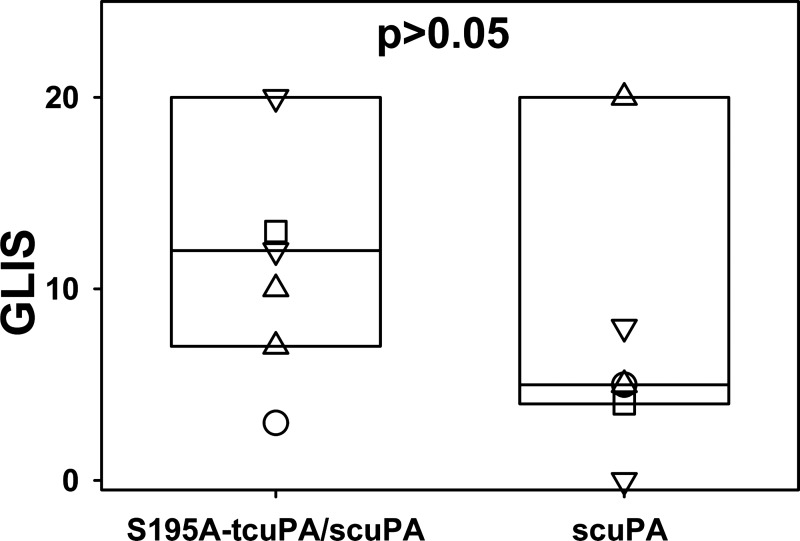
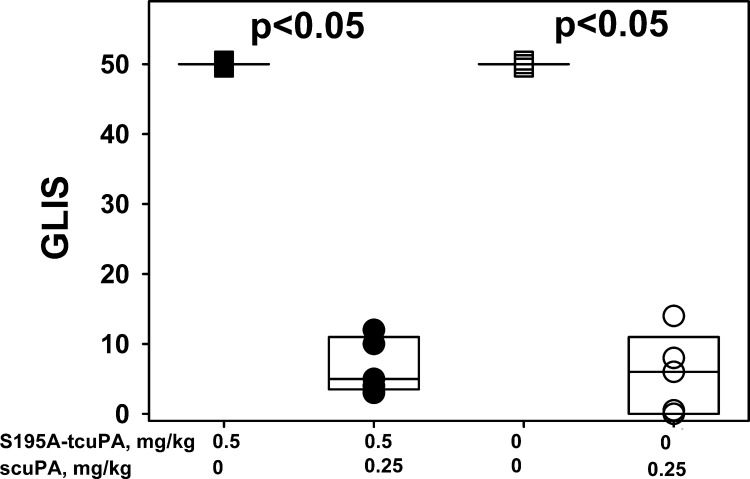
S195A-tcuPA, an enzymatically inactive mutant variant with alanine substitution of a serine residue of the catalytic triad [Schechter and Berger nomenclature (71)], binds active PAI-1 and its complex with Vn with nanomolar affinity, forming nonproductive tertiary molecular sandwich-type complex S195A-tcuPA/PAI-1/Vn (26) and thus competing for uPA with productive tertiary Michaelis complexes [dissociation constant (Kd) << Km, Fig. 1, mechanism II]. The reaction between S195A-tcuPA/PAI-1/Vn and uPA follows the inhibitory branch of the PAI-1 mechanism (Fig. 1, ki), with the rate of the reaction limited by the slow dissociation of S195A-tcuPA (Fig. 1, mechanism I, koff; 26). First, we have studied the effect of S195A-tcuPA on the outcome of IPFT in an experiment, when two groups of animals (n = 6 each) were treated with a variety of scuPA dosages (0.5, 0.25, 0.125, and 0.0625 mg/kg; n = 1, 2, 2, and 1, respectively) with or without S195A-tcuPA (0.1 mg/kg). IPFT with S195A-tcuPA alone and with the vehicle (PBS) were used as controls. GLIS values were calculated as described in experimental procedures and elsewhere (25, 42, 46, 48), and the data for both groups (scuPA alone and scuPA with 0.1 mg/kg of S195A-tcuPA) were expressed as a box plot (Fig. 6). In contrast to the effect of 179RHRGGS184→179AAAAAA184 mutation, which resulted in a trend toward improved IPFT (Fig. 3), the presence of S195A-tcuPA caused the opposite effect, increasing the GLIS (Fig. 6). Thus alternative mechanisms, different from scavenging active PAI-1 to a ternary S195A-tcuPA/PAI-1/Vn complex with a low koff (Fig. 1, mechanism II), also contribute to the outcome of IPFT in the presence of S195A-tcuPA. Next, to determine how competition with uPA for PAI-1 affects the results of IPFT, S195A-tcuPA (0.5 mg/kg) was used as an adjunct to a maximal ineffective dose of scuPA (0.25 mg/kg), as previously described (25). In control experiments, animals with TCN-induced pleural injury were treated with 0.25 mg/kg scuPA alone (n = 5), with 0.5 mg/kg S195A-tcuPA alone (n = 3), or with vehicle (PBS; n = 3). Aliquots of pleural fluid were collected for further analysis at 0, 10, 20, and 40 min and at 24 h after IPFT. IPFT outcomes were assessed at 24 h using GLIS, shown as a box plot in Fig. 7. There was no statistical difference (P > 0.05) between IPFT outcomes for TCN-injured animals treated with scuPA alone or in the presence of S195A-tcuPA (Fig. 7). In control experiments, treatment with S195A-tcuPA alone (0.5 mg/kg; n = 3) failed to improve the outcome of TCN-induced pleural injury at 24 h (Fig. 7); control animals, treated with either S195A-tcuPA or the vehicle (n = 3), had a GLIS of 50 (the maximal score for ineffective IPFT). Thus S195A-tcuPA alone or in combination with scuPA (Fig. 1, mechanism II) did not improve the severity of pleural injury or the outcomes of IPFT (Fig. 7; P > 0.05) in TCN-induced pleural injury in rabbits.
Fig. 6.
Addition of two-chain urokinase with Ser195Ala substitution (S195A-tcuPA; 0.1 mg/kg) to a bolus dose of prourokinase (scuPA; 0.0625–0.5 mg/kg) does not improve intrapleural fibrinolytic therapy (IPFT) outcomes in tetracycline (TCN)-induced pleural injury in rabbits. IPFT outcomes [expressed as gross lung injury score (GLIS) values (25, 48)] are given for two groups of animals (n = 6 animals per group) treated with scuPA [0.0625 (□), 0.125 (▽), 0.25 (△), and 0.5 (○) mg/kg] alone (box at left) or together with 0.1 mg/kg S195A-tcuPA (n = 1, 2, 2, and 1, respectively). IPFT was considered successful with a GLIS ≤ 10. The data are presented as a box plot (showing interquartile ranges). There was no statistically significant difference between these two groups (P > 0.05).
Fig. 7.
Two-chain urokinase with Ser195Ala substitution (S195A-tcuPA; 0.5 mg/kg) does not improve the outcomes of intrapleural fibrinolytic therapy (IPFT) with prourokinase (scuPA; 0.25 mg/kg). IPFT outcomes [expressed by gross lung injury score (GLIS; 25, 48)] in the treatment of tetracycline (TCN)-induced pleural injury with (from left to right) 0.5 mg/kg S195A-tcuPA alone (■; n = 3), S195A-tcuPA (0.5 mg/kg) and 0.25 mg/kg scuPA (●; n = 5), vehicle control (PBS; □; n = 3), and scuPA (0.25 mg/kg) alone (○; n = 5) are given. IPFT was considered successful with a GLIS ≤ 10. The data are presented as a box plot (showing interquartile ranges). There was no statistically significant difference between treatments with the scuPA alone and in combination with 0.5 mg/kg of S195A-tcuPA (P > 0.05). The differences in the median values between groups with and without scuPA were statistically significant (P < 0.05).
Presence of S195A-tcuPA did not affect intrapleural processing of scuPA.
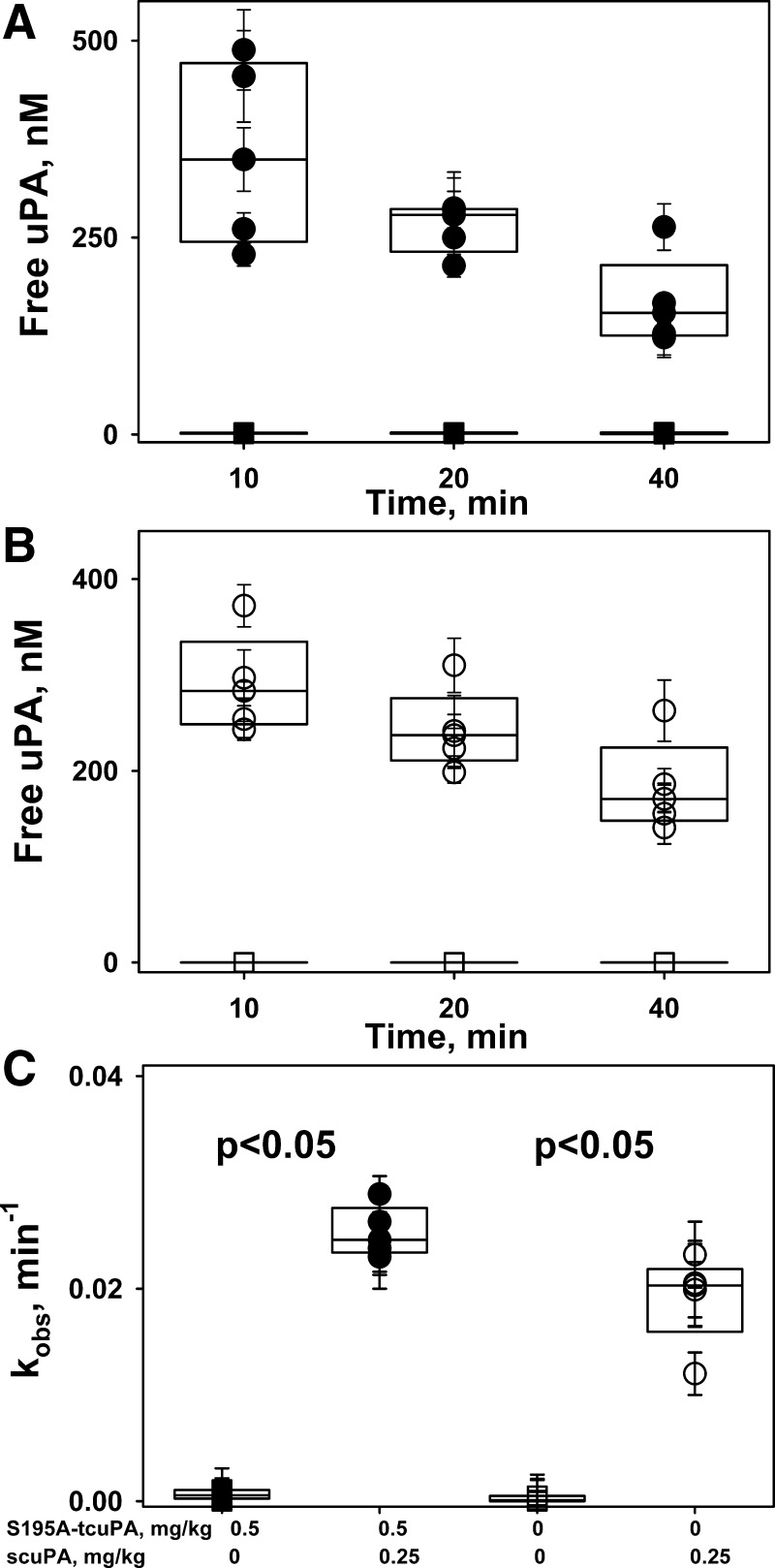
To determine the effects of S195A-tcuPA on the intrapleural processing of scuPA, aliquots of pleural fluid collected during IPFT were analyzed for amidolytic and plasminogen-activating activities and for levels of intrapleural αM/uPA complexes as previously described (46, 48). The rate constant (kobs) of PAI-1-independent intrapleural inactivation of uPA in the presence of 0.5 mg/kg of S195A-tcuPA (Fig. 8A) was similar to that observed for IPFT with scuPA alone (Fig. 8B) and twofold slower (P < 0.05) than that for IPFT with ΔDS-scuPA (Fig. 4C). Thus the similarity between kobs for IPFT with scuPA alone and with S195A-tcuPA (Fig. 8; 46) indicates that competition between wild-type enzyme and its inactive mutant variant for PAI-1 neither protects fibrinolysin from inactivation nor improves IPFT outcomes (P > 0.05). In control experiments (Fig. 8A), S195A-tcuPA alone (0.5 mg/kg; n = 3) failed to protect endogenous plasminogen activators, indicating that IPFT with exogenous fibrinolysin is required to resolve TCN-induced pleural injury in rabbits. Analysis of samples of pleural fluid withdrawn during IPFT with 0.25 mg/kg scuPA with S195A-tcuPA (0.5 mg/kg; Fig. 9A) for amidolytic activity in the presence of an excess of exogenous PAI-1 revealed the formation of αM/uPA complexes, similar to controls with scuPA alone (not shown). No αM/uPA complexes were detected in samples from animals treated with S195A-tcuPA (0.5 mg/kg) alone (Fig. 9A), indicating that this inactive mutant variant of uPA does not promote the interaction of endogenous uPA with αM. Neither the rate constant for αM/uPA formation (kapp; Fig. 9B) nor the maximal level of αM/uPA (not shown) were statistically different (P > 0.05) from samples collected from the control group treated with scuPA alone. Although there was a trend toward decreasing intrapleural concentrations of αM/uPA complexes at 24 h after IPFT with S195A-tcuPA (Fig. 9C), this difference was also not statistically significant (P > 0.05).
Fig. 8.
Two-chain urokinase with Ser195Ala substitution (S195A-tcuPA; 0.5 mg/kg) does not affect the rate of intrapleural inactivation of urokinase (uPA). A: time dependence of the amidolytic activity of intrapleural, free uPA during intrapleural fibrinolytic therapy (IPFT) with 0.5 mg/kg of S195A-tcuPA with (●; n = 5) and without (■; n = 3) 0.25 mg/kg of scuPA. The amidolytic activity of free uPA was determined in samples of pleural fluid withdrawn at 10–40 min after IPFT. The amidolytic activity of free uPA was calculated as the difference between total uPA activity and activity attributed to α-macroglobulin (αM)/uPA complexes, as previously described (46). There was a statistically significant difference (P < 0.05) between free uPA activity in animals treated with (○) and without (□) scuPA (0.25 mg/kg). B: time dependence of the amidolytic activity of free uPA during IPFT with scuPA (0.25 mg/kg, ○; n = 5) and vehicle control (PBS, □; n = 3). The amidolytic activity of free uPA was the difference between total uPA activity and activity of αM/uPA complexes. There was a statistically significant difference (P < 0.05) between free uPA activity in animals treated with (○) and without (□) scuPA (0.25 mg/kg). C: observed first-order rate constants (kobs) for the intrapleural inactivation of uPA with (●; n = 5) and without (○; n = 5) S195A-tcuPA (0.5 mg/kg). A single exponential equation was fit to the dependence of [free uPA] with respect to time to obtain the kobs of PAI-1-independent inactivation of uPA as previously described (46). There was a statistically significant difference (P < 0.05) between kobs in animals treated with (○) and without (□) scuPA (0.25 mg/kg). There was no statistically significant difference (P > 0.05) between the kobs observed for IPFT with scuPA (0.25 mg/kg) with (●) or without (○) S195A-tcuPA (0.5 mg/kg).
Fig. 9.
Effect of two-chain urokinase with Ser195Ala substitution (S195A-tcuPA; 0.5 mg/kg) on the accumulation of α-macroglobulin (αM)/urokinase (uPA) complexes during intrapleural fibrinolytic therapy (IPFT) with 0.25 mg/kg prourokinase (scuPA). A: time dependence of intrapleural levels of αM/uPA during IPFT with 0.5 mg/kg of S195A-tcuPA, with (●) and without (■) scuPA 0.25 mg/kg. Samples of pleural fluid withdrawn at 0–40 min after IPFT were supplemented with 100–200 nM of exogenous recombinant human plasminogen activator inhibitor-1 (PAI-1), and the amidolytic activity of uPA was measured as previously described (46). There was a statistically significant difference (P < 0.05) between αM/uPA in animals treated with S195A-tcuPA/scuPA and with S195A-tcuPA alone at 10, 20, and 40 min. B: values of the apparent first-order rate constants (kapp) for intrapleural accumulation of αM/uPA with (solid symbols) or without (open symbols) 0.5 mg/kg of S195A-tcuPA. A single exponential equation was fit to the dependence of [αM/uPA] for treatments with (A) or without (not shown) S195A-tcuPA on time as previously described (46). There was no statistically significant difference between the rates of accumulation of αM/uPA during IPFT with 0.25 mg/kg scuPA alone or in the presence of S195A-tcuPA (P > 0.05). C: levels of intrapleural αM/uPA “molecular cage”-type complexes at 24 h after IPFT with 0.25 mg/kg scuPA with (●) or without (○) S195A-tcuPA (0.5 mg/kg). Briefly, the amidolytic activity of uPA was measured after supplementation of samples of pleural fluid withdrawn at 24 h after IPFT with an excess (20–40 nM) of exogenous recombinant human PAI-1. There was no statistically significant difference between [αM/uPA] observed for treatment with 0.25 mg/kg scuPA with (●) or without (○) S195A-tcuPA (0.5 mg/kg; P > 0.05).
Since ΔDS-scuPA as a fibrinolysin (0.5–0.0625 mg/kg) or S195A-tcuPA (0.1 or 0.5 mg/kg) as an adjunct for wt-scuPA did not improve IPFT outcomes, slowing the interaction between PAI-1 and uPA (Fig. 1, mechanisms I and II) was ineffective for increasing the efficacy of IPFT in TCN-induced pleural injury in rabbits. Notably, IPFT outcomes with ΔDS-scuPA and S195A-tcuPA with wt-scuPA, compared with wt-scuPA alone, demonstrated opposite trends—toward a decrease (Fig. 3) and an increase (Figs. 6 and 8) in GLIS, respectively. We hypothesized that because of the slow rate of intrapleural fibrinolysis (4–8 h; 48), inactivation of PAI-1, but not a decrease in the rate of the reaction with uPA, increases the efficacy of IPFT. Moreover, accumulation of active PAI-1 in a stable ternary molecular sandwich-type complex could adversely affect IPFT in TCN-induced pleural injury in rabbits.
Whereas neutralization of endogenous PAI-1 improves IPFT outcomes, accumulation of PAI-1 in a stable ternary complex results in the opposite effect.
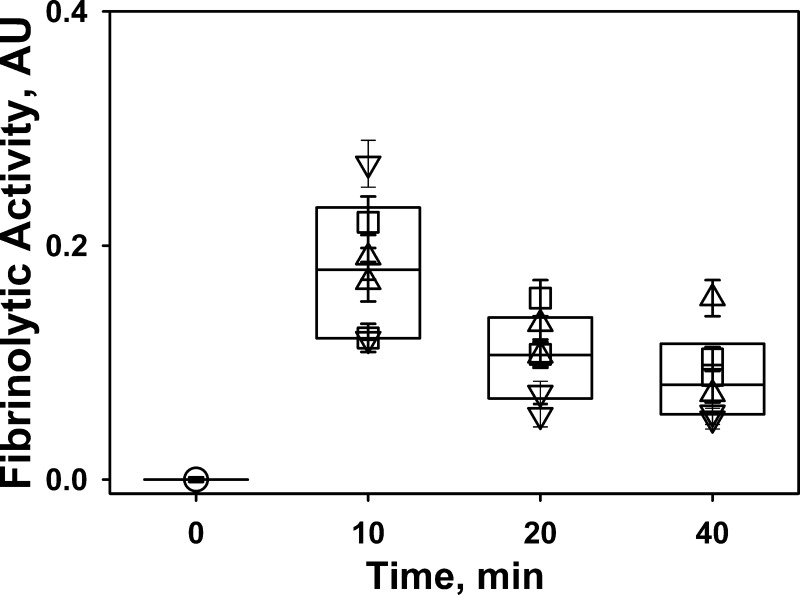
To test our hypothesis, two mAbs, MA-56A7C10 and MA-33B8, that target PAI-1 through mechanisms II and III (Fig. 1) were used as adjuncts in IPFT with wt-scuPA. mAbs possess higher intrapleural stability than uPA and remain present in the pleural fluids for up to 24 h after IPFT (25). MA-56A7C10 [forms stable, nonproductive molecular sandwich-type complexes with a low koff; (Fig. 1, mechanism II; 26)] and MA-33B8 [accelerates spontaneous inactivation of PAI-1 via the active-to-latent transition (Fig. 1, mechanism III; 84)] were administered with a maximal ineffective dose of scuPA (0.25 mg/kg; 36). In control experiments, animals were treated with mouse IgG (0.5 mg/kg) alone (n = 2) or with 0.25 mg/kg of scuPA (n = 2). Outcomes of IPFT at 24 h were expressed as GLIS (Table 1). IPFT with scuPA (0.25 mg/kg) was effective (GLIS ≤ 10) only in the presence of MA-33B8, which does not form molecular sandwich-type complexes and neutralizes endogenous PAI-1. IPFT was ineffective in the presence of MA-56A7C10 (GLIS = 50; maximal score for ineffective IPFT), which significantly stabilizes active PAI-1 and slows its transition to the latent form. Notably, IPFT outcomes with MA-56A7C10 were significantly worse than those in control animals treated with 0.25 mg/kg scuPA alone (Figs. 3 and 6) or in the presence of 0.5 mg/kg of mouse IgG (Table 1) and similar to those observed in vehicle-treated animal controls (Fig. 7). Samples of pleural fluid collected at 0, 10, 20, and 40 min and 24 h after IPFT were analyzed for fibrinolytic activity using a FITC film assay (49). Fibrinolytic activity was inhibited before IPFT (Fig. 10) and at 24 h (not shown). There was no statistically significant difference between levels of fibrinolytic activity at 10–40 min between different treatments (Fig. 10), indicating that intrapleural fibrinolysis does not depend on the type of adjunct present during the first 40 min of IPFT. Fibrinolytic activity was completely inhibited (not shown) at 0–24 h in samples from animals treated with mouse IgG (GLIS = 50; Table 1), indicating that activation of fibrinolysis by an exogenous fibrinolysin is required for successful IPFT. Similar to fibrinolysis, plasminogen-activating activity was also suppressed at 0 and 24 h (not shown) for all treatments, reflecting intrapleural accumulation of active PAI-1 before and 24 h after IPFT. Increased PAI-1 activity was found in pleural fluids of animals treated with 0.25 mg/kg scuPA in the presence of 0.5 mg/kg of MA-56A7C10 at 24 h after IPFT (Table 1). Thus newly expressed PAI-1 likely accumulates in a ternary MA-56A7C10/PAI-1/Vn molecular sandwich-type complex, which stabilizes the serpin in its active conformation and blocks spontaneous transition to its latent form (Fig. 1, klat). In contrast, levels of PAI-1 activity in pleural fluids of animals treated with scuPA and MA-33B8, which does not form molecular sandwich complexes with active PAI-1 (26), were considerably lower (Table 1). Intrapleural levels of αM/uPA at 24 h after treatment with 0.25 mg/kg of scuPA in the presence of MA-56A7C10 were decreased compared with those observed in animals treated with 0.25 mg/kg scuPA alone (Fig. 9C) or in the presence of MA-33B8 (Table 1). Thus the relatively fast inhibition of intrapleural uPA and accumulation of PAI-1 in the presence of MA-56A7C10 affects the formation and/or stability of αM/uPA complexes. Finally, the “footprint” of intrapleural MA-56A7C10/PAI-1/Vn molecular sandwich complexes was detected after supplementation of pleural fluids from animals treated with scuPA and MA-56A7C10 at 24 h after IPFT with uPA and its fluorogenic substrate (Fig. 11A, dashed trace). Slow inactivation of uPA by pleural fluids from animals treated with scuPA and MA-56A7C10 reflects the dissociation of active PAI-1 from a molecular sandwich complex at a rate similar to that observed in vitro for MA-56A7C10/PAI-1/Vn (26). In contrast, free active PAI-1 in samples of pleural fluid from animals treated with scuPA with (Fig. 11A; dotted trace) or without (not shown) MA-33B8 quickly interacts with a fraction of exogenous uPA, resulting in the linear trace that reflects the residual uPA activity.
Table 1.
Effects of MA-33B8 and MA-56A7C10 on the outcome of IPFT with 0.25 mg/kg scuPA and on the levels of active PAI-1 and αM/uPA complexes in the pleural fluids at 24 h after IPFT
| Pleural Fluids at 24 h |
|||||
|---|---|---|---|---|---|
| scuPA Treatment, mg/kg | Adjunct | Adjunct Dose, mg/kg | Outcome (GLIS)* | PAI-1, nM† | αM/uPA, nM |
| 0.25 | Mouse IgG | 0.5 | 17 | 2.4 ± 0.6 | 0.45 ± 0.08 |
| 0.25 | MA-33B8 | 0.5 | 3 | 2.1 ± 0.4 | 1.6 ± 0.6 |
| 0.25 | MA-56A7C10 | 0.5 | 50 | 6.7 ± 0.9 | 0.34 ± 0.07 |
| 0 | Mouse IgG | 0.5 | 50 | 3.2 ± 0.4 | <0.02 |
Levels of active PAI-1 and αM/uPA complexes are means ± SE; n = 2 for each group. IPFT, intrapleural fibrinolytic therapy; scuPA, prourokinase; αM, α-macroglobulin; uPA, urokinase.
Gross lung injury score (GLIS; 25, 48) was determined by counting number of strands, webs, and aggregates of intrapleural fibrin organizations to quantify the extent of the injury. GLIS from 0 to 10 and from 11 to 50 correspond to successful or unsuccessful IPFT, respectively. The differences in median values among the treatment groups were statistically significant (P = 0.025).
Plasminogen activator inhibitor-1 (PAI-1) activity was estimated from the results of inhibition of exogenous two-chain uPA (tcuPA; 0.5 nM), added to the samples of pleural fluids as previously described.
Fig. 10.
Outcomes of intrapleural fibrinolytic therapy (IPFT) do not correlate with the levels of fibrinolytic activity in the first 40 min. Aliquots of pleural fluid withdrawn before (○) and at 10, 20, and 40 min after IPFT with 0.25 mg/kg prourokinase (scuPA) in combination with mouse IgG [▽, n = 2; gross lung injury score (GLIS) = 17; Table 1], MA-56A7C10 (□, n = 2; GLIS = 50; Table 1), or MA-33B8 (△, n = 2; GLIS = 3; Table 1). Fibrinolytic activities in pleural fluids at 0–40 min were analyzed using a FITC-fibrin film assay (49) as described in experimental procedures. There was no statistically significant difference (P > 0.05) in fibrinolytic activity at 10, 20, or 40 min or among different treatments. A significant increase in the fibrinolytic activity after supplementation with exogenous urokinase (uPA; 5 nM; not shown) was observed in all baseline (0 min; ○) samples but not in samples collected at 10–40 min after initiation of IPFT. AU, arbitrary units.
Fig. 11.
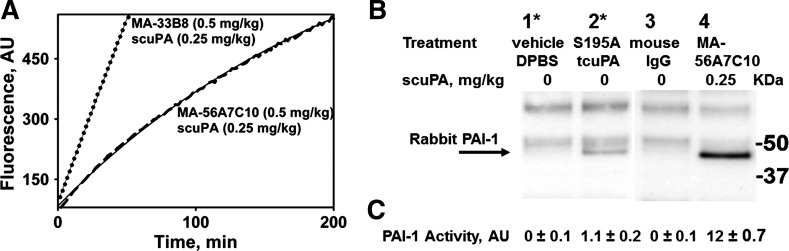
Accumulation of active plasminogen activator inhibitor-1 (PAI-1) in pleural fluids 24 h after intrapleural fibrinolytic therapy (IPFT). A: changes in the activity of exogenous two-chain urokinase (tcuPA; 0.5 nM) added to the samples of pleural fluid collected 24 h after IPFT with 0.25 mg/kg prourokinase (scuPA) and MA-56A7C10 (dashed line) or MA-33B8 (dotted line). Thin, solid lines represent the best fit of a single exponential [first-order rate constant of intrapleural uPA inactivation (kobs) = 4.5 × 10−3 min−1] and linear equation to the data, respectively. The levels of PAI-1 activity in the pleural fluid are shown in the Table 1. B: Western blot analysis of PAI-1 complexed with MA-56A7C10 and tcuPA with Ser195Ala substitution (S195A-tcuPA) isolated from pleural fluids of animals by immunoprecipitation. Complexes of endogenous active PAI-1 were precipitated with magnetic beads (Dynabeads M-280 with sheep anti-mouse IgG; Invitrogen by Thermo Fisher Scientific) per the manufacturer’s protocol, as described in experimental procedures. Western blot analysis detected rabbit PAI-1 in the precipitates obtained from pleural fluids of animals treated with MA-56A7C10 and scuPA (0.5 and 0.25 mg/kg, respectively; lane 4) or S195A-tcuPA (0.5 mg/kg; lane 2), but not in pleural fluids of animals treated with mouse IgG (0.5 mg/kg) or vehicle control [Dulbecco’s phosphate-buffered saline (DPBS); lanes 3 and 1, respectively]. Two pairs of lanes (lanes 1–4) represent parts of the same gel. The positioning of molecular weight markers is shown at right. Treatments are described in the table above the Western blot image. Bands on the Western blot other than rabbit PAI-1, which are present in every lane, represent nonspecific binding of secondary antibodies. *Pleural fluids of rabbits treated with DPBS and S195A-tcuPA (lanes 1 and 2, respectively) were supplemented with anti-human uPA monoclonal antibody (4–8 µg), 10 min before addition of magnetic beads. C: PAI-1 activity was precipitated by sheep anti-mouse IgG magnetic beads from pleural fluids of animals treated with MA-56A7C10 in combination with scuPA (0.5 and 0.25 mg/kg, respectively) and with S195A-tcuPA (0.5 mg/kg), but not from pleural fluids of animals treated with mouse IgG alone (0.5 mg/kg) or vehicle control. PAI-1 activity was measured by incubating an aliquot (5–10 µl) of the bead slurry with 0.2 nM uPA and fluorogenic substrate in DPBS with BSA (1 mg/ml). Amidolytic activity of uPA was measured using fluorogenic substrate as described in experimental procedures and elsewhere (42). Relative levels of active PAI-1 bound to the resin were estimated from decreases in the uPA activity. The levels of active PAI-1 (on average, 2 independent experiments) in pleural fluids were expressed in arbitrary units (AU).
IP with sheep anti-mouse IgG immobilized on magnetic beads was used to isolate complexes of active PAI-1 with MA-56A7C10 and S195A-tcuPA from pleural fluids (100–200 µl) collected at 24 h after IPFT. Pleural fluids of animals (n = 2) treated with scuPA and MA-56A7C10 (0.25 and 0.5 mg/kg, respectively) and S195A-tcuPA (0.5 mg/kg) were analyzed as described in experimental procedures. Pleural fluids (n = 2) after treatment with mouse IgG (0.5 mg/kg) and vehicle (DPBS) were used as controls. Anti-human uPA mAb (2–4 µg) was added to pleural fluids with S195A-tcuPA and vehicle control 10 min before addition to the magnetic beads. One aliquot of the magnetic bead slurry after incubation with pleural fluids and washing was subjected to SDS-PAGE under nonreducing conditions. Western blotting was used to visualize rabbit PAI-1 antigen (Fig. 11B). Another aliquot of the magnetic bead slurry was incubated with 0.2 nM tcuPA in the presence of the fluorogenic substrate as previously described (42). The concentration of active PAI-1 complexed with MA-56A7C10 and S195A-tcuPA was estimated from the loss of uPA activity. Neither rabbit PAI-1 antigen nor PAI-1 activity was detected in samples of magnetic beads incubated with pleural fluids from animals treated with mouse IgG or vehicle control (Fig. 11B). These results confirm in vivo formation of intrapleural complexes of endogenous active PAI-1 and MA-56A7C10 and S195A-tcuPA, which were used as adjuncts for IPFT. Moreover, the relative amounts of these complexes agree with the expected intrapleural stability of mAbs (25) and uPA (46) in TCN-induced pleural injury in rabbits.
Thus the results shown in Table 1 and in Fig. 11 demonstrate that whereas neutralization of endogenous PAI-1 (Fig. 1, mechanism III) is an effective strategy for improving IPFT outcomes in TCN-induced pleural injury in rabbits, stabilization of PAI-1 activity in a molecular sandwich-type complex (Fig. 1, mechanism II) adversely affects IPFT.
DISCUSSION
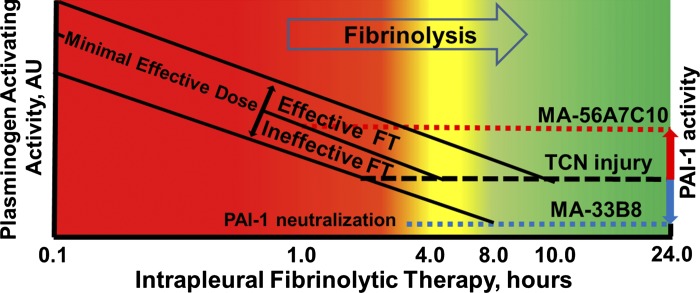
Recently, we described mechanisms by which PAI-1 affects intrapleural fibrinolysis and fibrinolysin processing in rabbits with TCN-induced pleural injury (36, 50) and found that active PAI-1 is a biomarker (42, 46) and a valid therapeutic target (25, 48) for IPFT. Figure 12 illustrates the effects of changing the level of endogenous PAI-1 activity (red and blue arrows) on IPFT outcomes under conditions of slow (4–8 h; 48) intrapleural fibrinolysis. Notably, the minimal amount of time necessary for effective fibrinolysis in the TCN-induced pleural injury model is comparable for minimal effective doses of scuPA and single-chain tissue plasminogen activator (sctPA; 0.5 and 0.145 mg/kg, respectively; 46). This indicates that intrapleural fibrinolysis depends more on the endogenous fibrinolytic system than on the origin of the plasminogen activator (uPA, tPA, or their derivatives). Since the quantities of exogenous plasminogen activators used for IPFT are orders of magnitude higher than the maximal amounts of PAI-1 present in pleural fluids, endogenous PAI-1 is completely quenched (Fig. 1, ki) soon after a bolus injection of the fibrinolysin. However, the intrapleural plasminogen-activating activity decreases rapidly (Fig. 12, solid lines) because of PAI-1-independent inactivation of uPA (Figs. 4, A and B, and 8, A and B) and formation of αM/uPA complexes (Figs. 5A and 9B). Both wt- and ΔDS-uPA, when complexed with αM, retain amidolytic activity toward LMW substrates and are resistant to PAI-1 (Figs. 5 and 9; 46, 50), forming an intrapleural reservoir of plasminogen-activating activity. Although intrapleural degradation of αM/uPA contributes to the plasminogen-activating activity observed, PAI-1-independent inactivation, which has an unknown mechanism, is irreversible (46). Once newly expressed PAI-1 (Fig. 12, black dashed line), in combination with PAI-1-independent inactivation of uPA, inhibits (at the intercept of dashed and solid lines) intrapleural plasminogen-activating activity, fibrinolysis stops. Since effective fibrinolysis in TCN-induced pleural injury in rabbits is slow and requires at least 4–8 h (46; Fig. 12, yellow zone), earlier termination (Fig. 12, red zone) makes IPFT ineffective, whereas maintaining fibrinolytic activity for a longer time (Fig. 12, yellow or green zones) results in successful IPFT. As a result, the minimal effective dose of the fibrinolysin is the one that maintains positive plasminogen-activating activity for 4–8 h after injection (Fig. 12, middle solid line). Thus doses of scuPA higher and lower than the minimal effective dose will result in effective and ineffective IPFT, respectively (Fig. 12). Inhibition of intrapleural PAI-1 (blue arrow) and a decrease in the level of active PAI-1 (blue dotted line) result in extended positive plasminogen-activating activity rendering otherwise ineffective doses of scuPA able to improve outcomes of IPFT in TCN-induced pleural injury. Indeed, delivering IPFT with PAI-1-neutralizing mAbs, which decrease the level of active PAI-1 by redirecting the reaction toward the substrate branch (Fig. 1, ks), resulted in an increase in the efficacy of IPFT (25). A similar increase in the efficacy (Table 1) of IPFT was observed when using MA-33B8 as an adjunct (Fig. 12); this mAb does not form molecular sandwich-type complexes (26), and it stabilizes the transient intermediate between active and latent conformations of the serpin and accelerates the inactivation of PAI-1 (Fig. 1, mechanism III) due to its transition into its latent form (84). Whereas PAI-1 neutralization improves IPFT outcomes, increasing active PAI-1 (Fig. 12, red arrow) results in the opposite effect, because of the relatively fast (before 4–8 h) inhibition of intrapleural plasminogen-activating activity (intercepts between the solid and red dotted lines) and fibrinolysis. Indeed, overexpression of human PAI-1 in TCN-induced pleural injury in rabbits exacerbated the pleural fibrosis and increased inhibition of the plasminogen activators during IPFT (42). Additionally, the decreased efficacy in IPFT with minimal effective doses of scuPA and sctPA observed in animals imaged with chest computed tomography coincided with an increase in intrapleural levels of active PAI-1 (48). Intrapleural concentrations of PAI-1 in rabbit S. pneumoniae and P. multocida models of acute empyema are higher than those observed in TCN-induced pleural injury (47). Notably, IPFT with minimal effective doses of scuPA and sctPA identified in TCN-induced pleural injury (36) failed in both empyema models (47), reflecting the adverse contribution of increased PAI-1 to IPFT outcomes in infectious pleural injury.
Fig. 12.
Changing plasminogen activator inhibitor-1 (PAI-1) activity affects intrapleural fibrinolytic therapy (IPFT) outcomes under conditions of slow fibrinolysis in the pleural space. Successful IPFT in tetracycline (TCN)-induced pleural injury in rabbits requires maintaining plasminogen-activating activity for 4–8 h (48). The minimal time necessary for effective fibrinolysis (4–8 h) is shown as a yellow zone between effective (green zone; >8 h) and ineffective (red zone; <4 h) IPFT outcomes. The rate of PAI-1-independent inactivation of uPA remains the same with different doses of prourokinase (scuPA; 46), shown as parallel solid lines, with the minimal effective dose in the middle. Fibrinolysis stops as soon as endogenous PAI-1 (black dashed line) inhibits the plasminogen activator(s) present (intercept of solid and dashed lines), which in turn determines outcomes for effective (yellow and green zones) and ineffective (red zone) IPFT. Neutralizing PAI-1 (blue arrow) decreases PAI-1 activity (blue dotted line). Consequently, an otherwise ineffective dose of scuPA (the lowest solid line) provides positive plasminogen-activating activity for >8 h (the intercept with the blue dotted line in the green zone), representing the increased efficacy of IPFT (decreasing the minimal effective dose). On the other hand, increasing the PAI-1 activity in the pleural space (red arrow) results in faster inhibition of intrapleural plasminogen activator (the intercept with the red dotted line in the red zone) and in ineffective IPFT with doses of scuPA that are normally effective. The efficacy of IPFT in tetracycline (TCN)-induced pleural injury was increased when PAI-1 was neutralized with MA-33B8 [Table 1; gross lung injury score (GLIS) = 3] or with monoclonal antibodies that redirect the PAI-1 mechanism toward the substrate branch (Fig. 1, ks; 25). The adverse effects of increased PAI-1 were observed during IPFT in the presence of MA-56A7C10 (Table 1; GLIS = 50), in animals subjected to serial computed chest tomography (48) and in rabbits with infectious pleural injury (empyema; 47). AU, arbitrary units; FT, fibrinolytic therapy.
A dramatic (P < 0.05) decrease in the efficacy of IPFT in the presence of MA-56A7C10 (Table 1) likely reflects the accumulation (Fig. 12, red arrow) of active PAI-1 in molecular sandwich-type complexes (26). These complexes between endogenous active PAI-1 and MA-56A7C10 or S195A-tcuPA were isolated from pleural fluid collected at 24 h after treatment using magnetic beads with sheep anti-mouse IgG bound to the surface, and PAI-1 was detected by Western blot. Moreover, both original pleural fluid and magnetic beads (Fig. 11, A and C) with immobilized complexes of active PAI-1 with MA-56A7C10 possessed plasminogen activator-inhibiting activity with kobs similar to that observed for the reaction with molecular sandwich complexes in vitro (26). Finally, the level of PAI-1 activity at 24 h in pleural fluids of animals treated with MA-56A7C10 in combination with scuPA (0.25 mg/kg) was significantly higher than that in animals treated with the same dose of scuPA and either mouse IgG or MA-33B8 (Table 1). These findings reflect the accumulation and stabilization [due to blocking spontaneous active-to-latent transition (Fig. 1, klat); 26] of endogenous PAI-1 complexed with MA-56A7C10 (Table 1 and Fig. 11). Thus intrapleural accumulation of active PAI-1 (Fig. 12, red arrow) in stable molecular sandwich-type complexes induces a dramatic decrease in the efficacy of IPFT (Table 1). These results strongly support our hypothesis (Fig. 12) that the neutralization of PAI-1 increases the efficacy of IPFT under conditions of slow fibrinolysis in TCN-induced pleural injury and that an increase in the level of active PAI-1 adversely affects the outcomes of IPFT.
Notably, the level of fibrinolytic activity at the beginning of IPFT depends on neither the level of plasminogen-activating activity (25) nor the presence of an adjunct (Fig. 10). Thus the intrapleural life span of the plasminogen activator (time of intercept of solid and dashed lines, Fig. 12) becomes critical for successful IPFT. As soon as plasminogen-activating activity is inhibited, fibrinolysis stops, and PAI-1 and plasminogen accumulate, being present at 24 h in pleural fluids in animals after both successful and unsuccessful IPFT (25, 48). Unlike MA-56A7C10, which binds and stabilizes PAI-1 in its active conformation (26), MA-33B8 does not form molecular sandwich-type complexes, possesses relatively low affinity to active PAI-1, but effectively binds latent and cleaved PAI-1, as well as PAI-1 in complexes with proteinases (84). Since MA-33B8 is not an abzyme (catalytic antibody; 84), the presence of active PAI-1 in pleural fluids at 24 h (Table 1) indicates a lack of uncomplexed mAb. In contrast to MA-33B8, MA-56A7C10 binds active PAI-1 with higher affinity than its inactive forms (10, 11). Thus high PAI-1 activity and low levels of αM/uPA at 24 h in the pleural fluids of animals treated with MA-56A7C10 (Table 1) likely reflect the relatively fast inhibition of intrapleural fibrinolysis, which results in ineffective IPFT. Interestingly, similar trends—a decrease both in the efficacy of IPFT (an increase in GLIS; Fig. 6) and in the level of αM/uPA at 24 h (Fig. 9C)—were observed when S195A-tcuPA was used as an adjunct. S195A-tcuPA binds active PAI-1, accommodating the reactive center loop of the serpin into the active site (56). Like MA-56A7C10, S195A-tcuPA forms a molecular sandwich-type ternary complex, S195A-tcuPA/PAI-1/Vn, that promotes the stabilization and intrapleural accumulation of active PAI-1 (26). However, in contrast to mAbs, which were detected in significant amounts at 24 h after IPFT (25), free uPA is rapidly cleared from the pleural space (48). Thus the adverse effect of S195A-tcuPA on IPFT outcomes (Figs. 6 and 7) was less severe than that observed with MA-56A7C10 (Table 1), reflecting the relative instability of S195A-tcuPA in the pleural space compared with mAb. The results of IP experiments (Fig. 11B) also demonstrated lower levels of endogenous PAI-1 complexed with S195A-tcuPA at 24 h compared with MA-56A7C10.
ΔDS-scuPA was effective in IPFT of TCN-induced pleural injury in rabbits but has not demonstrated better efficacy compared with the wild-type proenzyme. The mechanism of intrapleural processing is similar for both fibrinolysins. Like wt-scuPA, ΔDS-scuPA is converted to an active two-chain form within 10 min of intrapleural injection, quenches all endogenous active PAI-1, and activates any accumulated plasminogen to plasmin, initiating slow intrapleural fibrinolysis. A trend toward increased efficacy of IPFT with 0.25 and 0.0625 mg/kg ΔDS-scuPA (Fig. 3) could originate from increased levels of intrapleural αM/ΔDS-uPA molecular cage-type complexes and their contribution to intrapleural PA activity (46, 50). However, the possible benefits of a 60-fold decrease in the rate of inhibition of ΔDS-uPA by PAI-1 (Fig. 2) and the profibrinolytic effect of increased αM/ΔDS-uPA were hampered by rapid intrapleural inactivation of ΔDS-uPA (Fig. 4C), resulting in a statistically insignificant trend (P > 0.05) toward increased efficacy of IPFT (Fig. 3). Thus exosite interactions between PAI-1 and fibrinolysin may serve as a possible target for further development of adjuncts to modulate IPFT in pleural injury. Combining ΔDS-uPA with PAI-1-neutralizing mAbs or using DS-PAI-1 peptide EEIIMD (4, 5, 75) to protect wild-type enzyme could be among these approaches.
The results of the present study demonstrate that unlike PAI-1 inactivation (25), a decrease in the rate of formation of the inhibitory complex between PAI-1 and uPA without changes to the stoichiometry of the reaction (Fig. 1, ki) does not significantly improve the IPFT outcomes in TCN-induced pleural injury in rabbits, where the amount of time necessary for effective fibrinolysis is 4–8 h (47). Moreover, accumulation of PAI-1 activity in a ternary complex with the ligand (Fig. 1, mechanism II) adversely affects IPFT outcomes. On the other hand, a derivative of S195A-uPA was successfully used in a mouse model of thrombosis, where the time of thrombolysis (29) is considerably shorter than the minimal time for effective fibrinolysis observed for IPFT of TCN-induced pleural injury (48). Thus adjuncts such as S195A-tcuPA and MA-56A7C10 could potentially improve fibrinolytic therapy in diseases where effective fibrinolysis is reasonably fast, such as in mustard gas lung injury (82, 83), the rabbit model of thromboembolic stroke (62), or even myocardial infarction or stroke in humans, where the effective therapeutic window for fibrinolytic therapy is relatively narrow (2, 3, 20, 27, 59). On the other hand, treatment of human pleural parapneumonic empyema and complicated parapneumonic effusion requires multiple bolus injections of fibrinolytics over a period of 48–72 h (63, 65, 69), which suggests that relatively slow fibrinolysis takes place. Thus, out of four different mechanisms of modulation of endogenous PAI-1 activity (Fig. 1), intrapleural neutralization, either by mAb-mediated transition to the latent form or by redirection of the reaction to the substrate branch [Fig. 1, mechanism III and ks (25), respectively] are promising choices for PAI-1-targeting IPFT in TCN-induced and, likely, infectious pleural injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 107186-02 (to S. Idell and A. A. Komissarov) and 130402-01A1 (to G. Florova, S. Idell, and A. A. Komissarov) and the Texas Lung Injury Institute, The University of Texas Health Science Center at Tyler.
DISCLOSURES
S. Idell is the unpaid Chief Scientific Officer of Lung Therapeutics Incorporated, serves on its board of directors, and has an equity position in the company, which was created to develop and commercialize single-chain urokinase and other agents for use in lung and pleural disease. His work on single-chain urokinase and pleural injury has been supported by grants from the National Institutes of Health and philanthropy. G. Florova and A. A. Komissarov serve as coinvestigators on research involving intellectual property licensed to Lung Therapeutics. Their work on single-chain urokinase and pleural injury has been supported as well by grants from the National Institutes of Health and philanthropy.
AUTHOR CONTRIBUTIONS
G.F., S.I., and A.A.K. conceived and designed research; G.F., A.O.A., S.K., C.S., and A.A.K. performed experiments; G.F., S.I., and A.A.K. analyzed data; G.F. and A.A.K. interpreted results of experiments; G.F. and A.A.K. prepared figures; G.F. and A.A.K. drafted manuscript; G.F., A.O.A., S.K., S.V.Y., P.J.D., D.B.C., S.I., and A.A.K. edited and revised manuscript; G.F., A.O.A., S.K., C.S., S.V.Y., P.J.D., D.B.C., S.I., and A.A.K. approved final version of manuscript.
REFERENCES
- 1.Alemán C, Alegre J, Monasterio J, Segura RM, Armadans L, Anglés A, Varela E, Ruiz E, Fernández de Sevilla T. Association between inflammatory mediators and the fibrinolysis system in infectious pleural effusions. Clin Sci (Lond) 105: 601–607, 2003. doi: 10.1042/CS20030115. [DOI] [PubMed] [Google Scholar]
- 2.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC Jr; American College of Cardiology; American Heart Association . ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Can J Cardiol 20: 977–1025, 2004. doi: 10.1016/j.jacc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Antman EM, Cooper HA, Gibson CM, de Lemos JA, McCabe CH, Giugliano RP, Coussement P, Murphy S, Scherer J, Anderson K, Van de Werf F, Braunwald E; Thrombolysis in Myocardial Infarction (TIMI) 14 Investigators . Determinants of improvement in epicardial flow and myocardial perfusion for ST elevation myocardial infarction; insights from TIMI 14 and InTIME-II. Eur Heart J 23: 928–933, 2002. doi: 10.1053/euhj.2001.2964. [DOI] [PubMed] [Google Scholar]
- 4.Armstead WM, Riley J, Kiessling JW, Cines DB, Higazi AA. Novel plasminogen activator inhibitor-1-derived peptide protects against impairment of cerebrovasodilation after photothrombosis through inhibition of JNK MAPK. Am J Physiol Regul Integr Comp Physiol 299: R480–R485, 2010. doi: 10.1152/ajpregu.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstead WM, Riley J, Kiessling JW, Cines DB, Higazi AA. PAI-1-derived peptide EEIIMD prevents impairment of cerebrovasodilation by augmenting p38 MAPK upregulation after cerebral hypoxia/ischemia. Am J Physiol Heart Circ Physiol 299: H76–H80, 2010. doi: 10.1152/ajpheart.00185.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbato A, Panizzolo C, Monciotti C, Marcucci F, Stefanutti G, Gamba PG. Use of urokinase in childhood pleural empyema. Pediatr Pulmonol 35: 50–55, 2003. doi: 10.1002/ppul.10212. [DOI] [PubMed] [Google Scholar]
- 7.Barnes NP, Hull J, Thomson AH. Medical management of parapneumonic pleural disease. Pediatr Pulmonol 39: 127–134, 2005. doi: 10.1002/ppul.20127. [DOI] [PubMed] [Google Scholar]
- 8.Bender JM, Ampofo K, Sheng X, Pavia AT, Cannon-Albright L, Byington CL. Parapneumonic empyema deaths during past century, Utah. Emerg Infect Dis 15: 44–48, 2009. doi: 10.3201/eid1501.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bianchini MA, Ceccarelli PL, Repetto P, Durante V, Biondini D, Bergamini B, Cacciari A. Once-daily intrapleural urokinase treatment of complicated parapneumonic effusion in pediatric patients. Turk J Pediatr 52: 274–277, 2010. [PubMed] [Google Scholar]
- 10.Bijnens AP, Gils A, Stassen JM, Komissarov AA, Knockaert I, Brouwers E, Shore JD, Declerck PJ. The distal hinge of the reactive site loop and its proximity: a target to modulate plasminogen activator inhibitor-1 activity. J Biol Chem 276: 44912–44918, 2001. doi: 10.1074/jbc.M103077200. [DOI] [PubMed] [Google Scholar]
- 11.Bijnens AP, Ngo TH, Gils A, Dewaele J, Knockaert I, Stassen JM, Declerck PJ. Elucidation of the binding regions of PAI-1 neutralizing antibodies using chimeric variants of human and rat PAI-1. Thromb Haemost 85: 866–874, 2001. [PubMed] [Google Scholar]
- 12.Burgos J, Falcó V, Pahissa A. The increasing incidence of empyema. Curr Opin Pulm Med 19: 350–356, 2013. doi: 10.1097/MCP.0b013e3283606ab5. [DOI] [PubMed] [Google Scholar]
- 13.Burgos J, Lujan M, Falcó V, Sánchez A, Puig M, Borrego A, Fontanals D, Planes AM, Pahissa A, Rello J. The spectrum of pneumococcal empyema in adults in the early 21st century. Clin Infect Dis 53: 254–261, 2011. doi: 10.1093/cid/cir354. [DOI] [PubMed] [Google Scholar]
- 14.Byington CL, Spencer LY, Johnson TA, Pavia AT, Allen D, Mason EO, Kaplan S, Carroll KC, Daly JA, Christenson JC, Samore MH. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis 34: 434–440, 2002. doi: 10.1086/338460. [DOI] [PubMed] [Google Scholar]
- 15.Chaillan-Huntington CE, Gettins PGW, Huntington JA, Patston PA. The P6-P2 region of serpins is critical for proteinase inhibition and complex stability. Biochemistry 36: 9562–9570, 1997. doi: 10.1021/bi970651g. [DOI] [PubMed] [Google Scholar]
- 16.Colice GL, Idell S. Counterpoint: should fibrinolytics be routinely administered intrapleurally for management of a complicated parapneumonic effusion? No. Chest 145: 17–20, 2014. doi: 10.1378/chest.13-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coombs GS, Bergstrom RC, Madison EL, Corey DR. Directing sequence-specific proteolysis to new targets. The influence of loop size and target sequence on selective proteolysis by tissue-type plasminogen activator and urokinase-type plasminogen activator. J Biol Chem 273: 4323–4328, 1998. doi: 10.1074/jbc.273.8.4323. [DOI] [PubMed] [Google Scholar]
- 18.Corcoran JP, Hallifax R, Rahman NM. New therapeutic approaches to pleural infection. Curr Opin Infect Dis 26: 196–202, 2013. doi: 10.1097/QCO.0b013e32835d0b71. [DOI] [PubMed] [Google Scholar]
- 19.de Benedictis FM, De Giorgi G, Niccoli A, Troiani S, Rizzo F, Lemmi A. Treatment of complicated pleural effusion with intracavitary urokinase in children. Pediatr Pulmonol 29: 438–442, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 20.de Lemos JA, Antman EM, Giugliano RP, Morrow DA, McCabe CH, Cutler SS, Charlesworth A, Schroder R, Braunwald E; InTIME-II Investigators . Comparison of a 60- versus 90-minute determination of ST-segment resolution after thrombolytic therapy for acute myocardial infarction. Am J Cardiol 86: 1235–1237, 2000. doi: 10.1016/S0002-9149(00)01207-8. [DOI] [PubMed] [Google Scholar]
- 21.Debrock S, Declerck PJ. Neutralization of plasminogen activator inhibitor-1 inhibitory properties: identification of two different mechanisms. Biochim Biophys Acta 1337: 257–266, 1997. doi: 10.1016/S0167-4838(96)00173-2. [DOI] [PubMed] [Google Scholar]
- 22.Declerck PJ, Gils A. Three decades of research on plasminogen activator inhibitor-1: a multifaceted serpin. Semin Thromb Hemost 39: 356–364, 2013. doi: 10.1055/s-0033-1334487. [DOI] [PubMed] [Google Scholar]
- 23.Dewilde M, Van De Craen B, Compernolle G, Madsen JB, Strelkov S, Gils A, Declerck PJ. Subtle structural differences between human and mouse PAI-1 reveal the basis for biochemical differences. J Struct Biol 171: 95–101, 2010. doi: 10.1016/j.jsb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Fears R, Hibbs MJ, Smith RA. Kinetic studies on the interaction of streptokinase and other plasminogen activators with plasminogen and fibrin. Biochem J 229: 555–558, 1985. doi: 10.1042/bj2290555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Florova G, Azghani A, Karandashova S, Schaefer C, Koenig K, Stewart-Evans K, Declerck PJ, Idell S, Komissarov AA. Targeting of plasminogen activator inhibitor 1 improves fibrinolytic therapy for tetracycline-induced pleural injury in rabbits. Am J Respir Cell Mol Biol 52: 429–437, 2015. doi: 10.1165/rcmb.2014-0168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florova G, Karandashova S, Declerck PJ, Idell S, Komissarov AA. Remarkable stabilization of plasminogen activator inhibitor 1 in a “molecular sandwich” complex. Biochemistry 52: 4697–4709, 2013. doi: 10.1021/bi400470s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gersh BJ, Stone GW, White HD, Holmes DR Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA 293: 979–986, 2005. doi: 10.1001/jama.293.8.979. [DOI] [PubMed] [Google Scholar]
- 28.Gils A, Declerck PJ. Plasminogen activator inhibitor-1. Curr Med Chem 11: 2323–2334, 2004. doi: 10.2174/0929867043364595. [DOI] [PubMed] [Google Scholar]
- 29.Gong L, Proulle V, Fang C, Hong Z, Lin Z, Liu M, Xue G, Yuan C, Lin L, Furie B, Flaumenhaft R, Andreasen P, Furie B, Huang M. A specific plasminogen activator inhibitor-1 antagonist derived from inactivated urokinase. J Cell Mol Med 20: 1851–1860, 2016. doi: 10.1111/jcmm.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grijalva CG, Zhu Y, Nuorti JP, Griffin MR. Emergence of parapneumonic empyema in the USA. Thorax 66: 663–668, 2011. doi: 10.1136/thx.2010.156406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hekman CM, Loskutoff DJ. Kinetic analysis of the interactions between plasminogen activator inhibitor 1 and both urokinase and tissue plasminogen activator. Arch Biochem Biophys 262: 199–210, 1988. doi: 10.1016/0003-9861(88)90182-8. [DOI] [PubMed] [Google Scholar]
- 32.Hendrickson DJ, Blumberg DA, Joad JP, Jhawar S, McDonald RJ. Five-fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 27: 1030–1032, 2008. doi: 10.1097/INF.0b013e31817e5188. [DOI] [PubMed] [Google Scholar]
- 33.Horrevoets AJG, Pannekoek H, Nesheim ME. A steady-state template model that describes the kinetics of fibrin-stimulated [Glu1]- and [Lys78]plasminogen activation by native tissue-type plasminogen activator and variants that lack either the finger or kringle-2 domain. J Biol Chem 272: 2183–2191, 1997. doi: 10.1074/jbc.272.4.2183. [DOI] [PubMed] [Google Scholar]
- 34.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem 257: 2912–2919, 1982. [PubMed] [Google Scholar]
- 35.Idell S. The pathogenesis of pleural space loculation and fibrosis. Curr Opin Pulm Med 14: 310–315, 2008. doi: 10.1097/MCP.0b013e3282fd0d9b. [DOI] [PubMed] [Google Scholar]
- 36.Idell S, Azghani A, Chen S, Koenig K, Mazar A, Kodandapani L, Bdeir K, Cines D, Kulikovskaya I, Allen T. Intrapleural low-molecular-weight urokinase or tissue plasminogen activator versus single-chain urokinase in tetracycline-induced pleural loculation in rabbits. Exp Lung Res 33: 419–440, 2007. doi: 10.1080/01902140701703333. [DOI] [PubMed] [Google Scholar]
- 37.Idell S, Girard W, Koenig KB, McLarty J, Fair DS. Abnormalities of pathways of fibrin turnover in the human pleural space. Am Rev Respir Dis 144: 187–194, 1991. doi: 10.1164/ajrccm/144.1.187. [DOI] [PubMed] [Google Scholar]
- 38.Idell S, Mazar A, Cines D, Kuo A, Parry G, Gawlak S, Juarez J, Koenig K, Azghani A, Hadden W, McLarty J, Miller E. Single-chain urokinase alone or complexed to its receptor in tetracycline-induced pleuritis in rabbits. Am J Respir Crit Care Med 166: 920–926, 2002. doi: 10.1164/rccm.200204-313OC. [DOI] [PubMed] [Google Scholar]
- 39.Idell S, Pendurthi U, Pueblitz S, Koenig K, Williams T, Rao LV. Tissue factor pathway inhibitor in tetracycline-induced pleuritis in rabbits. Thromb Haemost 79: 649–655, 1998. [PubMed] [Google Scholar]
- 40.Iglesias D, Alegre J, Alemán C, Ruíz E, Soriano T, Armadans LI, Segura RM, Anglés A, Monasterio J, de Sevilla TF. Metalloproteinases and tissue inhibitors of metalloproteinases in exudative pleural effusions. Eur Respir J 25: 104–109, 2005. doi: 10.1183/09031936.04.00010504. [DOI] [PubMed] [Google Scholar]
- 41.Jo M, Takimoto S, Montel V, Gonias SL. The urokinase receptor promotes cancer metastasis independently of urokinase-type plasminogen activator in mice. Am J Pathol 175: 190–200, 2009. doi: 10.2353/ajpath.2009.081053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karandashova S, Florova G, Azghani AO, Komissarov AA, Koenig K, Tucker TA, Allen TC, Stewart K, Tvinnereim A, Idell S. Intrapleural adenoviral delivery of human plasminogen activator inhibitor-1 exacerbates tetracycline-induced pleural injury in rabbits. Am J Respir Cell Mol Biol 48: 44–52, 2013. doi: 10.1165/rcmb.2012-0183OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komissarov AA, Andreasen PA, Bødker JS, Declerck PJ, Anagli JY, Shore JD. Additivity in effects of vitronectin and monoclonal antibodies against alpha-helix F of plasminogen activator inhibitor-1 on its reactions with target proteinases. J Biol Chem 280: 1482–1489, 2005. doi: 10.1074/jbc.M408608200. [DOI] [PubMed] [Google Scholar]
- 44.Komissarov AA, Andreasen PA, Declerck PJ, Kamikubo Y, Zhou A, Gruber A. Redirection of the reaction between activated protein C and a serpin to the substrate pathway. Thromb Res 122: 397–404, 2008. doi: 10.1016/j.thromres.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Komissarov AA, Declerck PJ, Shore JD. Mechanisms of conversion of plasminogen activator inhibitor 1 from a suicide inhibitor to a substrate by monoclonal antibodies. J Biol Chem 277: 43858–43865, 2002. doi: 10.1074/jbc.M204110200. [DOI] [PubMed] [Google Scholar]
- 46.Komissarov AA, Florova G, Azghani A, Karandashova S, Kurdowska AK, Idell S. Active α-macroglobulin is a reservoir for urokinase after fibrinolytic therapy in rabbits with tetracycline-induced pleural injury and in human pleural fluids. Am J Physiol Lung Cell Mol Physiol 305: L682–L692, 2013. doi: 10.1152/ajplung.00102.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komissarov AA, Florova G, Azghani AO, Buchanan A, Boren J, Allen T, Rahman NM, Koenig K, Chamiso M, Karandashova S, Henry J, Idell S. Dose dependency of outcomes of intrapleural fibrinolytic therapy in new rabbit empyema models. Am J Physiol Lung Cell Mol Physiol 311: L389–L399, 2016. doi: 10.1152/ajplung.00171.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komissarov AA, Florova G, Azghani AO, Buchanan A, Bradley WM, Schaefer C, Koenig K, Idell S. The time course of resolution of adhesions during fibrinolytic therapy in tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol 309: L562–L572, 2015. doi: 10.1152/ajplung.00136.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komissarov AA, Florova G, Idell S. Effects of extracellular DNA on plasminogen activation and fibrinolysis. J Biol Chem 286: 41949–41962, 2011. doi: 10.1074/jbc.M111.301218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komissarov AA, Mazar AP, Koenig K, Kurdowska AK, Idell S. Regulation of intrapleural fibrinolysis by urokinase-α-macroglobulin complexes in tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol 297: L568–L577, 2009. doi: 10.1152/ajplung.00066.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komissarov AA, Zhou A, Declerck PJ. Modulation of serpin reaction through stabilization of transient intermediate by ligands bound to alpha-helix F. J Biol Chem 282: 26306–26315, 2007. doi: 10.1074/jbc.M702089200. [DOI] [PubMed] [Google Scholar]
- 52.Kvassman JO, Shore JD. Purification of human plasminogen activator inhibitor (PAI-1) from Escherichia coli and separation of its active and latent forms by hydrophobic interaction chromatography. Fibrinolysis 9: 215–221, 1995. doi: 10.1016/S0268-9499(08)80062-8. [DOI] [Google Scholar]
- 53.Kwon YS. Pleural infection and empyema. Tuberc Respir Dis (Seoul) 76: 160–162, 2014. doi: 10.4046/trd.2014.76.4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee MH, Nahm CH, Choi JW. Thrombin-antithrombin III complex, proinflammatory cytokines, and fibrinolytic indices for assessing the severity of inflammation in pleural effusions. Ann Clin Lab Sci 40: 342–347, 2010. [PubMed] [Google Scholar]
- 55.Lin FC, Chen YC, Chen FJ, Chang SC. Cytokines and fibrinolytic enzymes in tuberculous and parapneumonic effusions. Clin Immunol 116: 166–173, 2005. doi: 10.1016/j.clim.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 56.Lin Z, Jiang L, Yuan C, Jensen JK, Zhang X, Luo Z, Furie BC, Furie B, Andreasen PA, Huang M. Structural basis for recognition of urokinase-type plasminogen activator by plasminogen activator inhibitor-1. J Biol Chem 286: 7027–7032, 2011. doi: 10.1074/jbc.M110.204537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madison EL, Goldsmith EJ, Gerard RD, Gething MJ, Sambrook JF, Bassel-Duby RS. Amino acid residues that affect interaction of tissue-type plasminogen activator with plasminogen activator inhibitor 1. Proc Natl Acad Sci USA 87: 3530–3533, 1990. doi: 10.1073/pnas.87.9.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maskell NA, Davies CW, Nunn AJ, Hedley EL, Gleeson FV, Miller R, Gabe R, Rees GL, Peto TE, Woodhead MA, Lane DJ, Darbyshire JH, Davies RJ; First Multicenter Intrapleural Sepsis Trial (MIST1) Group . U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 352: 865–874, 2005. doi: 10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]
- 59.Minematsu K, Toyoda K, Hirano T, Kimura K, Kondo R, Mori E, Nakagawara J, Sakai N, Shiokawa Y, Tanahashi N, Yasaka M, Katayama Y, Miyamoto S, Ogawa A, Sasaki M, Suga S, Yamaguchi T.. Guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc Dis 22: 571–600, 2013. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Nieuwenhuizen W, Voskuilen M, Vermond A, Hoegee-de Nobel B, Traas DW. The influence of fibrin(ogen) fragments on the kinetic parameters of the tissue-type plasminogen-activator-mediated activation of different forms of plasminogen. Eur J Biochem 174: 163–169, 1988. doi: 10.1111/j.1432-1033.1988.tb14077.x. [DOI] [PubMed] [Google Scholar]
- 61.Philip-Joët F, Alessi MC, Philip-Joët C, Aillaud M, Barriere JR, Arnaud A, Juhan-Vague I. Fibrinolytic and inflammatory processes in pleural effusions. Eur Respir J 8: 1352–1356, 1995. doi: 10.1183/09031936.95.08081352. [DOI] [PubMed] [Google Scholar]
- 62.Phillips DA, Davis MA, Fisher M. Selective embolization and clot dissolution with tPA in the internal carotid artery circulation of the rabbit. AJNR Am J Neuroradiol 9: 899–902, 1988. [PMC free article] [PubMed] [Google Scholar]
- 63.Piccolo F, Pitman N, Bhatnagar R, Popowicz N, Smith NA, Brockway B, Nickels R, Burke AJ, Wong CA, McCartney R, Choo-Kang B, Blyth KG, Maskell NA, Lee YC. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 11: 1419–1425, 2014. doi: 10.1513/AnnalsATS.201407-329OC. [DOI] [PubMed] [Google Scholar]
- 64.Playfor SD, Smyth AR, Stewart RJ. Increase in incidence of childhood empyema. Thorax 52: 932, 1997. [PubMed] [Google Scholar]
- 65.Popowicz N, Piccolo F, Shrestha R, Lee YC. Two sequential tPA/DNase courses for noncommunicating loculated collections in pleural infection. Respirol Case Rep 2: 87–89, 2014. doi: 10.1002/rcr2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Preissner KT, Seiffert D. Role of vitronectin and its receptors in haemostasis and vascular remodeling. Thromb Res 89: 1–21, 1998. doi: 10.1016/S0049-3848(97)00298-3. [DOI] [PubMed] [Google Scholar]
- 67.Pretorius E, Humphries P, Ekpo OE, Smit E, van der Merwe CF. Comparative ultrastructural analyses of mouse, rabbit, and human platelets and fibrin networks. Microsc Res Tech 70: 823–827, 2007. doi: 10.1002/jemt.20482. [DOI] [PubMed] [Google Scholar]
- 68.Psallidas I, Corcoran JP, Rahman NM. Management of parapneumonic effusions and empyema. Semin Respir Crit Care Med 35: 715–722, 2014. doi: 10.1055/s-0034-1395503. [DOI] [PubMed] [Google Scholar]
- 69.Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, Peckham D, Davies CW, Ali N, Kinnear W, Bentley A, Kahan BC, Wrightson JM, Davies HE, Hooper CE, Lee YC, Hedley EL, Crosthwaite N, Choo L, Helm EJ, Gleeson FV, Nunn AJ, Davies RJ. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 365: 518–526, 2011. doi: 10.1056/NEJMoa1012740. [DOI] [PubMed] [Google Scholar]
- 70.Rees JH, Spencer DA, Parikh D, Weller P. Increase in incidence of childhood empyema in West Midlands, UK. Lancet 349: 402, 1997. doi: 10.1016/S0140-6736(97)80022-0. [DOI] [PubMed] [Google Scholar]
- 71.Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun 27: 157–162, 1967. doi: 10.1016/S0006-291X(67)80055-X. [DOI] [PubMed] [Google Scholar]
- 72.Sonnappa S, Jaffe A. Treatment approaches for empyema in children. Paediatr Respir Rev 8: 164–170, 2007. doi: 10.1016/j.prrv.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Stefanutti G, Ghirardo V, Barbato A, Gamba P. Evaluation of a pediatric protocol of intrapleural urokinase for pleural empyema: a prospective study. Surgery 148: 589–594, 2010. doi: 10.1016/j.surg.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 74.Strange C, Tomlinson JR, Wilson C, Harley R, Miller KS, Sahn SA. The histology of experimental pleural injury with tetracycline, empyema, and carrageenan. Exp Mol Pathol 51: 205–219, 1989. doi: 10.1016/0014-4800(89)90020-8. [DOI] [PubMed] [Google Scholar]
- 75.Tan Z, Li X, Kelly KA, Rosen CL, Huber JD. Plasminogen activator inhibitor type 1 derived peptide, EEIIMD, diminishes cortical infarct but fails to improve neurological function in aged rats following middle cerebral artery occlusion. Brain Res 1281: 84–90, 2009. doi: 10.1016/j.brainres.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomson AH, Hull J, Kumar MR, Wallis C, Balfour Lynn IM. Randomised trial of intrapleural urokinase in the treatment of childhood empyema. Thorax 57: 343–347, 2002. doi: 10.1136/thorax.57.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thorsen S, Philips M, Selmer J, Lecander I, Astedt B. Kinetics of inhibition of tissue-type and urokinase-type plasminogen activator by plasminogen-activator inhibitor type 1 and type 2. Eur J Biochem 175: 33–39, 1988. doi: 10.1111/j.1432-1033.1988.tb14162.x. [DOI] [PubMed] [Google Scholar]
- 78.Tomasini BR, Mosher DF. Vitronectin. Prog Hemost Thromb 10: 269–305, 1991. [PubMed] [Google Scholar]
- 79.Tucker T, Idell S. Plasminogen-plasmin system in the pathogenesis and treatment of lung and pleural injury. Semin Thromb Hemost 39: 373–381, 2013. doi: 10.1055/s-0033-1334486. [DOI] [PubMed] [Google Scholar]
- 80.Tucker TA, Jeffers A, Boren J, Quaid B, Owens S, Koenig KB, Tsukasaki Y, Florova G, Komissarov AA, Ikebe M, Idell S. Organizing empyema induced in mice by Streptococcus pneumoniae: effects of plasminogen activator inhibitor-1 deficiency. Clin Transl Med 5: 17, 2016. doi: 10.1186/s40169-016-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van De Craen B, Declerck PJ, Gils A. The biochemistry, physiology and pathological roles of PAI-1 and the requirements for PAI-1 inhibition in vivo. Thromb Res 130: 576–585, 2012. doi: 10.1016/j.thromres.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 82.Veress LA, Anderson DR, Hendry-Hofer TB, Houin PR, Rioux JS, Garlick RB, Loader JE, Paradiso DC, Smith RW, Rancourt RC, Holmes WW, White CW. Airway tissue plasminogen activator prevents acute mortality due to lethal sulfur mustard inhalation. Toxicol Sci 143: 178–184, 2015. doi: 10.1093/toxsci/kfu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Veress LA, Hendry-Hofer TB, Loader JE, Rioux JS, Garlick RB, White CW. Tissue plasminogen activator prevents mortality from sulfur mustard analog-induced airway obstruction. Am J Respir Cell Mol Biol 48: 439–447, 2013. doi: 10.1165/rcmb.2012-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verhamme I, Kvassman JO, Day D, Debrock S, Vleugels N, Declerck PJ, Shore JD. Accelerated conversion of human plasminogen activator inhibitor-1 to its latent form by antibody binding. J Biol Chem 274: 17511–17517, 1999. doi: 10.1074/jbc.274.25.17511. [DOI] [PubMed] [Google Scholar]
- 85.Wilkosz S, Edwards LA, Bielsa S, Hyams C, Taylor A, Davies RJ, Laurent GJ, Chambers RC, Brown JS, Lee YC. Characterization of a new mouse model of empyema and the mechanisms of pleural invasion by Streptococcus pneumoniae. Am J Respir Cell Mol Biol 46: 180–187, 2012. doi: 10.1165/rcmb.2011-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wiman B, Almquist A, Sigurdardottir O, Lindahl T. Plasminogen activator inhibitor 1 (PAI) is bound to vitronectin in plasma. FEBS Lett 242: 125–128, 1988. doi: 10.1016/0014-5793(88)80999-2. [DOI] [PubMed] [Google Scholar]
- 87.Wyseure T, Declerck PJ. Novel or expanding current targets in fibrinolysis. Drug Discov Today 19: 1476–1482, 2014. doi: 10.1016/j.drudis.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 88.Zamarron C, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by natural and recombinant tissue-type plasminogen activator. J Biol Chem 259: 2080–2083, 1984. [PubMed] [Google Scholar]
- 89.Zhang L, Strickland DK, Cines DB, Higazi AA. Regulation of single chain urokinase binding, internalization, and degradation by a plasminogen activator inhibitor 1-derived peptide. J Biol Chem 272: 27053–27057, 1997. doi: 10.1074/jbc.272.43.27053. [DOI] [PubMed] [Google Scholar]