Abstract
Heart failure (HF) is a global public health problem that, independent of its etiology [reduced (HFrEF) or preserved ejection fraction (HFpEF)], is characterized by functional impairments of cardiac function, chemoreflex hypersensitivity, baroreflex sensitivity (BRS) impairment, and abnormal autonomic regulation, all of which contribute to increased morbidity and mortality. Exercise training (ExT) has been identified as a nonpharmacological therapy capable of restoring normal autonomic function and improving survival in patients with HFrEF. Improvements in autonomic function after ExT are correlated with restoration of normal peripheral chemoreflex sensitivity and BRS in HFrEF. To date, few studies have addressed the effects of ExT on chemoreflex control, BRS, and cardiac autonomic control in HFpEF; however, there are some studies that have suggested that ExT has a beneficial effect on cardiac autonomic control. The beneficial effects of ExT on cardiac function and autonomic control in HF may have important implications for functional capacity in addition to their obvious importance to survival. Recent studies have suggested that the peripheral chemoreflex may also play an important role in attenuating exercise intolerance in HFrEF patients. The role of the central/peripheral chemoreflex, if any, in mediating exercise intolerance in HFpEF has not been investigated. The present review focuses on recent studies that address primary pathophysiological mechanisms of HF (HFrEF and HFpEF) and the potential avenues by which ExT exerts its beneficial effects.
Keywords: autonomic control, chemoreflex drive, exercise training, heart failure
INTRODUCTION
Heart failure (HF) is a global public health problem that affects ∼20% of people of >75 yr of age (3, 49). This progressive disease is generally characterized by an inability of the heart to pump sufficient blood to meet the metabolic demands of the tissues (41). Both HF with reduced ejection fraction (HFrEF; ejection fraction ≤ 40%) and HF with preserved ejection fraction (HFpEF; ejection fraction ≥ 50%) are highly prevalent (116) and are associated with similar morbidity and mortality rates (13). From an etiological standpoint, increases in sympathetic nerve activity, parasympathetic withdrawal, and activation of the renin-angiotensin system (RAS) initially act as an adaptive response to improve cardiac function (26, 120) but ultimately become maladaptive and contribute to progression of the disease (111).
Previous studies have indicated that a cardiac autonomic imbalance (sympathetic activation and vagal withdrawal) in HF results from biochemical alterations in central autonomic nuclei as well as altered function of peripheral autonomic reflexes (106). Several lines of evidence suggest that enhanced peripheral chemoreflex function as well as a decrease cardiac baroreflex sensitivity (BRS) contribute to the autonomic imbalance observed in HFrEF (4, 21, 65, 96, 109). Previous studies have indicated that an enhanced peripheral chemoreflex drive contributes to tonic sympathetic activation under eupneic conditions and that enhanced peripheral chemoreflex gain exacerbates sympathetic activation in response to repetitive apneas/hypopneas, which are common in HF patients (31, 98). Repetitive hypoxic/hypercapnic episodes associated with apneas and blood flow reduction to the carotid body (CB) secondary to the decrease in EF may further enhance chemoreflex gain and elicit plasticity in presympathetic neurons in the brain stem triggering sympathoexcitation and finally cardiac function deterioration (4, 21, 22, 109). In fact, previous studies have documented that chronic activation of presympathetic rostral ventrolateral medulla (RVLM) neurons in HFrEF rats is strongly associated with enhanced peripheral chemoreflex drive (21). The relative contribution of episodic hypoxia versus tonic chemoreflex activation to this phenomenon is undetermined. Mechanisms underlying autonomic imbalance in HFpEF are not as well characterized (4, 52, 106, 107). Recently, we showed that contrary to what has been described in HFrEF, central but not peripheral chemoreflex activation is a major contributor to the impairment of autonomic control in HFpEF (110). However, as well as what is observed in HFrEF, we have found increased oxidative stress, chronic hyperactivation of brain stem areas, and BRS impairment in HFpEF rats (5, 110). Hence, increased production of reactive oxidative species (ROS), which is likely associated with upregulation of the RAS, appears to contribute to sympathoexcitation in both types of HF, contributing to the progression of the disease (5, 21, 28, 29, 106, 108).
Despite the widespread use of antioxidant treatment, the relative efficacy of current therapies is limited. Thus, nonpharmacological approaches such as exercise training (ExT) may prove to be valuable adjuncts to standard therapy. Numerous studies in patients with HFrEF and in animal models have indicated that ExT has beneficial effects on cardiac function, quality of life, and survival and that these effects are associated with restoration of cardiac autonomic balance as well as attenuated oxidative stress in the brain stem (7, 10, 29, 40, 54, 71, 77, 88, 108, 117). It is unclear how changes in chemoreflex-mediated autonomic control relate to exercise tolerance in HF (81). This is of particular importance as HF patients that are exercise intolerant have a higher mortality risk (108). To date, few studies have addressed the therapeutic efficacy of ExT or the extent of exercise intolerance in HFpEF (116). This review will summarize the physiological effects of ExT in HF and discuss potential mechanisms by which ExT improves cardiac BRS, chemoreflex function, and autonomic control in HFrEF and HFpEF. In addition, we will also discuss potential mechanisms associated with exercise intolerance in HF.
AUTONOMIC ABNORMALITIES IN HF
The autonomic nervous system is composed of two complementary systems: the sympathetic nervous system and the parasympathetic nervous system (46). Functions of the sympathetic nervous system relevant to HF include contraction of vascular smooth muscle, acceleration of heart rate (HR), and increases in cardiac contractility. In contrast, activation of the parasympathetic nervous system decreases HR and cardiac contractility (46). Autonomic imbalance, characterized by increased sympathetic drive, is one of the major pathophysiological features of HF (4, 26, 85, 109, 111). A significant amount of research has focused on the contribution of central and peripheral chemoreceptors to this increased sympathetic drive in HF (84). Indeed, in HFrEF, it has been shown that central and peripheral chemoreflex activation contributes to increases in sympathetic outflow and to progression of the disease (11, 21, 31, 52, 64, 110). In contrast, very few studies have addressed the contribution of central and/or peripheral chemoreflexes to heightened sympathetic outflow in HFpEF (5, 110). Importantly, it has been shown that, after exercise training, HFrEF animals display improved peripheral chemoreflex control that is closely related to reductions in sympathetic tone (14, 58). On the other hand, Toledo et al. (110) showed that acute activation of central chemoreceptors contributes to abnormal cardiac autonomic control and cardiac dysfunction in HFpEF rats. Although several studies have shown beneficial effects of ExT on both chemoreflex and BRS in HFrEF, the potential positive effects of ExT on chemoreflex and BRS in HFpEF has not been studied extensively. Recently, we provided the first evidence showing that ExT improves cardiac baroreflex and autonomic control in HFpEF rats (5). In addition to these findings, previous studies have shown a salutary effect of ExT on chemoreflex function in HFrEF. The potential beneficial effects of ExT on central/peripheral chemoreflex control in HFpEF are unknown.
CARDIAC AUTONOMIC IMBALANCE IN HF
It has been shown that both HFrEF and HFpEF patients display high levels of circulating norepinephrine and decreases in HR variability (HRV), confirming that autonomic dysfunction is a hallmark of HF regardless of its etiology (49, 70, 110). Several hypothalamic and brain stem areas have been identified to contribute to the development of autonomic imbalance in HF (4, 5, 21, 26, 85, 108, 109). The paraventricular nucleus (PVN) of the hypothalamus, the subfornical organ (SFO), and the RVLM are all recognized as integration sites for neural activity that controls sympathetic outflow (35, 47, 60). Interestingly, in HFrEF rats, enhanced peripheral chemoreflex drive is strongly associated with chronic activation of RVLM presympathetic neurons (21). Furthermore, ablation of the peripheral chemoreceptors normalizes RVLM neuronal activity and restores normal autonomic control in HFrEF rats (21). In addition, it has been shown that peripheral chemoreceptor stimulation activates PVN neurons in healthy rats, suggesting that the PVN may also participate in the peripheral chemoreceptor-induced sympathoexcitation observed in HFrEF (53). However, causal evidence to support this hypothesis is lacking, and future studies should address this issue. Recently, we showed that RVLM neurons are chronically active in HFpEF rats (110); however, the pathophysiological contribution of autonomic hyperreflexia and/or chronic activation of the RVLM, PVN, or SFO to heightened sympathetic activity has not been established in HFpEF (Table 1).
Table 1.
Effect of exercise training on peripheral and central chemoreflex, brain stem oxidative stress and angiotensin II, autonomic control, and cardiac function in heart failure with reduced and preserved ejection fraction
| Chemoreflex |
Autonomic Control |
|||||||
|---|---|---|---|---|---|---|---|---|
| Peripheral | Baroreflex | Brainstem (Oxidative Stress) | Central | Heart rate variability | Nerve recording | Cardiac Function | Reference(s) | |
| Heart failure with reduced ejection fraction | ||||||||
| Patients | ND | ND | ↑ | ND | ↑ | ↑ | ↑ | 17, 33, 48, 54, 62, 63, 71, 88, 98, 114 |
| Animal models | ↑ | ND | ↑ | ↑ | ND | ↑ | ↑ | 14, 29, 58–60, 67, 82 |
| Heart failure with preserved ejection fraction | ||||||||
| Patients | ND | ND | ↑ | ND | ↑ | ND | ↑↓ | 6, 71, 91 |
| Animal models | ND | ND | ↑ | ↑ | ↑ | ND | ↑↓ | 5 |
↑, Improvement; ↓, worsening; ↑↓, controversial results; ND, not described in the literature.
On the other hand, parasympathetic withdrawal is also a characteristic present in both experimental and human HF. Contrary to what is known to be related to sympathetic control in HF, the contribution of parasympathetic withdrawal on autonomic regulation and deterioration of cardiac function in HF has been poorly studied. Evidence obtained in rats subjected to myocardial infarction (leading to HFrEF) showed that vagal nerve stimulation (10 s/min) significantly reduced mortality compared with unstimulated HF rats (57). Recently, Garrott et al. (30) showed that selective activation of parasympathetic neurons reduces myocyte hypertrophy, cardiac fibrosis, and cardiac systolic and diastolic function in HFrEF rats. Much less is known about the role of parasympathetic control on autonomic imbalance, cardiac arrhythmias, and cardiac function in HFpEF. Recently, we showed that, in HFpEF rats, ExT is an effective means to improve the vagal component of HRV, decrease cardiac arrhythmias, and improve cardiac function (5). Therefore, the beneficial effects of ExT on autonomic regulation in HF are not restricted to reductions in sympathetic outflow but may also improve parasympathetic control of the heart. Further studies will need to be performed to elucidate the mechanisms underlying parasympathetic withdrawal in HF.
CHEMOREFLEX AND BAROREFLEX DYSREGULATION CONTRIBUTES TO THE AUTONOMIC IMBALANCE IN HF
The Chemoreflex
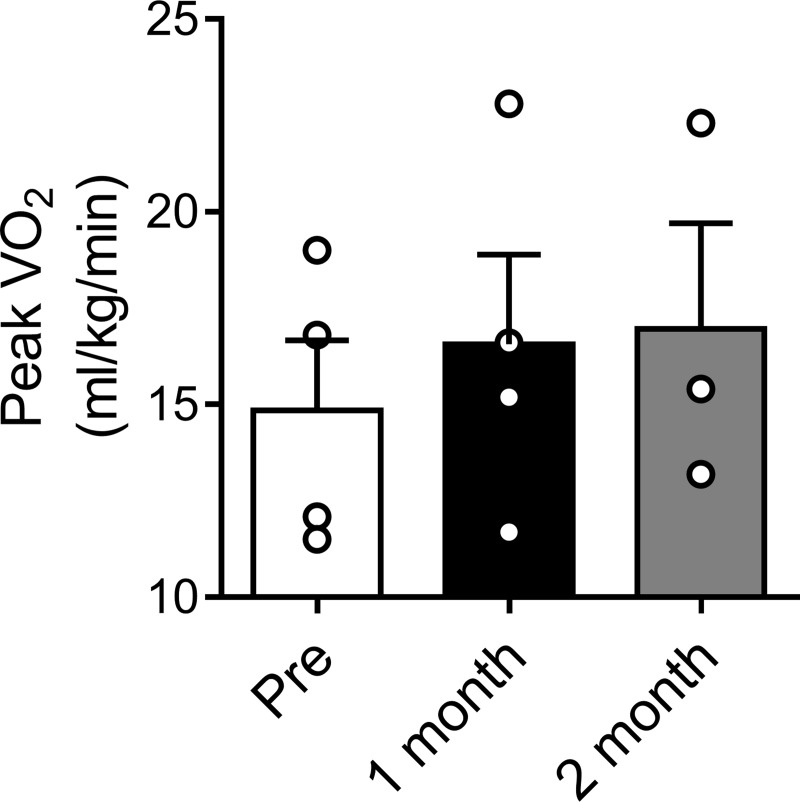
Peripheral and central chemoreceptors play a pivotal role in the maintenance of arterial blood gases, pH, and cardiovascular regulation (12, 87). The CBs are the main peripheral chemoreceptors (43, 44) and play an important role in the control of cardiorespiratory function at rest and during exercise (94). The CB responds to changes in arterial Po2, Pco2, pH, glucose, and blood flow (18, 22, 24, 43, 51). The activation of type I CB glomus cells triggers a reflex response that results in increases in pulmonary ventilation, arterial blood pressure, HR, and sympathetic activity (95, 97). Several studies have demonstrated that peripheral chemoreflex function is altered in HF (4, 94, 105, 110). In HFrEF, it has been shown that the CB-mediated chemoreflex is oversensitized and plays a fundamental role in the progression of the disease (21, 75, 84). Del Rio et al. (21) showed that, in myocardial infarcted HF rats, selective CB denervation decreased sympathetic outflow to the heart and significantly reduced mortality. Recently, Niewinski et al. (76) showed that, in patients with HFrEF, unilateral CB resection restored autonomic control and partially improved peak O2 consumption during the exercise capacity test (Fig. 1). These findings suggest that the peripheral chemoreflex plays an important role in the control of sympathetic outflow and the cardiovascular response to exercise in HFrEF.
Fig. 1.
Effect of unilateral carotid body resection on peak O2 consumption (V̇o2) during exercise in heart failure (HF) patients. Exercise capacity was assessed using treadmill spiroergometric test, and peak V̇o2 was calculated. Note that peak V̇o2 was increased at 1 and 2 mo after unilateral carotid body resection compared with baseline in patients with systolic heart failure (pre). [Data adapted from Niewinski et al. (76).]
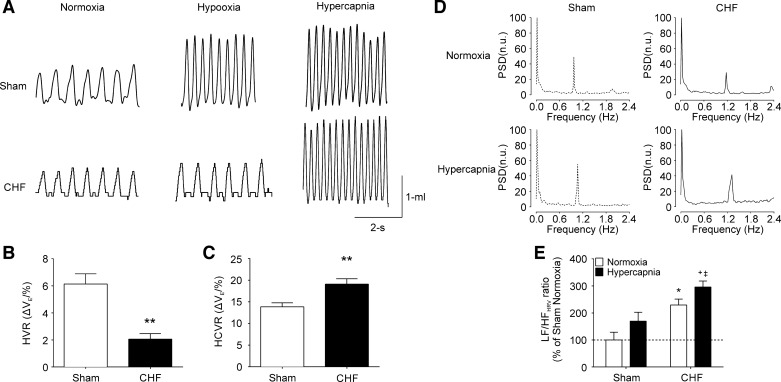
Central chemoreceptors are located throughout the brain. However, the retrotrapezoid nucleus (RTN) located on the ventral medullary surface of the brain stem represents a major chemosensitive area (73, 104). RTN chemoreceptor neurons are pH-sensitive cells secondary to enhanced Pco2 and regulate the activity of the respiratory pattern generator (35, 38, 70). Importantly, neurons from the RTN serve as an integration center for chemosensory information from other central and peripheral sites, including the CB (37). Several studies have shown that central chemoreflex function is enhanced in both animals and humans with HFrEF (52, 72). Central chemoreflex function in HFpEF is not well characterized; however, Giannoni et al. (31) showed that hypersensitivity to hypercapnia is a major predictor of mortality events in HF patients. In addition, Kristen et al. (52) showed that HFpEF rats displayed enhanced renal sympathetic nerve activity responses to hypercapnia, suggesting that an oversensitive central chemoreflex may contribute to autonomic imbalance in this HF type. Furthermore, we recently showed that central chemoreflex sensitivity is markedly increased in HFpEF rats without alterations in peripheral chemoreflex function. Perhaps more importantly, acute activation of the central chemoreflex (fraction of inspired CO2 7%) had adverse effects on cardiac autonomic control in HFpEF rats (Fig. 2) (110). Taken together, these results suggest that central chemoreceptors may contribute to exaggerated sympathoexcitation in HFpEF and that CB hyperreflexia is not enhanced, as it is in HFrEF.
Fig. 2.
Increase central chemoreflex sensitivity worsens cardiac autonomic imbalance in experimental heart failure (HF) with preserved ejection fraction (HFpEF). A: representative recordings of tidal volume (Vt) in normoxia (21% fraction of inspired O2), hypoxia (10% fraction of inspired O2), and hypercapnia (7% fraction of inspired O2) in one sham rat and one HF rat. B and C: gain of the hypercapnic ventilatory response (HCVR) but not the hypoxic ventilatory response (HVR) was increased in HFpEF rats. n = 6 rats. **P < 0.01 vs. the sham condition. D: representative heart rate variability (HRV) spectrums during normoxia (21% fraction of inspired O2) and during brief hypercapnic (7% fraction of inspired O2, 30 s) stimulation in one sham rat and one HF rat. E: summary data showing the effects of hypercapnia on the low-frequency (LF)/high-frequency (HF) ratio of HRV. Note that acute central chemoreflex activation worsens cardiac sympatho-vagal imbalance in HFpEF rats. n = 4 rats. *P < 0.01 vs. sham + normoxia; +P < 0.05 vs. chronic HF + hypercapnia; ‡P < 0.05 vs. sham + hypercapnia. [Modified from Toledo et al. (110) with permission.]
The Baroreflex
Baroreceptors are strectch receptors located in the aortic arch and carotid bifurcation and sense short-term fluctuations in blood pressure (3, 4, 19, 89). Activation of baroreceptors triggers a reflex response that results in increased parasympathetic tone and decreased sympathetic tone. In contrast, unloading of baroreceptors results in sympathoexcitation (2, 67). Several studies have shown that BRS is decreased in both HFrEF and HFpEF (5, 16, 115). More importantly, it has been shown that a reduced BRS is strongly associated with mortality rate in HF (99). Indeed, HF patients with <3 ms/mmHg BRS gain had decreased survival rate compared with HF patients that showed >3 ms/mmHg BRS gain (99). Although the mechanisms underlying BRS impairment are not completely known, it is well accepted that activation of chemoreceptors results in baroreflex inhibition in HFrEF. Indeed, Del Rio et al. (21) showed that CB denervation in rats with HFrEF completely restored normal BRS. Taken together, this evidence strongly suggests that hyperactivation of peripheral chemoreceptors promotes baroreflex control impairment (83) and that both may contribute to disease progression/maintenance in HFrEF.
In HFpEF, less is known about baroreflex control impairment. Nevertheless, possible interactions between peripheral chemoreflex and BRS are less likely since the peripheral chemoreflex is not enhanced in HFpEF rats (110). However, it has been shown that HFpEF rats displayed both an increase central chemoreflex drive (21, 111) and a decrease in BRS (5, 74). Therefore, it is plausible that, in HFpEF, interactions between central chemoreceptor areas and baroreflex control-related areas within the brain stem might account for the reduction in BRS in HFpEF. Further studies should address the precise mechanisms underpinning baroreflex impairment in HFpEF.
ExT: A NONPHARMACOLOGICAL APPROACH TO IMPROVE AUTONOMIC FUNCTION IN HF
Several studies have shown that ExT mitigates sympathetic activation in animals with HFrEF (29, 65, 67, 68, 86). Masson et al. (67) showed that the beneficial effects of ExT on autonomic function in HF rats are critically dependent on the exercise paradigm used. Indeed, 8 wk of endurance ExT (70% of maximal capacity/30 min per day) and moderate-interval ExT (5 min at 80% of maximal capacity followed by 5 min at 60% of maximal capacity/30 min per day) induced similar improvements in cardiac function, but only endurance ExT (low or moderate intensity and high volume) improved cardiac autonomic control (67). In concordance with these findings, improvements in frequency and time domain components of HRV are observed in patients with HFrEF that undertake an endurance ExT program (Fig. 3 and Table 1) (40, 71, 88, 100). These findings suggest that endurance ExT is a potential therapeutic strategy to improve autonomic imbalance and cardiac function in HFrEF patients.
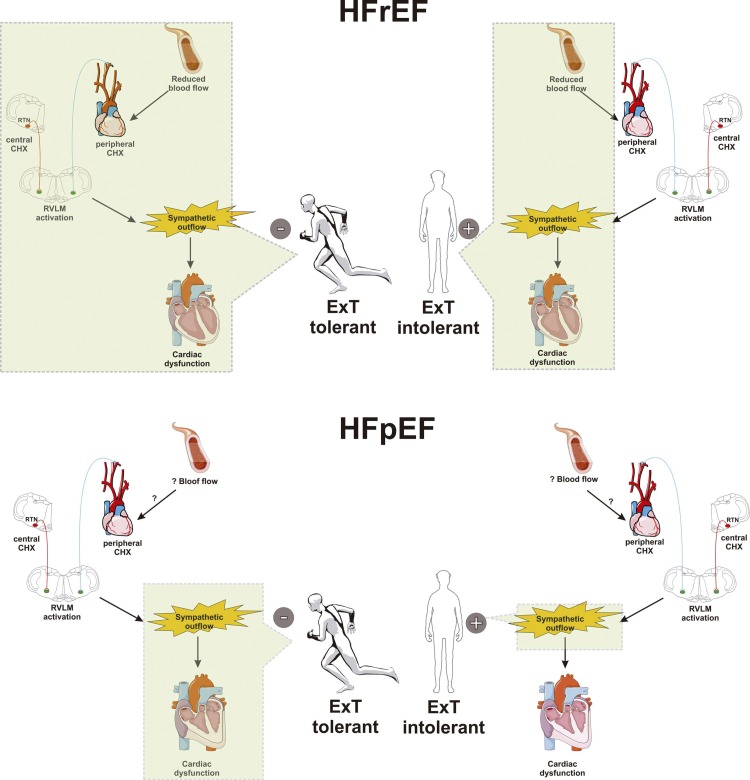
Fig. 3.
Effect of exercise training (ExT) on the pathophysiology of heart failure (HF) with reduced ejection fraction (HFrEF) and with preserved ejection fraction (HFpEF). HF is characterized by augmented central and peripheral chemoreflex drive, activation of brain stem autonomic control nuclei (i.e., rostral ventrolateral medulla), and sympathoexcitation, which all promote cardiac dysfunction, worsening quality of life and lifespan. In exercise-tolerant HFrEF animals and patients, ExT restores blood flow to the muscle, brain, and carotid body chemosensitive cells, normalizing peripheral chemoreflex drive. On the contrary, in the HFrEF exercise-intolerant population, no effect of ExT on blood flow is can be found, and peripheral chemoreflex drive remains potentiated, promoting sympathoexcitation and cardiac dysfunction. Less is known about the role of ExT on HFpEF progression. Indeed, the effect of ExT on blood flow and chemoreflex sensitivity has not been studied. However, recent evidence showed in experimental HFpEF that ExT restored autonomic control in exercise-tolerant animals. Interestingly, HFpEF patients with exercise intolerance display a further deterioration in cardiac autonomic imbalance during ExT. In summary, ExT may hasten HF pathophysiology in the exercise-intolerant population, an effect that is independent of HF ethiology. The mechanism associated with exercise tolerance in HF deserves future investigation.
The effects of ExT on autonomic control in HFpEF patients have not been studied extensively (Fig. 3 and Table 1) (1). Murad et al. (71) showed that ExT imparts less robust improvements in HRV (SD of all normal RR intervals) compared with its effects in HFrEF. This study strongly suggests that ExT (1 h/day, 3 days/wk per 16 wk at 60% of HR reserve) in groups with similar demographic characteristics (age: 68.0 ± 40.8 yr and NYHA: New york Heart Association II and III) but with different HF etiology (HFrEF and HFpEF) has a differential effect on autonomic function (71). Recently, we showed that 6 wk of endurance ExT results in improvements in cardiac systolic function and cardiac arrhythmogenesis in rats with HFpEF (5). We also observed that sympathetic activation, parasympathetic withdrawal, and BRS impairment were all restored after ExT (5). To date, there are no comprehensive studies addressing the effects of ExT on autonomic control in HFpEF patients; however, there have been a number of randomized control trials examining the general effect of ExT on HFpEF patients (23). Interestingly, several of these studies have suggested that ExT elicits only minor improvements in HFpEF and does not improve cardiac diastolic function or exercise capacity (23, 80). In concordance with this evidence, we observed no effect of ExT on cardiac diastolic function in experimental HFpEF (5). Importantly, Angadi et al. (6) showed that the beneficial effects of ExT in patients with HFpEF appear to be critically dependent on the exercise paradigm. Four weeks of high-intensity interval training (85–90% of peak HR/4 × 4 min followed by 3 min of active recovery) but not moderate-intensity aerobic continuous training (70% peak HR/30 min per day) resulted in a marked improvement in peak O2 consumption and diastolic function in HFpEF patients (6). Future studies should address the possible contribution of different ExT programs on autonomic regulation in HFpEF patients.
ExT MODULATES CHEMOREFLEX AND BAROREFLEX FUNCTION IN HF
Recent studies have indicated that altered chemoreceptor and baroreceptor functions, especially an enhanced peripheral chemoreflex and decreased BRS, both play an important role in the progression of HF (21, 31, 92, 99) and may contribute to exercise intolerance (75). Niewinski et al. (76) showed that unilateral CB resection results in decreased peripheral chemosensitivity, decreased sympathetic activity, and partial improvement in exercise tolerance (Fig. 1). Importantly, HF patients that underwent CB resection displayed a significant improvement in exercise times (566 ± 73 vs. 642 ± 70 s, P = 0.03, baseline vs. 6 mo after CB resection, respectively) (76). However, no change in BRS was observed (76). These results strongly suggest that peripheral chemoreflex dysfunction, and not baroreflex control, contributes to reductions in exercise tolerance in HFrEF. Despite the finding that CB resection is a feasible approach to treatment of HFrEF patients, it has been proposed that ExT may have better results than CB denervation since ExT also improves endothelial function and increases blood flow to many vascular beds (45). Moreover, previous studies have indicated that ExT can also reduce the increases in tonic peripheral chemoreflex activity and improve BRS (Table 1) (17, 58, 65, 86). Indeed, in rabbits with pacing-induced HFrEF, it has been shown that endurance ExT (8 m/min followed by 20 min at 13 m/min and a subsequent cooldown period of 8 m/min for 5 min, 5 days/wk) partially reverses the chemoreflex-mediated increases in sympathetic tone to the heart and to the kidney (58, 65). In addition, Calegari et al. (14) showed that in rats with myocardial infarction-induced HFrEF, the pressor response elicited by intravenous potassium cyanide, a peripheral chemoreceptor stimulant, was reduced after ExT (16 m/min, 60 m/day, 5 days/wk, 8 wk). Taken together, these studies suggest that the beneficial effects of ExT on autonomic control in HFrEF are partially mediated by the restoration of normal peripheral chemoreflex function. However, it is worth noting that these studies did not determine whether the effect of ExT on chemoreflex control and sympathetic activation occurred in tandem or whether one lead to the other. Contrary to what is known in HFrEF, the effect of ExT on peripheral and/or central chemoreflex function in HFpEF has not been studied extensively (Table 1) (4, 116).
Toledo et al. (110) previously reported that central chemoreflex function, but not peripheral chemoreflex function, is enhanced in HFpEF. Furthermore, they showed that acute activation of the central chemoreflex worsens cardiac autonomic function, cardiac arrhythmogenesis, and cardiac diastolic function in HFpEF rats (Fig. 2). Determining whether ExT has any effect on the enhanced central chemoreflex and/or cardiac autonomic dysfunction observed in HFpEF is an important area of study to be addressed in the future.
MECHANISMS UNDERLYING CHEMOREFLEX DYSFUNCTION IN HF
Some of the purported mechanisms underlying enhanced CB chemoreflex activation and consequently autonomic dysfunction in HFrEF include oxidative stress in the CB and both oxidative stress and chronic hyperactivation in RVLM-C1 neurons (21, 24, 58). It has been postulated that the primary stimulus for these biochemical changes in the CB and RVLM is the reduction of blood flow secondary to decreased cardiac output in HFrEF (24). In support of this notion, blood flow reduction per se is sufficient to recapitulate many physiological and biochemical aspects of the enhanced chemoreflex drive observed in HFrEF (24). In contrast, in rats with HFpEF, the primary mechanism associated with altered chemoreflex function is not related to blood flow restrictions to the CB (22). Indeed, we observed that HFpEF rats showed normal CB blood flow and no sensitization of the CB-mediated chemoreflex (22). Nevertheless, we found that HFpEF rats displayed an enhanced central chemoreflex drive, suggesting that factors other than blood flow reduction are involved in the altered chemoreflex drive and autonomic imbalance in HFpEF (110). Interestingly, Rosin et al. (91) showed bilateral anatomic connections between RTN and RVLM-C1 neurons. Several groups have shown that acute stimulation of central chemoreceptor triggers sympathoexcitation in humans (103), rats (79), and cats (69). Importantly, we showed that hypercapnic stimulation (fraction of inspired CO2: 7%) induces a HRV disturbance in HFpEF rats characterized by a shift in the spectral components toward a more sympathetic predominance (110). Therefore, it is plausible to hypothesize that in HFpEF, sympathoexcitation may result from increased activity of central RTN chemoreceptor neurons projecting to RVLM-C1 sympathetic neurons. Is important to note that ExT normalizes cardiac autonomic balance in HFpEF rats (5). Thus, it is plausible that ExT may also restore normal central chemoreflex sensitivity as a result of normalizing RTN/RVLM cross-talk in HFpEF. The effects of ExT on central chemoreflex drive in HFpEF require further study.
CENTRAL MECHANISMS CONTRIBUTING TO AUTONOMIC DYSFUNCTION IN HF
Oxidative stress plays an important role in the progression of HF (112). Increases in ROS formation in the brain stem are a major contributor to sympathoexcitation in HF (5, 111). Several studies have shown that, independent of HF etiology, there is a marked change in the balance between pro- and antioxidant enzyme expression and/or activation that contributes to increased systemic (9), cardiac (15), and central nervous system ROS production (29, 31, 110). Intracellular redox balance in the brain stem is significantly shifted in a prooxidative direction by alterations in pro-/antioxidant enzyme expression in HFrEF. NADPH oxidase (NOX) is upregulated, whereas CuZn-superoxide dismutase (CuZn-SOD) and manganese-superoxide dismutase (Mn-SOD) are both downregulated, in the RVLM of HFrEF animals (29, 93). Less is known about the cellular and molecular mechanisms associated with sympathoexcitation in HFpEF. We recently showed that ROS formation in RVLM neurons of HFpEF rats is increased (5, 110) and that this is associated with increased phosphorylation of the p47phox subunit of NOX (5). In contrast to findings in HFrEF rats, CuZn-SOD enzyme expression in the RVLM was not decreased in HFpEF rats (5). These results suggest that a prooxidative shift in redox balance within the RVLM of HFpEF rats is related to NOX activation rather than downregulation of CuZn-SOD. The effects of ExT on other antioxidant enzymes (i.e., Mn-SOD) during the progression of HFpEF deserve further study.
Besides the RVLM, other brain regions have been also described as potential contributors to sympathoexcitation in HF (8, 78, 119). Indeed, the nucleus of the tractus solitarius (NTS), which represents the primary central integration site for peripheral chemoreceptor and baroreceptor activity, has been identified as a master regulator of chemoreflex and baroreflex function (36). Interestingly, in both HFrEF and HFpEF, there is a significant overexpression of the angiotensin II type 1 receptor in the NTS, suggesting an inflammatory process and oxidative stress in this area (90, 101, 118). In addition, it has been shown that the PVN and SFO are both hyperactive in HFrEF and contribute to impairment of autonomic function (119). The role of the NTS, PVN, and SFO in HFpEF pathophysiology remains largely unknown. Future studies are needed to determine the contribution of these areas in the progression and maintenance of altered autonomic function in HFpEF.
ExT AND MUSCLE BLOOD FLOW REGULATION IN HF
It has been proposed that chronic reductions in blood flow to various tissues in the body contribute to autonomic dysregulation and the progression of both HFrEF and HFpEF (14, 24, 56). Reductions in blood flow in HF are mediated, at least in part, by reduced cardiac output and increases in peripheral vascular resistance associated with increased sympathetic outflow to blood vessels (2, 102). In addition to contributing to autonomic dysregulation, blood flow reduction to exercising muscle likely contributes to exercise intolerance in both HFrEF and HFpEF (25, 56). Indeed, leg blood flow during single-leg knee extension exercise is reduced in HFrEF patients (2). Thus, chronic blood flow reduction may contribute to enhanced peripheral and central chemoreflex drive in HFrEF and HFpEF, which in turn may further exacerbate reductions in muscle blood flow associated with reduced cardiac output. While there is some evidence to suggest that hyperactivation of the sympathetic nervous system in HF contributes to exercise intolerance, there is currently no evidence about the contribution of altered chemoreflex function on sympathetically mediated increases in peripheral vascular resistance in either HFrEF and HFpEF.
Mechanistically, the cellular and molecular effects of chronic blood flow reduction are likely due to altered expression of several transcription factors or activation of signaling pathways that are sensitive to shear stress. Kruppel-like factor 2 (KLF2) is a mechanosensitive transcription factor that responds to alterations in blood flow and shear stress (20), which may be a viable link between chronic blood flow reduction and biochemical changes that lead to autonomic dysregulation in HF. HFrEF rats have significantly lower KLF2 expression in tissue from the peripheral chemoreceptors (39). Importantly, statin treatment, a known inducer of KLF2 expression, restores CB KLF2 expression, reduces chemoreflex sensitivity, and normalizes autonomic dysfunction (39). This evidence strongly suggests that KLF2 expression plays a pivotal role in HFrEF pathophysiology. It is unknown whether or not expression of KLF2 or any of its downstream targets play an important role in mediating muscle blood flow during exercise or exercise tolerance in general.
Finally, it is well established that resting blood flow to several organs is improved after completion of an ExT program in HF (65, 86, 100); however, the effects of ExT on muscle blood flow in HFpEF are not well established. Based on our previous studies, it is reasonable to hypothesize that ExT exerts its beneficial effects on CB function in HFrEF through the upregulation of CB KLF2. Further investigations are needed to determine whether this pathway is a primary target of ExT. Currently, there are no studies addressing blood flow reduction in the central nervous system or any potential beneficial effect of ExT on central nervous system blood flow in HFpEF.
EXERCISE INTOLERANCE IN HF
It has been shown that both HFrEF and HFpEF patients have severe exercise intolerance (113), which is considered one of the initial points in the pathogenesis and diagnosis of HF (34). Exercise intolerance is characterized by reduced exercise capacity and the presence of dyspnea during daily activities, symptoms that worsen the quality of life (107). Importantly, Tabet et al. (108) showed that HFrEF patients who are unable to complete ExT have increased mortality rates compared with patients that were able to complete ExT. The ExT protocol consisted of 5 sessions/wk for 4−8 wk (30 min of segmental gymnastics and 40 min of cycling on a cycle ergometer). Patients that improved <2 ml·kg−1·min−1 in O2 consumption had a lower survival rate compared with the exercise-tolerant patients (108). Importantly, exercise tolerance appears to be independent of the degree of cardiac failure since tolerant and intolerant HF patients display similar initial values of HF deterioration (108). It is worth noting that no determinations of autonomic control and chemosensitivity between tolerant versus intolerant HF patients were addressed by Tabet et al. (108). Taking into account that unilateral CB resection improved peak O2 consumption and improved exercise capacity in HFrEF patients (75), it is possible to hypothesize that exercise intolerance in HF may be related to the peripheral chemoreflex dysfunction. Indeed, it has been suggested that impairments in O2 transport from the blood to muscle in both HFrEF and HFpEF play a key role in the development of exercise intolerance (25). Therefore, it is possible that increases in vascular resistance result from the enhanced peripheral/central chemoreflex drive in HF and that any ExT-mediated improvements in peripheral/central chemoreflex function could potentially improve exercise tolerance.
Another mechanism that may contribute to the development of exercise intolerance in HF is parasympathetic withdrawal. Indeed, recent evidence obtained in healthy animals revealed that optogenetic silencing of vagal neurons from the dorsal motor vagal nucleus significantly reduces exercise capacity (61). Importantly, studies of both experimental and human HF have shown a marked decrease in cardiac vagal tone. Thus, future studies should focus on the role of decreased parasympathetic activity in the development of exercise intolerance in HF.
Alterations in cerebral blood flow have been also proposed to participate in exercise tolerance in HF (12, 27, 32). HFrEF patients display brain hypoperfusion and reduced cerebral metabolism (55). Indeed, Koike et al. (50) demonstrated the presence of cerebral hypoperfusion during an incremental symptom-limited maximal exercise test in HF patients. In addition, Fu et al. (27) showed that altered cerebral hemodynamics were associated with the reduced functional capacity displayed by HF patients, suggesting that the attenuated cerebrovascular response to exercise contributes to the decline in functional capacity. Taking into account that brain hypoperfusion is related to sympathoexcitation (66), reductions in blood flow to the brain in HF may be mediated at least in part by the enhanced sympathetic activity that, in turn, contributes to exercise intolerance during the progression of the disease. Also, recent evidence obtained in healthy rats shows that purinergic signaling mediates RTN central chemoreceptor gain by regulating blood vessel dilation during hypercapnic stimulation (42). Interestingly, in HFpEF, there is a significant increase in central chemoreflex gain. Therefore, it is plausible to hypothesize that disruption of the normal purinergic signaling in the RTN may take place in HF to potentiate the central chemoreflex. Thus, ExT may improve central chemoreflex in HFpEF by normalizing vasodilation in the RTN. HF patients that are intolerant of ExT may not show exercise-induced vasodilation in the RTN and thus no restoration of normal chemoreflex drive or autonomic balance would be expected to occur. These novel hypotheses deserve further investigation.
FUTURE PERSPECTIVES
ExT has been shown to be an effective treatment to improve autonomic control, cardiac function, arrhythmogenesis, and survival in HFrEF patients (10, 29, 40). However, little is known about the effects of ExT on the outcome of HFpEF patients. Recently, we showed that ExT positively impacts autonomic control and cardiac function in HFpEF rats (5). Interestingly, the beneficial effects of ExT on HF are associated with the level of tolerance/intolerance to physical exercise (108). Exercise intolerance is a predictor of hospital readmission and mortality in HF (107). Common factors are involved in exercise intolerance that appear to be independent of HF etiology. Factors such as sympathovagal imbalance and reductions in brain and muscle blood flow have all been associated with exercise intolerance in patients and animal models of HF (12, 27, 56). The precise mechanism by which ExT improves HF outcome is a still a matter of debate. Future studies should address the effects of ExT on peripheral/chemoreflex function and brain stem autonomic control areas and their relationship with exercise tolerance in both HFrEF and HFpEF.
CONCLUSIONS
HFpEF or HFrEF is characterized by a marked impairment in cardiac autonomic control, which ultimately hastens cardiac function deterioration. ExT has been proposed as an effective means to restore/improve autonomic control. Indeed, it has been shown that ExT normalizes cardiac autonomic control in experimental HFrEF and HFpEF. The mechanisms associated with the beneficial effects of ExT on autonomic regulation are still a matter of debate, but it seems that in both HFrEF and HFpEF, ExT is able to significantly reduce oxidative stress in key brain stem areas related to sympathetic control. Other mechanisms (i.e., cerebral blood flow normalization) can certainly contribute to reduce sympathoexcitation in HF after ExT, but causal links are missing. Importantly, sympathoexcitation and parasympathetic withdrawal appear to be linked to an increased sensitivity of peripheral and central chemoreceptors and decreased BRS in HFrEF and HFpEF. Therefore, ExT may also exert beneficial effects on cardiac autonomic function through the normalization of both chemoreflex drive and cardiac BRS. Whether or not this effect is cause or consequence of the improvements in cardiac function after ExT still remains to be determined. Ultimately, exercise intolerance is of major importance in HF, and addressing the mechanisms underlying it may improve prognosis in HFrEF and HFpEF populations. Importantly, there is no evidence showing the precise mechanisms associated with exercise tolerance in HF. It is plausible that altered chemoreflex function in HF may provide an explanation for this phenomenon, but this hypothesis requires further study.
GRANTS
This work was supported by Fondo de Desarrollo Científico y Tecnológico Fondecyt 1140275 and Fondecyt 1180172 (to R. Del Rio). D. C. Andrade was supported by National Fund for Scientific and Technological Development of Chile 2015-21251230). M. Amann was supported by National Heart, Lung, and Blood Institute Grant HL-116579 and Veterans Affairs Award System Grant E1572P. N. J. Marcus was supported by National Heart, Lung, and Blood Institute Grant HL-138600-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.C.A., C.T., and H.SD prepared figures; D.C.A., A.A.-A., C.T., H.S.D., and R.D.R. drafted manuscript; D.C.A., A.A.-A., C.T., H.S.D., C.L., R.Q., H.D.S., N.J.M., M.A., and R.D.R. edited and revised manuscript; D.C.A., A.A.-A., C.T., H.S.D., C.L., R.Q., H.D.S., N.J.M., M.A., and R.D.R. approved final version of manuscript.
REFERENCES
- 1.Adams V, Alves M, Fischer T, Rolim N, Werner S, Schütt N, Bowen TS, Linke A, Schuler G, Wisloff U. High-intensity interval training attenuates endothelial dysfunction in a Dahl salt-sensitive rat model of heart failure with preserved ejection fraction. J Appl Physiol 119: 745–752, 2015. doi: 10.1152/japplphysiol.01123.2014. [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan Groot H, Walter Wray D, Stehlik J, Richardson RS. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol 174: 368–375, 2014. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosy AP, Gheorghiade M, Chioncel O, Mentz RJ, Butler J. Global perspectives in hospitalized heart failure: regional and ethnic variation in patient characteristics, management, and outcomes. Curr Heart Fail Rep 11: 416–427, 2014. doi: 10.1007/s11897-014-0221-9. [DOI] [PubMed] [Google Scholar]
- 4.Andrade DC, Lucero C, Toledo C, Madrid C, Marcus NJ, Schultz HD, Del Rio R. Relevance of the carotid body chemoreflex in the progression of heart failure. BioMed Res Int 2015: 467597, 2015. doi: 10.1155/2015/467597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade DC, Arce-Alvarez A, Toledo C, Diaz HS, Lucero C, Schultz HD, Marcus NJ, Del Rio R. Exercise training improve cardiac autonomic control, cardiac function and arrhythmogenesis in rats with preserved ejection fraction heart failure. J Appl Physiol. doi: 10.1152/japplphysiol.00189.2017. [DOI] [PubMed] [Google Scholar]
- 6.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol 119: 753–758, 2015. doi: 10.1152/japplphysiol.00518.2014. [DOI] [PubMed] [Google Scholar]
- 7.Antonicelli R, Spazzafumo L, Scalvini S, Olivieri F, Matassini MV, Parati G, Del Sindaco D, Gallo R, Lattanzio F. Exercise: a “new drug” for elderly patients with chronic heart failure. Aging (Albany NY) 8: 860–872, 2016. doi: 10.18632/aging.100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker BK, Tian C, Zucker IH, Wang HJ. Influence of brain-derived neurotrophic factor-tyrosine receptor kinase B signalling in the nucleus tractus solitarius on baroreflex sensitivity in rats with chronic heart failure. J Physiol 594: 5711–5725, 2016. doi: 10.1113/JP272318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belch JJ, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. Br Heart J 65: 245–248, 1991. doi: 10.1136/hrt.65.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besnier F, Labrunée M, Pathak A, Pavy-Le Traon A, Galès C, Sénard JM, Guiraud T. Exercise training-induced modification in autonomic nervous system: An update for cardiac patients. Ann Phys Rehabil Med 60: 27–35, 2017. doi: 10.1016/j.rehab.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol 588: 2455–2471, 2010. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brassard P, Gustafsson F. Exercise intolerance in heart failure: did we forget the brain? Can J Cardiol 32: 475–484, 2016. doi: 10.1016/j.cjca.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Brutsaert DL, De Keulenaer GW. Diastolic heart failure: a myth. Curr Opin Cardiol 21: 240–248, 2006. doi: 10.1097/01.hco.0000221587.02114.da. [DOI] [PubMed] [Google Scholar]
- 14.Calegari L, Mozzaquattro BB, Rossato DD, Quagliotto E, Ferreira JB, Rasia-Filho A, Dal Lago P. Exercise training attenuates the pressor response evoked by peripheral chemoreflex in rats with heart failure. Can J Physiol Pharmacol 94: 979–986, 2016. doi: 10.1139/cjpp-2015-0518. [DOI] [PubMed] [Google Scholar]
- 15.Cesselli D, Jakoniuk I, Barlucchi L, Beltrami AP, Hintze TH, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res 89: 279–286, 2001. doi: 10.1161/hh1501.094115. [DOI] [PubMed] [Google Scholar]
- 16.Chen JS, Wang W, Bartholet T, Zucker IH. Analysis of baroreflex control of heart rate in conscious dogs with pacing-induced heart failure. Circulation 83: 260–267, 1991. doi: 10.1161/01.CIR.83.1.260. [DOI] [PubMed] [Google Scholar]
- 17.Cider A, Tygesson H, Hedberg M, Seligman L, Wennerblom B, Sunnerhagen KS. Peripheral muscle training in patients with clinical signs of heart failure. Scand J Rehabil Med 29: 121–127, 1997. [PubMed] [Google Scholar]
- 18.Conde SV, Ribeiro MJ, Melo BF, Guarino MP, Sacramento JF. Insulin resistance: a new consequence of altered carotid body chemoreflex? J Physiol 595: 31–41, 2017. doi: 10.1113/JP271684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowley AW Jr, Liard JF, Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res 32: 564–576, 1973. doi: 10.1161/01.RES.32.5.564. [DOI] [PubMed] [Google Scholar]
- 20.Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol 167: 609–618, 2005. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62: 2422–2430, 2013. doi: 10.1016/j.jacc.2013.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Rio R, Andrade DC, Toledo C, Diaz HS, Lucero C, Arce-Alvarez A, Marcus NJ, Schultz HD. Carotid Body-Mediated Chemoreflex Drive in The Setting of low and High Output Heart Failure. Sci Rep 7: 8035, 2017. doi: 10.1038/s41598-017-08142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dieberg G, Ismail H, Giallauria F, Smart NA. Clinical outcomes and cardiovascular responses to exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta-analysis. J Appl Physiol 119: 726–733, 2015. doi: 10.1152/japplphysiol.00904.2014. [DOI] [PubMed] [Google Scholar]
- 24.Ding Y, Li YL, Schultz HD. Role of blood flow in carotid body chemoreflex function in heart failure. J Physiol 589: 245–258, 2011. doi: 10.1113/jphysiol.2010.200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol 55: 1945–1954, 2010. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J 36: 1974–1982, 2015. doi: 10.1093/eurheartj/ehv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu TC, Wang CH, Hsu CC, Cherng WJ, Huang SC, Wang JS. Suppression of cerebral hemodynamics is associated with reduced functional capacity in patients with heart failure. Am J Physiol Heart Circ Physiol 300: H1545–H1555, 2011. doi: 10.1152/ajpheart.00867.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 29.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation 115: 3095–3102, 2007. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- 30.Garrott K, Dyavanapalli J, Cauley E, Dwyer MK, Kuzmiak-Glancy S, Wang X, Mendelowitz D, Kay MW. Chronic activation of hypothalamic oxytocin neurons improves cardiac function during left ventricular hypertrophy-induced heart failure. Cardiovasc Res 113: 1318–1328, 2017. doi: 10.1093/cvr/cvx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giannoni A, Emdin M, Bramanti F, Iudice G, Francis DP, Barsotti A, Piepoli M, Passino C. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J Am Coll Cardiol 53: 1975–1980, 2009. doi: 10.1016/j.jacc.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 32.González-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol 557: 331–342, 2004. doi: 10.1113/jphysiol.2004.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bolla G, Mancia G. Effects of hypertension and obesity on the sympathetic activation of heart failure patients. Hypertension 42: 873–877, 2003. doi: 10.1161/01.HYP.0000098660.26184.63. [DOI] [PubMed] [Google Scholar]
- 34.Gupte AA, Hamilton DJ. Exercise intolerance in heart failure with preserved ejection fraction. Methodist DeBakey Cardiovasc J 12: 105–109, 2016. doi: 10.14797/mdcj-12-2-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG, Kanbar R. Central CO2 chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol 108: 995–1002, 2010. doi: 10.1152/japplphysiol.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guyenet PG, Abbott SB, Stornetta RL. The respiratory chemoreception conundrum: light at the end of the tunnel? Brain Res 1511: 126–137, 2013. doi: 10.1016/j.brainres.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guyenet PG, Bayliss DA, Stornetta RL, Ludwig MG, Kumar NN, Shi Y, Burke PG, Kanbar R, Basting TM, Holloway BB, Wenker IC. Proton detection and breathing regulation by the retrotrapezoid nucleus. J Physiol 594: 1529–1551, 2016. doi: 10.1113/JP271480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haack KK, Marcus NJ, Del Rio R, Zucker IH, Schultz HD. Simvastatin treatment attenuates increased respiratory variability and apnea/hypopnea index in rats with chronic heart failure. Hypertension 63: 1041–1049, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu CY, Hsieh PL, Hsiao SF, Chien MY. Effects of exercise training on autonomic function in chronic heart failure: systematic review. BioMed Res Int 2015: 591708, 2015. doi: 10.1155/2015/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW; American College of Cardiology Foundation; American Heart Association . 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the diagnosis and management of heart failure in adults a report of the American College of Cardiology Foundation/American Heart Association task force on Practice Guidelines Developed in Collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 53: e1–e90, 2009. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Hawkins VE, Takakura AC, Trinh A, Malheiros-Lima MR, Cleary CM, Wenker IC, Dubreuil T, Rodriguez EM, Nelson MT, Moreira TS, Mulkey DK. Purinergic regulation of vascular tone in the retrotrapezoid nucleus is specialized to support the drive to breathe. eLife 6: e25232, 2017. doi: 10.7554/eLife.25232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iturriaga R, Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Brain Res Rev 47: 46–53, 2004. doi: 10.1016/j.brainresrev.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Iturriaga R, Del Rio R, Idiaquez J, Somers VK. Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease. Biol Res 49: 13, 2016. doi: 10.1186/s40659-016-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson BD, Joyner MJ. Carotid body denervation: too soon to get breathless about heart failure? J Am Coll Cardiol 62: 2431–2432, 2013. doi: 10.1016/j.jacc.2013.08.718. [DOI] [PubMed] [Google Scholar]
- 46.Johnson JO, Hemmings HC, Talmage DE. Autonomic nervous system physiology. In: Pharmacology and Physiology for Anesthesia. Philadelphia, PA: Saunders, 2013, p. 208–217. doi: 10.1016/B978-1-4377-1679-5.00012-0. [DOI] [Google Scholar]
- 47.Kang YM, Yang Q, Yu XJ, Qi J, Zhang Y, Li HB, Su Q, Zhu GQ. Hypothalamic paraventricular nucleus activation contributes to neurohumoral excitation in rats with heart failure. Regen Med Res 2: 2, 2014. doi: 10.1186/2050-490X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiilavuori K, Toivonen L, Näveri H, Leinonen H. Reversal of autonomic derangements by physical training in chronic heart failure assessed by heart rate variability. Eur Heart J 16: 490–495, 1995. doi: 10.1093/oxfordjournals.eurheartj.a060941. [DOI] [PubMed] [Google Scholar]
- 49.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 288: 2144–2150, 2002. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 50.Koike A, Itoh H, Oohara R, Hoshimoto M, Tajima A, Aizawa T, Fu LT. Cerebral oxygenation during exercise in cardiac patients. Chest 125: 182–190, 2004. doi: 10.1378/chest.125.1.182. [DOI] [PubMed] [Google Scholar]
- 51.Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 49: 1434–1442, 2000. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- 52.Kristen AV, Just A, Haass M, Seller H. Central hypercapnic chemoreflex modulation of renal sympathetic nerve activity in experimental heart failure. Basic Res Cardiol 97: 177–186, 2002. doi: 10.1007/s003950200009. [DOI] [PubMed] [Google Scholar]
- 53.Kubo T, Yanagihara Y, Yamaguchi H, Fukumori R. Excitatory amino acid receptors in the paraventricular hypothalamic nucleus mediate pressor response induced by carotid body chemoreceptor stimulation in rats. Clin Exp Hypertens 19: 1117–1134, 1997. doi: 10.3109/10641969709083208. [DOI] [PubMed] [Google Scholar]
- 54.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 106: 945–949, 2002. doi: 10.1161/01.CIR.0000027565.12764.E1. [DOI] [PubMed] [Google Scholar]
- 55.Lee CW, Lee JH, Kim JJ, Park SW, Hong MK, Kim ST, Lim TH, Park SJ. Cerebral metabolic abnormalities in congestive heart failure detected by proton magnetic resonance spectroscopy. J Am Coll Cardiol 33: 1196–1202, 1999. doi: 10.1016/S0735-1097(98)00701-3. [DOI] [PubMed] [Google Scholar]
- 56.Lee JF, Barrett-O’Keefe Z, Nelson AD, Garten RS, Ryan JJ, Nativi-Nicolau JN, Richardson RS, Wray DW. Impaired skeletal muscle vasodilation during exercise in heart failure with preserved ejection fraction. Int J Cardiol 211: 14–21, 2016. doi: 10.1016/j.ijcard.2016.02.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109: 120–124, 2004. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 58.Li YL, Ding Y, Agnew C, Schultz HD. Exercise training improves peripheral chemoreflex function in heart failure rabbits. J Appl Physiol (1985) 105: 782–790, 2008. doi: 10.1152/japplphysiol.90533.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation 102: 1854–1862, 2000. doi: 10.1161/01.CIR.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 60.Llewellyn TL, Sharma NM, Zheng H, Patel KP. Effects of exercise training on SFO-mediated sympathoexcitation during chronic heart failure. Am J Physiol Heart Circ Physiol 306: H121–H131, 2014. doi: 10.1152/ajpheart.00534.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machhada A, Trapp S, Marina N, Stephens RC, Whittle J, Lythgoe MF, Kasparov S, Ackland GL, Gourine AV. Vagal determinants of exercise capacity. Nat Commun 8: 15097, 2017. doi: 10.1038/ncomms15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malfatto G, Facchini M, Bragato R, Branzi G, Sala L, Leonetti G. Short and long term effects of exercise training on the tonic autonomic modulation of heart rate variability after myocardial infarction. Eur Heart J 17: 532–538, 1996. doi: 10.1093/oxfordjournals.eurheartj.a014905. [DOI] [PubMed] [Google Scholar]
- 63.Malfatto G, Branzi G, Riva B, Sala L, Leonetti G, Facchini M. Recovery of cardiac autonomic responsiveness with low-intensity physical training in patients with chronic heart failure. Eur J Heart Fail 4: 159–166, 2002. doi: 10.1016/S1388-9842(01)00221-5. [DOI] [PubMed] [Google Scholar]
- 64.Mansukhani MP, Kara T, Caples SM, Somers VK. Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr Hypertens Rep 16: 476, 2014. doi: 10.1007/s11906-014-0476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marcus NJ, Pügge C, Mediratta J, Schiller AM, Del Rio R, Zucker IH, Schultz HD. Exercise training attenuates chemoreflex-mediated reductions of renal blood flow in heart failure. Am J Physiol Heart Circ Physiol 309: H259–H266, 2015. doi: 10.1152/ajpheart.00268.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marina N, Tang F, Figueiredo M, Mastitskaya S, Kasimov V, Mohamed-Ali V, Roloff E, Teschemacher AG, Gourine AV, Kasparov S. Purinergic signalling in the rostral ventro-lateral medulla controls sympathetic drive and contributes to the progression of heart failure following myocardial infarction in rats. Basic Res Cardiol 108: 317, 2013. doi: 10.1007/s00395-012-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masson GS, Borges JP, da Silva PP, da Nóbrega AC, Tibiriçá E, Lessa MA. Effect of continuous and interval aerobic exercise training on baroreflex sensitivity in heart failure. Auton Neurosci 197: 9–13, 2016. doi: 10.1016/j.autneu.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Michelini LC, Stern JE. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp Physiol 94: 947–960, 2009. doi: 10.1113/expphysiol.2009.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Millhorn DE. Neural respiratory and circulatory interaction during chemoreceptor stimulation and cooling of ventral medulla in cats. J Physiol 370: 217–231, 1986. doi: 10.1113/jphysiol.1986.sp015931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mulkey DK, Wenker IC, Kréneisz O. Current ideas on central chemoreception by neurons and glial cells in the retrotrapezoid nucleus. J Appl Physiol 108: 1433–1439, 2010. doi: 10.1152/japplphysiol.01240.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murad K, Brubaker PH, Fitzgerald DM, Morgan TM, Goff DC Jr, Soliman EZ, Eggebeen JD, Kitzman DW. Exercise training improves heart rate variability in older patients with heart failure: a randomized, controlled, single-blinded trial. Congest Heart Fail 18: 192–197, 2012. doi: 10.1111/j.1751-7133.2011.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Narkiewicz K, Pesek CA, van de Borne PJ, Kato M, Somers VK. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation 100: 262–267, 1999. doi: 10.1161/01.CIR.100.3.262. [DOI] [PubMed] [Google Scholar]
- 73.Nattie E, Li A. Central chemoreceptors: locations and functions. Compr Physiol 2: 221–254, 2012. doi: 10.1002/cphy.c100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niebauer M, Zucker IH. Static and dynamic responses of carotid sinus baroreceptors in dogs with chronic volume overload. J Physiol 369: 295–310, 1985. doi: 10.1113/jphysiol.1985.sp015902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niewinski P. Carotid body modulation in systolic heart failure from the clinical perspective. J Physiol 595: 53–61, 2017. doi: 10.1113/JP271692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Piesiak P, Jazwiec P, Banasiak W, Fudim M, Sobotka PA, Javaheri S, Hart EC, Paton JF, Ponikowski P. Carotid body resection for sympathetic modulation in systolic heart failure: results from first-in-man study. Eur J Heart Fail 19: 391–400, 2017. doi: 10.1002/ejhf.641. [DOI] [PubMed] [Google Scholar]
- 77.Nolte K, Herrmann-Lingen C, Wachter R, Gelbrich G, Düngen HD, Duvinage A, Hoischen N, von Oehsen K, Schwarz S, Hasenfuss G, Halle M, Pieske B, Edelmann F. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex-DHF-P trial. Eur J Prev Cardiol 22: 582–593, 2015. doi: 10.1177/2047487314526071. [DOI] [PubMed] [Google Scholar]
- 78.Ogundele OM, Rosa FA, Dharmakumar R, Lee CC, Francis J. Systemic sympathoexcitation was associated with paraventricular hypothalamic phosphorylation of synaptic CaMKIIα and MAPK/ErK. Front Neurosci 11: 447, 2017. doi: 10.3389/fnins.2017.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oikawa S, Hirakawa H, Kusakabe T, Nakashima Y, Hayashida Y. Autonomic cardiovascular responses to hypercapnia in conscious rats: the roles of the chemo- and baroreceptors. Auton Neurosci 117: 105–114, 2005. doi: 10.1016/j.autneu.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 8: 33–40, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ; American Heart Association Committee on exercise, rehabilitation, and prevention . Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 107: 1210–1225, 2003. doi: 10.1161/01.CIR.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 82.Pliquett RU, Cornish KG, Patel KP, Schultz HD, Peuler JD, Zucker IH. Amelioration of depressed cardiopulmonary reflex control of sympathetic nerve activity by short-term exercise training in male rabbits with heart failure. J Appl Physiol 95: 1883–1888, 2003. doi: 10.1152/japplphysiol.00486.2003. [DOI] [PubMed] [Google Scholar]
- 83.Ponikowski P, Chua TP, Piepoli M, Ondusova D, Webb-Peploe K, Harrington D, Anker SD, Volterrani M, Colombo R, Mazzuero G, Giordano A, Coats AJ. Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation 96: 2586–2594, 1997. doi: 10.1161/01.CIR.96.8.2586. [DOI] [PubMed] [Google Scholar]
- 84.Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJ. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation 104: 544–549, 2001. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- 85.Porter TR, Eckberg DL, Fritsch JM, Rea RF, Beightol LA, Schmedtje JF Jr, Mohanty PK. Autonomic pathophysiology in heart failure patients. Sympathetic-cholinergic interrelations. J Clin Invest 85: 1362–1371, 1990. doi: 10.1172/JCI114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pügge C, Mediratta J, Marcus NJ, Schultz HD, Schiller AM, Zucker IH. Exercise training normalizes renal blood flow responses to acute hypoxia in experimental heart failure: role of the α1-adrenergic receptor. J Appl Physiol 120: 334–343, 2016. doi: 10.1152/japplphysiol.00320.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C. Breathing without CO(2) chemosensitivity in conditional Phox2b mutants. J Neurosci 31: 12880–12888, 2011. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ricca-Mallada R, Migliaro ER, Piskorski J, Guzik P. Exercise training slows down heart rate and improves deceleration and acceleration capacity in patients with heart failure. J Electrocardiol 45: 214–219, 2012. doi: 10.1016/j.jelectrocard.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 89.Robertson D, Diedrich A, Chapleau MW. Editorial on arterial baroreflex issue. Auton Neurosci 172: 1–3, 2012. doi: 10.1016/j.autneu.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosârio LB, Rocha I, Silva-Carvalho L. Effect of losartan microinjections into the NTS on the cardiovascular components of chemically evoked reflexes in a rabbit model of acute heart ischemia. Adv Exp Med Biol 536: 423–431, 2003. doi: 10.1007/978-1-4419-9280-2_54. [DOI] [PubMed] [Google Scholar]
- 91.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499: 64–89, 2006. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- 92.Sarmento AO, Antunes-Correa LM, Lima DM, Piovezani BE, Ivani Credidio I, Nunes MJ, Brandão MU, Rondon E, Campos ML, Mady C, Negrao CE Ianni BM. Exercise training increases baroreflex control of heart rate and decreases muscle sympathetic nerve activity in chronic chagasic cardiomyopathy patients. FASEB J 30: 995.4, 2016. [Google Scholar]
- 93.Saku K, Tohyama T, Shinoda M, Kishi T, Hosokawa K, Nishikawa T, Oga Y, Sakamoto T, Tsutsui H, Miyamoto T, Sunagawa K. Central chemoreflex activation induces sympatho-excitation without altering static or dynamic baroreflex function in normal rats. Physiol Rep 5: e13406, 2017. doi: 10.14814/phy2.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sawyer DB. Oxidative stress in heart failure: what are we missing? Am J Med Sci 342: 120–124, 2011. doi: 10.1097/MAJ.0b013e3182249fcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schultz HD, Sun SY. Chemoreflex function in heart failure. Heart Fail Rev 5: 45–56, 2000. doi: 10.1023/A:1009846123893. [DOI] [PubMed] [Google Scholar]
- 96.Schultz HD, Marcus NJ, Del Rio R. Role of the carotid body in the pathophysiology of heart failure. Curr Hypertens Rep 15: 356–362, 2013. doi: 10.1007/s11906-013-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schultz HD, Marcus NJ, Del Rio R. Mechanisms of carotid body chemoreflex dysfunction during heart failure. Exp Physiol 100: 124–129, 2015a. doi: 10.1113/expphysiol.2014.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schultz HD, Marcus NJ, Del Rio R. Role of the carotid body chemoreflex in the pathophysiology of heart failure: a perspective from animal studies. Adv Exp Med Biol 860: 167–185, 2015b. doi: 10.1007/978-3-319-18440-1_19. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz PJ, La Rovere MT, De Ferrari GM, Mann DL. Autonomic modulation for the management of patients with chronic heart failure. Circ Heart Fail 8: 619–628, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001964. [DOI] [PubMed] [Google Scholar]
- 100.Selig SE, Carey MF, Menzies DG, Patterson J, Geerling RH, Williams AD, Bamroongsuk V, Toia D, Krum H, Hare DL. Moderate-intensity resistance exercise training in patients with chronic heart failure improves strength, endurance, heart rate variability, and forearm blood flow. J Card Fail 10: 21–30, 2004. doi: 10.1016/S1071-9164(03)00583-9. [DOI] [PubMed] [Google Scholar]
- 101.Shigematsu H, Hirooka Y, Eshima K, Shihara M, Tagawa T, Takeshita A. Endogenous angiotensin II in the NTS contributes to sympathetic activation in rats with aortocaval shunt. Am J Physiol Regul Integr Comp Physiol 280: R1665–R1673, 2001. doi: 10.1152/ajpregu.2001.280.6.R1665. [DOI] [PubMed] [Google Scholar]
- 102.Shoemaker JK, Naylor HL, Hogeman CS, Sinoway LI. Blood flow dynamics in heart failure. Circulation 99: 3002–3008, 1999. doi: 10.1161/01.CIR.99.23.3002. [DOI] [PubMed] [Google Scholar]
- 103.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol 67: 2101–2106, 1989. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 104.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol 86: 1264–1272, 1999. doi: 10.1152/jappl.1999.86.4.1264. [DOI] [PubMed] [Google Scholar]
- 106.Suzuki H, Maehara K, Yaoita H, Maruyama Y. Altered effects of angiotensin ii type 1 and type 2 receptor blockers on cardiac norepinephrine release and inotropic responses during cardiac sympathetic nerve stimulation in aorto-caval shunt rats. Circ J 68: 683–690, 2004. doi: 10.1253/circj.68.683. [DOI] [PubMed] [Google Scholar]
- 107.Szlachcic J, Masse BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol 55: 1037–1042, 1985. doi: 10.1016/0002-9149(85)90742-8. [DOI] [PubMed] [Google Scholar]
- 108.Tabet JY, Meurin P, Beauvais F, Weber H, Renaud N, Thabut G, Cohen-Solal A, Logeart D, Ben Driss A. Absence of exercise capacity improvement after exercise training program: a strong prognostic factor in patients with chronic heart failure. Circ Heart Fail 1: 220–226, 2008. doi: 10.1161/CIRCHEARTFAILURE.108.775460. [DOI] [PubMed] [Google Scholar]
- 109.Toledo C, Andrade DC, Lucero C, Schultz HD, Marcus N, Retamal M, Madrid C, Del Rio R. Contribution of peripheral and central chemoreceptors to sympatho-excitation in heart failure. J Physiol 595: 43–51, 2017. doi: 10.1113/JP272075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Toledo C, Andrade DC, Lucero C, Arce-Alvarez A, Díaz HS, Aliaga V, Schultz HD, Marcus NJ, Manríquez M, Faúndez M, Del Rio R. Cardiac diastolic and autonomic dysfunction are aggravated by central chemoreflex activation in heart failure with preserved ejection fraction rats. J Physiol 595: 2479–2495, 2017. doi: 10.1113/JP273558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54: 1747–1762, 2009. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 112.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301: H2181–H2190, 2011. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 113.Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol 12: 294–304, 2015. doi: 10.11909/j.issn.1671-5411.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van de Borne P, Montano N, Pagani M, Oren R, Somers VK. Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation 95: 1449–1454, 1997. doi: 10.1161/01.CIR.95.6.1449. [DOI] [PubMed] [Google Scholar]
- 115.Wang W, Zhu GQ, Gao L, Tan W, Qian ZM. Baroreceptor reflex in heart failure. Sheng Li Xue Bao 56: 269–281, 2004. [PubMed] [Google Scholar]
- 116.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128: 1810–1852, 2013. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 117.Yeh GY, McCarthy EP, Wayne PM, Stevenson LW, Wood MJ, Forman D, Davis RB, Phillips RS. Tai chi exercise in patients with chronic heart failure: a randomized clinical trial. Arch Intern Med 171: 750–757, 2011. doi: 10.1001/archinternmed.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoshimura R, Sato T, Kawada T, Shishido T, Inagaki M, Miyano H, Nakahara T, Miyashita H, Takaki H, Tatewaki T, Yanagiya Y, Sugimachi M, Sunagawa K. Increased brain angiotensin receptor in rats with chronic high-output heart failure. J Card Fail 6: 66–72, 2000. doi: 10.1016/S1071-9164(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 119.Yu Y, Wei SG, Weiss RM, Felder RB. TNF-α receptor 1 knockdown in the subfornical organ ameliorates sympathetic excitation and cardiac hemodynamics in heart failure rats. Am J Physiol Heart Circ Physiol 313: H744–H756, 2017. doi: 10.1152/ajpheart.00280.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zucker IH, Schultz HD, Patel KP, Wang H. Modulation of angiotensin II signaling following exercise training in heart failure. Am J Physiol Heart Circ Physiol 308: H781–H791, 2015. doi: 10.1152/ajpheart.00026.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]