Abstract
Human rhinovirus (HRV) is the most common virus contributing to acute exacerbations of chronic obstructive pulmonary disease (COPD) nearly year round, but the mechanisms have not been well elucidated. Recent clinical studies suggest that high levels of growth differentiation factor 15 (GDF15) protein in the blood are associated with an increased yearly rate of all-cause COPD exacerbations. Therefore, in the current study, we investigated whether GDF15 promotes HRV infection and virus-induced lung inflammation. We first examined the role of GDF15 in regulating host defense and HRV-induced inflammation using human GDF15 transgenic mice and cultured human GDF15 transgenic mouse tracheal epithelial cells. Next, we determined the effect of GDF15 on viral replication, antiviral responses, and inflammation in human airway epithelial cells with GDF15 knockdown and HRV infection. Finally, we explored the signaling pathways involved in airway epithelial responses to HRV infection in the context of GDF15. Human GDF15 protein overexpression in mice led to exaggerated inflammatory responses to HRV, increased infectious particle release, and decreased IFN-λ2/3 (IL-28A/B) mRNA expression in the lung. Moreover, GDF15 facilitated HRV replication and inflammation via inhibiting IFN-λ1/IL-29 protein production in human airway epithelial cells. Lastly, Smad1 cooperated with interferon regulatory factor 7 (IRF7) to regulate airway epithelial responses to HRV infection partly via GDF15 signaling. Our results reveal a novel function of GDF15 in promoting lung HRV infection and virus-induced inflammation, which may be a new mechanism for the increased susceptibility and severity of respiratory viral (i.e., HRV) infection in cigarette smoke-exposed airways with GDF15 overproduction.
Keywords: epithelial cells, GDF15, human rhinovirus, inflammation, lung
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) affects more than 15.7 million adults and now is the third leading cause of death in the United States (58). Acute exacerbations of COPD lead to pronounced airway inflammation and accelerated lung function decline, which remarkably increases the morbidity, mortality, and substantial health care costs (66). Emerging evidence has shown that respiratory viral infection is the main cause of COPD exacerbations and linked to increased inflammatory cytokines (e.g., IL-6 and IL-8) and neutrophils in the lung during COPD exacerbations (1, 5, 22, 29, 36, 42, 43). Among the viral species identified, human rhinovirus (HRV) is the most common virus accounting for COPD exacerbations (up to 50%) nearly year round (15, 59, 67). However, there are currently no approved antiviral therapies for HRVs, and the development of effective HRV vaccines has been hindered by the existence of high antigenic variation in over 150 serotypes (19). Therefore, the mechanism underlying the increased susceptibility and severity of HRV infection in COPD airways needs to be better defined for developing new prevention and treatment approaches for HRV-induced COPD exacerbations.
Growth differentiation factor 15 (GDF15) is a member of the transforming growth factor-β (TGF-β) superfamily (8). Our previous studies have demonstrated that GDF15 is upregulated in airway epithelial cells of COPD smokers. Cigarette smoke exposure induces GDF15 production to promote mucus production and cellular senescence in human airway epithelial cells (60, 61). Importantly, recent clinical studies suggest that high GDF15 protein levels in the blood are associated with an increased yearly rate of all-cause COPD exacerbations (16, 23, 32). However, little is known about the role of GDF15 in regulating host defense and inflammatory responses during HRV infection, which may contribute to the disease pathogenesis and progression of HRV-induced COPD exacerbations.
Airway epithelial cells are the primary site for virus entry and replication, representing the first line of host defense against HRVs and an important source of inflammatory mediators in the lung (30, 49). Cultures of bronchial epithelial cells from COPD patients produce higher levels of inflammatory cytokines such as IL-6, IL-8, and interferon-γ-inducible protein 10 (IP-10) at baseline and especially after infection of a major group virus HRV-39 (41). It is important to note that IP-10 increases from baseline to exacerbation in HRV-positive COPD exacerbations and correlates with sputum HRV load (3, 37). Moreover, IFN-λ1/IL-29 is the predominant IFN subtype produced by human airway epithelial cells after infection of HRVs (24, 26). Abnormal IFN-λ1/IL-29 production has been reported in COPD bronchial epithelial cells although the underlying mechanisms remain to be determined (41).
In the present study, we hypothesized that overproduction of GDF15 promotes HRV infection and subsequently enhances virus-induced inflammation in the lung. To test this hypothesis, we first examined the role of GDF15 in the regulation of host defense and HRV-induced airway inflammation by using human GDF15 transgenic (hGDF15 Tg+) mice and cultures of hGDF15 Tg+ mouse tracheal epithelial cells. Next, we determined the effect of GDF15 on viral replication, antiviral responses, and inflammation in human bronchial epithelial (HBE) cells with GDF15 knockdown and HRV infection. Finally, we explored the signaling pathways involved in the responses of airway epithelial cells to HRV infection in the context of GDF15.
MATERIALS AND METHODS
HRV preparation.
HRV-1B and HRV-16 (American Type Culture Collection, Manassas, VA) were propagated in H1-Hela cells (CRL-1958; American Type Culture Collection), purified, and titrated as described previously (63).
Animals.
The human GDF15 transgenic (hGDF15 Tg+) mouse line 1398 on a C57/BL6 background was generated to ubiquitously and constitutively express human GDF15 protein under the control of a chicken β-actin promoter (CAG) in Cre-protamine transgenic mice as described previously (2). The male hGDF15 Tg+ mice were bred to wild-type (WT) C57BL/6 females (The Jackson Laboratory, Bar Harbor, ME) for generating hGDF15 Tg+ mice and WT littermate controls under pathogen-free conditions in the animal facility at National Jewish Health and the University of Colorado Anschutz Medical Campus. All the animal procedures were approved by the Institutional Animal Care and Use Committee at National Jewish Health and the University of Colorado Denver.
HRV-1B infection in mice.
Mouse models for HRV infection have been established, but only the minor group virus HRV-1B can infect mouse tissues (4). Age-matched and gender-matched hGDF15 Tg+ mice and WT littermates (8–12 wk) were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) and intranasally inoculated with 50 μl of PBS (control) or HRV-1B at 1 × 107 plaque-forming units (PFU) per mouse for 24 h. At death, mouse lungs were lavaged with 1 ml of sterile saline and bronchoalveolar lavage (BAL) was collected. Cell-free BAL fluid was stored at −80°C until use. Cytospins of BAL cells were stained with a Diff-Quick Kit (IMEB, San Marcos, CA). Cell differentials were determined as the percentage of 500 counted leukocytes. The lung tissues were processed for histological analysis and RNA extraction.
Histology.
Formalin-fixed, paraffin-embedded mouse lungs were cut into 5-μm thickness sections and stained with hematoxylin and eosin. The slides were evaluated under the light microscope using a histopathologic inflammatory scoring system in a double-blinded fashion as previously described (13). Image processing was performed with Nikon Eclipse 80i microscope and imaging software NIS-Elements AR (Nikon). Ten to twenty airways were counted per mouse section. A final score per mouse (both infected and uninfected) on a scale of 0 to 26 (least to most severe) was obtained based on an assessment of the quantity and quality of peribronchiolar and peribronchial inflammatory infiltrates, luminal exudates, perivascular infiltrates, and parenchymal pneumonia.
HRV-1B infection in well-differentiated primary mouse airway epithelial cells.
All the materials for primary mouse tracheal epithelial cell culture were from Sigma-Aldrich (St. Louis, MO) unless specifically indicated. Tracheas from naïve hGDF15 Tg+ mice and WT littermates (8–12 wk) were cut longitudinally and digested with ice-cold DMEM (GIBCO, Grand Island, NY) supplemented with 0.1% protease solution and amphotericin B (50 μg/ml) at 4°C for 6 h. The released cells were plated onto collagen-coated 12-well Transwell inserts (8 × 104 cells/Transwell) in complete PneumaCult-ALI medium (STEMCELL Technologies, Vancouver, Canada) with mouse epidermal growth factor (15 ng/ml). Culture medium was refreshed every other day until cells reached 100% confluence under immersed culture. Cells were then shifted to air-liquid interfecae (ALI) culture for additional 14 days to induce epithelial mucociliary differentiation. Twenty-four hours before HRV-1B infection, hydrocortisone was removed from the culture medium, and this hydrocortisone-free medium was used for subsequent HRV-1B infection and postinfection culture. The apical surface of ALI cultures was gently washed three times in PBS to remove accumulated mucus and infected with HRV-1B at a multiplicity of infection (MOI) of 1 or PBS (control) at 37°C, 5% CO2 for 2 h. After free viral particles were removed by washing, ALI cultures were incubated at 37°C, 5% CO2 for additional 24 h. At the end of culture, apical supernatants were brought up to 500 μl in PBS. Cells were lysed in RLT buffer for RNA extraction and in RIPA buffer for Western blot analysis.
Chromatin immunoprecipitation followed by next-generation sequencing.
Human tracheobronchial epithelial (hTBE) cells were isolated from tracheas and bronchi of deidentified organ donors as previously described (62). The collection of hTBE cells was approved by the Institutional Review Board at National Jewish Health. Normal hTBE cells at passage 2 were seeded onto collagen-coated 60-mm tissue culture dishes (4 × 105 cells/dish) in bronchial epithelial cell growth medium (BEGM) with SingleQuots supplements (Lonza, Walkersville, MD) at 37°C, 5% CO2. At 80–90% confluence, cells were treated with 0.1% BSA-HCl (control) or rhGDF15 (25 ng/ml, rhGDF15 protein was reconstituted in PBS containing 0.1% BSA and 4 mM HCl; R&D Systems, Minneapolis, MN) for 2 h. The maximal activation of the Smad1 pathway was observed at 2 h with rhGDF15 (25 ng/ml) stimulation in submerged cultures of normal hTBE cells in our previous study (61).
The pSmad1 chromatin immunoprecipitation (ChIP) assay followed by next-generation sequencing, and analysis were performed as previously described (50). Briefly, the cross-linked chromatin from 3 × 106 cells was sheared into 100- to 300-bp fragments using Covaris S2 sonicator and immunoprecipitated with phospho-Smad1/Smad5/Smad9 (D5B10) rabbit mAb (No. 11971; Cell Signaling, Danvers, MA). The enriched DNA was processed for preparing next-generation sequencing libraries using Ion Plus Fragment Library Kit (Life Technologies) and sequenced with Ion Proton Sequencer (Life Technologies) at National Jewish Health Genomics Facility. An average depth of 13-20 million reads was generated per sample and aligned to the human reference genome (hg19) using Ion Torrent Suite software. The peak calling was performed using the Model-based Analysis for ChIP-Sequencing (MACS2) software with a false discovery rate cutoff of 0.001. The pSmad1-binding peak regions were assigned to the nearest genes (within 2 kb of a gene body) using the RefSeq and UCSC Known Genes annotation databases. Identification of putative transcription factor binding sites for pSmad1 peak-associated genes and gene ontology enrichment analysis were performed using GATHER Duke Bioinformatics System. The sequencing data are available in the National Ccenter for Biotechnology Information Gene Expression Omnibus database with Accession No. GSE100625.
HRV-16 infection in well-differentiated primary human airway epithelial cells with lentivirus-mediated gene knockdown.
The MISSION lentiviral vector containing human GDF15 shRNA (TRCN000005839, pLKO.1-shRNA-hGDF15, shGDF15) or control shRNA (SHC001, shControl) was purchased from Sigma-Aldrich, and lentiviruses were generated as previously described (52). Lentiviruses encoding human Smad1 shRNA (sc-29483-v, shSmad1) or control shRNA (sc-108080, shControl) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
HBE cells were collected by endobronchial brushings via fiberoptic bronchoscopy from healthy nonsmokers (n = 4) as previously reported (21). The protocols were approved by the Institutional Review Board at National Jewish Health, and all subjects provided written informed consent. Normal HBE cells at passage 1 were seeded onto collagen-coated 60-mm tissue culture dishes (1 × 105 cells) in BEGM with SingleQuots supplements and transduced with shGDF15, shSmad1, or shControl lentiviruses at MOI 1. Cells were selected with puromycin (1 µg/ml) for 48 h before being transferred onto collagen-coated 12-well Transwell inserts (4 × 104 cells/Transwell) in DMEM/BEGM (1:1) with SingleQuots supplements. Culture medium was refreshed every other day until cells reached 100% confluence under immersed culture. Cells were then shifted to ALI culture for additional 28 days to induce epithelial mucociliary differentiation. Target gene expression at mRNA and/or protein levels was examined, and only those subjects with a satisfactory knockdown of target gene were applied to the treatments.
To examine if HRV infection induces interferon regulatory factor 7 (IRF7) expression through GDF15 signaling, shGDF15 or shControl lentivirus-transduced ALI cultures were infected with HRV-16 (MOI 1) or PBS (control) as previously described (63). To examine if Smad1 directly regulates IRF7 expression during GDF15 stimulation and/or HRV infection, shSmad1 or shControl lentivirus-transduced ALI cultures were pretreated with 0.1% BSA-HCl (control) or rhGDF15 (25 ng/ml) for 72 h and infected with HRV-16 (MOI 1) or PBS (control) as previously described (63). Briefly, the apical surface of ALI cultures was infected with HRV-16 or PBS at 37°C, 5% CO2 for 24 h. After free viral particles were removed by washing, ALI cultures were incubated at 37°C, 5% CO2 for additional 24 h. At the end of culture, apical supernatants were brought up to 500 μl in PBS. Cells were lysed in RLT buffer for RNA extraction and in RIPA buffer for Western blot analysis.
Quantitative real-time RT-PCR.
TaqMan gene expression assays for human GDF15 (Hs03986124_s1), mouse IFN-λ2/3 (IL-28A/B) (Mm04204158-gH), 18S rRNA (4310893E), and human GAPDH (4352934E) were obtained from Thermo Fisher Scientific (Foster City, CA). Other specific primers and probes were: HRV (forward: 5′-CCT CCG GCC CCT GAA T-3′; reverse: 5′-GGT CCC ATC CCG CAA TT-3′; probe: 5′-CTA ACC TTA AAC CTG CAG CCA-3′); IFN-λ1/IL-29 (forward: 5′-GGG AAC CTG TGT CTG AGA ACG T-3′; reverse: 5′-GAG TAG GGC TCA GCG CAT AAA TA-3′; probe: 5′-CTG AGT CCA CCT GAC ACC CCA CAC C-3′). Housekeeping gene GAPDH (for human samples) or 18S rRNA (for mouse samples) was evaluated as an endogenous control. The comparative cycle of threshold (ΔΔCt) method was used to demonstrate the relative mRNA levels of target genes.
Plaque assay.
Viral titers in the cell-free BAL fluid from HRV-infected mice or apical supernatants from HRV-infected cell cultures were determined by plaque assay as previously described (63).
ELISA.
Cell-free BAL fluid or cell culture supernatants were measured with the appropriate ELISA development kit from R&D Systems for mouse GDF15 (DY6385), mouse IL-6 (DY406), mouse KC (DY453), mouse IP-10 (DY466), mouse IFN-λ2/3 (IL-28A/B) (DY1789B), human GDF15 (DY957), human IL-8 (DY208), human IP-10 (DY266), human IFN-λ1/IL-29 (DY7246), and human IL-6 (eBioscience, San Diego, CA) per manufacturer's instructions.
Western blot analysis.
The equal amount (50 µg/lane) of cell lysates were separated by 10% SDS-PAGE, transferred onto PVDF membranes, and probed with anti-human GDF15 (Sigma-Aldrich), anti-IRF7 and anti-GAPDH (Santa Cruz Biotechnology), anti-phosphorylated Smad1 (pSmad1), anti-Smad1, or anti-β-actin (Cell Signaling). After being incubated with appropriate horseradish peroxidase-linked secondary antibodies, membranes were subjected to ECL substrate and analyzed with the FOTO/Analyst Luminary/FX imaging workstation (Fotodyne, Hartland, WI).
Statistical analysis.
Data are presented as means ± SE. One-way ANOVA was used for multiple comparisons, and a Tukey’s post hoc test was applied where appropriate. Student’s t-test was used when only two groups were compared. P < 0.05 was considered significant.
RESULTS
Overexpressing human GDF15 protein in mice enhances lung HRV infection.
The hGDF15 Tg+ mice ubiquitously and constitutively express the human GDF15 protein in most tissues and are used to examine the in vivo biological activity of human GDF15 in different diseases (2, 6, 11, 12, 27, 44, 54, 56). To elucidate the effect of human GDF15 on host defense to HRV infection, hGDF15 Tg+ mice and WT littermates were infected with HRV-1B for 24 h to examine human and mouse GDF15 protein, viral load, and type III IFN in the lung. In humans, type III IFN family has three members, IFN-λ1/IL-29, IFN-λ2/IL-28A, and IFN-λ3/IL-28B. However, an active IFN-λ1/IL-29 gene is absent in mice and sequence homology between mouse IFN-λ2/IL-28A and IFN-λ3/IL-28B is 97% (53). Thus we determined the expression of IFN-λ2/3 (IL-28A/B) in our mouse model.
As expected, only hGDF15 Tg+ mice, but not WT littermates, express the human GDF15 protein in BAL fluid, which was not notably affected by HRV-1B infection (Fig. 1A). Although the absolute mouse GDF15 concentrations were low, HRV-1B infection significantly induced mouse GDF15 protein in WT controls (Fig. 1B). In PBS-treated hGDF15 Tg+ mice, high mouse GDF15 protein signals were observed, presumably related to the cross reactivity between human and mouse GDF15 proteins since they share 84% sequence similarity (31).
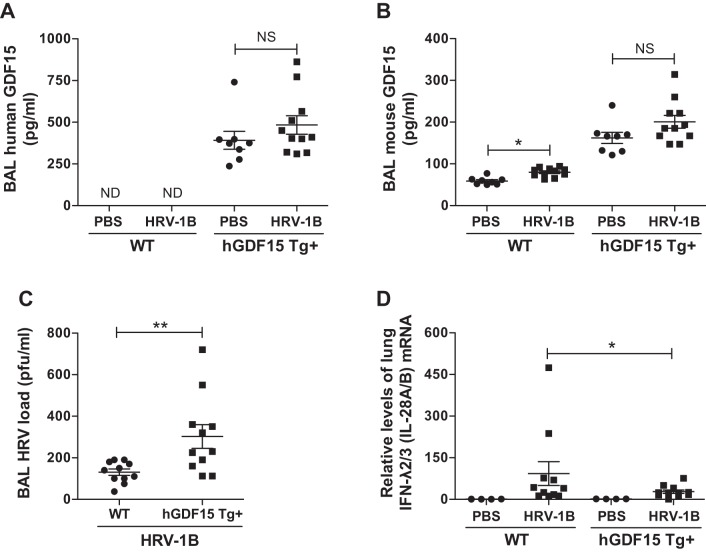
Fig. 1.
Overexpressing human growth differentiation factor 15 (GDF15) in mice enhances lung viral infection and inhibits IFN-λ2/3 (IL-28A/B) expression during human rhinovirus (HRV)-1B infection. Human growth differentiation factor 15 transgenic (hGDF15 Tg+) mice or wild-type (WT) littermates (8–12 wk) were intranasally infected with HRV-1B at 107 plaque-forming units (PFU)/mouse or PBS (control) for 24 h. A and B: human GDF15 protein (A) and mouse GDF15 (B) protein in bronchoalveolar lavage (BAL) were assessed by ELISA. C: the release of infectious virus particles in BAL fluid of HRV-infected mice was examined by plaque assay. D: lung IFN-λ2/3 (IL-28A/B) mRNA levels were measured by quantitative real-time RT-PCR. Relative levels of mRNA expression were normalized to 18S rRNA levels and calculated via the ΔΔCt method. Data are presented as means ± SE from 2 independent experiments (PBS: n = 3–5 mice/group; HRV-1B: n = 4–7 mice/group). *P < 0.05, **P < 0.01, compared with PBS controls or HRV-infected WT mice; NS, not significant.
Following HRV infection, the release of infectious viral particles was significantly higher in BAL fluid of hGDF15 Tg+ mice (up to 2.3-fold, P = 0.002) than WT littermates (Fig. 1C). Moreover, hGDF15 Tg+ mice infected with HRV-1B exhibited ~41% decrease in lung IFN-λ2/3 (IL-28A/B) mRNA expression (P = 0.04) as compared with HRV-infected WT littermates (Fig. 1D). The induction of IFN-λ2/3 (IL-28A/B) protein in BAL fluid was slightly reduced in HRV-infected hGDF15 Tg+ mice (21 ± 6 pg/ml in Tg+ vs. 43 ± 12 pg/ml in WT, P = 0.18).
Overexpressing human GDF15 protein in mice increases HRV-induced lung inflammation.
Having shown overexpressing human GDF15 protein in mice enhances HRV infection and inhibits IFN-λ2/3 (IL-28A/B) expression in the lung, we then determined if human GDF15 protein affects lung inflammatory responses during HRV infection. As compared with WT littermates, human GDF15 overexpression in mice did not significantly alter the total number of BAL leukocytes while hGDF15 Tg+ mice tended to have more cells at baseline (2.5 ± 0.9 × 104 cells/ml in Tg+ vs. 1.3 ± 0.6 × 104 cells/ml in WT, P = 0.2) and upon HRV-1B infection (28.5 ± 8.9 × 104 cells/ml in Tg+ vs. 16.0 ± 2.9 × 104 cells/ml in WT, P = 0.1). Moreover, there was no difference in the number of BAL macrophages (Fig. 2A) and lymphocytes (Fig. 2B) at baseline and after HRV-1B infection between hGDF15 Tg+ mice and WT littermates. However, neutrophils were the predominant cells recovered in BAL fluid after HRV-1B infection in both hGDF15 Tg+ mice and WT littermates (Fig. 2C). After HRV-1B infection, hGDF15 Tg+ mice had significantly increased neutrophil numbers in the BAL compared with WT littermates.
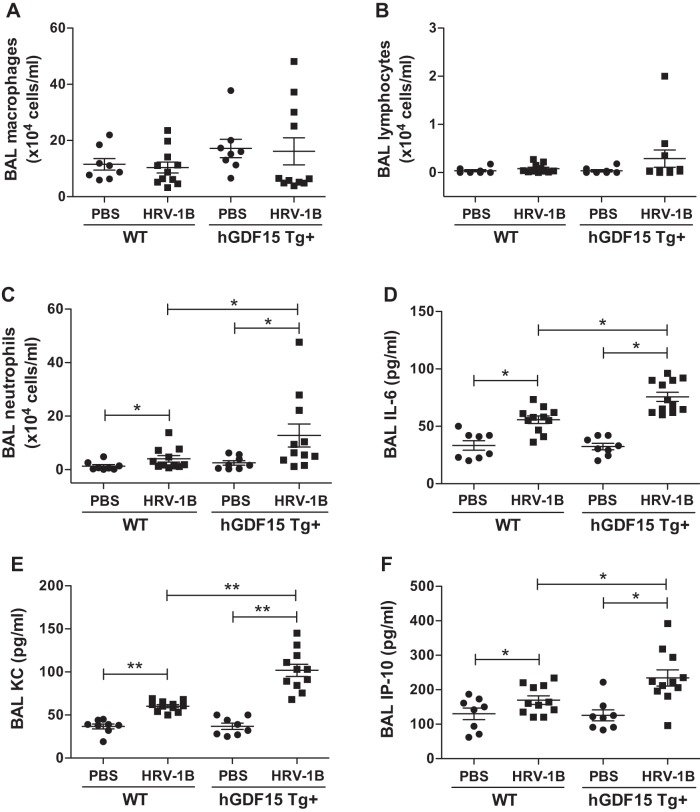
Fig. 2.
Overexpressing human growth differentiation factor 15 (GDF15) in mice increases lung inflammatory responses during HRV-1B infection. Human GDF15 transgenic (hGDF15 Tg+) mice or wild-type (WT) littermates were intranasally infected with HRV-1B at 107 PFU/mouse or PBS (control) for 24 h. Macrophages (A), lymphocytes (B), and neutrophils (C) counts in bronchoalveolar lavage (BAL). Protein levels of IL-6 (D), KC (E), and IP-10 (F) in BAL fluid were assessed by ELISA. Data are presented as means ± SE from 2 independent experiments (PBS: n = 3–5 mice/group; HRV-1B: n = 4–7 mice/group). *P < 0.05, **P < 0.01, compared with PBS controls or HRV-infected WT mice.
In addition to the BAL inflammatory cell profiles, we measured the protein levels of proinflammatory cytokines (i.e., IL-6, KC, and IP-10) commonly implicated in the lung neutrophil recruitment and activation. We found that HRV-1B infection significantly induced production of IL-6 (Fig. 2D), KC (Fig. 2E), and IP-10 (Fig. 2F) in BAL fluid of hGDF15 Tg+ mice and WT littermates. Importantly, compared with WT littermates, HRV-induced IL-6, KC, and IP-10 protein levels were significantly higher in hGDF15 Tg+ mice, which was associated with the higher viral load and BAL neutrophil counts during HRV infection as shown above.
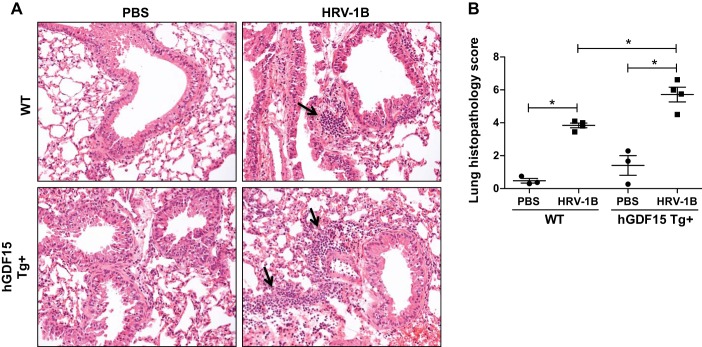
Lastly, we evaluated the lung tissue inflammation in hGDF15 Tg+ mice and WT littermates infected with HRV-1B by using a histopathological scoring system on hematoxylin and eosin-stained mouse lung sections. Compared with PBS controls, HRV-1B infection resulted in patchy peribronchial and perivascular inflammatory infiltrates in both hGDF15 Tg+ mice and WT littermates (Fig. 3A). In line with the BAL inflammation data, hGDF15 Tg+ mice exhibited a greater extent of inflammatory infiltration, representing by a higher histopathological score at baseline (1.4 ± 0.6 in Tg+ vs. 0.5 ± 0.2 in WT, P = 0.25) and particularly following HRV-1B infection (5.7 ± 0.4 in Tg+ vs. 3.8 ± 0.1 in WT, P = 0.02) (Fig. 3B).
Fig. 3.
Lung histopathology. Overexpressing human growth differentiation factor 15 (GDF15) in mice increases the infiltration of inflammatory cells into the lung during HRV-1B infection. Human GDF15 transgenic (hGDF15 Tg+) mice or wild-type (WT) littermates were intranasally infected with HRV-1B at 107 PFU/mouse or PBS (control) for 24 h. A: representative photomicrographs of hematoxylin and eosin stained lung tissue sections for peribronchial inflammatory infiltrates (black arrow, original magnification, 200×). B: quantitative histopathology scores. Data are presented as means ± SE (n = 3–4 mice/group). *P < 0.05, compared with PBS controls or HRV-infected WT mice.
Overexpressing human GDF15 protein in mouse airway epithelial cells promotes viral release during HRV-1B infection.
ALI cultures of primary tracheal epithelial cells from naïve hGDF15 Tg+ mice and WT littermates were infected with HRV-1B for 24 h to study the effect of human GDF15 protein on airway epithelial responses to HRV. We found that cultured hGDF15 Tg+ mouse tracheal epithelial cells consistently produced high levels of human GDF15 protein. Moreover, human GDF15 protein levels were not significantly affected by HRV-1B infection (490 ± 13 pg/ml in PBS-treated hGDF15 Tg+ cells vs. 467 ± 6 pg/ml in HRV-infected hGDF15 Tg+ cells). In contrast, in WT cells, HRV-1B induced mouse GDF15 protein production although the absolute levels were low (59 ± 3 pg/ml in PBS-treated WT cells vs. 78 ± 5 pg/ml in HRV-infected WT cells, P = 0.0475). The release of infectious virus particles was significantly higher in the apical side of hGDF15 Tg+ cells following HRV-1B infection compared with WT cells (27 ± 9 × 103 PFU/transwell in hGDF15 Tg+ cells vs. 6 ± 2 × 103 PFU/transwell in WT cells, P = 0.03). However, in cultured hGDF15 Tg+ mouse tracheal epithelial cells, we could not reproduce the inhibition of IFN-λ2/3 (IL-28A/B) and the increased production of KC and IP-10, which were observed in HRV-infected hGDF15 Tg+ mice.
GDF15 facilitates viral replication and inflammation via inhibiting production of IFN-λ1/IL-29 during HRV-16 infection in primary human airway epithelial cells.
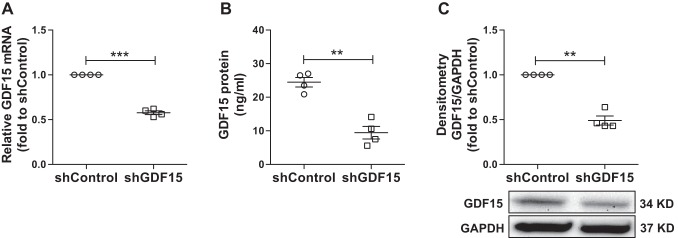
HBE cells are the major host cells for HRV entry and replication to initiate effective host defense (34). Induction of IL-8, IP-10, and type III IFNs significantly depends on active HRV replication (41, 48). HRV-16 is a widely used experimental challenge rhinovirus for studying disease exacerbations of chronic lung diseases (i.e., COPD) and replicates more readily in human airway epithelial cells (17). Thus ALI cultures of normal HBE cells with or without GDF15 knockdown were infected with HRV-16 for 24 h to further evaluate the effect of GDF15 on viral replication, production of IFN-λ1/IL-29, and inflammation. The successful GDF15 knockdown was confirmed at both mRNA and protein levels in shGDF15 cells by quantitative real-time RT-PCR, ELISA, and Western blot before HRV-16 infection (Fig. 4).
Fig. 4.
Successful knockdown of growth differentiation factor 15 (GDF15) in well-differentiated human primary airway epithelial cells. Normal human bronchial epithelial cells transduced with lentiviruses encoding human GDF15 shRNA (shGDF15) or control shRNA (shControl) were grown at air-liquid interface for 28 days to examine GDF15 expression. A: GDF15 mRNA levels were measured by quantitative real-time RT-PCR and normalized to GAPDH mRNA levels via the ΔΔCt method. B: GDF15 protein levels in basolateral supernatants were assessed by ELISA. C, top: quantitative Western blot densitometry data of GDF15 and GAPDH protein (loading control). C, bottom: representative Western blot pictures. Data are presented as means ± SE from 4 independent experiments. **P < 0.01, ***P < 0.001.
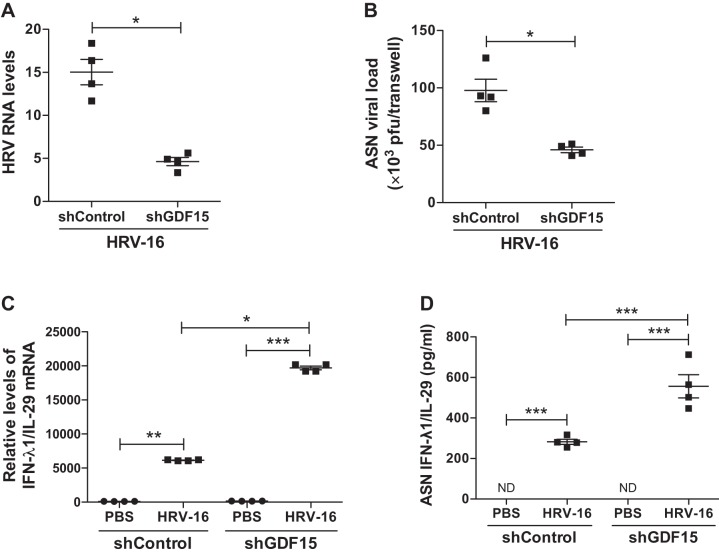
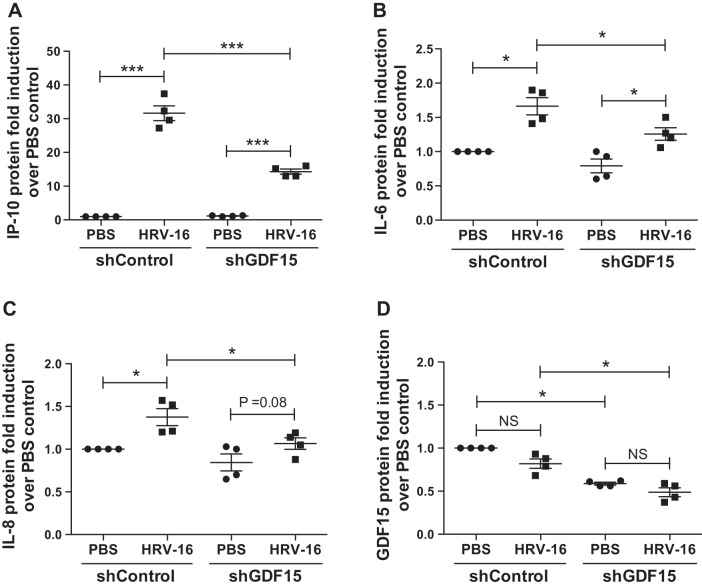
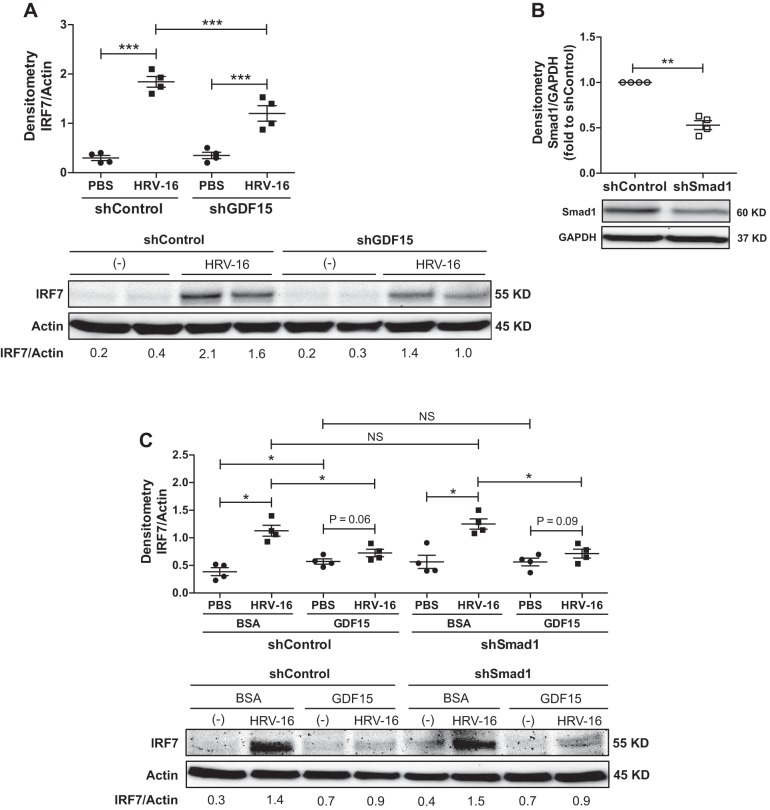
Compared with HRV-infected shControl cells, knockdown of GDF15 significantly reduced HRV-16 RNA levels in cells (74% decrease, P = 0.047, Fig. 5A) and the release of infectious virus particles in the apical side of ALI cultures (53% decrease, P = 0.03, Fig. 5B). This reduction of viral load was accompanied by an enhanced IFN-λ1/IL-29 mRNA expression (Fig. 5C) and protein production (Fig. 5D) in cells with GDF15 knockdown and HRV-16 infection compared with HRV-infected shControl cells. Moreover, HRV-16 infection significantly induced the production of IP-10, IL-6, and IL-8 protein, which was attenuated after GDF15 knockdown (Fig. 6, A–C). Interestingly, HRV-16 infection resulted in a comparably marginal reduction in GDF15 protein in both shControl and shGDF15 cells (Fig. 6D).
Fig. 5.
Knockdown of growth differentiation factor 15 (GDF15) reduces viral load and enhances IFN-λ1/IL-29 expression during HRV-16 infection in human airway epithelial cells. Brushed normal human bronchial epithelial cells transduced with lentiviruses encoding human GDF15 shRNA (shGDF15) or control shRNA (shControl) were grown at air-liquid interface and infected with HRV-16 [multiplicity of infection (MOI) 1] or PBS (control) for 24 h. A: HRV RNA levels were measured by quantitative real-time RT-PCR. B: released virus particles in apical supernatants (ASN) were assessed by plaque assay. C: IFN-λ1/IL-29 mRNA levels were measured by quantitative real-time RT-PCR. D: IFN-λ1/IL-29 protein levels in ASN were assessed by ELISA. Relative levels of mRNA or RNA expression were normalized to GAPDH mRNA levels and calculated via the ΔΔCt method. Data are presented as means ± SE from 4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, compared with PBS controls or shControl cells; ND, Not detectable.
Fig. 6.
Knockdown of growth differentiation factor 15 (GDF15) reduces virus-induced inflammation during HRV-16 infection in human airway epithelial cells. Normal human bronchial epithelial cells transduced with lentiviruses encoding human GDF15 shRNA (shGDF15) or control shRNA (shControl) were grown at air-liquid interface and infected with HRV-16 [multiplicity of infection (MOI) 1] or PBS (control) for 24 h. Protein levels of IP-10 (A), IL-6 (B), IL-8 (C), and GDF15 (D) in apical supernatants (ASN) were assessed by ELISA. Data are presented as means ± SE from four independent experiments. *P < 0.05, ***P < 0.001, compared with PBS controls or shControl cells; NS, not significant.
Smad1 cooperates with IRF7 in response to GDF15 stimulation and HRV infection in airway epithelial cells.
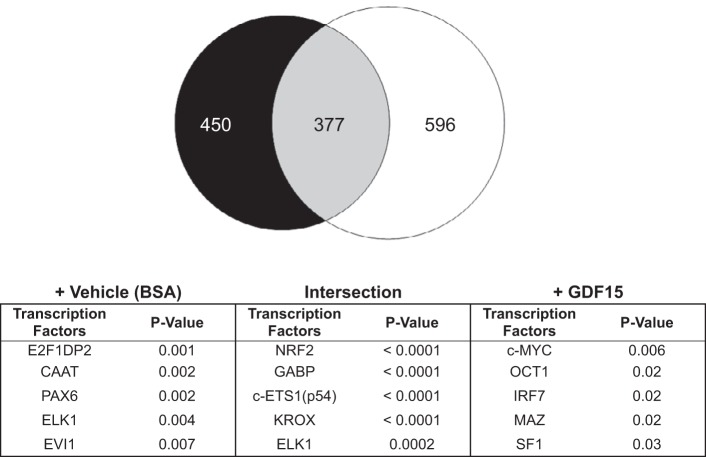
Our previous study shows that GDF15 activates the Smad1 pathway in human airway epithelial cells (61). To elucidate the genome-wide transcriptional regulation by Smad1 associated with GDF15, we performed the ChIP assay with a pSmad1 antibody followed by next-generation sequencing using normal hTBE cells treated with GDF15 or BSA for 2 h. As shown in Fig. 7, we found 450 peak-associated genes were enriched by pSmad1 ChIP without GDF15 treatment (BSA control), 596 peak-associated genes were enriched by pSmad1 ChIP after GDF15 treatment, and 377 peak-associated genes enriched by pSmad1 ChIP were related to both BSA and GDF15 treatments. Intriguingly, upon GDF15 stimulation, identified pSmad1 peaks were not associated with any known host defense or inflammatory response gene. However, 137 of pSmad1 peak-associated genes in GDF15-stimulated cells were predicted to bear IRF7 binding site, a key transcription factor in host immune responses to HRV infection (51). These potential IRF7-regulated pSmad1-binding targets were enriched into gene ontology terms related to defense response, antimicrobial humoral response, inflammatory response, and regulation of apoptosis (Table 1).
Fig. 7.
Chromatin immunoprecipitation followed by next-generation sequencing (ChIP-seq) defines genome-wide transcriptional regulation by pSmad1 protein upon growth differentiation factor 15 (GDF15) in human airway epithelial cells. Normal human tracheobronchial epithelial cells in submerged culture were treated with 0.1% BSA-HCl (control) or recombinant human GDF15 (25 ng/ml) for 2 h to perform the phosphorylated-Smad1 (pSmad1) ChIP-seq analysis.
Table 1.
Enriched gene ontology biological processes for the potential interferon regulatory factor 7-regulated pSmad1-binding targets in growth differentiation factor 15-stimulated human airway epithelial cells
| GO ID and Term | pSmad1 Peak-Associated Genes |
|---|---|
| GO:0006952 [5]: defense response | ADA BLM COLEC11 GTPBP1 IFI27 TACR1 ULBP1 |
| GO:0006955 [4]: immune response | ADA BLM COLEC11 GTPBP1 IFI27 TACR1 ULBP1 |
| GO:0009613 [5]: response to pest, pathogen or parasite | ADA BLM COLEC11 IFI27 TACR1 |
| GO:0006959 [5]: humoral immune response | ADA BLM COLEC11 |
| GO:0019735 [7]: antimicrobial humoral response | ADA BLM |
| GO:0006954 [5]: inflammatory response | TACR1 |
| GO:0043065 [7]: positive regulation of apoptosis | MDM4 PRKCE PRODH |
GO, gene ontology.
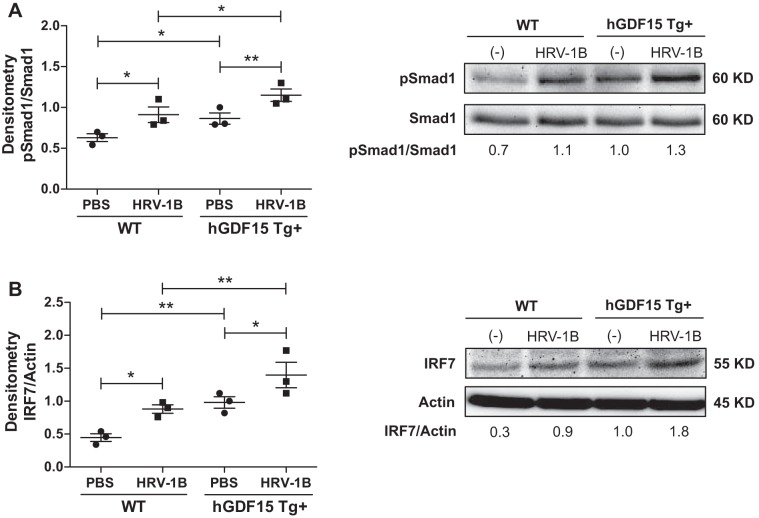
To validate the ChIP-sequencing data, we first examined Smad1 activation and IRF7 expression in ALI cultures of hGDF15 Tg+ mouse tracheal epithelial cells with or without HRV-1B infection. Without HRV-1B infection, hGDF15 Tg+ cells already exhibited increased Smad1 phosphorylation (Fig. 8A) and IRF7 expression (Fig. 8B) at baseline as compared with WT cells. HRV-1B infection further enhanced Smad1 phosphorylation, associated with upregulation of IRF7 protein in both hGDF15 Tg+ and WT cells. Next, we measured the IRF7 protein levels in ALI cultures of HBE cells with or without GDF15 knockdown and HRV-16 infection. IRF7 protein was significantly induced by HRV-16, which was reduced when GDF15 was knocked down (Fig. 9A). Lastly, we examined IRF7 protein levels in ALI cultures of HBE cells with or without Smad1 knockdown in the absence or presence of HRV-16 infection and GDF15 stimulation. The successful knockdown of Smad1 protein in shSmad1 cells was confirmed by Western blot analysis (Fig. 9B). As HRV-16 markedly induced IRF7 protein in shControl and shSmad1 cells, rhGDF15 at 25 ng/ml only triggered a weak induction of IRF7 protein in shControl cells. Neither HRV-induced nor GDF15-induced IRF7 protein was affected by Smad1 knockdown (Fig. 9C).
Fig. 8.
Overexpressing human growth differentiation factor 15 (GDF15) enhances activation of Smad1 and interferon regulatory factor 7 (IRF7) in mouse airway epithelial cells. Primary tracheal epithelial cells from naïve human GDF15 transgenic (hGDF15 Tg+) mice or wild-type (WT) littermates were grown at air-liquid interface and infected with HRV-1B [multiplicity of infection (MOI) 1] or PBS (control) for 24 h. A, left: quantitative Western blot densitometry data of phosphorylated-Smad1 (pSmad1) and total Smad1 protein. A, right: representative Western blot pictures; B, left: quantitative Western blot densitometry data of IRF7 and β-actin protein (loading control). B, right: representative Western blot pictures. Data are presented as means ± SE from three independent experiments. *P < 0.05, **P < 0.01, compared with PBS controls or HRV-infected WT cells.
Fig. 9.
Smad1 cooperates with interferon regulatory factor 7 (IRF7) in response to HRV infection via growth differentiation factor 15 (GDF15) signaling in human airway epithelial cells. A: normal human bronchial epithelial cells transduced with lentiviruses encoding human GDF15 shRNA (shGDF15) or control shRNA (shControl) were grown at air-liquid interface and infected with HRV-16 [multiplicity of infection (MOI) 1] or PBS (control) for 24 h. A, top: quantitative Western blot densitometry data of IRF7 and β-actin protein (loading control). A, bottom: representative Western blot pictures. B, top: normal human tracheobronchial epithelial cells transduced with lentiviruses encoding human Smad1 shRNA (shSmad1) or control shRNA (shControl) were grown at air-liquid interface for 28 days to examine Smad1 protein expression. B, bottom: quantitative Western blot densitometry data of Smad1 and GAPDH protein (loading control). C: normal human tracheobronchial epithelial cells transduced with lentiviruses encoding human Smad1 shRNA (shSmad1) or control shRNA (shControl) were grown at air-liquid interface, pretreated with 0.1% BSA-HCl (control) or recombinant human GDF15 (25 ng/ml) for 72 h, and infected with HRV-16 (MOI 1) or PBS for 24 h. C, top: quantitative Western blot densitometry data of IRF7 and β-actin protein (loading control). C, bottom: representative Western blot pictures. Data are presented as means ± SE from 4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, compared with PBS controls or shControl cells; NS, not significant.
DISCUSSION
Our study has identified a novel function of GDF15 in promoting lung virus infection and virus-associated inflammation during HRV infection and the underlying mechanism. First, overexpressing human GDF15 protein in WT mice exaggerates inflammatory responses with increased virus release and a moderate decrease of IFN-λ2/3 (IL-28A/B) in the lung. Second, GDF15 facilitates viral replication and inflammation via inhibiting IFN-λ1/IL-29 production against HRV in human airway epithelial cells. Lastly, Smad1 may cooperate with IRF7 in regulating the responses of airway epithelial cells to HRV infection partly via GDF15 signaling.
While increased GDF15 is suggested as an independent predictor of all-cause COPD exacerbations, it remains to be determined whether GDF15 regulates airway epithelial responses to respiratory viral (i.e., HRV) infection contributing to the pathogenesis of COPD exacerbations (16, 23, 32). In the present study, we used a unique hGDF15 Tg+ mouse strain that mimics upregulation of GDF15 in COPD airways to examine its role in regulating host defense and lung inflammation during HRV infection. Upon HRV-1B infection, hGDF15 Tg+ mice had more releases of infectious viral particles in the BAL with decreased lung IFN-λ2/3 (IL-28A/B) mRNA expression. Increased neutrophilic inflammation is a characteristic feature of COPD in both stable-state and virus-induced exacerbations (38). Indeed, hGDF15 Tg+ mice exhibited increased BAL neutrophil numbers, coupled with a greater extent of inflammatory infiltration, at baseline and particularly after HRV-1B infection. However, there was no difference observed in BAL macrophages and lymphocytes between hGDF15 Tg+ mice and WT controls at 24 h following HRV-1B infection. This is not surprising since HRV-1B infection is mild and transient in naïve adult WT mice, with a predominance of neutrophils in the BAL at 24 h and increase of BAL lymphocytes at late time points (>2 days) postinfection (4, 33, 47).
Besides, HRV-infected hGDF15 Tg+ mice showed an enhanced production of inflammatory cytokines (i.e., IL-6, KC, IP-10) involved in neutrophil recruitment and activation. As we are aware, prior studies show that hGDF15 Tg+ mice exhibit a reduced inflammatory response (i.e., KC and IL-6) to a systemic LPS injection (27, 55). The possible reasons for this discrepancy are due to different delivery routes, the complexity of live HRV, and involvement of distinct Toll-like receptor signaling in host immune responses. Activated airway epithelial cells are the major cell source of production of IP-10 following HRV infection (46, 48, 57), but activated neutrophils also secrete IP-10 to orchestrate the adaptive immune response (10, 39). Therefore, the enhanced IP-10 production in HRV-infected hGDF15 Tg+ mice is more likely as a consequence of both airway epithelial response to the increased virus load and activated neutrophil accumulation in the lung. Moreover, IP-10 recruits inflammatory cells to the tissue to perpetuate inflammation. Although no difference of BAL lymphocytes was observed at 24 h postinfection between hGDF15 Tg+ mice and WT littermates, we could not exclude the effect of GDF15 on the recruitment of lymphocytes through IP-10 at later time points following HRV-1B infection. Future studies in hGDF15 Tg+ mice with depletion of a particular cell type across multiple time points are needed to precisely clarify the cell-specific contribution to IP-10 production and its role in regulating host immune responses during HRV infection. Collectively, GDF15 overexpression leads to increased lung viral release and inflammation during HRV infection, suggesting a potential link between GDF15 upregulation and greater HRV infection in the airways. Future studies to characterize the relationship of GDF15 levels and HRV infection are required to establish such causality in cigarette smoke-exposed mouse models and COPD smokers.
Airway epithelial cells are the primary site of HRV infection and represent the first line of host defense. As anticipated, we can reproduce the enhanced HRV-1B release in well-differentiated hGDF15 Tg+ mouse tracheal epithelial cells. However, the enhancement of KC and IP-10 production and inhibition of IFN-λ2/3 (IL-28A/B) expression were not replicated in vitro. This is possible because of the low HRV-1B infection efficiency in well-differentiated mouse tracheal epithelial cell cultures and more importantly a limited HRV-1B replication and a quick decline of HRV-1B titer within 24 h postinfection as reported previously (25). HBE cells are the primary host cells for viral entry and replication and express a high baseline production of GDF15 protein at ALI cultures in vitro (60). Therefore, in our current study, we chose to use ALI cultures of normal HBE cells with GDF15 knockdown as a model system to evaluate the direct role of GDF15 in viral replication and airway epithelial responses. A marginal GDF15 protein reduction is observed in our well-differentiated human airway epithelial cell cultures following HRV-16 infection, likely due to cell detachment via apoptosis or infected-cell shedding induced by live virus replication (14, 20, 28, 40). GDF15 knockdown significantly reduces viral replication and the released infectious viruses, associated with an enhanced IFN-λ1/IL-29 production and attenuated release of IP-10, IL-6, and IL-8 protein. Our results are consistent with a prior study showing that GDF15 overexpression increases virus replication, whereas GDF15 knockdown inhibits virus replication in human hepatoma cells with hepatitis C virus infection (45). Moreover, our findings partially support a prior study showing an increased HRV titer and IP-10 production in COPD bronchial epithelial cells after HRV-39 infection (41). Of note, while HRV-induced IFN-λ1/IL-29 and IP-10 production are consistently observed in human airway epithelial cells, HRV-induced IP-10 production is not dependent on the production of type III IFNs but requires HRV internalization and replication (48, 65). Thus IP-10 reduction in GDF15-knockdown cells is most likely the consequence of a decreased HRV replication and could be independent of IFN-λ1/IL-29 production. The disagreement of IFN-λ1/IL-29 production following HRV infection between these two studies is possibly related to the experimentation differences (i.e., disease condition, cell source, virus strain, and infection method) and requires future investigation. Moreover, we are currently developing the experimental model of chronic cigarette smoke exposure in well-differentiated HBE cells with GDF15 knockdown and HRV-16 infection to study the function of cigarette smoke-induced GDF15 in airway epithelial responses to HRV infection. Interestingly, although type III IFN-λs are best known for their antiviral activity, recent studies suggest that they have an unexpected anti-inflammatory role in mice with autoimmune and airway inflammatory diseases (7, 35, 64). Taken together, our findings indicate that GDF15 overproduction promotes lung HRV infection and inflammation partly through impairing IFN-λ1/IL-29 production in airway epithelial cells. GDF15-mediated type III IFN inhibition may not only contribute to the impaired antiviral defense but also the exaggerated inflammation in response to HRV infection. Further research is required to fully understand the complex roles of type III IFN in the regulation of airway antiviral defense and inflammation following HRV infection with or without GDF15 overproduction.
Our previous study shows that GDF15 activates Smad1 signaling in human airway epithelial cells (61). In the current study, our pSmad1 ChIP-sequencing data confirm that target genes bound by pSmad1 are significantly affected by GDF15 stimulation in human airway epithelial cells. Interestingly, the potential IRF7 coregulated pSmad1-binding target genes (e.g., ADA, BLM, COLEC11, GTPBP1, IFI27, TACR1, ULBP1, MDM4, PRKCE, and PRODH) are associated with host defense response, inflammatory response, and cell apoptosis upon GDF15 in human airway epithelial cells. In agreement with the ChIP-seq data, hGDF15 Tg+ mouse tracheal epithelial cells exhibit Smad1 phosphorylation at baseline, which is enhanced by HRV-1B infection. The phosphorylation of Smad1 is linked to IRF7 induction at baseline and after HRV-1B infection. Moreover, GDF15 knockdown leads to a significant reduction in HRV-16-induced IRF7 protein in well-differentiated human airway epithelial cells. We could not detect Smad1 phosphorylation after HRV-16 infection in this experiment since cells were harvested at a very late time point (48 h after starting HRV infection). All these results suggest that HRV infection upregulates IRF7 protein partly through GDF15 signaling in airway epithelial cells.
Finally, we show that Smad1 does not directly regulate IRF7 expression since induction of IRF7 protein by HRV-16 and GDF15 is not affected by Smad1 knockdown in well-differentiated human airway epithelial cells. Although rhGDF15 protein at 25 ng/ml is a physiologically relevant dose determined from GDF15 protein levels in epithelial lining fluid of cigarette smoke-exposed human airways (60), it did not induce IRF7 protein efficiently because of a high baseline GDF15 production in well-differentiated human airway epithelial cells at ALI cultures. Additionally, we could not conclude if IRF7 directly or indirectly regulates airway epithelial responses after HRV infection with the current data. Previous studies suggest that knockdown of IRF7 decreases IL-6 and IP-10 expression in response to HRV-16 infection in undifferentiated human airway epithelial cells (9). Knockdown of IRF7 reduces lung neutrophil infiltration in HRV-1B-infected WT mice (18). Therefore, we speculate that IRF7 may promote inflammation (i.e., IL-6, IL-8, and IP-10) in the lung during HRV infection, especially when GDF15 is upregulated. Future studies with Smad1 and IRF7 knockdown in well-differentiated HBE cells are warranted to dissect their exact role and interaction in regulating airway epithelial inflammation with GDF15 overproduction and HRV infection. All these findings provide the first evidence that HRV infection activates Smad1 signaling and upregulates IRF7 protein to promote inflammation in airway epithelial cells, partly through GDF15 signaling. IRF7 may work as one of the cooperative binding factors recruited to complex with pSmad1 for regulating its target genes during GDF15 overproduction and HRV infection.
In conclusion, our data for the first time suggest a novel function of GDF15 in promoting lung HRV infection and virus-associated inflammation, which may serve as a new mechanism contributing to the increased susceptibility and severity of respiratory viral (i.e., HRV) infection in cigarette smoke-exposed airways. Future studies are required to confirm our findings in well-differentiated HBE cell cultures and mouse models with cigarette smoke exposure and HRV infection and to evaluate the link between GDF15 upregulation and greater HRV infection in COPD smokers. All these efforts will provide necessary insights to define GDF15 signaling as a new target to prevent and treat respiratory viral (i.e., HRV) infection in COPD exacerbations.
GRANTS
This study was supported by the Flight Attendant Medical and Research Institute Young Clinical Scientist Award Grant No. 123254 (to Q. Wu). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.W. conceived and designed research; Q.W., D.J., N.R.S., L.H., and B.P.O. performed experiments; Q.W., L.H., B.P.O., and T.E. analyzed data; Q.W., L.H., B.P.O., T.E., and H.W.C. interpreted results of experiments; Q.W., D.J., and L.H. prepared figures; Q.W. drafted manuscript; Q.W., D.J., N.R.S., L.H., B.P.O., T.E., O.E., and H.W.C. edited and revised manuscript; Q.W., D.J., N.R.S., L.H., B.P.O., T.E., O.E., and H.W.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bicheng Zhang, Kristina Ternyak, and Jennifer L. Matsuda at National Jewish Health Mouse Genetics Core Facility for excellent service in mouse genotyping.
REFERENCES
- 1.Aaron SD, Angel JB, Lunau M, Wright K, Fex C, Le Saux N, Dales RE. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163: 349–355, 2001. doi: 10.1164/ajrccm.163.2.2003122. [DOI] [PubMed] [Google Scholar]
- 2.Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, Mishina Y, Martin DW, Shoieb A, McEntee MF, Eling TE. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology 131: 1553–1560, 2006. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, Lindblad K, Patel H, Rugman P, Dodson P, Jenkins M, Saunders M, Newbold P, Green RH, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 184: 662–671, 2011. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, Zhu J, Glanville N, Choy KJ, Jourdan P, Burnet J, Tuthill TJ, Pedrick MS, Hurle MJ, Plumpton C, Sharp NA, Bussell JN, Swallow DM, Schwarze J, Guy B, Almond JW, Jeffery PK, Lloyd CM, Papi A, Killington RA, Rowlands DJ, Blair ED, Clarke NJ, Johnston SL. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med 14: 199–204, 2008. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 55: 114–120, 2000. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder AK, Kosak JP, Janardhan KS, Moser G, Eling TE, Korach KS. Expression of human NSAID activated gene 1 in mice leads to altered mammary gland differentiation and impaired lactation. PLoS One 11: e0146518, 2016. [Erratum. PLoS One 11: e0151504, 2016.] doi: 10.1371/journal.pone.0146518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blazek K, Eames HL, Weiss M, Byrne AJ, Perocheau D, Pease JE, Doyle S, McCann F, Williams RO, Udalova IA. IFN-λ resolves inflammation via suppression of neutrophil infiltration and IL-1β production. J Exp Med 212: 845–853, 2015. doi: 10.1084/jem.20140995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA 94: 11514–11519, 1997. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosco A, Wiehler S, Proud D. Interferon regulatory factor 7 regulates airway epithelial cell responses to human rhinovirus infection. BMC Genomics 17: 76, 2016. doi: 10.1186/s12864-016-2405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassatella MA, Gasperini S, Calzetti F, Bertagnin A, Luster AD, McDonald PP. Regulated production of the interferon-gamma-inducible protein-10 (IP-10) chemokine by human neutrophils. Eur J Immunol 27: 111–115, 1997. doi: 10.1002/eji.1830270117. [DOI] [PubMed] [Google Scholar]
- 11.Cekanova M, Lee SH, Donnell RL, Sukhthankar M, Eling TE, Fischer SM, Baek SJ. Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prev Res (Phila) 2: 450–458, 2009. doi: 10.1158/1940-6207.CAPR-09-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chrysovergis K, Wang X, Kosak J, Lee SH, Kim JS, Foley JF, Travlos G, Singh S, Baek SJ, Eling TE. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int J Obes 38: 1555–1564, 2014. doi: 10.1038/ijo.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu HW, Honour JM, Rawlinson CA, Harbeck RJ, Martin RJ. Effects of respiratory Mycoplasma pneumoniae infection on allergen-induced bronchial hyperresponsiveness and lung inflammation in mice. Infect Immun 71: 1520–1526, 2003. doi: 10.1128/IAI.71.3.1520-1526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deszcz L, Gaudernak E, Kuechler E, Seipelt J. Apoptotic events induced by human rhinovirus infection. J Gen Virol 86: 1379–1389, 2005. doi: 10.1099/vir.0.80754-0. [DOI] [PubMed] [Google Scholar]
- 15.Djamin RS, Uzun S, Snelders E, Kluytmans JJ, Hoogsteden HC, Aerts JG, Van Der Eerden MM. Occurrence of virus-induced COPD exacerbations during four seasons. Infect Dis (Lond) 47: 96–100, 2015. doi: 10.3109/00365548.2014.968866. [DOI] [PubMed] [Google Scholar]
- 16.Freeman CM, Martinez CH, Todt JC, Martinez FJ, Han MK, Thompson DL, McCloskey L, Curtis JL. Acute exacerbations of chronic obstructive pulmonary disease are associated with decreased CD4+ & CD8+ T cells and increased growth & differentiation factor-15 (GDF-15) in peripheral blood. Respir Res 16: 94, 2015. doi: 10.1186/s12931-015-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fullen DJ, Murray B, Mori J, Catchpole A, Borley DW, Murray EJ, Balaratnam G, Gilbert A, Mann A, Hughes F, Lambkin-Williams R. A tool for investigating asthma and COPD exacerbations: a newly manufactured and well characterised GMP wild-type human rhinovirus for use in the human viral challenge model. PLoS One 11: e0166113, 2016. doi: 10.1371/journal.pone.0166113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girkin J, Hatchwell L, Foster P, Johnston SL, Bartlett N, Collison A, Mattes J. CCL7 and IRF-7 mediate hallmark inflammatory and IFN responses following rhinovirus 1B infection. J Immunol 194: 4924–4930, 2015. doi: 10.4049/jimmunol.1401362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glanville N, Johnston SL. Challenges in developing a cross-serotype rhinovirus vaccine. Curr Opin Virol 11: 83–88, 2015. doi: 10.1016/j.coviro.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Griggs TF, Bochkov YA, Basnet S, Pasic TR, Brockman-Schneider RA, Palmenberg AC, Gern JE. Rhinovirus C targets ciliated airway epithelial cells. Respir Res 18: 84, 2017. doi: 10.1186/s12931-017-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross CA, Bowler RP, Green RM, Weinberger AR, Schnell C, Chu HW. beta2-agonists promote host defense against bacterial infection in primary human bronchial epithelial cells. BMC Pulm Med 10: 30, 2010. doi: 10.1186/1471-2466-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunawardana N, Finney L, Johnston SL, Mallia P. Experimental rhinovirus infection in COPD: implications for antiviral therapies. Antiviral Res 102: 95–105, 2014. doi: 10.1016/j.antiviral.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husebø GR, Grønseth R, Lerner L, Gyuris J, Hardie JA, Bakke PS, Eagan TM. Growth differentiation factor-15 is a predictor of important disease outcomes in patients with COPD. Eur Respir J 49: 1601298, 2017. doi: 10.1183/13993003.01298-2016. [DOI] [PubMed] [Google Scholar]
- 24.Ioannidis I, Ye F, McNally B, Willette M, Flaño E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J Virol 87: 3261–3270, 2013. doi: 10.1128/JVI.01956-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs SE, Lamson DM, St. George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev 26: 135–162, 2013. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, Papi A, Stanciu LA, Kotenko SV, Johnston SL. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy 64: 375–386, 2009. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim JM, Kosak JP, Kim JK, Kissling G, Germolec DR, Zeldin DC, Bradbury JA, Baek SJ, Eling TE. NAG-1/GDF15 transgenic mouse has less white adipose tissue and a reduced inflammatory response. Mediators Inflamm 2013: 641851, 2013. doi: 10.1155/2013/641851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, Finkbeiner WE, Dolganov GM, Widdicombe JH, Boushey HA, Avila PC. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol 123: 1384–90.e2, 2009. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, Papi A, Stanciu LA, Kon OM, Johnson M, Johnston SL. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 183: 734–742, 2011. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosser AG, Vrtis R, Burchell L, Lee WM, Dick CR, Weisshaar E, Bock D, Swenson CA, Cornwell RD, Meyer KC, Jarjour NN, Busse WW, Gern JE. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med 171: 645–651, 2005. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 31.Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, Beck SC, South VJ, Dinh TQ, Cash-Mason TD, Cavanaugh CR, Nelson S, Huang C, Hunter MJ, Rangwala SM. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med 23: 1150–1157, 2017. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- 32.Mutlu LC, Altintas N, Aydin M, Tulubas F, Oran M, Kucukyalin V, Kaplan G, Gurel A. Growth differentiation factor-15 is a novel biomarker predicting acute exacerbation of chronic obstructive pulmonary disease. Inflammation 38: 1805–1813, 2015. doi: 10.1007/s10753-015-0158-5. [DOI] [PubMed] [Google Scholar]
- 33.Nagarkar DR, Bowman ER, Schneider D, Wang Q, Shim J, Zhao Y, Linn MJ, McHenry CL, Gosangi B, Bentley JK, Tsai WC, Sajjan US, Lukacs NW, Hershenson MB. Rhinovirus infection of allergen-sensitized and -challenged mice induces eotaxin release from functionally polarized macrophages. J Immunol 185: 2525–2535, 2010. doi: 10.4049/jimmunol.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, Meyer J, Lackie PM, Sanderson G, Holgate ST, Johnston SL. Rhinoviruses infect the lower airways. J Infect Dis 181: 1875–1884, 2000. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 35.Qiu C, Li Y, Zhou M, Liu J, Li M, Wu Y, Xu D, Li M. Hydrodynamic delivery of IL-28B (IFN-λ3) gene ameliorates lung inflammation induced by cigarette smoke exposure in mice. Biochem Biophys Res Commun 447: 513–519, 2014. doi: 10.1016/j.bbrc.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, Jeffery PK. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 168: 968–975, 2003. doi: 10.1164/rccm.200208-794OC. [DOI] [PubMed] [Google Scholar]
- 37.Quint JK, Donaldson GC, Goldring JJ, Baghai-Ravary R, Hurst JR, Wedzicha JA. Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. Chest 137: 812–822, 2010. doi: 10.1378/chest.09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quint JK, Wedzicha JA. The neutrophil in chronic obstructive pulmonary disease. J Allergy Clin Immunol 119: 1065–1071, 2007. doi: 10.1016/j.jaci.2006.12.640. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Perez N, Schiavi E, Frei R, Ferstl R, Wawrzyniak P, Smolinska S, Sokolowska M, Sievi NA, Kohler M, Schmid-Grendelmeier P, Michalovich D, Simpson KD, Hessel EM, Jutel M, Martin-Fontecha M, Palomares O, Akdis CA, O’Mahony L. Altered fatty acid metabolism and reduced stearoyl-coenzyme a desaturase activity in asthma. Allergy 72: 1744–1752, 2017. doi: 10.1111/all.13180. [DOI] [PubMed] [Google Scholar]
- 40.Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med 178: 1271–1281, 2008. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider D, Ganesan S, Comstock AT, Meldrum CA, Mahidhara R, Goldsmith AM, Curtis JL, Martinez FJ, Hershenson MB, Sajjan U. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 332–340, 2010. doi: 10.1164/rccm.200911-1673OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, Wedzicha JA. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164: 1618–1623, 2001. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 43.Seemungal TA, Harper-Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J 16: 677–683, 2000. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu S, Kadowaki M, Yoshioka H, Kambe A, Watanabe T, Kinyamu HK, Eling TE. Proteasome inhibitor MG132 induces NAG-1/GDF15 expression through the p38 MAPK pathway in glioblastoma cells. Biochem Biophys Res Commun 430: 1277–1282, 2013. doi: 10.1016/j.bbrc.2012.11.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Si Y, Liu X, Cheng M, Wang M, Gong Q, Yang Y, Wang T, Yang W. Growth differentiation factor 15 is induced by hepatitis C virus infection and regulates hepatocellular carcinoma-related genes. PLoS One 6: e19967, 2011. doi: 10.1371/journal.pone.0019967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singanayagam A, Glanville N, Walton RP, Aniscenko J, Pearson RM, Pinkerton JW, Horvat JC, Hansbro PM, Bartlett NW, Johnston SL. A short-term mouse model that reproduces the immunopathological features of rhinovirus-induced exacerbation of COPD. Clin Sci (Lond) 129: 245–258, 2015. doi: 10.1042/CS20140654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song DJ, Miller M, Beppu A, Rosenthal P, Das S, Karta M, Vuong C, Mehta AK, Croft M, Broide DH. Rhinovirus infection of ORMDL3 transgenic mice is associated with reduced rhinovirus viral load and airway inflammation. J Immunol 199: 2215–2224, 2017. doi: 10.4049/jimmunol.1601412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol 289: L85–L95, 2005. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- 49.Thompson AB, Robbins RA, Romberger DJ, Sisson JH, Spurzem JR, Teschler H, Rennard SI. Immunological functions of the pulmonary epithelium. Eur Respir J 8: 127–149, 1995. doi: 10.1183/09031936.95.08010127. [DOI] [PubMed] [Google Scholar]
- 50.Tong Q, Weaver MR, Kosmacek EA, O’Connor BP, Harmacek L, Venkataraman S, Oberley-Deegan RE. MnTE-2-PyP reduces prostate cancer growth and metastasis by suppressing p300 activity and p300/HIF-1/CREB binding to the promoter region of the PAI-1 gene. Free Radic Biol Med 94: 185–194, 2016. doi: 10.1016/j.freeradbiomed.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Troy NM, Bosco A. Respiratory viral infections and host responses; insights from genomics. Respir Res 17: 156, 2016. doi: 10.1186/s12931-016-0474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Meeteren LA, Thorikay M, Bergqvist S, Pardali E, Stampino CG, Hu-Lowe D, Goumans MJ, ten Dijke P. Anti-human activin receptor-like kinase 1 (ALK1) antibody attenuates bone morphogenetic protein 9 (BMP9)-induced ALK1 signaling and interferes with endothelial cell sprouting. J Biol Chem 287: 18551–18561, 2012. doi: 10.1074/jbc.M111.338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wack A, Terczyńska-Dyla E, Hartmann R. Guarding the frontiers: the biology of type III interferons. Nat Immunol 16: 802–809, 2015. doi: 10.1038/ni.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Baek SJ, Eling TE. The diverse roles of nonsteroidal anti-inflammatory drug activated gene (NAG-1/GDF15) in cancer. Biochem Pharmacol 85: 597–606, 2013. doi: 10.1016/j.bcp.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Chrysovergis K, Kosak J, Eling TE. Lower NLRP3 inflammasome activity in NAG-1 transgenic mice is linked to a resistance to obesity and increased insulin sensitivity. Obesity (Silver Spring) 22: 1256–1263, 2014. doi: 10.1002/oby.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Chrysovergis K, Kosak J, Kissling G, Streicker M, Moser G, Li R, Eling TE. hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling. Aging (Albany NY) 6: 690–704, 2014. doi: 10.18632/aging.100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wark PA, Grissell T, Davies B, See H, Gibson PG. Diversity in the bronchial epithelial cell response to infection with different rhinovirus strains. Respirology 14: 180–186, 2009. doi: 10.1111/j.1440-1843.2009.01480.x. [DOI] [PubMed] [Google Scholar]
- 58.Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC) . Employment and activity limitations among adults with chronic obstructive pulmonary disease–United States, 2013. MMWR Morb Mortal Wkly Rep 64: 289–295, 2015. [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkinson TMA, Aris E, Bourne S, Clarke SC, Peeters M, Pascal TG, Schoonbroodt S, Tuck AC, Kim V, Ostridge K, Staples KJ, Williams N, Williams A, Wootton S, Devaster JM; AERIS Study Group . A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax 72: 919–927, 2017. doi: 10.1136/thoraxjnl-2016-209023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Q, Jiang D, Chu HW. Cigarette smoke induces growth differentiation factor 15 production in human lung epithelial cells: implication in mucin over-expression. Innate Immun 18: 617–626, 2012. doi: 10.1177/1753425911429837. [DOI] [PubMed] [Google Scholar]
- 61.Wu Q, Jiang D, Matsuda JL, Ternyak K, Zhang B, Chu HW. Cigarette smoke induces human airway epithelial senescence via growth differentiation factor 15 production. Am J Respir Cell Mol Biol 55: 429–438, 2016. doi: 10.1165/rcmb.2015-0143OC. [DOI] [PubMed] [Google Scholar]
- 62.Wu Q, Jiang D, Smith S, Thaikoottathil J, Martin RJ, Bowler RP, Chu HW. IL-13 dampens human airway epithelial innate immunity through induction of IL-1 receptor-associated kinase M. J Allergy Clin Immunol 129: 825–833.e2, 2012. doi: 10.1016/j.jaci.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Q, van Dyk LF, Jiang D, Dakhama A, Li L, White SR, Gross A, Chu HW. Interleukin-1 receptor-associated kinase M (IRAK-M) promotes human rhinovirus infection in lung epithelial cells via the autophagic pathway. Virology 446: 199–206, 2013. doi: 10.1016/j.virol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan B, Chen F, Xu L, Wang Y, Wang X. Interleukin-28B dampens airway inflammation through up-regulation of natural killer cell-derived IFN-γ. Sci Rep 7: 3556, 2017. doi: 10.1038/s41598-017-03856-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaheer RS, Wiehler S, Hudy MH, Traves SL, Pelikan JB, Leigh R, Proud D. Human rhinovirus-induced ISG15 selectively modulates epithelial antiviral immunity. Mucosal Immunol 7: 1127–1138, 2014. doi: 10.1038/mi.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zdrenghea MT, Mallia P, Johnston SL. Immunological pathways in virus-induced COPD exacerbations: a role for IL-15. Eur J Clin Invest 42: 1010–1015, 2012. doi: 10.1111/j.1365-2362.2012.02672.x. [DOI] [PubMed] [Google Scholar]
- 67.Zwaans WA, Mallia P, van Winden ME, Rohde GG. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—a systematic review. J Clin Virol 61: 181–188, 2014. doi: 10.1016/j.jcv.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]