Abstract
Postprandial hyperglycemia has deleterious effects on endothelial function. Restricting carbohydrate intake and postmeal walking have each been shown to reduce postprandial hyperglycemia, but their combination and subsequent effects on endothelial function have not been investigated. Here, we sought to examine the effect of blunting postprandial hyperglycemia by following a low-carbohydrate diet, with or without postmeal walking exercise, on markers of vascular health in type 2 diabetes (T2D). In a randomized crossover design, individuals with T2D (n = 11) completed three 4-day controlled diet interventions consisting of 1) low-carbohydrate diet alone (LC), 2) low-carbohydrate diet with 15-min postmeal walks (LC + Ex), and 3) low-fat control diet (CON). Fasting blood samples and brachial artery flow-mediated dilation (%FMD) were measured before and after each intervention. Total circulating microparticles (MPs), endothelial MPs, platelet MPs, monocyte-platelet aggregates, and adhesion molecules were assessed as biomarkers of vascular health. There was a significant condition × time interaction for %FMD (P = 0.01), with post hoc tests revealing improved %FMD after LC + Ex (+0.8 ± 1.0%, P = 0.02), with no change after LC or CON. Endothelial MPs were significantly reduced with the LC diet by ~45% (from 99 ± 60 to 44 ± 31 MPs/μl, P = 0.02), with no change after LC + Ex or CON (interaction: P = 0.04). Total MPs were lower (main effect time: P = 0.02), whereas monocyte-platelet aggregates were higher (main effect time: P < 0.01) after all interventions. Plasma adhesion molecules and C-reactive protein were unaltered. Attenuating postprandial hyperglycemic excursions using a low-carbohydrate diet combined with postmeal walking appears to be an effective strategy to improve endothelial function in individuals with T2D.
NEW & NOTEWORTHY Carbohydrate restriction and postmeal walking lower postprandial hyperglycemia in individuals with type 2 diabetes. Here, we show that the combination significantly improved endothelial function and that carbohydrate restriction alone reduced circulating endothelial microparticles in individuals with type 2 diabetes.
Listen to this article’s corresponding podcast at http://ajpheart.podbean.com/e/low-carb-diet-and-exercise-improve-endothelial-health/.
Keywords: flow-mediated dilation, glucose, glycemic control, high fat, low-carbohydrate high-fat diet, microparticles, walking
INTRODUCTION
Prevention of cardiovascular disease in individuals with type 2 diabetes (T2D) is a major treatment goal (29, 42). Within this, diet and exercise remain the cornerstone lifestyle therapies (42). Separately, and in combination, diet and exercise interventions significantly improve cardiovascular risk factors (24, 54, 71). Increased risk for cardiovascular disease (CVD) in individuals with T2D is attributed to a multitude of factors including hyperglycemia, inflammation, oxidative stress, and dyslipidemia (59). In addition, impaired flow-mediated dilation (FMD), a measure of endothelial function, is an early manifestation of CVD that disproportionately affects individuals with T2D (36). Markers of endothelial activation are also elevated in individuals with T2D (45); for example, endothelial microparticles (EMPs) and platelet-derived microparticles (MPs; extracellular vesicles, which are released from apoptotic or activated cells) and monocyte platelet aggregates (MPAs; which reflect platelet activation and inflammation) are markedly elevated under hyperglycemic conditions and are important novel pathogenic markers of vascular disease (3, 7, 43).
Postprandial hyperglycemia has emerged as an independent risk factor for the development of diabetes complications, including vascular disease (8, 10). Postprandial hyperglycemia is particularly detrimental to endothelial function, as various studies have shown that postprandial glucose excursions can directly promote oxidative stress, activate inflammatory pathways, reduce nitric oxide bioavailability, and impair FMD (11, 13, 50). Combined epidemiological and experimental studies have suggested that hyperglycemia-induced endothelial dysfunction could be a mechanistic link between postprandial glucose spikes and CVD risk (10).
Dietary carbohydrate restriction is reemerging as an effective approach for glycemic control (1, 26). Given that the rise in blood glucose concentration after a meal is largely dependent on the carbohydrate composition (57), reducing exogenous carbohydrate intake at each meal is a logical strategy to lower postprandial glucose and insulin responses (31, 35, 53). However, despite the immediate improvements in hyperglycemia observed with carbohydrate restriction (52, 61, 65), there is strong apprehension surrounding the adoption of a low-carbohydrate diet because it is typically high in fat [i.e., a low-carbohydrate high-fat (LCHF) diet] (46, 68). After a high-fat meal, several studies have observed a transient impairment in endothelial function and increase in endothelial MPs, which is typically attributed to postprandial hypertriglyceridemia (25, 64, 66, 70). Thus, by targeting one risk factor for endothelial dysfunction and CVD risk (i.e., postprandial hyperglycemia), a LCHF diet may introduce another (postprandial hypertriglyceridemia). However, studies showing detrimental effects of dietary fat on the endothelium have been acute single-meal studies, often involving combined high-fat and high-carbohydrate loads using shakes and/or fast food meals (25, 64, 66, 70). The response to several days of meals reflecting contemporary LCHF meals in T2D patients is unknown.
Exercise has well-established benefits for vascular and overall health (34) and thus may be an attractive addition to a LCHF diet to maximize glucose-lowering effects while mitigating any potential impairments caused by increasing dietary fat. Specifically, postmeal walking has been shown to markedly reduce postprandial hyperglycemia (22, 54) and lipemia (38, 56) in individuals with, and at risk for, T2D. However, to the best of our knowledge, no study has combined these two lifestyle approaches in an effort to optimize a lifestyle strategy for T2D.
The aim of the present study was to examine the effects of 4 days of a low-carbohydrate diet, with or without daily postmeal walking, on endothelial function and biomarkers of vascular health in individuals with T2D. Given that carbohydrate restriction leads to significant weight loss and metabolic adaptations in the first few weeks to months, we chose a short-term (4 days) intervention in this initial study to reduce the confounding effect of these factors on endothelial function outcomes (6, 16).
METHODS
Overview
Individuals with physician-diagnosed T2D [HbA1c > 6.5%, fasting plasma glucose > 7.0 mmol/l, or 2-h glucose oral glucose tolerance text > 11.1 mmol/l, Canadian Diabetes Association (32)] were recruited to complete three short-term controlled intervention periods in a randomized crossover design. Interventions for this proof-of-concept study were conducted across 4 days to reduce the confounding influence of body composition changes and to improve compliance and standardization. All food was provided to participants, and diets were matched for energy and protein content. Accelerometers (Actigraph wGTX3+) were worn to monitor activity for all conditions and confirm the completion of postmeal walks. This trial was registered with clinicaltrials.gov (NCT02683135) and approved by the University of British Columbia Clinical Research Ethics Board. Before study commencement, participants provided written informed consent.
Participants
Sixteen (n = 8 men and 8 women) aged between 48 and 72 yr, not on exogenous insulin and without diagnosed cardiovascular, kidney, or any other diabetes complications, were recruited from the local community. Individuals currently involved in a regular exercise routine (>3 days of structured exercise/wk for last 3 mo), following a low-carbohydrate diet, or unwilling to consume the provided meat-containing diets were excluded. Five of sixteen participants did not complete all three conditions due to family reasons (n = 1), inability or unwilling to follow study diets (n = 3), and change of medications (n = 1, addition of sodium-glucose cotransporter 2 inhibitor after completing one condition). Therefore, 11 participants (4 men and 7 women who were all postmenopausal) were included in analyses, and their baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of participants
| Value | |
|---|---|
| Age, yr | 64 ± 8 |
| Body mass, kg | 91 ± 18 |
| Body mass index, kg/m2 | 34 ± 8 |
| Waist circumference, cm | 105 ± 13 |
| HbA1c, % | 7 ± 1 |
| Years of diagnosis | 6 ± 4 |
| Blood pressure, mmHg | 93 ± 4 |
| Medications | |
| Metformin | 9 |
| Glucagon-like peptide-1 | 1 |
| Dipeptidyl peptidase-4 | 1 |
| Sulfonylurea | 1 |
| Statin | 3 |
Values are means ± SD; n = 11 participants.
Experimental Protocol
Each participant attended a baseline screening session, which included the measurement of anthropometrics, a physical activity readiness questionnaire (PARQ+), and a Godin leisure time exercise questionnaire followed by the three 4-day interventions in a random order. The day before each intervention diet and activity were standardized, and a washout of 9–14 days occurred between each intervention where participants were asked to return to their normal diet and physical activity habits. Fasting blood for biomarkers of vascular health and brachial artery FMD were measured before and after each 4-day intervention at the same time of day after consumption of a standardized mixed meal on the evening before the start of each intervention. During each intervention, a continuous glucose monitor (CGM; iPro Professional CGM, Medtronic) was worn to measure postprandial glucose responses. The CGM was inserted by a trained researcher on the first morning of each intervention, calibrated 4 times/day by the participant using finger stick glucose values (OneTouch UltraMini Blood Glucose Meter, LifeScan), and downloaded using the Medtronic CareLink Pro software to construct glucose curves for the 4-day period. The incremental area under the curve (iAUC) was calculated using the trapezoid method (44). All intervention diets were isoenergetic, with calories estimated using the Harris-Benedict equation (39) and habitual intake (i.e., matched from first trial). An example meal plan for 1 day of each diet is shown in Table 2. For the low-carbohydrate plus exercise condition (LC + Ex), the estimated individualized energy utilization for the postmeal walking (~70 kcal) (48) was added to each meal to assure energy equilibrium among the three diets.
Table 2.
Example meal plan showing the three meals provided for 1 day of each intervention
| Diet |
|||
|---|---|---|---|
| CON | LC | LC + Ex | |
| Goal macronutrient ratio (carbohydrate:protein:fat), % | 55:25:20 | 10:25:65 | 10:25:65 |
| Breakfast | 95 g Oats; 25 g whey; 30 g blueberries; 30 g raspberries | 150 g Whole egg; 110 g egg whites; 55 g avocado; 30 g peppers; 40 g onions; 40 g carrots; 10 g almonds | 150 g Whole egg; 110 g egg whites; 55 g avocado; 30 g peppers; 40 g onions; 40 g carrots; 10 g almonds |
| Lunch | 105 g Chicken breast; 230 g yams; 40 g green beans; 17 g cashews | 105 g Ground turkey; 38 g cashews; 15 g olive oil; 35 g spinach; 30 g carrots; 30 g cucumber | 105 g Ground turkey; 38 g cashews; 15 g olive oil; 35 g spinach; 30 g carrots; 30 g cucumber |
| Dinner | 100 g Turkey; 85 g brown rice; 14 g cashews; 40 g broccoli | 100 g Steak (ribeye); 30 g cashews; 13 g olive oil; 30 g apple; 30 g spinach; 50 g cucumber | 100 g Steak (ribeye); 30 g cashews; 13 g olive oil; 30 g apple; 30 g spinach; 50 g cucumber |
Each meal contained 500 kcal/meal. CON diet, control low-fat moderate-carbohydrate diet; LC diet, low-carbohydrate diet; LC + Ex diet, low-carbohydrate diet plus postmeal walking.
Low-fat control diet.
Participants were provided a diet [control (CON)] with meals based on the current dietary guidelines for adults with T2D comprising low-fat, low-glycemic index whole foods (23). Each meal comprised ~55% of total energy from carbohydrate (predominately from low-glycemic index and high-fiber carbohydrate sources), 20% energy from fat (aiming for <7% saturated fatty acids), and 25% protein (primarily from lean meats).
LCHF diet.
Participants consumed a diet that provided the same energy content as the CON diet but with carbohydrates reduced to ~10% of total energy (LC diet). The percent protein was matched at ~25%, with the remainder of the energy coming from fat (~65% of total kcal).
LC + Ex.
Participants performed 15 min of walking beginning ~30 min after breakfast, lunch, and dinner. A similar strategy has been previously shown to reduce postprandial hyperglycemia in individuals with impaired glucose tolerance (22). The exercise intensity of the postmeal walking was light to moderate, which was confirmed on day 1 of the intervention by having participants walk on a horizontal treadmill in the laboratory at a comfortable pace that elicited a rating of perceived exertion (CR-10 scale) of 3 (“moderate”; equating to ~60% of maximal heart rate). Participants were instructed to replicate this pace at home for each of the 15-min postmeal walks for the remainder of the intervention. Accelerometers were worn to confirm compliance and intensity. Participants consumed the same diet as the LC intervention but with the addition of ~70 kcal to each meal to account for the estimated energy expenditure of 15 min of walking.
Physiological Measures
Brachial artery FMD.
Endothelial function was assessed with brachial artery FMD using high-resolution ultrasound (Terason 3200) according to current guidelines (17, 62). First, a longitudinal section, 2–3 cm from the antecubital fossa of the brachial artery, was imaged for 1 min using B-mode ultrasound (insonation angle maintained at 60°). A rapid inflation cuff positioned 1–2 cm distal from the olecranon process of the forearm was then inflated to >60 mmHg above systolic blood pressure for 5 min. Simultaneous diameter and velocity measurements continued throughout and were recorded 30 s before and for 3 min after the cuff was rapidly deflated. Brachial blood pressure was measured using a manual sphygmomanometer and stethoscope. Mean arterial blood pressure (MAP) was calculated as 1/3 × systolic blood pressure (SBP) + 2/3 × diastolic blood pressure (DBP).
Analyses for the synchronized diameter and velocity measures were performed using edge detection software (33, 72). FMD was expressed as the percent change in artery diameter from baseline [%FMD = 100 × (post – preocclusionmean diameter/preocclusionmean diameter].
Biomarkers of Vascular Health
Collection of blood samples.
For adhesion molecules, venous blood was collected into EDTA-containing tubes (BD Vacutainer) and plasma was obtained after centrifugation at 1,550 g for 15 min at 4°C. For MPA and MP analyses, venous blood was collected from the antecubital vein by venipuncture into sodium citrate tubes (BD Vacutainer). MPAs were analyzed from whole blood 10 min after blood collection (details below). Plasma was generated by centrifugation for 15 min at 1,550 g and stored at −80° before batch analyses were performed (described below).
MPAs.
Exactly 10 min after blood collection, 90 μl of whole blood were transferred from the sodium citrate vacutainer into a TruCount tube (BD Biosciences). Ten microliters of FcR blocking reagent (Miltenyi Biotec) were added, and the sample was then incubated in the dark at room temperature for 10 min. After this, 2 μl of CD14-Vioblue (Miltenyi Biotec) and 10 μl of CD42b-APC (BD Biosciences) were added and gently mixed before incubation under the same conditions. Finally, 1 ml of red blood cell lysis buffer was added before incubation on a rocking platform for 15 min. The sample was then analyzed on a flow cytometer (Miltenyi Biotec MACSQuant Analyzer) with MPAs defined as events positive for both CD14 and CD42b (51). Fluorescence-minus-one controls were used to determine positive staining for both CD14 and CD42b events.
Circulating MPs.
MPs were characterized using flow cytometry, as previously described (4, 30). For the quantification of total MP, EMP, and platelet microparticle (PMP) subspecies, plasma samples were centrifuged a 13,000 g for 2 min and 200 μl of platelet-free plasma were then transferred to a TruCount tube (BD Biosciences). MP size threshold was established using Megamix-Plus SSC calibrator beads (Megamix-Plus SSC beads, Biocytex, Marseille, France), and only events < 1 μm in size were counted. Total MPs were defined as events falling within the Megamix-Plus SSC established size range (0.16, 0.20, 0.24, and 0.5 μm). Cellular specific MP lineage was determined by flourochrome staining for endothelial (CD62e)- and platelet (CD62p)-specific antibodies and falling within the respective MP size range (BioLegend, San Diego, CA). Samples were incubated with antibodies for 20 min in the dark at room temperature. After incubation, samples were fixed with 2% paraformaldehyde (ChemCruz Biochemicals, Santa Cruz, CA), diluted with PBS, and analyzed using BD Biosciences FACSAria I High Speed Cell sorter and flow cytometer (University of Colorado Anschutz Medical Campus Allergy and Clinical Immunology/Infectious Disease Flow Core). Concentrations of total MPs, EMPs, and PMPs were determined using the following formula: [(number of events in region containing MPs/number of events in absolute count bead region) × (total number of beads per test/total volume of sample)].
Adhesion molecules, C-reactive protein, and serum amyloid A.
Plasma was thawed, mixed, and diluted 1,000-fold before analyses of ICAM-1, VCAM-1, C-reactive protein (CRP), and serum amyloid A (SAA) were made using the V-PLEX Vascular Injury Panel 2 Human Kit (Meso Scale Discovery) according to the manufacturer’s instructions. Measures were made in duplicate and analyzed on a MESO QuickPlex SQ 120 (Meso Scale Discovery), with an intra-assay coefficient of variation of 3.7%.
Statistics
All data were first tested for normality using Q-Q plots and are reported as means ± SD or 95% confidence intervals. Data were analyzed using linear mixed model, with repeated measures of condition (CON, LC, and LC + Ex) and time (pre and post) as fixed factors with SPSS 22.0 (SPSS, Chicago, IL). Post hoc analyses using Tukey’s procedure were used to evaluate within condition changes (i.e., pre vs. post) following significant interactions. Statistical significance was set at P < 0.05. Magnitude-based inference analyses were performed according to contemporary views on statistical reporting, allowing for clinically meaningful inference (5) using the spreadsheet available from http://www.sportsci.org. The smallest clinically beneficial threshold for %FMD was +1%, based on a recent meta-analyses that showed a 13% reduced risk of future cardiovascular events for every 1% improvement in %FMD (95% confidence interval: 9–17%) (41). For measurements with an unknown smallest clinical change threshold, the default Cohen’s d of 0.2 was used.
RESULTS
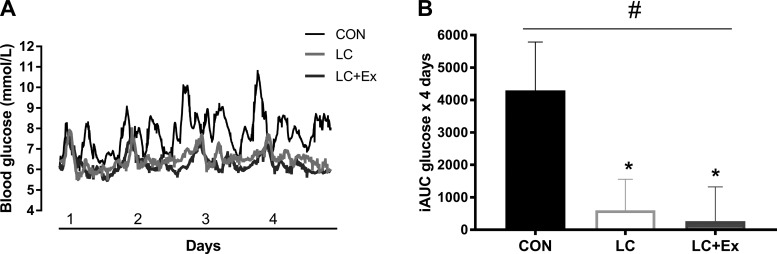
Figure 1A shows the 24-h continuous glucose monitor curves for CON, LC, and LC + Ex conditions (n = 11). LC and LC + Ex for 4 days reduced the iAUC by ~86 ± 21% and ~94 ± 22%, respectively, compared with CON (P = 0.01; Fig. 1B). The change in body mass was not different between interventions (−1.9 ± 0.8 kg, main effect of time, P < 0.01). However, the change in body mass was not different between conditions (interaction: P = 0.82), supporting successful matching of energy intake across diets.
Fig. 1.
Continuous blood glucose data (n = 11) showing the blood glucose excursions across 4 days of a control low-fat diet (CON), low-carbohydrate diet (LC), and low-carbohydrate plus exercise (LC + Ex) (A) and the incremental area under the curve (AUC) for each 4-day condition (B). #P < 0.05 interaction; *P < 0.05 post hoc.
%FMD
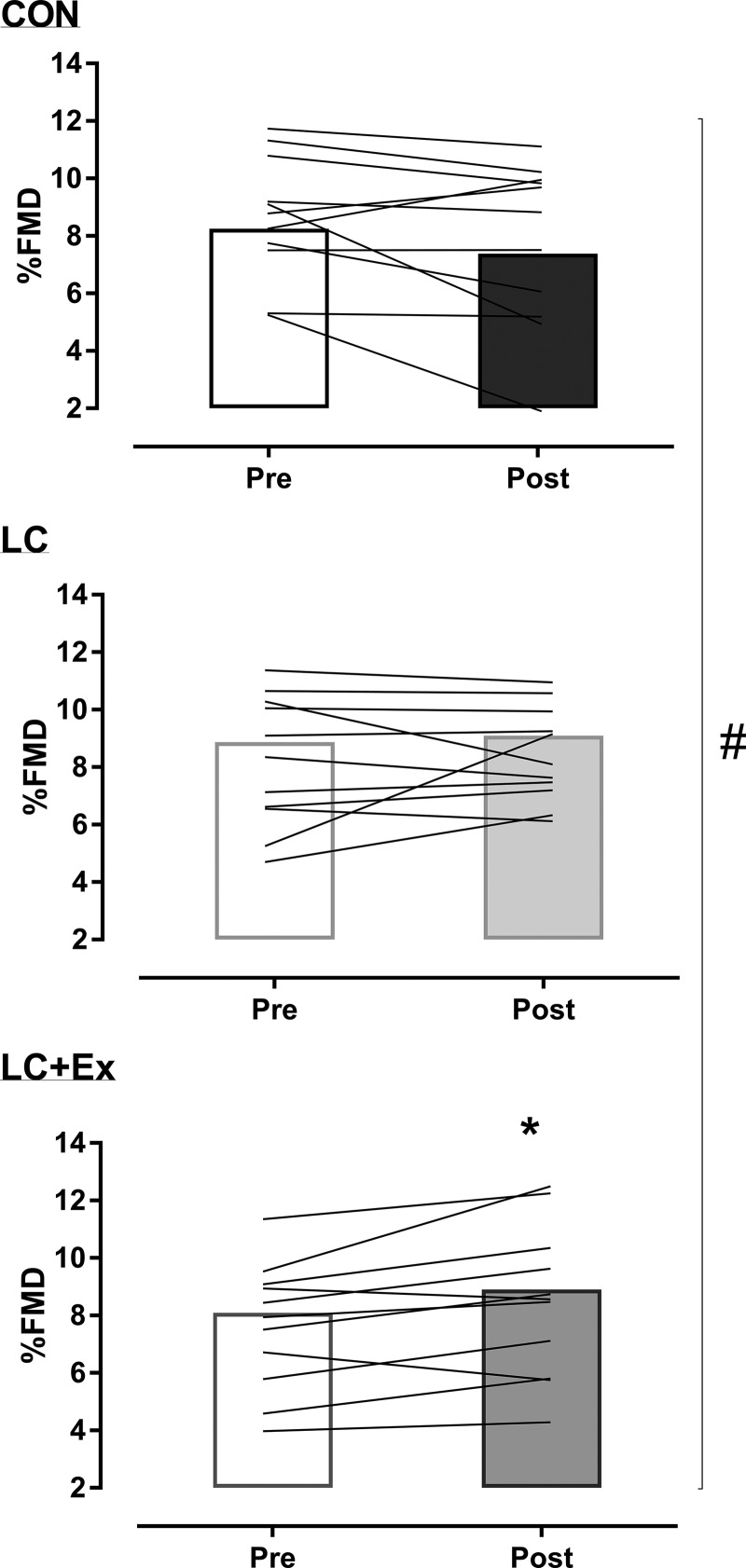
There was a significant condition × time interaction (P = 0.01) for the change in %FMD. %FMD was significantly increased after LC + Ex (by +0.81 ± 0.95%, P = 0.02; Fig. 2), with no change after CON and LC (both P > 0.12; Fig. 2). The probability that the change in %FMD with LC + Ex is beneficial/negligible/harmful based on the clinically meaningful change (+1%) was 25/75/0% (95% confidence interval: 1.6, 0.04%), respectively. For the CON and LC conditions, the probability was 0/61/40% and 11/88/1%, beneficial/negligible/harmful, respectively. Baseline diameter and time to peak diameter did not change across time or between conditions (Table 3).
Fig. 2.
Changes in flow-mediated dilation (FMD) before and after short-term control low-fat diet (CON), low-carbohydrate diet (LC), and low-carbohydrate plus exercise (LC + Ex) conditions. Group mean (bar: n = 11) and individual data (lines). #P < 0.05 interaction; *P < 0.05 post hoc.
Table 3.
Flow-mediated dilation, microparticles, monocyte-platelet aggregates, adhesion molecules, serum amyloid A, and C-reactive protein data before and after each 4-day condition
| CON Diet |
LC Diet |
LC + Ex Diet |
P Value |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Interaction | Main effect | |
| Baseline diameter, mm | 0.39 ± 0.07 | 0.41 ± 0.09 | 0.39 ± 0.06 | 0.40 ± 0.06 | 0.40 ± 0.06 | 0.40 ± 0.07 | 0.15 | 0.11 |
| Peak diameter, mm | 0.42 ± 0.07 | 0.44 ± 0.08 | 0.42 ± 0.06 | 0.44 ± 0.07 | 0.43 ± 0.06 | 0.44 ± 0.06 | 0.33 | 0.11 |
| Time to peak, s | 51.3 ± 35.3 | 50.0 ± 26.7 | 51.1 ± 27.1 | 47.2 ± 30.4 | 44.6 ± 36.9 | 60.1 ± 47.1 | 0.48 | 0.67 |
| Platelet microparticles, microparticles/μl | 42.1 ± 26.5 | 46.1 ± 31.7 | 33.2 ± 30.8 | 33.9 ± 21.5 | 50.8 ± 20.0 | 28.8 ± 25.6 | 0.07 | 0.34 |
| Total monocyte-platelet aggregates, cells/μl | 77.5 ± 21.7 | 95.5 ± 22.7 | 80.2 ± 20.5 | 92.7 ± 27.9 | 77.3 ± 20.0 | 87.1 ± 19.0 | 0.15 | <0.01 |
| Monocyte-platelet aggregates, % | 29.8 ± 5.5 | 31.3 ± 5.5 | 31.0 ± 6.4 | 32.7 ± 5.4 | 31.0 ± 6.4 | 31.3 ± 7.8 | 0.78 | 0.08 |
| C-reactive protein, mg/l | 8.0 ± 8.3 | 7.9 ± 8.5 | 5.3 ± 4.9 | 8.2 ± 8.4 | 7.3 ± 7.2 | 9.3 ± 11.4 | 0.12 | 0.29 |
| Serum amyloid A, mg/l | 5.7 ± 4.2 | 6.4 ± 4.3 | 3.9 ± 2.5 | 5.3 ± 5.6 | 5.3 ± 3.4 | 5.1 ± 4.2 | 0.72 | 0.30 |
| ICAM-1, ng/ml | 461 ± 89 | 478 ± 83 | 368 ± 111 | 402 ± 77 | 389 ± 102 | 422 ± 89 | 0.44 | 0.19 |
| VCAM-1, ng/ml | 392 ± 249 | 441 ± 255 | 320 ± 208 | 395 ± 217 | 389 ± 252 | 400 ± 248 | 0.48 | 0.06 |
Data are means ± SD; n = 11 for flow-mediated dilation; n = 9 for microparticles; n = 11 for monocyte platelet aggregates, adhesion molecules, serum amyloid A, and C-reactive protein. CON diet, control low-fat moderate-carbohydrate diet; LC diet, low-carbohydrate diet; LC + Ex diet, low-carbohydrate diet plus postmeal walking; Pre and Post, before and after each 4-day condition, respectively.
Microparticles
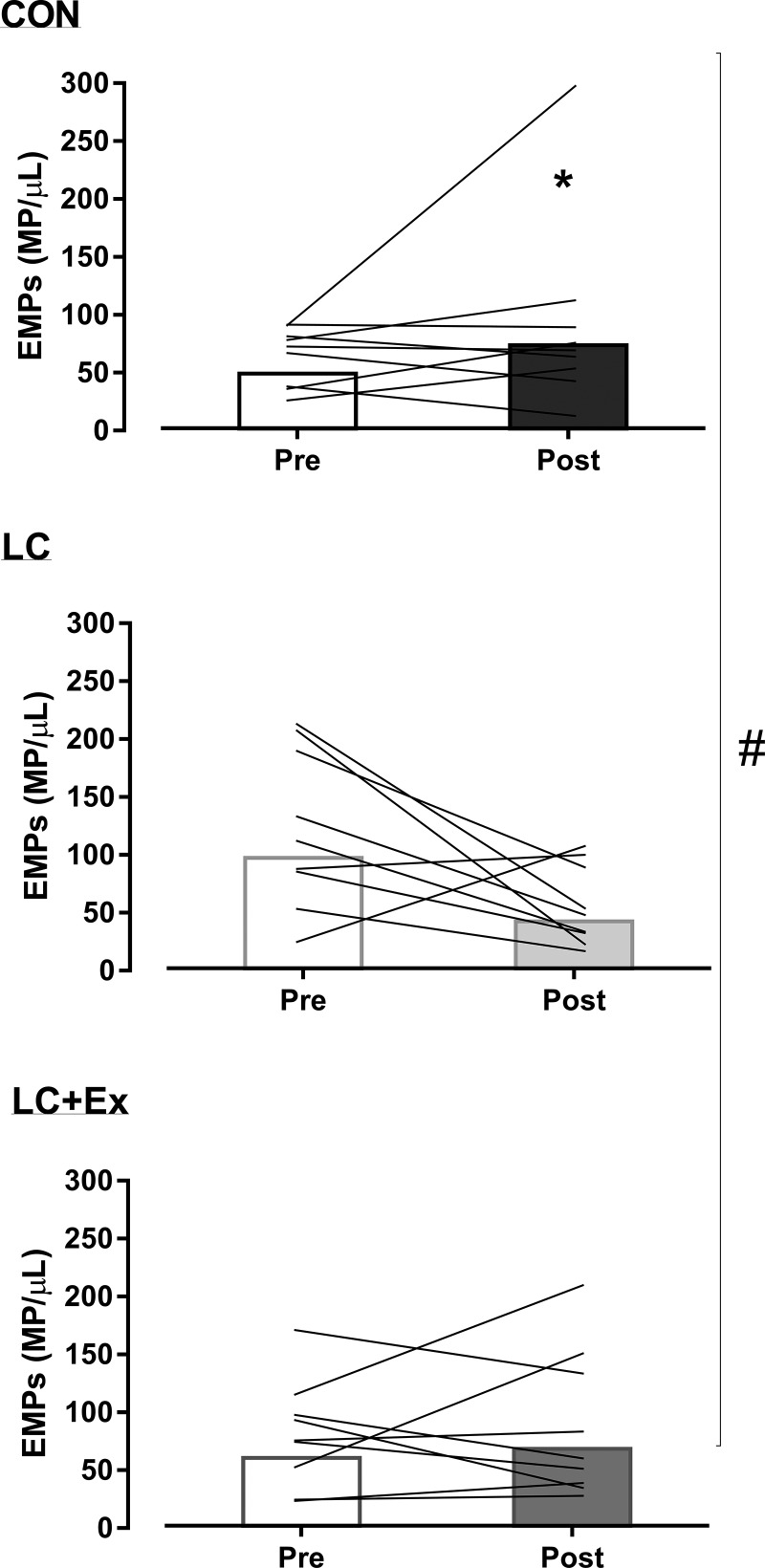
There was a significant condition × time interaction (P = 0.04) for the change in EMPs. EMPs were significantly reduced after the LC condition (by 65%, P = 0.04; Fig. 3), with no change after CON and LC + Ex (both P > 0.12; Fig. 3). The probability that the change in EMPs with LC is beneficial/negligible/harmful was 95/4/1%, respectively (Cohen’s d: 0.81). For the CON and LC + Ex conditions, the probability was 6/26/68% and 17/42/41% beneficial/negligible/harmful, respectively. A significant main effect of time (P = 0.02) revealed a collective 45% reduction in total MPs (from 8,790 ± 6,036 to 5,865 ± 4,327 MP/μl). The probability that the change in circulating MPs is beneficial/negligible/harmful was 94/6/0%, respectively (Cohen’s d: 0.52; Table 4). PMPs did not differ significantly between conditions (interaction: P = 0.07; Table 3).
Fig. 3.
Changes in endothelial microparticles (EMPs) before and after short-term control low-fat diet (CON), low-carbohydrate diet (LC), and low-carbohydrate plus exercise (LC + Ex) conditions. Group mean (bar: n = 9) and individual data (lines). #P < 0.05 interaction; *P < 0.05 post hoc.
Table 4.
Pairwise comparisons using magnitude-based inferences for the change in selected markers of vascular health measured before and after each 4-day condition
| CON Diet | LC Diet | LC + Ex Diet | |
|---|---|---|---|
| Total microparticles | |||
| Cohen's d | 0.4 | 0.9 | 0.6 |
| Beneficial/trivial/harmful, % | 65/22/13 | 96/3/1 | 88/10/2 |
| Qualitative inference | Possibly beneficial | Very likely beneficial | Likely beneficial |
| Platelet microparticles | |||
| Cohen's d | 0.1 | 0.8 | 0.1 |
| Beneficial/trivial/harmful, % | 23/40/37 | 97/3/0 | 23/37/40 |
| Qualitative inference | Unclear | Very likely beneficial | Unclear |
| Monocyte platelet aggregate count | |||
| Cohen's d | 0.9 | 0.6 | 0.5 |
| Beneficial/trivial/harmful, % | 0/3/97 | 2/11/88 | 2/15/83 |
| Qualitative inference | Very likely harmful | Likely harmful | Likely harmful |
| Monocyte platelet aggregates, % | |||
| Cohen's d | 0.6 | 0.3 | 0.2 |
| Beneficial/trivial/harmful, % | 2/11/88 | 8/33/59 | 9/41/50 |
| Qualitative inference | Likely harmful | Unclear | Unclear |
| C-reactive protein | |||
| Cohens d | 0.0 | 0.5 | 0.2 |
| Beneficial/trivial/harmful, % | 31/46/23 | 3/17/80 | 10/35/55 |
| Qualitative inference | Unclear | Likely harmful | Unclear |
| Serum amyloid A | |||
| Cohen's d | 0.3 | 0.3 | 0.1 |
| Beneficial/trivial/harmful, % | 11/29/60 | 8/31/61 | 40/34/25 |
| Qualitative inference | Unclear | Unclear | Unclear |
| ICAM | |||
| Cohen's d | 0.3 | 0.3 | 0.3 |
| Beneficial/trivial/harmful, % | 11/29/60 | 6/29/65 | 8/32/60 |
| Qualitative inference | Unclear | Unclear | Unclear |
| VCAM | |||
| Cohen's d | 0.6 | 0.9 | 0.1 |
| Beneficial/trivial/harmful, % | 2/10/88 | 1/3/96 | 21/41/39 |
| Qualitative inference | Likely harmful | Very likely harmful | Unclear |
CON diet, control low-fat moderate-carbohydrate diet; LC diet, low-carbohydrate diet; LC + Ex diet, low-carbohydrate diet plus postmeal walking.
MPAs, CRP, and Adhesion Molecules
A significant main effect of time (P < 0.01) revealed a collective 14% increase in total (count/ml) MPAs (interaction: P = 0.15; Table 4). The probability that the change in MPAs is beneficial/negligible/harmful was 1/5/94%, respectively (Cohen’s d: 0.71; Table 4). Percent MPA (interaction: P = 0.78; time: P = 0.08; Table 3), plasma adhesion molecules (ICAM-1 and VCAM-1), SAA, and CRP (all P > 0.12) were not significantly changed after all conditions (Table 3). Pairwise comparisons for the magnitude-based inference for each condition are shown in Table 4.
DISCUSSION
The present study examined the short-term effects of a low-carbohydrate diet, with and without postmeal walking exercise, on endothelial function and markers of vascular health in individuals with T2D. We tested whether the attenuation of postprandial hyperglycemia with LC + Ex might represent an optimal strategy for improving endothelial function and markers of vascular health. The main findings of the present study were that 1) LC + Ex significantly improved endothelial function as assessed by FMD and 2) LC alone lowered circulating EMPs. No changes were observed in the selected measures of vascular health after a low-fat CON diet based on current diabetes guidelines (2, 14). Additionally, total circulating MPs were reduced; however, MPAs were slightly increased, after all short-term conditions. The present study shows that attenuating postprandial hyperglycemia by restricting carbohydrates and postmeal walking can improve vascular health in individuals with T2D. The addition of postmeal walking to a LCHF diet may mitigate the purported deleterious effects of high-fat meals on endothelial function seen in some (19, 25, 66, 70), but not all (67), investigations, and further research is warranted to determine this in larger, longer interventions.
Postprandial hyperglycemia, exacerbated by carbohydrate consumption at meals, contributes to the excess CVD risk in individuals with T2D (8, 11, 18). Elevated blood glucose levels after an oral glucose load (13) or oscillating glucose infusion (11) impair endothelial function. However, antioxidant, statin, and/or insulin therapies that reduce glycemia and oxidative stress can restore endothelial function, at least in an acute setting (12). In the present study, the aim was to reduce postprandial hyperglycemic excursions with lifestyle interventions, namely carbohydrate restriction and postmeal walking. Continuous glucose monitoring confirmed the reduction in postprandial hyperglycemia with LC alone and in combination with postmeal walking compared with the currently recommended low-fat diet (CON). Indeed, postprandial hyperglycemia as assessed by iAUC was reduced by 86% and 94%, respectively, with short-term LC and LC + Ex. Furthermore, endothelial function was increased after LC + Ex. The observed reduction in postprandial hyperglycemia with LC + Ex was larger than that typically seen with Acarbose treatment (a drug to delay carbohydrate digestion and thus postprandial hyperglycemia) (20). Acute Acarbose administration before a sucrose load has been shown to mitigate postprandial endothelial dysfunction (69). In addition, long-term trials show that Acarbose treatment reduces the incidence of diabetes and prevents CVD (15, 37). However, there is a high prevalence of adverse gastrointestinal symptoms with Acarbose use (15, 37). Here, we show, for the first time, that lowering postprandial hyperglycemia with the nonpharmacological combination of a LCHF diet and postmeal walking improves endothelial function in individuals with T2D. Therefore, this lifestyle combination may be effective for reducing vascular dysfunction in T2D.
The postprandial period is associated with a cascade of proatherogenic events, including endothelial and immune cell activation (9). Circulating MPs are biologically active submicron particles that are shed from the membrane of cells under conditions of stress/injury (21). Studies have shown that circulating MPs are indicative of endothelial dysfunction in individuals with T2D (27). Indeed, EMPs carry and express endothelial proteins such as adhesion molecules and integrins and thus disturb vascular homeostasis (21). Acutely, previous studies have shown an increase in EMPs after a high-fat meal, indicating endothelial activation (28, 60). However, in the present study, EMPs were reduced after 4 days of a LCHF diet. This is likely attributed to the reduction in postprandial hyperglycemic excursions with carbohydrate restriction compared with the low-fat diet in individuals with T2D. Indeed, oscillating blood glucose is more deleterious for oxidative stress than constant high glucose in those with T2D (11). However, it is unclear why the same decrease in EMPs was not seen after the LC + Ex condition. There is some evidence to suggest that this may be due to exercise-induced shear stress, which mediates MP shedding from the vascular wall (49). After a single session of exercise, studies have reported a transient increase in MPs and have attributed this to increased shear stress and/or oxidative stress (47, 49, 58). In this regard, acute exercise before a high-fat meal may not acutely lower EMPs (40); however, repetitive exposure to exercise over several weeks may improve defense systems. Indeed, regular exercise improves endothelial function and vascular health, which appears to be primarily mediated by shear stress and increased nitric oxide bioavailability (55, 63). Thus, it is possible that the addition of three postmeal walks in the LC + Ex condition was promoting vascular remodeling in the previously inactive T2D participants, which may have led to a different EMP response compared with the LC diet alone.
The present study did not include an exercise-only low-fat CON condition. Although it is hypothesized that the reduced postprandial hyperglycemia with the LC diet combined with exercise has additive and may have unique effects on the vasculature, further research is needed to compare exercise alone to low-carbohydrate diet approaches. Previous research has shown that postmeal walking with a low-fat diet reduces 24-h blood glucose (22) and postprandial iAUC (54) by ~11%. The present study shows that the combination of low-carbohydrate diet and postmeal exercise lowers 4-day iAUC by 94% compared with low-fat CON diet alone. A low-carbohydrate diet alone lowered the 4-day iAUC by ~86%. Therefore, it appears that a low-carbohydrate diet approach is much more potent than postmeal walking for lowering glucose levels, but our study cannot ascertain the independent and combined effects of each approach.
Interestingly, the total MP count was reduced after all conditions, suggesting that all diets altered circulating MP concentration. This is most likely driven by the decrease in EMPs after the LC condition and PMPs after the LC + Ex condition. However, the CON diet, which was composed of low-fat low-glycemic index whole foods (23), is likely a less-processed and “healthier” diet than typically consumed by participants. Thus, this improvement in overall food quality may underlie the resulting decrease in MPs after all conditions (independent of changes in glycemic control). The reduction in body mass experienced after all three short-term diet conditions generally supports that the provided diets were healthier or lower in calories and this could have played a role. It is unknown whether the observed change in MP count might be the result of altered MP production and/or clearance, which are areas that require further research. Furthermore, the MP species, cargo, and subsequent physiological signaling could be different regardless of total MP concentration (73).
In conclusion, in individuals with T2D, postprandial hyperglycemia is particularly concerning as it contributes to CVD risk through impairing endothelial function, increasing oxidative stress, and promoting inflammation (11, 13, 50). The results of the present study show that controlling postprandial hyperglycemia with a LCHF diet combined with postmeal walking exercise improves endothelial function in individuals with T2D. MPs, as markers of endothelial activation, were reduced with short-term carbohydrate restriction. Carbohydrate restriction and postmeal exercise may therefore represent an effective strategy to mitigate the negative effects of postprandial hyperglycemia and reduce CVD risk in individuals with T2D. Further research is needed to elucidate the long-term impact of carbohydrate restriction and postmeal exercise on CVD risk factors in individuals with, and at risk for, T2D.
GRANTS
J. P. Little is supported by Canadian Institutes of Health Research New Investigator Salary Award MSH-141980 and Michael Smith Foundation for Health Research Scholar Award 16890. Continuous glucose monitors and sensors were provided in kind from Investigator-Initiated Grant NERP15-016 from Medtronic.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.E.F., E.M.-C., and J.P.L. conceived and designed research; M.E.F., E.M.-C., T.D.B., C.D., and H.N. performed experiments; M.E.F., E.M.-C., T.D.B., C.D., H.N., and C.A.D. analyzed data; M.E.F., T.D.B., C.D., C.A.D., and J.P.L. interpreted results of experiments; M.E.F. prepared figures; M.E.F. drafted manuscript; M.E.F., E.M.-C., T.D.B., C.D., H.N., and J.P.L. edited and revised manuscript; M.E.F., T.D.B., C.D., H.N., C.A.D., and J.P.L. approved final version of manuscript.
REFERENCES
- 1.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML; American Diabetes Association . Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 31, Suppl 1: S61–S78, 2008. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association American Diabetes Association Complete Guide to Diabetes. New York: Bantam, 2003. [Google Scholar]
- 3.Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ, Mendez A, Ferreira A, de Marchena E, Ahn YS. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol 98: 70–74, 2006. doi: 10.1016/j.amjcard.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 4.Bain AR, Ainslie PN, Bammert TD, Hijmans JG, Sekhon M, Hoiland RL, Flück D, Donnelly J, DeSouza CA. Passive heat stress reduces circulating endothelial and platelet microparticles. Exp Physiol 102: 663–669, 2017. doi: 10.1113/EP086336. [DOI] [PubMed] [Google Scholar]
- 5.Batterham AM, Hopkins WG. Making meaningful inferences about magnitudes. Int J Sports Physiol Perform 1: 50–57, 2006. doi: 10.1123/ijspp.1.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Berk KA, Oudshoorn TP, Verhoeven AJ, Mulder MT, Roks AJ, Dik WA, Timman R, Sijbrands EJ. Diet-induced weight loss and markers of endothelial dysfunction and inflammation in treated patients with type 2 diabetes. Clin Nutr ESPEN 15: 101–106, 2016. doi: 10.1016/j.clnesp.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Boden G, Rao AK. Effects of hyperglycemia and hyperinsulinemia on the tissue factor pathway of blood coagulation. Curr Diab Rep 7: 223–227, 2007. doi: 10.1007/s11892-007-0035-1. [DOI] [PubMed] [Google Scholar]
- 8.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 9.Ceriello A. The post-prandial state and cardiovascular disease: relevance to diabetes mellitus. Diabetes Metab Res Rev 16: 125–132, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 54: 1–7, 2005. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57: 1349–1354, 2008. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 12.Ceriello A, Kumar S, Piconi L, Esposito K, Giugliano D. Simultaneous control of hyperglycemia and oxidative stress normalizes endothelial function in type 1 diabetes. Diabetes Care 30: 649–654, 2007. doi: 10.2337/dc06-2048. [DOI] [PubMed] [Google Scholar]
- 13.Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, Da Ros R, Motz E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation 106: 1211–1218, 2002. doi: 10.1161/01.CIR.0000027569.76671.A8. [DOI] [PubMed] [Google Scholar]
- 14.Cheng AY; Canadian Diabetes Association Clinical Practice Guidelines Expert Committee . Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can J Diabetes 37, Suppl 1: S1–S3, 2013. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group . Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 290: 486–494, 2003. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 16.Clifton PM, Keogh JB, Foster PR, Noakes M. Effect of weight loss on inflammatory and endothelial markers and FMD using two low-fat diets. Int J Obes 29: 1445–1451, 2005. doi: 10.1038/sj.ijo.0803039. [DOI] [PubMed] [Google Scholar]
- 17.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 18.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 108: 1527–1532, 2003. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 19.Cuevas AM, Guasch V, Castillo O, Irribarra V, Mizon C, San Martin A, Strobel P, Perez D, Germain AM, Leighton F. A high-fat diet induces and red wine counteracts endothelial dysfunction in human volunteers. Lipids 35: 143–148, 2000. doi: 10.1007/BF02664763. [DOI] [PubMed] [Google Scholar]
- 20.Derosa G, Salvadeo SA, D’Angelo A, Ferrari I, Mereu R, Palumbo I, Maffioli P, Randazzo S, Cicero AF. Metabolic effect of repaglinide or acarbose when added to a double oral antidiabetic treatment with sulphonylureas and metformin: a double-blind, cross-over, clinical trial. Curr Med Res Opin 25: 607–615, 2009. doi: 10.1185/03007990802711024. [DOI] [PubMed] [Google Scholar]
- 21.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 31: 27–33, 2011. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 22.DiPietro L, Gribok A, Stevens MS, Hamm LF, Rumpler W. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care 36: 3262–3268, 2013. doi: 10.2337/dc13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dworatzek PD, Arcudi K, Gougeon R, Husein N, Sievenpiper JL, Williams SL. Nutrition therapy. Can J Diabetes 37: 45–55, 2013. doi: 10.1016/j.jcjd.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes Metab 12: 204–209, 2010. doi: 10.1111/j.1463-1326.2009.01151.x. [DOI] [PubMed] [Google Scholar]
- 25.Esposito K, Ciotola M, Sasso FC, Cozzolino D, Saccomanno F, Assaloni R, Ceriello A, Giugliano D. Effect of a single high-fat meal on endothelial function in patients with the metabolic syndrome: role of tumor necrosis factor-α. Nutr Metab Cardiovasc Dis 17: 274–279, 2007. doi: 10.1016/j.numecd.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, Accurso A, Frassetto L, Gower BA, McFarlane SI, Nielsen JV, Krarup T, Saslow L, Roth KS, Vernon MC, Volek JS, Wilshire GB, Dahlqvist A, Sundberg R, Childers A, Morrison K, Manninen AH, Dashti HM, Wood RJ, Wortman J, Worm N. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 31: 1–13, 2015. doi: 10.1016/j.nut.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Feng B, Chen Y, Luo Y, Chen M, Li X, Ni Y. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis 208: 264–269, 2010. doi: 10.1016/j.atherosclerosis.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira AC, Peter AA, Mendez AJ, Jimenez JJ, Mauro LM, Chirinos JA, Ghany R, Virani S, Garcia S, Horstman LL, Purow J, Jy W, Ahn YS, de Marchena E. Postprandial hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation 110: 3599–3603, 2004. doi: 10.1161/01.CIR.0000148820.55611.6B. [DOI] [PubMed] [Google Scholar]
- 29.Fox CS, Coady S, Sorlie PD, D’Agostino RB Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 115: 1544–1550, 2007. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 30.Francois ME, Little JP. The impact of acute high-intensity interval exercise on biomarkers of cardiovascular health in type 2 diabetes. Eur J Appl Physiol 117: 1607–1616, 2017. doi: 10.1007/s00421-017-3649-2. [DOI] [PubMed] [Google Scholar]
- 31.Gannon MC, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 53: 2375–2382, 2004. doi: 10.2337/diabetes.53.9.2375. [DOI] [PubMed] [Google Scholar]
- 32.Goldenberg R, Punthakee Z; Canadian Diabetes Association Clinical Practice Guidelines Expert Committee . Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes 37, Suppl 1: S8–S11, 2013. doi: 10.1016/j.jcjd.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Green D, Cheetham C, Reed C, Dembo L, O’Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol 93: 361–368, 2002. doi: 10.1152/japplphysiol.00051.2002. [DOI] [PubMed] [Google Scholar]
- 34.Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97: 495–528, 2017. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutniak M, Grill V, Efendić S. Effect of composition of mixed meals–low- versus high-carbohydrate content−on insulin, glucagon, and somatostatin release in healthy humans and in patients with NIDDM. Diabetes Care 9: 244–249, 1986. doi: 10.2337/diacare.9.3.244. [DOI] [PubMed] [Google Scholar]
- 36.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 3: 853–876, 2007. [PMC free article] [PubMed] [Google Scholar]
- 37.Hanefeld M, Schaper F, Koehler C. Effect of acarbose on vascular disease in patients with abnormal glucose tolerance. Cardiovasc Drugs Ther 22: 225–231, 2008. doi: 10.1007/s10557-008-6091-1. [DOI] [PubMed] [Google Scholar]
- 38.Hardman AE, Aldred HE. Walking during the postprandial period decreases alimentary lipaemia. J Cardiovasc Risk 2: 71–78, 1995. doi: 10.1097/00043798-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA 4: 370–373, 1918. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison M, Murphy RP, O’Connor PL, O’Gorman DJ, McCaffrey N, Cummins PM, Moyna NM. The endothelial microparticle response to a high fat meal is not attenuated by prior exercise. Eur J Appl Physiol 106: 555–562, 2009. doi: 10.1007/s00421-009-1050-5. [DOI] [PubMed] [Google Scholar]
- 41.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 42.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 55: 1577–1596, 2012. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 43.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Bidot CJ, Ahn YS. Endothelial microparticles (EMP) as vascular disease markers. Adv Clin Chem 39: 131–157, 2005. doi: 10.1016/S0065-2423(04)39005-0. [DOI] [PubMed] [Google Scholar]
- 44.Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve. Methodological aspects. Diabetes Care 13: 172–175, 1990. doi: 10.2337/diacare.13.2.172. [DOI] [PubMed] [Google Scholar]
- 45.Leroyer AS, Tedgui A, Boulanger CM. Microparticles and type 2 diabetes. Diabetes Metab 34, Suppl 1: S27–S32, 2008. doi: 10.1016/S1262-3636(08)70100-9. [DOI] [PubMed] [Google Scholar]
- 46.Lustig RH, Schmidt LA, Brindis CD. Public health: the toxic truth about sugar. Nature 482: 27–29, 2012. doi: 10.1038/482027a. [DOI] [PubMed] [Google Scholar]
- 47.Marsh SA, Coombes JS. Exercise and the endothelial cell. Int J Cardiol 99: 165–169, 2005. doi: 10.1016/j.ijcard.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Mikus CR, Oberlin DJ, Libla J, Boyle LJ, Thyfault JP. Glycaemic control is improved by 7 days of aerobic exercise training in patients with type 2 diabetes. Diabetologia 55: 1417–1423, 2012. doi: 10.1007/s00125-012-2490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, Komiyama Y, Fujimura Y, Ikeda Y, Fukuhara S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 88: 3456–3464, 1996. [PubMed] [Google Scholar]
- 50.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295: 1681–1687, 2006. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 51.Harding SA, Din JN, Sarma J, Jessop A, Weatherall M, Fox KA, Newby DE. Flow cytometric analysis of circulating platelet-monocyte aggregates in whole blood: methodological considerations. Thromb Haemost 98: 451–456, 2007. [PubMed] [Google Scholar]
- 52.Nielsen JV, Joensson E. Low-carbohydrate diet in type 2 diabetes. Stable improvement of bodyweight and glycemic control during 22 months follow-up. Nutr Metab (Lond) 3: 22, 2006. doi: 10.1186/1743-7075-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papakonstantinou E, Triantafillidou D, Panagiotakos DB, Iraklianou S, Berdanier CD, Zampelas A. A high protein low fat meal does not influence glucose and insulin responses in obese individuals with or without type 2 diabetes. J Hum Nutr Diet 23: 183–189, 2010. doi: 10.1111/j.1365-277X.2009.01020.x. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds AN, Mann JI, Williams S, Venn BJ. Advice to walk after meals is more effective for lowering postprandial glycaemia in type 2 diabetes mellitus than advice that does not specify timing: a randomised crossover study. Diabetologia 59: 2572–2578, 2016. doi: 10.1007/s00125-016-4085-2. [DOI] [PubMed] [Google Scholar]
- 55.Rush JW, Denniss SG, Graham DA. Vascular nitric oxide and oxidative stress: determinants of endothelial adaptations to cardiovascular disease and to physical activity. Can J Appl Physiol 30: 442–474, 2005. doi: 10.1139/h05-133. [DOI] [PubMed] [Google Scholar]
- 56.Schlierf G, Dinsenbacher A, Kather H, Kohlmeier M, Haberbosch W. Mitigation of alimentary lipemia by postprandial exercise–phenomena and mechanisms. Metabolism 36: 726–730, 1987. doi: 10.1016/0026-0495(87)90107-7. [DOI] [PubMed] [Google Scholar]
- 57.Sheard NF, Clark NG, Brand-Miller JC, Franz MJ, Pi-Sunyer FX, Mayer-Davis E, Kulkarni K, Geil P. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the american diabetes association. Diabetes Care 27: 2266–2271, 2004. doi: 10.2337/diacare.27.9.2266. [DOI] [PubMed] [Google Scholar]
- 58.Sossdorf M, Otto GP, Claus RA, Gabriel HH, Lösche W. Cell-derived microparticles promote coagulation after moderate exercise. Med Sci Sports Exerc 43: 1169–1176, 2011. doi: 10.1249/MSS.0b013e3182068645. [DOI] [PubMed] [Google Scholar]
- 59.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 16: 434–444, 1993. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 60.Strohacker K, Breslin WL, Carpenter KC, Davidson TR, Agha NH, McFarlin BK. Moderate-intensity, premeal cycling blunts postprandial increases in monocyte cell surface CD18 and CD11a and endothelial microparticles following a high-fat meal in young adults. Appl Physiol Nutr Metab 37: 530–539, 2012. doi: 10.1139/h2012-034. [DOI] [PubMed] [Google Scholar]
- 61.Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, Yancy WS Jr, Brinkworth GD. A very low-carbohydrate, low-saturated fat diet for type 2 diabetes management: a randomized trial. Diabetes Care 37: 2909–2918, 2014. doi: 10.2337/dc14-0845. [DOI] [PubMed] [Google Scholar]
- 62.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 64.Tushuizen ME, Nieuwland R, Scheffer PG, Sturk A, Heine RJ, Diamant M. Two consecutive high-fat meals affect endothelial-dependent vasodilation, oxidative stress and cellular microparticles in healthy men. J Thromb Haemost 4: 1003–1010, 2006. doi: 10.1111/j.1538-7836.2006.01914.x. [DOI] [PubMed] [Google Scholar]
- 65.Unwin D, Unwin J. Low carbohydrate diet to achieve weight loss and improve HbA1c in type 2 diabetes and pre‐diabetes: experience from one general practice. Pract Diabetes 31: 76–79, 2014. doi: 10.1002/pdi.1835. [DOI] [Google Scholar]
- 66.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 79: 350–354, 1997. doi: 10.1016/S0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 67.Volek JS, Ballard KD, Silvestre R, Judelson DA, Quann EE, Forsythe CE, Fernandez ML, Kraemer WJ. Effects of dietary carbohydrate restriction versus low-fat diet on flow-mediated dilation. Metabolism 58: 1769–1777, 2009. doi: 10.1016/j.metabol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Steffen LM, Zhou X, Harnack L, Luepker RV. Consistency between increasing trends in added-sugar intake and body mass index among adults: the Minnesota Heart Survey, 1980-1982 to 2007-2009. Am J Public Health 103: 501–507, 2013. doi: 10.2105/AJPH.2011.300562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wascher TC, Schmoelzer I, Wiegratz A, Stuehlinger M, Mueller-Wieland D, Kotzka J, Enderle M. Reduction of postchallenge hyperglycaemia prevents acute endothelial dysfunction in subjects with impaired glucose tolerance. Eur J Clin Invest 35: 551–557, 2005. doi: 10.1111/j.1365-2362.2005.01550.x. [DOI] [PubMed] [Google Scholar]
- 70.Tsai WC, Li YH, Lin CC, Chao TH, Chen JH. Effects of oxidative stress on endothelial function after a high-fat meal. Clin Sci (Lond) 106: 315–319, 2004. doi: 10.1042/CS20030227. [DOI] [PubMed] [Google Scholar]
- 71.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369: 145–154, 2013. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]
- 73.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066, 2015. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]