Abstract
Aging is associated with increased peripheral chemoreceptor activity, reduced nitric oxide (NO) bioavailability, and attenuation of cardiovagal baroreflex sensitivity (BRS), collectively increasing the risk of cardiovascular disease. Evidence suggests that NO may attenuate peripheral chemoreflex sensitivity and increase BRS. Exogenous inorganic nitrate () increases NO bioavailability via the --NO pathway. Our hypothesis was that inorganic supplementation would attenuate peripheral chemoreflex sensitivity and enhance spontaneous cardiovagal BRS in older adults. We used a randomized, placebo-controlled crossover design in which 13 older (67 ± 3 yr old) adults ingested beetroot powder containing (BRA) or devoid of (BRP) and daily over 4 wk. Spontaneous cardiovagal BRS was assessed over 15 min of rest and was quantified using the sequence method. Chemoreflex sensitivity was assessed via ~5 min of hypoxia (10% fraction of inspired O2) and reported as the slope of the relationship between O2 saturation (%) and minute ventilation (in l/min) or heart rate (in beats/min). Ventilatory responsiveness to hypoxia was reduced after BRA (from −0.14 ± 0.04 to −0.05 ± 0.02 l·min−1·%−1, P = 0.01) versus BRP (from −0.10 ± 0.05 to −0.11 ± 0.05 l·min−1·%−1, P = 0.80), with no differences in heart rate responsiveness (BRA: from −0.47 ± 0.06 to −0.33 ± 0.04 beats·min−1·%−1, BRP: from −0.48 ± 0.07 to −0.42 ± 0.06 beats·min−1·%−1) between conditions (interaction effect, P = 0.41). Spontaneous cardiovagal BRS was unchanged after BRA and BRP (interaction effects, P = 0.69, 0.94, and 0.39 for all, up, and down sequences, respectively), despite a reduction in resting systolic and mean arterial blood pressure in the experimental (BRA) group (P < 0.01 for both). These findings illustrate that inorganic supplementation attenuates peripheral chemoreflex sensitivity without concomitant change in spontaneous cardiovagal BRS in older adults.
NEW & NOTEWORTHY Exogenous inorganic nitrate supplementation attenuates ventilatory, but not heart rate, responsiveness to abbreviated hypoxic exposure in older adults. Additionally, inorganic nitrate reduces systolic and mean arterial blood pressure without affecting spontaneous cardiovagal baroreflex sensitivity. These findings suggest that inorganic nitrate may attenuate sympathetically oriented pathologies associated with aging.
Keywords: inorganic nitrate, nitric oxide, peripheral chemoreflex sensitivity, cardiovagal baroreflex sensitivity
INTRODUCTION
Synthesis of nitric oxide (NO) has historically been characterized as a product of l-arginine metabolism via endothelial NO synthase. However, the --NO pathway has emerged over the past decade as an alternative mechanism to boost NO bioavailability independent of the endothelium. Briefly, dietary is reduced to in saliva (via oral commensal bacteria) and absorbed into the circulation. Once absorbed, can be reduced further to NO through reductase enzymes such as xanthine oxidoreductase (26). Interestingly, xanthine oxidoreductase activity is enhanced during hypoxia, a peripheral chemoreflex agonist (32). The peripheral chemoreflex appears to be attenuated by NO, as exogenous NO sources inhibit activity of the carotid sinus nerve in cats (20) and rats (15). Furthermore, a reduction in NO bioavailability may lead to peripheral chemoreflex overactivity, as observed with chronic heart failure (8, 41) and obstructive sleep apnea (1).
Chemoreceptors located within the carotid bodies are responsible for augmenting ventilation (V̇e) in response to falling O2 concentrations through a polysynaptic neural network containing peripheral sensory cells, neurons, the medulla oblongata, and effector organs (16). Hypersensitivity (overactivity) of these receptors may contribute to hypertension and other pathologies (34). Studies investigating the effects of aging on peripheral chemoreflex sensitivity have yielded inconclusive results in cats (20, 44), rabbits (8, 41), rats (9, 15, 43), and humans (2, 5, 12, 23, 24, 40). Evidence from animal models suggests that NO may suppress peripheral chemoreflex sensitivity (20, 43, 44); interestingly, NO bioavailability is reduced with age (42). Indeed, exaggerated chemoreflex sensitivity paralleling reductions in NO bioavailability yields a captivating, age-associated paradigm with clinical implications.
It is widely accepted that blood pressure (BP) increases with aging (10). While BP is regulated by numerous physiological mechanisms (e.g., endocrinal, neurological, and renal), alterations in cardiovagal baroreflex sensitivity (BRS) are postulated to influence age-associated elevations in BP (7, 9, 28, 31). Matsuda et al. (27) showed that BRS was improved with administration of S-nitrosocysteine, a NO donor. Supplementation of inorganic reduces resting BP in young and older adults (39). Collectively, these data suggest that the reduction in BP associated with inorganic supplementation may be attributable to changes in BRS. Moreover, peripheral chemoreflex sensitivity and BRS gains share an inverse relationship in humans (19) and rats (9), such that an attenuation of chemoreflex sensitivity may enhance BRS.
Despite evidence illustrating the pathological ramifications associated with an exaggerated chemoreflex (1, 8, 41), the age-associated decline in BRS (7, 9, 31), and the numerous cardiovascular benefits associated with boosting NO bioavailability (32), the effects of prolonged inorganic supplementation on peripheral chemoreflex sensitivity and spontaneous cardiovagal BRS in older adults are unknown. Therefore, the purpose of this study was to assess how peripheral chemoreflex sensitivity and BRS are influenced by 4 wk of daily inorganic supplementation in older adults. We hypothesized that 4 wk of daily inorganic supplementation will attenuate V̇e and heart rate (HR) responsiveness to hypoxia (e.g., peripheral chemoreflex sensitivity) and increase spontaneous cardiovagal BRS in older adults.
METHODS
Subjects.
A total of 13 healthy older (67 ± 3 yr old) adults (7 men and 6 women), along with a young (25 ± 4 yr old) reference cohort (5 men and 5 women), participated in the study. All subjects were free from cardiovascular, respiratory, and metabolic diseases, were nonobese (body mass index ≤ 30 kg/m2), and did not smoke. All older women were postmenopausal and excluded if they took hormone replacement therapy. Young female subjects were studied during the early follicular phase of their menstrual cycle or, if they were taking an oral contraceptive, during the placebo phase to control for the possible influences of sex hormones on outcome measures. Experiments were performed in the morning after an overnight fast. Subjects abstained from exercise and alcohol and caffeine consumption for 24 h before visiting the laboratory. All subjects provided written informed consent, and study protocols were approved by the Institutional Review Board of the University of Iowa. Additionally, this study is registered with clinicaltrials.gov (NCT02593305).
Experimental design.
Each of the older subjects was enrolled in the study for ∼3 mo and completed four study visits. Young subjects completed a single study visit. Experimental protocols were identical across all study days, which began with a venous blood draw followed by 15 min of rest with the subject in the supine position. After rest, brachial BP was assessed in duplicate using an automated cuff (Cardiocap/5, Datex-Ohmeda, Louisville, CO). If BP values differed by 5 mmHg, a third measurement was taken. HR and beat-by-beat systemic BP were monitored continuously via three-lead electrocardiogram (Cardiocap/5, Datex-Ohmeda) and finger plethysmography (Nexfin, Edwards Lifesciences, Irvine, CA), respectively. Spontaneous cardiovagal BRS and peripheral chemoreceptor sensitivity were then assessed using protocols described below. Briefly, subjects were outfitted with a facemask and pneumotach (model VMM-401, Interface Associates, Confluent Medical Technologies, Fremont, CA) to measure tidal volume (VT; in ml) while hemodynamics were measured continuously.
Nitrate supplementation.
Ingestion of beetroot juice increases plasma concentration ([]) and concentration ([]) and, subsequently, NO bioavailability (22, 45); therefore, we used beetroot powder dissolved in water as an exogenous source of inorganic . This study used a randomized, double-blind, placebo-controlled crossover design, such that subjects (older) assigned to the active group during the first arm of the study (study days 1 and 2) received a placebo during the second arm (study days 3 and 4), or vice versa, to serve as their own control group. Ten grams of active beetroot powder (BRA; Superbeets, HumanN) dissolved in 120–180 ml of water were self-administered daily; each serving contained 250 mg (~4.03 mmol) of and 20 mg (~0.29 mmol) of . Placebo beetroot powder (BRP) supplementation was identical to its active counterpart; however, the powder used during the placebo arm was devoid of and . Subjects were instructed to abstain from the use of mouthwash before and 2 h after supplementation because of its ability to inhibit the commensal bacteria responsible for reducing to (14). The active and placebo arms of the study lasted 28 days. Additionally, a ≥28-day washout period separated each arm of the study. The study duration, the length of the washout period, and the dose of were consistent with previous works (21, 46).
Blood sampling.
Venous blood samples were obtained from an antecubital vein via aseptic techniques during each study visit for the determination of NO metabolites ([] and []). Blood was collected in tubes containing EDTA and immediately centrifuged at 3,000 rpm for 15 min. Aliquots of plasma were then added to Eppendorf tubes and immediately frozen at −80°C for later analysis. Plasma [] and [] were measured by their addition to vanadium III chloride in hydrochloric acid at 90°C and to potassium iodide in acetic acid at room temperature, respectively, within 30 min of thawing using a chemiluminescence NO analyzer (model NOA 280i, Sievers Instruments, Boulder, CO). Additionally, baseline fasting glucose and lipid concentrations were determined from the blood sample obtained on study day 1 in all subjects. Blood for this analysis was collected in tubes containing lithium heparin and analyzed by the University of Iowa Diagnostic Laboratory (University of Iowa Hospitals and Clinics, Iowa City, IA) using standardized hospital protocols.
Spontaneous cardiovagal BRS.
For quantification of spontaneous cardiovagal BRS, 15 min of continuous resting HR, BP, and Vt data were recorded with the subject in the supine position. Measurement of Vt was used to identify large respiratory excursions, which potentially confound cardiovascular measurements. Beat-to-beat time series of systolic BP and R-R intervals were analyzed using the sequence technique (33) for estimation of spontaneous cardiovagal BRS (Cardioseries, Ribeirao Preto, São Paulo, Brazil). Spontaneous cardiovagal BRS was determined from all sequences of three or more successive heart beats in which there were concordant increases or decreases in systolic BP and R-R intervals. The overall gain (in ms/mmHg) was determined from the average of all up (increase systolic BP-increase R-R interval) and down (decrease systolic BP-decrease R-R interval) sequences quantified using linear regression analysis. A Pearson correlation of ≥0.8, as well as a threshold of a 1.0-mmHg change in BP, was used for the inclusion of sequences.
Peripheral chemoreflex sensitivity.
Peripheral chemoreflex sensitivity was quantified as V̇e and HR responses to acute hypoxia. Subjects began the protocol with a 1-min baseline period, during which they inhaled 20.9% compressed O2. After baseline measurements, pulse O2 saturation (%), measured via pulse oximetry (Cardiocap/5, Datex-Ohmeda), was decreased to ≤80% using an isocapnic self-regulated, partial-rebreathe system in concert with 10.0% inspired O2 fraction. The transition from baseline to target % lasted 3–5 min and was repeated after a 15-min washout period. Peripheral chemoreflex sensitivity was quantified using a seven-breath average, with the slope of the linear regression line for each dependent variable, V̇e (in l/min) and HR (in beats/min), versus % serving as a surrogate measure of peripheral chemoreflex sensitivity (25). Average slope for each dependent variable (V̇e and HR) between the two trials was used for analysis.
Data analysis.
Data were collected at 143 Hz and analyzed offline with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH). Mean arterial BP (MAP) was derived from Nexfin pressure waveforms; V̇e was calculated as the product of respiratory rate and Vt. Independent-sample t-tests were used to compare young and older adults; data collected from the older subjects during their first study visit were used in this analysis. Dependent-sample t-tests were performed to compare baseline visits (study days 1 and 3). Two older subjects were excluded from chemoreflex analysis: one subject due to technical difficulties and the other subject due to inadequate desaturation (failure to reach ≤80% after 5 min). One of the 13 older subjects who completed the study was excluded from BRS analysis due to technical difficulties. A second older subject was removed from BRS analysis due to frequent ectopic beats. Two-way repeated-measures ANOVA was used to compare V̇e and HR slopes (n = 11) as well as all three BRS sequences (n = 11) between study visits. When significant F ratios were detected, pairwise comparisons were made using Tukey’s post hoc analysis. All statistical analyses were deemed significant a priori at α = 0.05 and were completed using SigmaPlot version 11.0 (Systat Software, San Jose, CA).
RESULTS
Older subjects’ demographic data and baseline blood values collected on study day 1 are shown in Table 1. Additionally, there were no differences in outcome variables between baseline visits (study days 1 and 3).
Table 1.
Characteristics and blood chemistry of older subjects at baseline
| Characteristic | Value |
|---|---|
| Age, yr | 67 ± 3 |
| Body mass index, kg/m2 | 26 ± 3 |
| Systolic blood pressure, mmHg | 123 ± 10 |
| Diastolic blood pressure, mmHg | 76 ± 6 |
| Mean arterial pressure, mmHg | 92 ± 6 |
| Glucose, mg/dl | 97 ± 5 |
| Cholesterol, mg/dl | 178 ± 37 |
| High-density lipoproteins, mg/dl | 52 ± 20 |
| Low-density lipoproteins, mg/dl | 104 ± 33 |
| Triglycerides, mg/dl | 112 ± 72 |
Values are means ± SD; n = 13.
Plasma [] and [].
Four weeks of BRA supplementation increased plasma [] from 40.1 ± 7.0 to 75.8 ± 13.2 µM and [] from 289.0 ± 27.1 to 412.8 ± 37.1 nM (P < 0.01 for both). Plasma [] and [] remained unchanged after BRP (from 39.0 ± 5.8 to 39.3 ± 4.6 µM and from 303.1 ± 31.3 to 301.7 ± 25.1 nM, P = 0.97 and 0.95, respectively).
Resting BP.
Resting brachial systolic BP and MAP decreased in older adults after 4 wk of BRA supplementation (from 125 ± 4 to 120 ± 3 mmHg and from 93 ± 3 to 89 ± 2 mmHg, respectively, P < 0.01 for both). After BRP, systolic BP (from 122 ± 3 to 124 ± 4 mmHg) and MAP (from 91 ± 2 to 92 ± 3 mmHg) were unchanged (P = 0.16 and 0.27, respectively). The change in diastolic BP did not differ after each respective intervention (BRA: from 78 ± 3 to 73 ± 2 mmHg and BRP: from 74 ± 2 to 76 ± 2 mmHg, condition × time interaction, P = 0.13). Additionally, resting HR was unchanged after BRA (from 63 ± 2 to 63 ± 2 beats/min) and BRP (from 63 ± 3 to 61 ± 2 beats/min, condition × time interaction, P = 0.38).
Peripheral chemoreflex sensitivity.
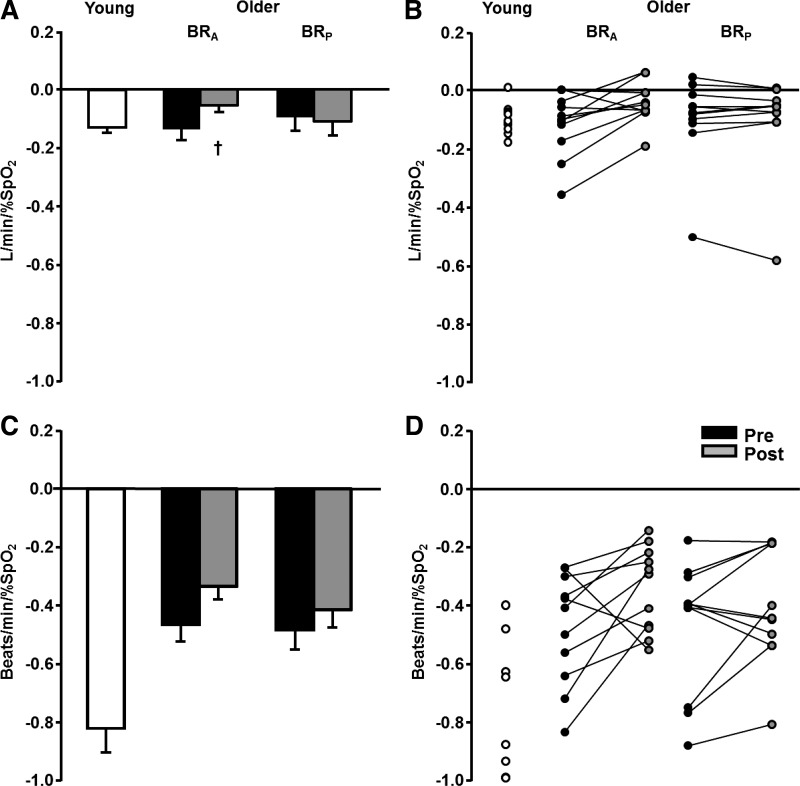
A representative V̇e slope from an older subject’s first study visit is shown in Fig. 1. Figure 2A shows mean and individual ventilatory responses to acute hypoxia before and after BRA and BRP supplementation in older subjects as well as a young reference cohort. Ventilatory responsiveness to acute hypoxia did not differ between older subjects and the young cohort (P = 0.79). However, older subjects demonstrated an attenuated HR response to the hypoxic stimulus compared with their young counterparts (P < 0.01). Systemic BP was unchanged from baseline in both young and older subjects during hypoxia (P > 0.05); however, V̇e and HR increased during hypoxia above baseline (P < 0.05 for both variables) in young and older adults.
Fig. 1.
Representative slope of the ventilatory response to a fall in percent O2 pulse saturation (peripheral chemoreflex sensitivity).
Fig. 2.
A and C: mean gain of ventilatory and heart rate responses, respectively, to acute hypoxia in young subjects (n = 10, 5 men and 5 women) and older subjects (n = 11, 7 men and 4 women). Values are means ± SE. B and D: individual ventilatory and heart rate responses, respectively. Pre, baseline; Post, after supplementation; BRA, beetroot containing nitrate and nitrite; BRP, beetroot devoid of nitrate and nitrite. Two-way repeated-measures ANOVA showed that the ventilatory response to acute hypoxia was reduced with inorganic nitrate (P < 0.05), whereas it was unchanged after placebo supplementation (P = 0.80). The heart rate response to acute hypoxia was unchanged by BRA or BRP supplementation (interaction, P = 0.41). †P < 0.05 vs. Pre.
V̇e responsiveness was attenuated after BRA (P = 0.01), but not BRP (P = 0.80), in older adults. HR responsiveness to acute hypoxia was unchanged after BRA or BRP (condition × time interaction, P = 0.41; Fig. 2B). Table 2 shows absolute changes in V̇e, HR, and % during hypoxia before and after BRA and BRP. The stimulus, acute hypoxia, did not differ between study visits (% condition × time interaction, P = 0.62). Additionally, there were no differences in the absolute change (baseline to peak response) in HR after BRA or BRP (condition × time interaction, P = 0.11); however, the absolute change in V̇e was lower after BRA (P = 0.02) compared with BRP (P = 0.10).
Table 2.
Absolute changes in response to acute hypoxia across all study visits
| BRA |
BRP |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Change in pulse O2 saturation, % | −16 ± 2 | −17 ± 2 | −17 ± 2 | −17 ± 2 |
| Change in minute ventilation, l/min | 2.9 ± 2.0 | 1.9 ± 1.4* | 2.7 ± 2.8 | 3.3 ± 3.6 |
| Change in heart rate, beats/min | 10 ± 4 | 8 ± 4 | 10 ± 4 | 9 ± 5 |
| Change in mean arterial pressure, mmHg | 0.3 ± 4.0 | 1.3 ± 4.0 | 0.8 ± 3.2 | −0.3 ± 3.3 |
Values are means ± SD; n = 11. BRA, beetroot containing nitrate and nitrite; BRP, beetroot devoid of nitrate and nitrite; Pre, baseline; Post, after supplementation.
P < 0.05 vs. Pre.
Spontaneous cardiovagal BRS.
Figure 3 shows mean and individual responses of overall spontaneous cardiovagal BRS before and after BRA and BRP supplementation in older subjects as well as the young reference cohort. Older subjects had an attenuated BRS responsiveness for all, up, and down sequences compared with young subjects (P < 0.01 for all). Four weeks of daily BRA or BRP supplementation did not elicit changes in overall cardiovagal BRS (condition × time interaction, P = 0.69) in older adults. Up and down sequences of cardiovagal BRS also did not differ between BRA and BRP (condition × time interaction, P = 0.94 and 0.39, respectively). Furthermore, individual changes in resting BP after BRA were not related to changes in cardiovagal BRS (change in systolic BP: r = −0.19, P = 0.56; change in diastolic BP: r = −0.16, P = 0.61; and change in MAP: r = −0.19, P = 0.56) in older adults.
Fig. 3.
Gain of all sequences from spontaneous cardiovagal baroreflex sensitivity (BRS) analysis in young subjects (n = 10, 5 men and 5 women) and older subjects (n = 11, 6 men and 5 women). A: group data. Values are means ± SE. B: individual responses. Pre, baseline; Post, after supplementation; BRA, beetroot containing nitrate and nitrite; BRP, beetroot devoid of nitrate and nitrite. Two-way repeated-measures ANOVA showed that nitrate supplementation did not change overall cardiovagal BRS compared with placebo (P = 0.69). *P < 0.05 vs. older subjects on study day 1.
DISCUSSION
The present study is the first to investigate the impact of daily inorganic supplementation on peripheral chemoreflex sensitivity and spontaneous cardiovagal BRS in older adults. Our primary findings were that 4 wk of daily inorganic supplementation 1) attenuated the V̇e, but not HR, response to acute hypoxia and 2) did not change any sequence of spontaneous cardiovagal BRS in older adults. Adjunct to these findings, our study supports previous notions that inorganic supplementation increases plasma [] and [] while reducing BP in older adults (13).
Previous investigations of peripheral chemoreflex sensitivity in older human and animal models have yielded inconclusive results: increased (5, 9, 24), decreased (17, 23, 37), or no change (2, 40) with aging. While these studies lack a cohesive conclusion, a “hypersensitive” chemoreflex response is characteristic of pathological conditions such as chronic heart failure and obstructive sleep apnea (1, 8, 34, 41). Sun et al. (41) showed that carotid sinus nerve activity was inhibited after l-arginine (a NO precursor) administration and was elevated in response to Nω-nitro-l-arginine (a NO synthase inhibitor) administration in rabbits with chronic heart failure. Our current data extend these findings and provide novel evidence that increasing NO bioavailability via inorganic supplementation may decrease peripheral chemoreflex sensitivity in older humans. Furthermore, our findings illustrate that the reduction in peripheral chemoreflex sensitivity can be achieved independently of endothelium-derived NO production, supporting previous findings that illustrate beneficial effects of inorganic supplementation in populations with clearly defined endothelial dysfunction (e.g., hypertension) (4, 21). Additionally, attenuation of chemoreflex sensitivity may enhance exercise tolerance in certain clinical populations (e.g., heart failure), as respiratory muscle workload would be reduced (30). While a hypersensitive chemoreflex may promote sympathetically oriented pathologies, adequate ventilatory responsiveness to hypoxia is undoubtedly essential to sustaining life. In this regard, the “ideal” peripheral chemoreflex gain remains undefined. The ventilatory response to hypoxia on study day 1 in our older subjects was similar to that of the young reference cohort.
Contrary to our hypothesis, the reduction in resting BP after inorganic supplementation was not accompanied by an increased spontaneous cardiovagal BRS. Indeed, this finding may be supported by previous suggestions that the operating point of arterial baroreceptors is fluid (18, 35). The mechanism responsible for resetting the operating point of the BRS is not yet defined, although some data suggest that the central command possesses this capability, at least during stimuli such as exercise (11, 36). Nevertheless, our data illustrate that inorganic supplementation seemingly stimulates a leftward shift in the BRS curve, as BRA supplementation led to a reduction in resting BP without a concomitant change in resting HR. However, it should be noted that increasing NO bioavailability through the --NO pathway has a direct vasodilatory effect that lowers BP (13).
A reduction in sympathetic nerve activity (SNA) and/or an enhanced arterial sympathetic BRS after inorganic supplementation may also explain the decline in BP in the present study. In support of this notion, Notay et al. (29) recently demonstrated that a single dose of inorganic reduces muscle SNA (MSNA) in young adults; however, BP and spontaneous arterial sympathetic BRS were unchanged (29). Aside from the age difference of the subjects between the two studies, resting systolic (106 vs. 122 mmHg) and diastolic (64 vs. 76 mmHg) BPs were noticeably lower in the study of Notay et al. than in the present study. The absence of a BP-lowering effect, despite reductions in MSNA, may be simply explained by the low baseline BP before the inorganic intervention (22). In this context, vasodilator drugs are less effective in reducing BP in individuals with the lowest pretreatment values (3). Collectively, these data suggest that daily inorganic supplementation may attenuate SNA and reduce BP without stimulating changes in spontaneous cardiovagal BRS. Interestingly, chemosensitivity and BRS have demonstrated an inverse relationship, such that activation of one attenuates activity of the other (6, 19). While our study did not seek to identify the impact of inorganic on this relationship, our results suggest that inorganic -derived NO may have a preferential inhibitory effect on peripheral chemoreflex sensitivity over BRS (when measured as spontaneous cardiovagal BRS).
Experimental considerations.
We recognize several limitations in our present study. As previously mentioned, BRS was measured during resting conditions; therefore, this approach does not provide insight into sympathetic BRS. Additionally, our assessment of BRS did not include a spectrum of pressures, as observed during the modified Oxford technique, further limiting the scope of our analysis.
Second, the present study investigated only a single dose of and . While no study has investigated the potential for a dose-response relationship involving inorganic on cardiovascular reflexes, dose-response reductions in BP are clearly evident (22, 38, 46). Additionally, our sample population was of modest size (n = 13), was healthy, and had relatively low ventilatory slopes in response to hypoxia. While studying this population provides novel insights and proof of concept, it is unclear if these findings can be extrapolated to diseased cohorts. In this context, a hypersensitive chemoreflex response is observed in some patients with chronic heart failure (8, 34, 41) and obstructive sleep apnea (1, 6). Nonetheless, our results, in concert with previous work (32), present a compelling argument for the therapeutic potential of inorganic for improving cardiovascular reflexes.
Conclusions and perspectives.
The attenuation in peripheral chemoreflex sensitivity without concurrent change in cardiovagal BRS suggests that inorganic has more of a preferential effect on peripheral chemoreflex sensitivity than does BRS in older adults. Furthermore, the beneficial effect of inorganic supplementation on resting BP does not appear to originate from overt increases in spontaneous cardiovagal BRS. However, it is possible that long-term inorganic supplementation may cause a leftward shift in the BRS operating point, as evidenced by a reduction in resting BP without concomitant changes in resting HR. We therefore propose that these findings potentially hold clinical implications for patients with chronic heart failure or obstructive sleep apnea, who characteristically demonstrate a hypersensitive chemoreflex response, lower BRS, and/or hypertension, as these symptoms may be attenuated with inorganic supplementation.
GRANTS
This work was funded by HumanN, Incorporated.
DISCLOSURES
N. S. Bryan is the Founder and Shareholder at HumanN and receives royalties from patents from the University of Texas Health Science Center at Houston, TX.
AUTHOR CONTRIBUTIONS
D.P.C., N.S.B., and J.K.L. conceived and designed research; J.M.B., A.C.S., W.E.H., K.U., and D.P.C. performed experiments; J.M.B., A.C.S., W.E.H., and D.P.C., analyzed data; J.M.B., J.K.L., and D.P.C. interpreted results; J.M.B. prepared figures and drafted manuscript; J.M.B., J.K.L., A.C.S., W.E.H., N.S.B., K.U., and D.P.C. edited and revised manuscript; J.M.B., J.K.L., A.C.S., W.E.H., N.S.B., K.U., and D.P.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Reginald Hochstedler for technical assistance during data collection and for help with subject recruitment. The authors also thank the laboratory of Dr. Garry R. Buettner for use of equipment for nitrate and nitrite analysis.
REFERENCES
- 1.Abboud F, Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest 124: 1454–1457, 2014. doi: 10.1172/JCI70420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed M, Giesbrecht GG, Serrette C, Georgopoulos D, Anthonisen NR. Ventilatory response to hypoxia in elderly humans. Respir Physiol 83: 343–351, 1991. doi: 10.1016/0034-5687(91)90053-L. [DOI] [PubMed] [Google Scholar]
- 3.Anand IS, Tam SW, Rector TS, Taylor AL, Sabolinski ML, Archambault WT, Adams KF, Olukotun AY, Worcel M, Cohn JN. Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 49: 32–39, 2007. doi: 10.1016/j.jacc.2006.04.109. [DOI] [PubMed] [Google Scholar]
- 4.Carlström M, Persson AE, Larsson E, Hezel M, Scheffer PG, Teerlink T, Weitzberg E, Lundberg JO. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc Res 89: 574–585, 2011. doi: 10.1093/cvr/cvq366. [DOI] [PubMed] [Google Scholar]
- 5.Chapman KR, Cherniack NS. Aging effects on the interaction of hypercapnia and hypoxia as ventilatory stimuli. J Gerontol 42: 202–209, 1987. doi: 10.1093/geronj/42.2.202. [DOI] [PubMed] [Google Scholar]
- 6.Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J Physiol 557: 1055–1065, 2004. doi: 10.1113/jphysiol.2004.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dauphinot V, Kossovsky MP, Gueyffier F, Pichot V, Gosse P, Roche F, Barthélémy JC. Impaired baroreflex sensitivity and the risks of new-onset ambulatory hypertension, in an elderly population-based study. Int J Cardiol 168: 4010–4014, 2013. doi: 10.1016/j.ijcard.2013.06.080. [DOI] [PubMed] [Google Scholar]
- 8.Ding Y, Li YL, Schultz HD. Downregulation of carbon monoxide as well as nitric oxide contributes to peripheral chemoreflex hypersensitivity in heart failure rabbits. J Appl Physiol 105: 14–23, 2008. doi: 10.1152/japplphysiol.01345.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini KG, Moreira ED, Ida F, Krieger EM. Alterations in the cardiovascular control by the chemoreflex and the baroreflex in old rats. Am J Physiol Regul Integr Comp Physiol 270: R310–R313, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Franklin SS, Wilkinson IB, McEniery CM. Unusual hypertensive phenotypes: what is their significance? Hypertension 59: 173–178, 2012. doi: 10.1161/HYPERTENSIONAHA.111.182956. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher KM, Fadel PJ, Smith SA, Strømstad M, Ide K, Secher NH, Raven PB. The interaction of central command and the exercise pressor reflex in mediating baroreflex resetting during exercise in humans. Exp Physiol 91: 79–87, 2006. doi: 10.1113/expphysiol.2005.032110. [DOI] [PubMed] [Google Scholar]
- 12.García-Río F, Villamor A, Gómez-Mendieta A, Lores V, Rojo B, Ramírez T, Villamor J. The progressive effects of ageing on chemosensitivity in healthy subjects. Respir Med 101: 2192–2198, 2007. doi: 10.1016/j.rmed.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Gee LC, Ahluwalia A. Dietary nitrate lowers blood pressure: epidemiological, pre-clinical experimental and clinical trial evidence. Curr Hypertens Rep 18: 17, 2016. doi: 10.1007/s11906-015-0623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19: 333–337, 2008. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Gozal D, Torres JE, Gozal YM, Littwin SM. Effect of nitric oxide synthase inhibition on cardiorespiratory responses in the conscious rat. J Appl Physiol 81: 2068–2077, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart EC, Charkoudian N, Joyner MJ, Barnes JN, Curry TB, Casey DP. Relationship between sympathetic nerve activity and aortic wave reflection characteristics in postmenopausal women. Menopause 20: 967–972, 2013. doi: 10.1097/GME.0b013e3182843b59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heesch CM, Carey LA. Acute resetting of arterial baroreflexes in hypertensive rats. Am J Physiol Heart Circ Physiol 253: H974–H979, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Heistad DD, Abboud FM, Mark AL, Schmid PG. Interaction of baroreceptor and chemoreceptor reflexes. Modulation of the chemoreceptor reflex by changes in baroreceptor activity. J Clin Invest 53: 1226–1236, 1974. doi: 10.1172/JCI107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iturriaga R, Mosqueira M, Villanueva S. Effects of nitric oxide gas on cat carotid body chemosensory response to hypoxia. Brain Res 855: 282–286, 2000. doi: 10.1016/S0006-8993(99)02369-0. [DOI] [PubMed] [Google Scholar]
- 21.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65: 320–327, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 56: 274–281, 2010. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 23.Kronenberg RS, Drage CW. Attenuation of the ventilatory and heart rate responses to hypoxia and hypercapnia with aging in normal men. J Clin Invest 52: 1812–1819, 1973. doi: 10.1172/JCI107363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lhuissier FJ, Canouï-Poitrine F, Richalet JP. Ageing and cardiorespiratory response to hypoxia. J Physiol 590: 5461–5474, 2012. doi: 10.1113/jphysiol.2012.238527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limberg JK, Johnson BD, Holbein WW, Ranadive SM, Mozer MT, Joyner MJ. Interindividual variability in the dose-specific effect of dopamine on carotid chemoreceptor sensitivity to hypoxia. J Appl Physiol 120: 138–147, 2016. doi: 10.1152/japplphysiol.00723.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 37: 395–400, 2004. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda T, Bates JN, Lewis SJ, Abboud FM, Chapleau MW. Modulation of baroreceptor activity by nitric oxide and S-nitrosocysteine. Circ Res 76: 426–433, 1995. doi: 10.1161/01.RES.76.3.426. [DOI] [PubMed] [Google Scholar]
- 28.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol 281: H284–H289, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Notay K, Incognito AV, Millar PJ. Acute beetroot juice supplementation on sympathetic nerve activity: a randomized, double-blind, placebo-controlled proof-of-concept study. Am J Physiol Heart Circ Physiol 313: H59−H65, 2017. doi: 10.1152/ajpheart.00163.2017. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell DE, D’Arsigny C, Raj S, Abdollah H, Webb KA. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med 160: 1804–1811, 1999. doi: 10.1164/ajrccm.160.6.9808134. [DOI] [PubMed] [Google Scholar]
- 31.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension 59: 98–104, 2012. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omar SA, Webb AJ, Lundberg JO, Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J Intern Med 279: 315–336, 2016. doi: 10.1111/joim.12441. [DOI] [PubMed] [Google Scholar]
- 33.Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension 12: 214–222, 1988. doi: 10.1161/01.HYP.12.2.214. [DOI] [PubMed] [Google Scholar]
- 34.Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61: 5–13, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 35.Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 265: H1928–H1938, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Raven PB, Fadel PJ, Smith SA. The influence of central command on baroreflex resetting during exercise. Exerc Sport Sci Rev 30: 39–44, 2002. doi: 10.1097/00003677-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Rocha BS, Gago B, Pereira C, Barbosa RM, Bartesaghi S, Lundberg JO, Radi R, Laranjinha J. Dietary nitrite in nitric oxide biology: a redox interplay with implications for pathophysiology and therapeutics. Curr Drug Targets 12: 1351–1363, 2011. doi: 10.2174/138945011796150334. [DOI] [PubMed] [Google Scholar]
- 38.Rosenbaek JB, Therwani SA, Jensen JM, Mose FH, Wandall-Frostholm C, Pedersen EB, Bech JN. Effect of sodium nitrite on renal function and sodium and water excretion and brachial and central blood pressure in healthy subjects: a dose-response study. Am J Physiol Renal Physiol 313: F378−F387, 2017. doi: 10.1152/ajprenal.00400.2016. [DOI] [PubMed] [Google Scholar]
- 39.Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J Nutr 143: 818–826, 2013. doi: 10.3945/jn.112.170233. [DOI] [PubMed] [Google Scholar]
- 40.Smith WD, Poulin MJ, Paterson DH, Cunningham DA. Dynamic ventilatory response to acute isocapnic hypoxia in septuagenarians. Exp Physiol 86: 117–126, 2001. doi: 10.1113/eph8602006. [DOI] [PubMed] [Google Scholar]
- 41.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol 86: 1273–1282, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. doi: 10.1161/01.HYP.38.2.274. [DOI] [PubMed] [Google Scholar]
- 43.Trzebski A, Sato Y, Suzuki A, Sato A. Inhibition of nitric oxide synthesis potentiates the responsiveness of carotid chemoreceptors to systemic hypoxia in the rat. Neurosci Lett 190: 29–32, 1995. doi: 10.1016/0304-3940(95)11492-F. [DOI] [PubMed] [Google Scholar]
- 44.Valdés V, Mosqueira M, Rey S, Del Rio R, Iturriaga R. Inhibitory effects of NO on carotid body: contribution of neural and endothelial nitric oxide synthase isoforms. Am J Physiol Lung Cell Mol Physiol 284: L57–L68, 2003. doi: 10.1152/ajplung.00494.2001. [DOI] [PubMed] [Google Scholar]
- 45.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784–790, 2008. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol 115: 325–336, 2013. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]