Abstract
When oxygen delivery to active muscle is insufficient to meet the metabolic demand during exercise, metabolites accumulate and stimulate skeletal muscle afferents, inducing a reflex increase in blood pressure, termed the muscle metaboreflex. In healthy individuals, muscle metaboreflex activation (MMA) during submaximal exercise increases arterial pressure primarily via an increase in cardiac output (CO), as little peripheral vasoconstriction occurs. This increase in CO partially restores blood flow to ischemic muscle. However, we recently demonstrated that MMA induces sympathetic vasoconstriction in ischemic active muscle, limiting the ability of the metaboreflex to restore blood flow. In heart failure (HF), increases in CO are limited, and metaboreflex-induced pressor responses occur predominantly via peripheral vasoconstriction. In the present study, we tested the hypothesis that vasoconstriction of ischemic active muscle is exaggerated in HF. Changes in hindlimb vascular resistance [femoral arterial pressure ÷ hindlimb blood flow (HLBF)] were observed during MMA (via graded reductions in HLBF) during mild exercise with and without α1-adrenergic blockade (prazosin, 50 µg/kg) before and after induction of HF. In normal animals, initial HLBF reductions caused metabolic vasodilation, while reductions below the metaboreflex threshold elicited reflex vasoconstriction, in ischemic active skeletal muscle, which was abolished after α1-adrenergic blockade. Metaboreflex-induced vasoconstriction of ischemic active muscle was exaggerated after induction of HF. This heightened vasoconstriction impairs the ability of the metaboreflex to restore blood flow to ischemic muscle in HF and may contribute to the exercise intolerance observed in these patients. We conclude that sympathetically mediated vasoconstriction of ischemic active muscle during MMA is exaggerated in HF.
NEW & NOTEWORTHY We found that muscle metaboreflex-induced vasoconstriction of the ischemic active skeletal muscle from which the reflex originates is exaggerated in heart failure. This results in heightened metaboreflex activation, which further amplifies the reflex-induced vasoconstriction of the ischemic active skeletal muscle and contributes to exercise intolerance in patients.
Keywords: exercise pressor reflex, ischemic muscle blood flow, sympathetically mediated vasoconstriction, β2-mediated vasodilation, metabolic vasodilation
INTRODUCTION
Ischemia in exercising skeletal muscle causes accumulation of metabolites (e.g., protons, diprotonated phosphate, and lactic acid) that activate the chemosensitive muscle afferents, eliciting a reflex pressor response, termed the muscle metaboreflex (1, 5, 18, 38). Muscle metaboreflex activation in healthy individuals increases heart rate (HR), cardiac output (CO), mean arterial pressure (MAP), ventricular contractility, and right atrial pressure (9, 13, 35, 36, 40, 45) and results in some regional vasoconstriction (4, 6, 24, 25).
During submaximal dynamic exercise in normal subjects, the muscle metaboreflex-induced pressor response, primarily driven by an increase in CO (4, 9, 16, 23), partially restores blood flow to ischemic active muscle (12, 30, 35). Metaboreflex activation also induces epinephrine release, resulting in β2-adrenergic-mediated vasodilation within the skeletal muscle (20), which is another viable mechanism for blood flow restoration to ischemic active muscle. However, we recently showed that ischemic active muscle, from which the metaboreflex originates, is itself a target vasculature for sympathetically mediated vasoconstriction during muscle metaboreflex activation (19). This functionally limits the ability of the metaboreflex to restore blood flow to the ischemic active muscle.
The strength and mechanisms of metaboreflex activation are altered in heart failure (HF) (7, 14, 26, 39, 41). HF is characterized by low resting CO and heightened sympathetic nerve activity (SNA). Inasmuch as metaboreflex-induced increases in CO and ventricular contractility are substantially attenuated in HF, the pressor response occurs primarily via enhanced vasoconstriction of the peripheral vasculature (7, 8, 14, 29). Whether ischemic muscle itself undergoes exaggerated reflex vasoconstriction in HF is unknown. Therefore, in this study, we investigated whether muscle metaboreflex-induced vasoconstriction of ischemic active muscle is exacerbated in HF.
METHODS
Experimental subjects.
Six adult mongrel canines (~19–25 kg) of either sex were selected for their willingness to walk on a motor-driven treadmill. All methods and procedures were approved by the Institutional Animal Care and Use Committee of Wayne State University and complied with the National Institutes of Health Guide to the Care and Use of Laboratory Animals. All animals were acclimatized to the laboratory surroundings and exercised voluntarily during experimentation; no negative reinforcement techniques were used.
Surgical procedures.
Before each surgery, animals received acepromazine (0.4–0.5 mg/kg im) for tranquilization. After 30 min, anesthesia was induced with ketamine (5 mg/kg iv) and diazepam (0.2–0.3 mg/kg iv) and maintained with isoflurane gas (1–3%). Animals received preoperative analgesics [fentanyl (75–125 µg/h for 72 h transdermally), buprenorphine (0.01–0.03 mg/kg im), and carprofen (4.4 mg/kg iv)]. Acepromazine (0.2–0.3 mg/kg iv) and buprenorphine (0.01–0.03 mg/kg im) were given during postoperative care. To avoid acute postoperative infections, cefazolin (antibiotic, 30 mg/kg iv) was administered pre- and postoperatively. Cephalexin (antibiotic, 30 mg/kg po bid) was administered prophylactically throughout the experimental protocol.
In the first surgical procedure under sterile conditions, the heart was exposed via a left thoracotomy (third/fourth intercostal space) approach. A blood flow transducer (18 or 20 PAU, Transonic Systems) was placed around the ascending aorta to measure CO. A telemetry pressure transmitter (model TA11 PA-D70, Data Sciences) was tethered subcutaneously, and its tip was secured inside the left ventricle to measure left ventricular pressure. Three stainless steel pacing electrodes (0-Flexon, Ethicon) were secured to the right ventricle. All wires were tunneled subcutaneously and exteriorized between the scapulae. The chest was closed in layers after reapproximation of the pericardium.
After ≥2 wk of recovery, the second surgical procedure, exposure of the abdominal aorta via a left flank incision cranial to the iliac crest, was performed under sterile conditions. A 10-mm blood flow transducer (Transonic Systems) was placed around the terminal aorta to measure hindlimb blood flow (HLBF), and two hydraulic occluders (8–10 mm, DocXS Biomedical Products) were placed just distal to the transducer. All side branches of the terminal aorta between the iliac arteries and HLBF probe were ligated. A 19-gauge polyvinyl catheter (Tygon, S54-HL, Norton) was inserted into a side branch of the aorta to measure systemic arterial pressure. A second 19-gauge polyvinyl catheter was inserted into a side branch of the aorta caudal to the occluders to measure arterial pressure below the occluders (n = 2). When catheterization of a caudal aortic branch was not possible in this surgery, a catheter was placed into a side branch of the femoral artery in a separate procedure performed ≥2 wk after the second surgery (n = 4). All instrumentation was tunneled subcutaneously and exteriorized between the scapulae. The abdomen was closed in layers. For experiments unrelated to this study, a 4-mm flow probe was placed around the left renal artery during the second surgery, and hydraulic occluders were placed around common carotid arteries in a separate procedure.
Data acquisition.
Animals recovered for ≥2 wk after each surgery. Before each experiment, the animal was brought into the laboratory and allowed to roam freely and acclimate for ~10–20 min. The animal was then led to the treadmill, arterial catheters were connected to pressure transducers (Transpac IV, ICU Medical), the left ventricular telemetry implant was turned on, and flow probes were connected to flowmeters (Transonic Systems). All hemodynamic variables were monitored as real-time waveforms by a data acquisition system (LabScribe2, iWorx) and recorded for subsequent offline analysis.
Experimental procedures.
Experiments were performed after the animals had fully recovered from surgery (i.e., ≥2 wk after surgery and when animals displayed a good appetite). During each experiment, resting steady-state hemodynamic parameters were recorded with the animal standing on the treadmill. After steady-state data were collected at rest, the treadmill was turned on and the speed was gradually increased to 3.2 km/h at 0% grade (mild exercise). The muscle metaboreflex was activated via graded reductions in HLBF (by partial inflation of terminal aortic occluders) during mild exercise, as shown in Fig. 1. The maximal reduction in HLBF was 50–60% of the free-flow exercising levels in each animal. Free-flow exercise and each reduction in HLBF were maintained until all hemodynamic parameters reached steady state (~3–5 min). Control experiments were repeated in the animals after α1-adrenergic blockade (prazosin, 50 µg/kg ia). Prazosin was administered 20–30 min before the experiment, and no subsequent experiments were performed for the next 48 h. In each experiment, the dose of prazosin was sufficient to abolish the large vasoconstrictor effect of phenylephrine (4 µg/kg). Before prazosin infusion, a phenylephrine bolus infusion increased MAP by 15.9 ± 1.4 mmHg. This response was virtually abolished after prazosin infusion: change in MAP 1.1 ± 1.6 mmHg before and 0.9 ± 1.0 mmHg after the metaboreflex experiment. After completion of the experiments in normal animals, HF was induced via rapid ventricular pacing (220–240 beats/min) for a group average of 32 ± 4 days. The development of HF was established by resting tachycardia and decreases in resting MAP, CO, left ventricular contractility [maximal rate of left ventricular contraction (dP/dtmax)], and lusitropy [maximal rate of left ventricular relaxation (dP/dtmin)] (Table 1). Control and α1-blockade experiments were repeated in the same animals after the induction of HF.
Fig. 1.
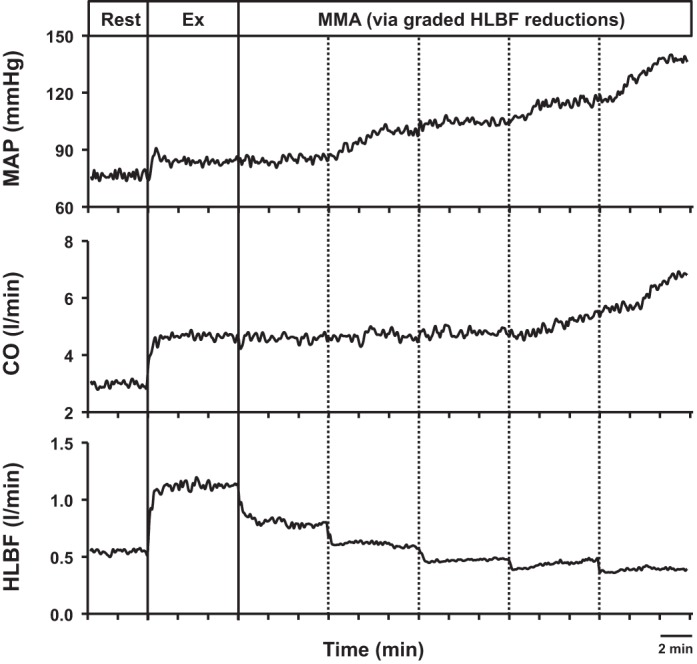
Data from 1 control experiment showing mean arterial pressure (MAP), cardiac output (CO), and hindlimb blood flow (HLBF) during rest, free-flow mild exercise (Ex), and muscle metaboreflex activation (MMA) induced via graded reductions in HLBF.
Table 1.
Mean hemodynamic values during rest, mild exercise, and maximal MMA with and without α1-adrenergic blockade in normal and heart failure animals
| Normal |
Heart Failure |
|||
|---|---|---|---|---|
| Control | α1-Blockade | Control | α1-Blockade | |
| Mean arterial pressure, mmHg | ||||
| Rest | 89.1 ± 1.8 | 80.9 ± 1.5† | 77.0 ± 0.6‡ | 71.1 ± 1.6 |
| Exercise | 91.5 ± 1.9* | 83.8 ± 1.4*† | 79.6 ± 2.0‡ | 72.3 ± 1.0 |
| MMA | 142.6 ± 1.5* | 117.5 ± 2.4*† | 111.2 ± 3.8‡ | 105.3 ± 3.8 |
| Cardiac output, l/min | ||||
| Rest | 2.78 ± 0.16 | 2.96 ± 0.21 | 2.26 ± 0.11‡ | 2.53 ± 0.13† |
| Exercise | 4.31 ± 0.22* | 4.48 ± 0.22*† | 3.38 ± 0.14*‡ | 4.00 ± 0.17*† |
| MMA | 6.16 ± 0.13* | 6.37 ± 0.19*† | 3.84 ± 0.22‡ | 5.57 ± 0.17*† |
| Heart rate, beats/min | ||||
| Rest | 75.2 ± 4.1 | 89.8 ± 5.3 | 105.0 ± 5.8 | 102.0 ± 4.0 |
| Exercise | 105.4 ± 3.4* | 119.6 ± 3.4* | 137.2 ± 5.8* | 139.6 ± 6.0* |
| MMA | 140.3 ± 3.2* | 159.9 ± 10.1* | 165.5 ± 6.6* | 173.6 ± 5.0*† |
| Nonischemic vascular conductance, ml·min−1·mmHg−1 | ||||
| Rest | 24.9 ± 1.7 | 29.5 ± 2.4† | 24.5 ± 1.6 | 29.2 ± 2.1† |
| Exercise | 35.4 ± 2.2* | 40.6 ± 2.6*† | 33.1 ± 1.3* | 41.0 ± 2.6*† |
| MMA | 40.2 ± 1.2* | 49.6 ± 1.7*† | 29.9 ± 1.2*‡ | 48.0 ± 2.2*† |
| Hindlimb blood flow, l/min | ||||
| Rest | 0.57 ± 0.05 | 0.58 ± 0.04 | 0.40 ± 0.02‡ | 0.49 ± 0.02† |
| Exercise | 1.06 ± 0.05* | 1.08 ± 0.07* | 0.85 ± 0.05*‡ | 1.03 ± 0.08*† |
| MMA | 0.54 ± 0.05* | 0.58 ± 0.07* | 0.48 ± 0.04*‡ | 0.57 ± 0.06*† |
| dP/dtmax, mmHg/s | ||||
| Rest | 1,854 ± 61 | 1,827 ± 69† | 792 ± 20‡ | 882 ± 39 |
| Exercise | 2,030 ± 93* | 2,120 ± 71*† | 911 ± 57*‡ | 1,064 ± 53* |
| MMA | 2,892 ± 104* | 3,211 ± 145*† | 1,266 ± 24*‡ | 1,640 ± 136*† |
| dP/dtmin, mmHg/s | ||||
| Rest | −1,593 ± 83 | −1,435 ± 42 | −786 ± 31‡ | −882 ± 56 |
| Exercise | −1,653 ± 84 | −1,513 ± 30* | −863 ± 52‡ | −1,033 ± 32† |
| MMA | −2,585 ± 89* | −2,219 ± 128* | −1,200 ± 9*‡ | −1,521 ± 89*† |
Values are means ± SE. MMA, muscle metaboreflex activation; dP/dtmax, maximal rate of left ventricular contraction; dP/dtmin maximal rate of left ventricular relaxation.
P < 0.05 vs. the previous setting in the same experiment;
P < 0.05 vs. control (without α1-blockade);
P < 0.05 vs. normal.
Data analysis.
MAP, femoral arterial pressure (FAP), HR, CO, left ventricular pressure, and HLBF were continuously recorded during each experimental procedure. Hindlimb vascular resistance was calculated as FAP ÷ HLBF. Vascular conductance to all vascular beds except the hindlimb [nonischemic vascular conductance (NIVC)] was calculated as NIVC = (CO − HLBF) ÷ MAP. One-minute averages of all variables were taken during steady states at rest, during free-flow mild exercise, and at each graded reduction in HLBF. Inasmuch as the HLBF during mild exercise in canines must be reduced below a clear threshold before the muscle metaboreflex is activated (4, 37, 48), initial reductions in HLBF did not evoke metaboreflex responses, while reductions in HLBF below the threshold resulted in a significant pressor response. Therefore, the data were approximated to two linear regression lines: 1) an initial slope line that includes free-flow exercise and each reduction in blood flow that did not elicit a reflex response and 2) a pressor slope line that includes reductions in HLBF that resulted in a large reflex-induced pressor response (Fig. 2). The threshold for metaboreflex activation was approximated as the intersection of the initial and pressor slope lines. Several experiments were performed on each animal in each condition. For each experiment, the values at rest and during free-flow exercise, initial and pressor slopes, and maximal metaboreflex activation were determined. These values within a given condition were averaged across experiments for each animal. These average values were then averaged across animals to determine the mean hemodynamic values for the population studies in each condition. Therefore, each animal only contributed once to the population mean (Table 1) and served as its own control.
Fig. 2.
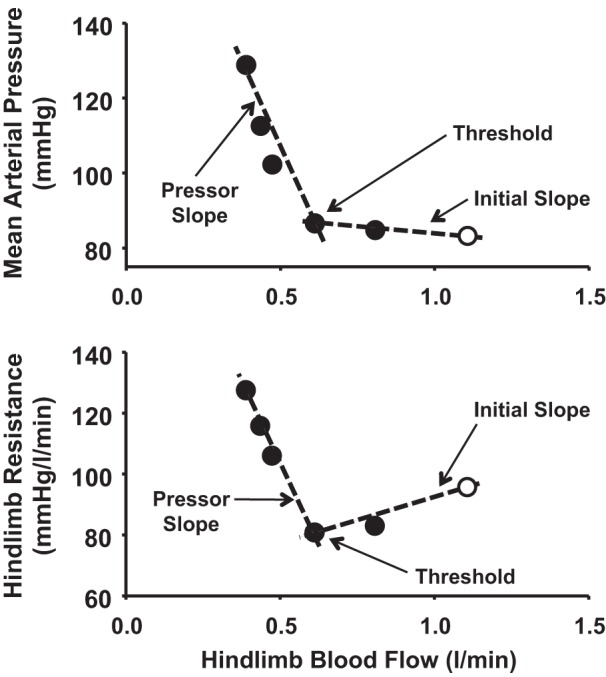
Initial and pressor slopes of mean arterial pressure (top) and hindlimb resistance (bottom) obtained by using a dual linear regression model in a control experiment. Free-flow exercise (○) was followed by graded reductions in hindlimb blood flow (●), eliciting the muscle metaboreflex during mild exercise.
Statistical analysis.
All hemodynamic data are reported as means ± SE. An α-level of P < 0.05 was used to determine statistical significance. Averaged responses for each animal were analyzed with Systat software (Systat 11.0). Two-way ANOVA with repeated measures was used to compare hemodynamic data for time and/or condition effects between control and α1-adrenergic blockade experiments and between control experiments before and after HF. In the event of a significant time-condition interaction, individual means were compared using the test for simple effects.
RESULTS
Figure 1 shows MAP, CO, and HLBF in a control experiment during rest, mild exercise, and muscle metaboreflex activation induced by graded reductions in HLBF (data are averaged over every 5 s for clarity). From rest to exercise, there was a small increase in MAP, CO, and HLBF. With initial graded reductions in HLBF, there was a very small increase in MAP and CO. When further reductions in HLBF elicited the muscle metaboreflex, marked increases in MAP and CO occurred. Figure 2 shows the relationships between MAP and HLBF and between hindlimb resistance and HLBF during the control experiment. Initial reductions in HLBF caused a small increase in MAP and a decrease in hindlimb resistance. Once the metaboreflex threshold was reached, further reductions in HLBF caused large reflex increases in MAP and hindlimb resistance.
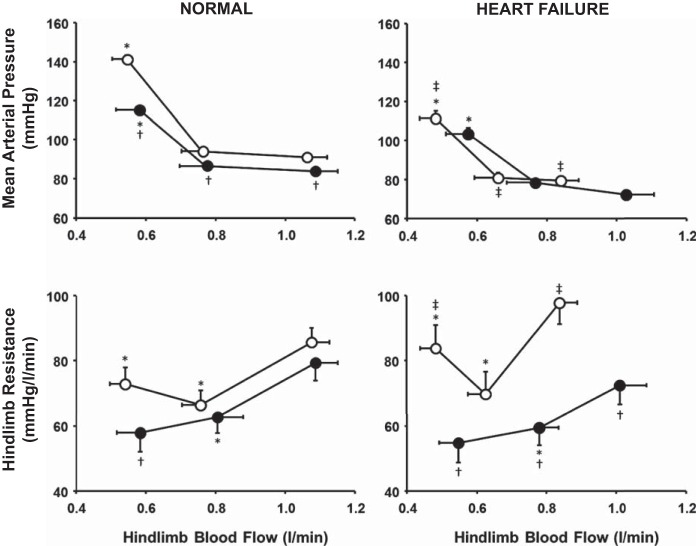
Figure 3 shows average MAP and hindlimb resistance responses as a function of HLBF in control and α1-adrenergic blockade experiments before and after induction of HF. During control experiments in normal animals, initial reductions in HLBF did not change MAP; however, once HLBF was reduced below the metaboreflex threshold, there was a large significant increase in MAP. After α1-blockade in normal animals, MAP was significantly lower at all time points, and the metaboreflex-induced increase in MAP was substantially attenuated compared with control. During control experiments in healthy animals, initial reductions in HLBF caused a significant decrease in hindlimb vascular resistance, indicating vasodilation in the hindlimb skeletal muscle. Once HLBF was reduced below threshold, there was a significant increase in hindlimb resistance, indicating vasoconstriction of the ischemic skeletal muscle. After α1-blockade, initial HLBF reductions caused a decrease in hindlimb resistance similar to control. However, once HLBF was reduced below threshold, the vasoconstriction observed in control experiments was completely abolished, and hindlimb resistance continued to fall with further reductions in HLBF. At maximal metaboreflex activation, hindlimb resistance after α1-blockade was significantly lower than control.
Fig. 3.
Average mean arterial pressure (top) and hindlimb resistance (bottom) responses (n = 6) during free-flow exercise, metaboreflex threshold, and maximal metaboreflex activation in control (○) and α1-adrenergic blockade (●) experiments in normal animals (left) and after induction of heart failure (right). *P < 0.05 vs. mean arterial pressure or hindlimb resistance at the previous setting in the same experiment. †P < 0.05 vs. control. ‡P < 0.05 vs. normal.
After induction of HF in the same animals, MAP and HLBF during free-flow exercise were significantly lower than before induction of HF, and HLBF resided much closer to the metaboreflex threshold, as observed in several previous studies (3, 14, 15) (see HLBF data in Table 1). With reduction of HLBF below the metaboreflex threshold, there was a significant increase in MAP, although it was significantly attenuated compared with the pressor response in normal animals. MAP responses after α1-adrenergic blockade in HF were not different from control levels in HF. After induction of HF, hindlimb resistance during free-flow exercise in control experiments was significantly higher than in normal animals. Hindlimb resistance decreased with initial reductions in HLBF and markedly increased in response to metaboreflex activation. Hindlimb resistance at maximal metaboreflex activation was significantly higher in HF than healthy animals. After α1-blockade in HF, hindlimb resistance during free-flow exercise was significantly lower and HLBF was markedly higher than during the control experiment in HF. Initial reductions in HLBF led to a decrease in hindlimb resistance, indicating vasodilation. After muscle metaboreflex activation, the large vasoconstriction observed in control experiments in HF was completely abolished following α1-blockade.
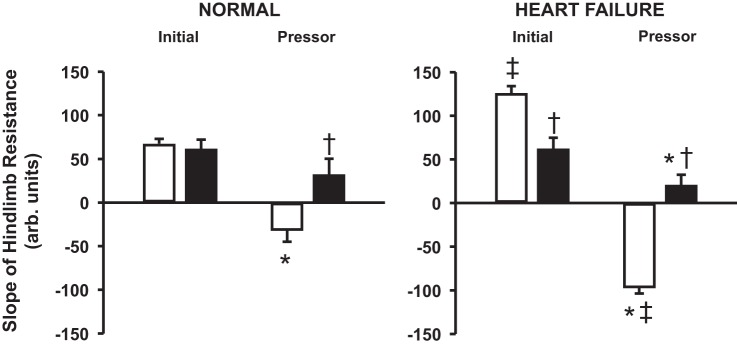
Figure 4 shows average slopes of the relationship between hindlimb vascular resistance and HLBF during control and α1-adrenergic blockade experiments before and after induction of HF. During control experiments in healthy animals, the initial slope was significantly different from the pressor slope. After α1-blockade, the initial and pressor slopes were not significantly different from each other. The initial slope after α1-blockade was similar to that in control experiments, while the pressor slope was reversed and significantly different from that in control experiments. After induction of HF, the initial and pressor slopes were significantly different from each other and markedly larger than in normal animals. After α1-blockade in HF animals, the two slopes were significantly different from each other and from the control experiments in HF.
Fig. 4.
Average initial and pressor slope values of hindlimb resistance (n = 6) during control (open bars) and α1-adrenergic blockade (filled bars) experiments in normal animals (left) and after induction of heart failure (right). *P < 0.05 vs. initial slope. †P < 0.05 vs. control. ‡P < 0.05 vs. normal.
Table 1 shows mean hemodynamic data during rest, mild exercise, and maximal muscle metaboreflex activation in control and α1-adrenergic blockade experiments before and after induction of HF in the same animals. From rest to exercise in control experiments, there were significant increases in MAP, CO, HR, NIVC, HLBF, and ventricular contractility. With muscle metaboreflex activation, there were further significant increases in MAP, CO, HR, NIVC, and indexes of ventricular inotropic and lusitropic state. After α1-blockade in normal animals, resting MAP was significantly lower, whereas NIVC and contractility were significantly higher, than in control experiments. With mild dynamic exercise, there was a significant increase in all variables, and MAP, CO, NIVC, and dP/dtmax were markedly higher than control exercise levels. Muscle metaboreflex activation following α1-blockade resulted in substantial increases in MAP, CO, HR, NIVC, dP/dtmax, and dP/dtmin. After α1-blockade in normal animals, the reflex-induced pressor response was significantly attenuated compared with control, while CO, NIVC, and ventricular contractility during maximal metaboreflex activation were substantially higher than during control.
After induction of HF in the same animals, resting levels of MAP, CO, HLBF, dP/dtmax, and dP/dtmin were significantly lower. Although CO, HR, NIVC, HLBF, and ventricular contractility increased from rest to exercise, these parameters, with the exception of HR, were significantly lower than in normal animals. After metaboreflex activation in HF animals, increases in MAP, CO, dP/dtmax, and dP/dtmin were significantly attenuated, and there was significant vasoconstriction in the nonischemic vasculature. After α1-adrenergic blockade, resting CO, HLBF, and NIVC increased. From rest to exercise, significant increases in CO, HR, NIVC, HLBF, and ventricular contractility were observed. Muscle metaboreflex activation following α1-blockade in HF animals significantly increased CO, HR, NIVC, dP/dtmax, and dP/dtmin, and these parameters were significantly improved compared with metaboreflex responses during the control condition in HF.
DISCUSSION
The major new finding of this study is that muscle metaboreflex-induced vasoconstriction of the ischemic active skeletal muscle is exaggerated in HF. Previous studies have shown that the metaboreflex-induced increase in MAP partially restores blood flow to ischemic active muscle in healthy subjects. Whether the muscle metaboreflex-induced increase in SNA causes vasoconstriction of the ischemic muscle from which it originates has been controversial (17, 28, 30). We have recently shown that metaboreflex activation during submaximal exercise induces α-adrenergic-mediated sympathetic vasoconstriction within the ischemic active muscle, limiting the capability of the metaboreflex to restore blood flow to the muscle (19). In HF, the ability of the reflex to increase CO is markedly limited, and the metaboreflex-induced pressor response occurs primarily via peripheral vasoconstriction. Inability to increase CO in HF limits restoration of blood flow to the ischemic muscle, and exaggerated vasoconstriction of the ischemic muscle would further limit blood supply and enhance muscle ischemia. This generates a positive-feedback loop that potentially contributes to exercise intolerance in HF.
Muscle metaboreflex responses in HF.
The small increase in CO with metaboreflex activation in HF attenuates the rise in MAP and, consequently, limits the ability of the metaboreflex to partially restore blood flow to the ischemic muscle. Inasmuch as heightened coronary vasoconstriction in HF limits cardiac function (7) and the metaboreflex-induced increase in CO is quite small, the mechanism of the muscle metaboreflex shifts from a CO-mediated to a peripheral vasoconstriction-induced pressor response (8, 14, 29). Vasoconstriction of inactive beds plays a very small role in generating the pressor response, as blood flow to inactive beds constitutes only a small proportion of CO and, thereby, total vascular conductance (27). Thus, with active skeletal muscle comprising the largest fraction of total vascular conductance during metaboreflex activation, it is the primary target vasculature for vasoconstriction, capable of generating large pressor responses. This is the first study to show that metaboreflex-induced vasoconstriction of the ischemic muscle is significantly exaggerated in HF. Ischemic muscle vasoconstriction was abolished after α1-adrenergic blockade, indicating a sympathetic origin of vasoconstriction. In addition, α1-blockade also markedly improved HLBF during free-flow exercise, revealing a potential therapeutic target for exercise intolerance in HF.
If activation of the muscle metaboreflex induces sympathetically mediated vasoconstriction of the ischemic muscle from which it originates, active ischemic muscle is both the origin and the target of the reflex. On the afferent side, metabolic accumulation in the muscle stimulates group III/IV nerves and elicits the muscle metaboreflex, which in turn increases CO, redistributes blood flow, and causes vasoconstriction of the ischemic muscle on the efferent side. Vasoconstriction of the already ischemic muscle would further limit oxygen delivery to the muscle, causing more ischemia and heightened metaboreflex activation. We have previously shown that, in healthy subjects, metaboreflex-induced vasoconstriction of the ischemic active muscle acts as a limiting positive feedback, where afferent and efferent responses progressively become smaller and plateau at an amplified level (19). The slope of the relationship between hindlimb resistance and HLBF in normal animals is −32.6 units compared with −97.9 units after induction of HF. Therefore, for every 1-l/min reduction in HLBF beyond the metaboreflex threshold, hindlimb resistance increases by 32.6 units in normal animals and 97.9 units after induction of HF. At constant pressure, this would result in an additional 0.54-l/min reduction in HLBF in normal animals, which increases substantially to 0.84 l/min after induction of HF. Thus, for every 1-l/min reduction in flow beyond the metaboreflex threshold, the reflex vasoconstriction further reduces blood flow by an additional 0.84 l/min in HF, which yields a reflex gain of 0.84 in terms of the strength of the reflex in its ability to elicit vasoconstriction in the ischemic muscle. In animals with HF, this additional decrease in HLBF would cause further ischemia and increase hindlimb resistance by another 82.1 mmHg·l−1·min−1, which in turn would reduce HLBF by 0.2 l/min, and so forth. Thus, as in the normal animals, the stimulus and the response become smaller with each cycle of positive feedback and will eventually reach a plateau. Therefore, muscle metaboreflex activation during mild exercise in moderate HF is still a limiting positive-feedback reflex, although with a markedly amplified response compared with normal subjects. Importantly, this high gain of reflex vasoconstriction in the ischemic active muscle in HF approaches the value of 1. If gain exceeds 1, the reflex vasoconstriction will cause a larger reduction in HLBF than the initial stimulus, and thus the vasoconstriction occurring with each cycle becomes greater and greater, ultimately causing total vasoconstriction. Previous studies have shown that a larger metaboreflex-induced vasoconstriction of the peripheral vasculature occurs with heavier workloads (4, 15). With increased workload, the metaboreflex could easily become a runaway feedback loop, where the stimulus and the response increase with each cycle, rapidly reaching infinite resistance. This scenario could contribute to exercise intolerance in HF.
Mechanisms for heightened SNA in HF.
Heightened SNA and impaired muscle blood flow in HF could result from various pathways and vascular factors. In healthy subjects, the arterial baroreflex buffers about one-half of the metaboreflex-induced pressor response by restraining peripheral vasoconstriction (22). However, the strength of the baroreflex in HF is impaired at rest (31) and during exercise (21). Reduced baroreflex buffering of the muscle metaboreflex in HF potentially contributes to elevated SNA and peripheral vasoconstriction. Respiratory muscles also play an important role in metaboreflex activation during dynamic exercise (33). Low CO in HF causes hypoperfusion of all tissues, including respiratory muscles. During exercise in HF, increased exertion of the respiratory muscles likely enhances afferent activation, causing augmented metaboreflex-induced responses. A recent study also implicated aberrant activation of cardiac sympathetic afferents in HF, which contributes to elevated SNA at rest (47). Enhanced arterial chemoreflex activation has also been shown in HF (43). Neurogenic vasoconstriction of the active skeletal muscle is attenuated by the metabolic by-products in the muscle, termed functional sympatholysis (32). However, functional sympatholysis is impaired in pathophysiological states such as HF and hypertension (44, 46), potentially contributing to augmented vasoconstriction in these disease states. Moreover, impaired endothelium-mediated vasodilation and elevated circulating levels of catecholamines, vasopressin, and angiotensin II could further increase the peripheral vasoconstriction in HF (10, 15). Some studies have shown reduced oxidative type I fibers and increased glycolytic type IIb fibers in HF subjects, which could reduce their exercise capacity and lead to exercise intolerance (11, 42).
Perspectives
The muscle metaboreflex is not tonically active at a mild workload in canines, as HLBF must be reduced below the threshold flow before metaboreflex responses are observed. Because of lower perfusion in HF, muscle blood flow during even mild exercise resides much closer to the metaboreflex threshold, and reflex activation occurs earlier during graded exercise and likely contributes to heightened sympathetic activation in HF as workload increases and tonic activity of the reflex ensues (4). The extent to which the muscle metaboreflex approaches runaway positive-feedback levels with higher-intensity exercise is unknown. Importantly, there is evidence that reflexes arising from skeletal muscle afferents are tonically active in humans during even relatively mild exercise (2, 34). The exaggerated vasoconstriction within the ischemic active muscle together with limited reflex restoration of blood flow to the ischemic muscle likely impairs exercise ability and contributes to exercise intolerance in HF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-55473 and HL-126706.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K. and D.S.O'L. conceived and designed research; J.K., D.S., A.C.K., H.W.H., A.A., T.M.M., and D.S.O'L. performed experiments; J.K. analyzed data; J.K., D.S., A.C.K., H.W.H., T.M.M., and D.S.O'L. interpreted results of experiments; J.K. prepared figures; J.K. drafted manuscript; J.K., D.S., A.C.K., H.W.H., A.A., T.M.M., and D.S.O'L. edited and revised manuscript; J.K., D.S., A.C.K., H.W.H., A.A., T.M.M., and D.S.O'L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jody Helme-Day and Audrey Nelson for expert technical assistance and animal care.
REFERENCES
- 1.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustyniak RA, Ansorge EJ, Kim JK, Sala-Mercado JA, Hammond RL, Rossi NF, O’Leary DS. Cardiovascular responses to exercise and muscle metaboreflex activation during the recovery from pacing-induced heart failure. J Appl Physiol 101: 14–22, 2006. doi: 10.1152/japplphysiol.00072.2006. [DOI] [PubMed] [Google Scholar]
- 4.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O’Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Boushel R, Madsen P, Nielsen HB, Quistorff B, Secher NH. Contribution of pH, diprotonated phosphate and potassium for the reflex increase in blood pressure during handgrip. Acta Physiol Scand 164: 269–275, 1998. doi: 10.1046/j.1365-201X.1998.00429.x. [DOI] [PubMed] [Google Scholar]
- 6.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O’Leary DS. Muscle metaboreflex-induced coronary vasoconstriction functionally limits increases in ventricular contractility. J Appl Physiol 109: 271–278, 2010. doi: 10.1152/japplphysiol.01243.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O’Leary DS. Muscle metaboreflex-induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am J Physiol Heart Circ Physiol 304: H1029–H1037, 2013. doi: 10.1152/ajpheart.00879.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- 9.Crisafulli A, Piras F, Filippi M, Piredda C, Chiappori P, Melis F, Milia R, Tocco F, Concu A. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J Physiol Sci 61: 385–394, 2011. doi: 10.1007/s12576-011-0163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drexler H, Hayoz D, Münzel T, Hornig B, Just H, Brunner HR, Zelis R. Endothelial function in chronic congestive heart failure. Am J Cardiol 69: 1596–1601, 1992. doi: 10.1016/0002-9149(92)90710-G. [DOI] [PubMed] [Google Scholar]
- 11.Drexler H, Riede U, Münzel T, König H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 85: 1751–1759, 1992. doi: 10.1161/01.CIR.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 12.Eiken O, Bjurstedt H. Dynamic exercise in man as influenced by experimental restriction of blood flow in the working muscles. Acta Physiol Scand 131: 339–345, 1987. doi: 10.1111/j.1748-1716.1987.tb08248.x. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH, Fadel PJ. Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. J Physiol 588: 1117–1127, 2010. doi: 10.1113/jphysiol.2009.185470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O’Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O’Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol 90: 55–61, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Ichinose MJ, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, O’Leary DS. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol 298: H245–H250, 2010. doi: 10.1152/ajpheart.00909.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joyner MJ. Does the pressor response to ischemic exercise improve blood flow to contracting muscles in humans? J Appl Physiol 71: 1496–1501, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol Respir Environ Exerc Physiol 57: 644–650, 1984. [DOI] [PubMed] [Google Scholar]
- 19.Kaur J, Machado TM, Alvarez A, Krishnan AC, Hanna HW, Altamimi YH, Senador D, Spranger MD, O’Leary DS. Muscle metaboreflex activation during dynamic exercise vasoconstricts ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 309: H2145–H2151, 2015. doi: 10.1152/ajpheart.00679.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O’Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in β2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. doi: 10.1152/ajpheart.00648.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JK, Augustyniak RA, Sala-Mercado JA, Hammond RL, Ansorge EJ, Rossi NF, O’Leary DS. Heart failure alters the strength and mechanisms of arterial baroreflex pressor responses during dynamic exercise. Am J Physiol Heart Circ Physiol 287: H1682–H1688, 2004. doi: 10.1152/ajpheart.00358.2004. [DOI] [PubMed] [Google Scholar]
- 22.Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O’Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol 288: H1374–H1380, 2005. doi: 10.1152/ajpheart.01040.2004. [DOI] [PubMed] [Google Scholar]
- 23.McNulty CL, Moody WE, Wagenmakers AJ, Fisher JP. Effect of muscle metaboreflex activation on central hemodynamics and cardiac function in humans. Appl Physiol Nutr Metab 39: 861–870, 2014. doi: 10.1139/apnm-2013-0414. [DOI] [PubMed] [Google Scholar]
- 24.Mittelstadt SW, Bell LB, O’Hagan KP, Clifford PS. Muscle chemoreflex alters vascular conductance in nonischemic exercising skeletal muscle. J Appl Physiol 77: 2761–2766, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Mittelstadt SW, Bell LB, O’Hagan KP, Sulentic JE, Clifford PS. Muscle chemoreflex causes renal vascular constriction. Am J Physiol Heart Circ Physiol 270: H951–H956, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol 280: H969–H976, 2001. [DOI] [PubMed] [Google Scholar]
- 27.O’Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol 260: H632–H637, 1991. [DOI] [PubMed] [Google Scholar]
- 28.O’Leary DS. Point: the muscle metaboreflex does restore blood flow to contracting muscles. J Appl Physiol 100: 357–358, 2006. doi: 10.1152/japplphysiol.01222.2005. [DOI] [PubMed] [Google Scholar]
- 29.O’Leary DS, Sala-Mercado JA, Augustyniak RA, Hammond RL, Rossi NF, Ansorge EJ. Impaired muscle metaboreflex-induced increases in ventricular function in heart failure. Am J Physiol Heart Circ Physiol 287: H2612–H2618, 2004. doi: 10.1152/ajpheart.00604.2004. [DOI] [PubMed] [Google Scholar]
- 30.O’Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Olivier NB, Stephenson RB. Characterization of baroreflex impairment in conscious dogs with pacing-induced heart failure. Am J Physiol Regul Integr Comp Physiol 265: R1132–R1140, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962. doi: 10.1161/01.RES.11.3.370. [DOI] [PubMed] [Google Scholar]
- 33.Rodman JR, Henderson KS, Smith CA, Dempsey JA. Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol 95: 1159–1169, 2003. doi: 10.1152/japplphysiol.00258.2003. [DOI] [PubMed] [Google Scholar]
- 34.Rowell LB, Savage MV, Chambers J, Blackmon JR. Cardiovascular responses to graded reductions in leg perfusion in exercising humans. Am J Physiol Heart Circ Physiol 261: H1545–H1553, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O’Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006. doi: 10.1152/ajpheart.00869.2005. [DOI] [PubMed] [Google Scholar]
- 36.Sheriff DD, Augustyniak RA, O’Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987. [DOI] [PubMed] [Google Scholar]
- 38.Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, Miller G, Moore RL. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. Am J Physiol Heart Circ Physiol 263: H1499–H1505, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- 40.Spranger MD, Sala-Mercado JA, Coutsos M, Kaur J, Stayer D, Augustyniak RA, O’Leary DS. Role of cardiac output versus peripheral vasoconstriction in mediating muscle metaboreflex pressor responses: dynamic exercise versus postexercise muscle ischemia. Am J Physiol Regul Integr Comp Physiol 304: R657–R663, 2013. doi: 10.1152/ajpregu.00601.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation 84: 2034–2039, 1991. doi: 10.1161/01.CIR.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 81: 518–527, 1990. doi: 10.1161/01.CIR.81.2.518. [DOI] [PubMed] [Google Scholar]
- 43.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol 86: 1264–1272, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Thomas GD, Zhang W, Victor RG. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: role of oxidative stress. Circ Res 88: 816–823, 2001. doi: 10.1161/hh0801.089341. [DOI] [PubMed] [Google Scholar]
- 45.Victor RG, Seals DR. Reflex stimulation of sympathetic outflow during rhythmic exercise in humans. Am J Physiol Heart Circ Physiol 257: H2017–H2024, 1989. [DOI] [PubMed] [Google Scholar]
- 46.Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol 589: 1209–1220, 2011. doi: 10.1113/jphysiol.2010.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 64: 745–755, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983. [DOI] [PubMed] [Google Scholar]