Abstract
Endothelium-dependent, nitric oxide-mediated dilatation is impaired in aging arteries. The dysfunction reflects increased production of reactive oxygen species (ROS), is reversed by inhibiting superoxide with superoxide dismutase (SOD) mimics, and is assumed to reflect superoxide-mediated inactivation of nitric oxide. However, the dysfunction also reflects Src-dependent degradation and loss of vascular-endothelial (VE)-cadherin from adherens junctions, resulting in a selective impairment in the ability of the junctions to amplify endothelial dilatation. Experiments therefore tested the hypothesis that SOD mimics might restore endothelial dilation in aging arteries by inhibiting Src and protecting endothelial adherens junctions. Tail arteries from young and aging Fisher 344 rats were processed for functional (pressure myograph), biochemical (immunoblot), and morphological (immunofluorescence) analyses. Cell-permeable SOD mimics [manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP) or tempol] did not affect acetylcholine-induced dilatation in young arteries but increased responses and restored normal dilator function in aging arteries. In aging arteries, MnTMPyP decreased Src activity (immunoblots of Tyr416 phosphorylated compared with total Src), increased the intensity and width of VE-cadherin staining at endothelial junctions, and increased VE-cadherin levels in Triton X-100-insoluble lysates, which represents the junctional protein. Because of aging-induced junctional disruption, inhibiting VE-cadherin clustering at adherens junctions with a function-blocking antibody does not affect acetylcholine-induced dilatation in aging arteries. However, the antibody prevented SOD mimics from restoring acetylcholine-induced dilatation in aging arteries. Therefore, SOD mimics improve impaired adherens junctions in aging endothelium, which is essential for SOD mimics to restore endothelium-dependent dilatation in aging arteries. The results suggest an important new pathological role for ROS in aging endothelium, namely, disruption of adherens junctions.
NEW & NOTEWORTHY Aging-induced endothelial dysfunction is reversed by SOD mimics. This study demonstrates that they improve impaired adherens junctions in aging endothelium and that their restoration of endothelial dilatation is dependent on increased junctional activity. The results suggest a novel role for oxygen radicals in vascular aging, namely, disruption of adherens junctions.
Keywords: aging, adherens junctions, endothelium, nitric oxide, vascular-endothelial cadherin
INTRODUCTION
Aging disrupts the normal protective activity of arterial endothelial cells, including nitric oxide (NO)-mediated endothelium-dependent dilatation (4, 6, 8). This aging-induced endothelial dysfunction contributes to structural and functional deterioration of the arterial system and to the amplification of cardiovascular diseases (8). Aging increases the vascular production of reactive oxygen species (ROS), and the impaired endothelium-dependent dilatation of aging arteries can be restored by inhibiting superoxide with superoxide dismutase (SOD) or cell-permeable SOD mimics (3, 6, 18). It has therefore been assumed that the aging-induced decrease in endothelium-dependent dilatation reflects direct inactivation of NO by superoxide.
The clustering of vascular-endothelial (VE)-cadherin at adherens junctions stimulates assembly of a macromolecular signaling complex that regulates endothelial cell signaling, including amplifying endothelium-dependent dilatation to acetylcholine (4, 8, 10). In aging rat arteries, Src-dependent internalization and degradation of VE-cadherin disrupts the structure and function of endothelial adherens junctions (4). Indeed, inhibition of adherens junctions by a VE-cadherin function-blocking antibody had no effect on endothelium-dependent dilation to acetylcholine in aging rat tail arteries but inhibited responses in young tail arteries and mimicked the aging process (4). Furthermore, pharmacological inhibition of Src fully restored endothelial dilatation in aging rat tail arteries, which was dependent on the improvement in endothelial adherens junctions (4). The results suggested that the diminution in endothelial NO-mediated dilatation occurring in aging rat tail arteries reflected a selective loss in the contribution of adherens junctions to endothelium-dependent dilatation (4).
As with other aging arteries (3, 6, 18), aging rat tail arteries have increased production of endothelial ROS, which appear to contribute to the impairment in endothelium-dependent dilatation (9). If so, that would suggest an important linkage between ROS activity and endothelial adherens junctions in aging arteries. Indeed, in cultured endothelial cells, increased production of ROS in response to inflammatory mediators causes disruption of adherens junctions through the internalization and loss of VE-cadherin from the junctions (1, 19, 20, 23), which can reflect ROS-dependent activation of Src (1, 19, 23). The present experiments were therefore performed to determine whether inhibiting superoxide restores endothelial dilatation in aging rat tail arteries and, if so, to determine the role of endothelial adherens junctions in that restoration.
MATERIALS AND METHODS
Ethical approval and animals.
Young (3–4 mo) and aging (22–25 mo) male Fisher 344 rats were obtained from Charles River and their National Institute on Aging colony and euthanized by CO2 asphyxiation. Animals were housed in our institution’s animal facilities and fed regular rodent chow until the experimental day. For experiments comparing the effects of treatments on arteries of young and aging animals, animals were studied concurrently. Particular animals and arteries for each experiment were selected randomly. Animal use was approved by the Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Tail arteries were rapidly removed and placed in cold Krebs-Ringer bicarbonate solution (4, 9). Tail arteries were selected for the study because they represent a well-studied peripheral arterial system, the effects of aging on these arteries is representative of other arteries, and because of our previous research on these aging arteries (4, 9). As with our previous studies on vascular aging, a limitation of our approach is that only male animals were analyzed.
Functional responses.
Tail arteries were cannulated with micropipettes, secured within a microvascular chamber, and maintained at a transmural pressure (PTM) of 60 mmHg in the absence of flow (Living Systems, VT) (4, 9). Arteries were superfused with Krebs-Ringer bicarbonate solution (37°C, pH 7.4, 16% O2-5% CO2-balance N2, control solution), and the chamber was placed on the stage of an inverted microscope. Arterial diameter was continuously monitored and recorded (BIOPAC, Santa Barbara, CA) (4, 9). Concentration-effect curves to acetylcholine were obtained during constriction to phenylephrine, and both of these stimuli were administered in the abluminal superfusate. The concentration of phenylephrine was increased gradually until the arteries were constricted to ~70% of the baseline diameter. There was no significant difference in the concentration of phenylephrine required to constrict young or aged arteries [log(M) of −6.80 ± 0.03 and −6.82 ± 0.05, respectively, n = 12]. There were also no significant effects of any of the drug or antibody treatments on the concentration of phenylephrine required to constrict the arteries. When analyzing the effects of the SOD mimics manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP; 50 μM) or tempol (1 mM) or the endothelial NO synthase (eNOS) inhibitor N-nitro-l-arginine methyl ester (l-NAME; 100 μM), arteries were incubated with or without these drugs for 60 min in the superfusate before and during exposure to acetylcholine. When assessing the effects of polyethylene glycol (PEG)-catalase (250 U/ml), it was present in the intraluminal perfusate and in the superfusate for 60 min before and during exposure to acetylcholine. To determine the role of VE-cadherin clustering at adherens junctions, the arterial endothelium was treated via the intraluminal perfusate to a function-blocking antibody to VE-cadherin (BV13, 50 μg/ml, eBioscience) for 2.5 h before responses to acetylcholine were analyzed (4, 10). The antibody targets an extracellular domain of VE-cadherin resulting in disruption of VE-cadherin interactions and dispersion of the protein from adherens junctions (see below) (4, 10).
Immunofluorescence.
Tail arteries were mounted in specialized “flipper” microvascular chambers (Living Systems) that enabled the blood vessel assembly to be rapidly (~1 s) transferred from control solution to paraformaldehyde (3%, 4°C, 30 min). Arteries were treated with or without MnTMPyP (50 μM) at PTM of 60 mmHg for 60 min or to a function-blocking antibody to VE-cadherin (BV13, 50 μg/ml) or a control antibody (50 μg/ml, eBioscience) for 2.5 h, as described above. They were then fixed with paraformaldehyde, permeabilized in Triton X-100 (TTX), and incubated in donkey serum to reduce nonspecific binding, as previously described (4, 10). They were incubated overnight with a goat polyclonal antibody to VE-cadherin directed against an intracellular COOH-terminus epitope (C-19, 1:500 dilution, Santa Cruz Biotechnology) (4, 10). Arteries were rinsed (PBS) and then incubated (120 min) with rhodamine-labeled donkey anti-goat antibody (1:200, Jackson ImmunoResearch) and Draq5 (5 μM, nuclear stain, Biostatus). The endothelium was imaged (1024 × 1024 pixels) with a Leica SP5 LSM using a ×63 objective (numerical aperture: 1.4), pinhole of 1 Airy unit, scan speed of 400 Hz, 6-line averaging, optical zoom of 3.0, and excitation/emission settings for rhodamine (543 nm/555–620 nm) and Draq (633 nm/650–750 nm) (4, 10). For quantitative comparison, control and treated arteries were processed at the same time using the same instrument settings. Z-stacks were obtained for the complete endothelial layer (0.25-μm steps). The width and intensity of adherens junctions were assessed using line profiles of VE-cadherin fluorescent staining, as previously described (4, 10).
Immunoblot analysis.
Tail arteries were mounted in specialized “flipper” microvascular chambers (Living Systems) and treated with or without MnTMPyP (50 μM) at PTM of 60 mmHg for 60 min, as described above. They were then rapidly (~1 s) transferred to ice-cold control solution. Arteries were processed using single or sequential lysis, with the latter providing TTX-soluble and TTX-insoluble fractions, as previously described (4). Protein concentrations were determined using BCA protein assay (Pierce; Rockford, IL), and protein samples were boiled in Laemmli sample buffer for 5 min. VE-cadherin and other proteins were assessed using agarose (1%)-acrylamide (4%) composite gels as previously described (4). Proteins were transferred to nitrocellulose membranes (Bio-Rad) and incubated with the following primary antibodies (18 h, 4°C): rat monoclonal VE-cadherin antibody (BV13, 1:400, eBioscience), rabbit monoclonal Src (1:2,000), rabbit monoclonal phosphorylated Src (Tyr416, 1:2,000, Cell Signaling Technology), or mouse monoclonal eNOS (1:2,000, BD Transduction Laboratories) (4). Throughout this report, “Src” refers to the Src family of kinases (4). After being extensively washed, membranes were incubated with peroxidase-conjugated goat secondary antibodies (Jackson ImmunoResearch Laboratories) for 60 min. Blots were developed using enhanced chemiluminescence and quantified using ImageJ.
Drugs.
Acetylcholine, l-NAME, PEG-catalase, and phenylephrine were from Sigma-Aldrich (St Louis, MO), MnTMPyP was from Axxora (Farmingdale, NY), and tempol was from Tocris (Minneapolis, MN).
Data analysis.
Vasomotor responses are expressed as percent changes in baseline diameter (4, 9). For vasomotor analyses, agonist concentrations causing 50% dilatation of the phenylephrine constriction (EC50) were calculated by regression analysis and compared as log EC50, and maximum dilator responses were determined as the maximal observed dilatation of the constriction to phenylephrine (4, 9). For immunofluorescence, intensity is expressed in absolute fluorescence detector units, and width is expressed in micrometers. In immunoblot analyses, levels of phosphorylated Src (Tyr416) are expressed relative to total Src. When the effects of MnTMPyP on VE-cadherin were analyzed, protein levels are expressed relative to eNOS to correct for any changes in endothelial content of lysates, which might have resulted from processing of the arterial samples. All data for immunoblots are expressed as a percentages of the average data for the corresponding control group, which enabled calculation of means ± SE for all groups. Data are expressed as means ± SE for n numbers of arteries and animals studied in the vasomotor analysis, for n numbers of adherens junctions studied in image analysis (arteries from at least three different animals), and n numbers of arterial lysates in the biochemical analyses (from at least three different animals) (4). Statistical evaluation of the data was performed using Student's t-test for paired or unpaired observations, and when more than two means were compared, ANOVA was used (repeated-measures ANOVA for paired and ordinary one-way ANOVA for unpaired observations, GraphPad Instat 3 software). If a significant F value was found in ANOVA, then the Tukey-Kramer test for multiple comparisons was used to identify differences among groups (GraphPad Instat 3 software). When the difference between maximal response and EC50 values across treatment (MnTMPyP or tempol) and age (young or old) was analyzed, a linear mixed model with added random effect for subject was used (15). Where appropriate, the Bonferroni correction for multiple testing were applied by multiplying P values by the number of multiple comparisons. The mixed-model analyses were performed using R version 3.2.2 (R foundation for Statistical Computing, Vienna, Austria). Values were considered to be statistically different when P < 0.05, and all tests were two-sided.
RESULTS
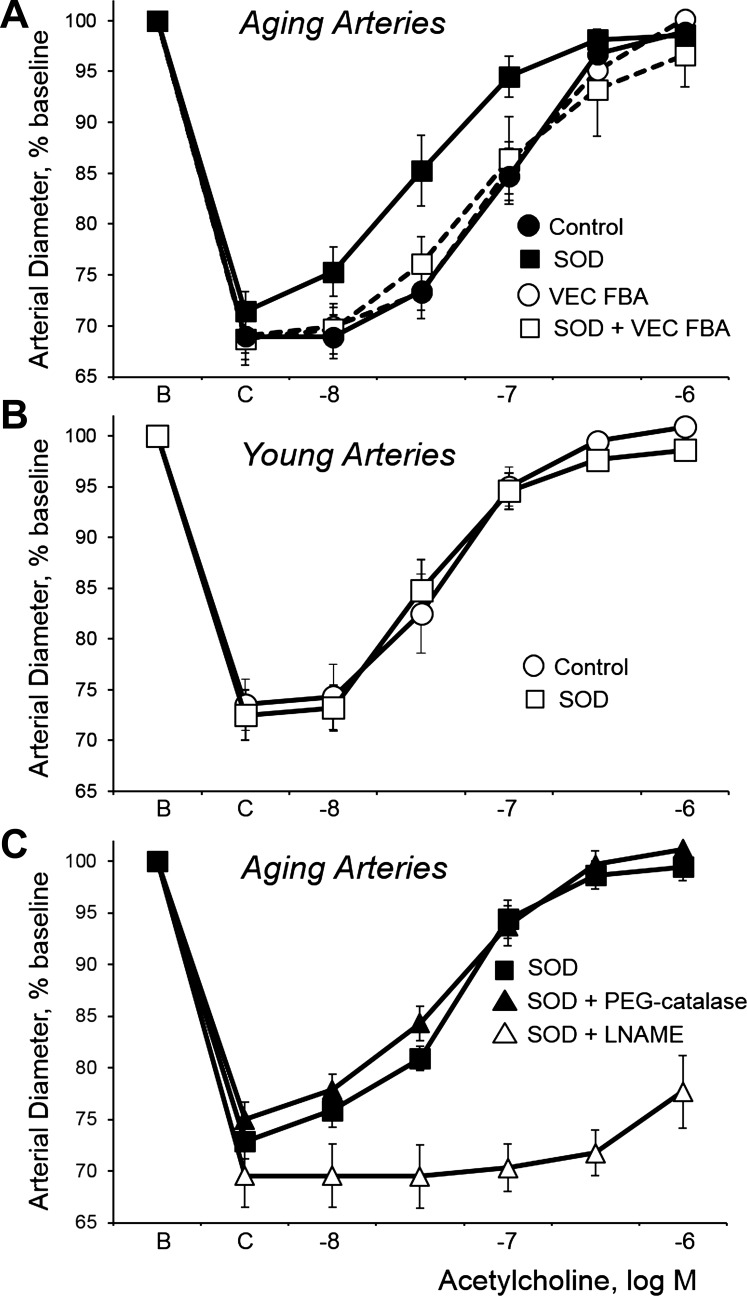
As previously described (4), endothelium-dependent dilatation to acetylcholine was inhibited in aging compared with young tail arteries, resulting in a significant rightward shift of the concentration-effect curve to the agonist (Fig. 1, A and B, and Table 1). The cell-permeable SOD mimics MnTMPyP (50 μM) or tempol (1 mM) did not affect dilatation to acetylcholine in young arteries (Fig. 1B and Table 1) but increased dilatation in aging arteries, causing significant leftward shifts in the concentration-effect curve (Fig. 1A and Table 1). Indeed, after treatment with the SOD mimics, there was no longer a significant difference in dilatation to acetylcholine between young and aging arteries (Fig. 1, A and B, and Table 1). The dilatation to acetylcholine in young and aging tail arteries is mediated predominantly by endothelium-derived NO and is reduced by inhibition of eNOS with l-NAME (100 μM) (4). After treatment with MnTMPyP (50 μM), the augmented dilatation to acetylcholine in aging arteries was still markedly inhibited by l-NAME (100 μM; Fig. 1C). In contrast, in aging arteries treated with MnTMPyP, PEG-catalase (250 U/ml) did not significantly affect dilatation to acetylcholine (Fig. 1C and Table 1), suggesting that H2O2 does not contribute to the enhanced dilatation.
Fig. 1.
Effects of the superoxide dismutase (SOD) mimic manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP; 50 μM, 60 min; SOD curve) on dilatation of isolated young and aging rat tail arteries to the endothelial agonist acetylcholine. Dilatation to acetylcholine was analyzed in pressurized arteries (transmural pressure of 60 mmHg) during stable constriction to phenylephrine (C). Data are expressed relative to the baseline diameter (B) and presented as means + SE; n = 6. See Table 1 for statistical analyses. Panel A: aging arteries. SOD increased acetylcholine-induced dilatation in aging arteries. However, after treatment with a function-blocking antibody to vascular-endothelial (VE)-cadherin (50 μg/ml, 2.5 h; VEC FBA), which did not by itself affect dilatation to acetylcholine in these aging arteries (see also Ref. 4), SOD no longer increased acetylcholine-induced dilatation. (Please note that the VEC FBA acetylcholine concentration-effect curve is mostly hidden behind the control concentration-effect curve.) Panel B: young arteries. SOD did not significantly affect dilatation to acetylcholine in young arteries. Panel C: aging arteries. In aging arteries treated with SOD, inhibition of endothelial nitric oxide synthase with l-NAME (100 μM, 60 min) markedly inhibited dilatation to acetylcholine, whereas polyethylene glycol (PEG)-catalase (250 U/ml, 60 min) had no significant effect on the dilatation.
Table 1.
Vasodilatation of young and old rat tail arteries to acetylcholine
| Treatment | Maximal Effect | Log EC50 |
|---|---|---|
| Old, control | 96.5 ± 1.9 | −6.98 ± 0.10* |
| Old, MnTMPyP | 93.7 ± 4.2 | −7.50 ± 0.12§ |
| Young, control | 103.8 ± 1.6 | −7.38 ± 0.10 |
| Young, MnTMPyP | 95.8 ± 3.8 | −7.42 ± 0.09 |
| Old, VE-cadherin FBA | 100.6 ± 2.0 | −7.05 ± 0.06 |
| Old, VE-cadherin FBA + MnTMPyP | 89.9 ± 9.7 | −7.00 ± 0.23 |
| Old, MnTMPyP | 98.0 ± 5.3 | −7.25 ± 0.07 |
| Old, MnTMPyP + polyethylene glycol-catalase | 105.6 ± 5.5 | −7.30 ± 0.11 |
| Old, control | 89.1 ± 5.2† | −6.63 ± 0.10† |
| Old, tempol | 101.4 ± 2.6‡ | −7.06 ± 0.07§ |
| Young, control | 106.6 ± 2.7 | −7.16 ± 0.12 |
| Young, tempol | 100.0 ± 3.7 | −7.17 ± 0.12 |
| Old, VE-cadherin FBA | 93.4 ± 4.6 | −6.76 ± 0.12 |
| Old, VE-cadherin FBA + tempol | 89.1 ± 5.9 | −6.77 ± 0.11 |
Data are presented as means ± SE; n = 6. MnTmPyP, manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin; FBA, function-blocking antibody.
Statistically significant difference compared with the corresponding group of young arteries: *P < 0.05 and †P < 0.01;
Statistically significant difference compared with the matching “control” group: ‡P < 0.05 and §P < 0.01. (For the Old, VE-cadherin FBA + MnTMPyP or Old, VE-cadherin FBA + tempol groups, the matching control group is the corresponding Old, VE-cadherin FBA group; likewise, for the Old, MnTMPyP + catalase group, the matching control group is the corresponding Old, MnTMPyP group.)
Clustering of VE-cadherin at endothelial adherens junctions amplifies endothelium-dependent dilatation (4, 8, 10), and inhibition of adherens junctions using a function-blocking antibody to VE-cadherin decreases acetylcholine-induced dilatation in young tail arteries (4). Because of an aging-induced disruption of endothelial adherens junctions, the antibody does not affect dilatation in aging arteries (Fig. 1A) (4). However, the function-blocking antibody to VE-cadherin abolished the ability of the SOD mimics MnTMPyP (50 μM) and tempol (1 mM) to increase acetylcholine-induced dilatation in aging arteries (Fig. 1A and Table 1). This suggests that clustering of VE-cadherin and formation of adherens junctions is required for the SOD mimics to restore endothelium-dependent dilatation in aging rat tail arteries.
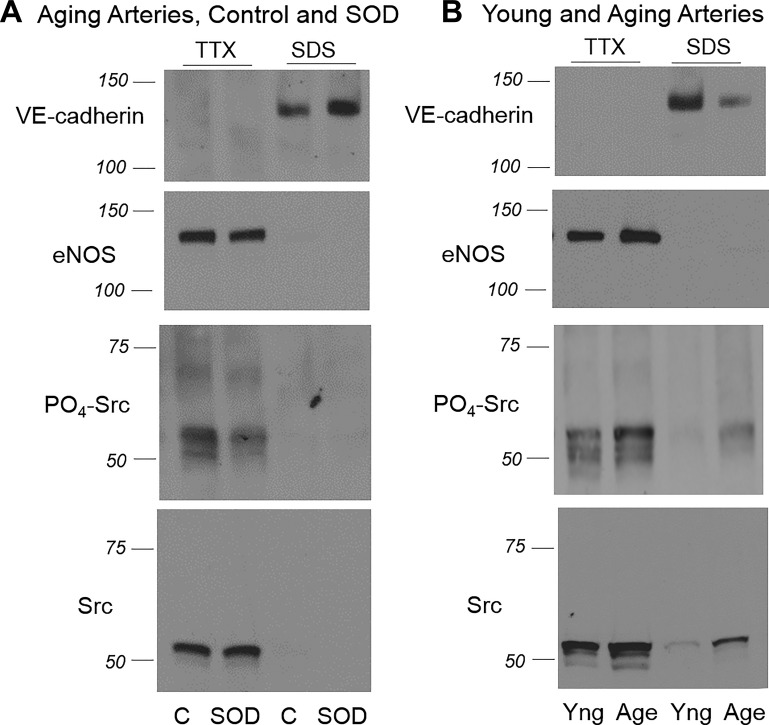
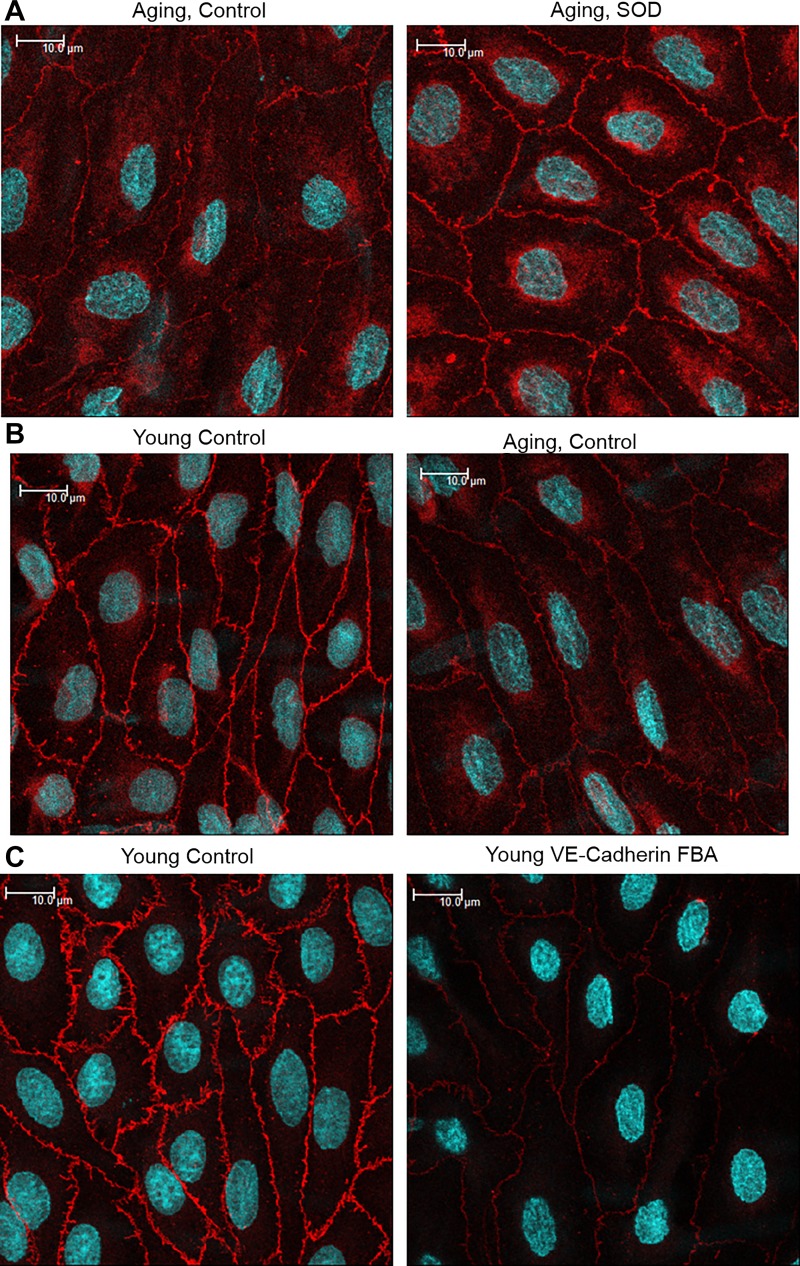
Src activity, assessed by immunoblot analysis of Tyr416 phosphorylated compared with total Src, was increased in aging compared with young arteries (from 100.0 ± 7.0% in aging arteries to 52.4 ± 5.2% in young arteries, expressed as percentage of activity in aging arteries, n = 10, P < 0.001; Fig. 2) (4) and is responsible for the increased internalization and degradation of VE-cadherin, loss of the protein from adherens junctions, and thinning and disruption of the junctions (4). Treatment of aging arteries with MnTMPyP (50 μM) significantly decreased Src activity in aging tail arteries, which was assessed by immunoblot analysis of Tyr416 phosphorylated relative to total Src, from 100.0 ± 5.4% to 66.6 ± 6.6% (n = 10, P < 0.01; Fig. 2). The increase in internalization of VE-cadherin in the aging endothelium was evident by decreased levels of a 140-kDa VE-cadherin species in TTX-insoluble arterial lysates (from 100.0 ± 3.6% in young arteries to 28.8 ± 5.0% in aging arteries, expressed as a percentage of expression in young arteries, n = 6, P < 0.001; Fig. 2) (4), which represents the protein localized to adherens junctions, and with reduced intensity of VE-cadherin staining at endothelial junctions and reduced width of the junctions (from 1.4 to 1.2 μm; Fig. 3) (4). MnTMPyP (50 μM) significantly increased levels of the 140-kDa VE-cadherin species in TTX-insoluble lysates from 100.0 ± 16.8% to 172.5 ± 25.7% (n = 10, P < 0.01; Fig. 2), significantly increased the width of the junctions from 1.10 ± 0.02 to 1.38 ± 0.03 μm (n = 288 junctions, P < 0.001; Fig. 3), and increased the intensity of VE-cadherin staining at the junctions from 2219.1 ± 30.7 to 2966.7 ± 28.6 fluorescent units (n = 288 junctions, P < 0.001; Fig. 3). The effects of MnTMPyP on VE-cadherin at adherens junctions were similar to those observed after direct pharmacological inhibition of Src activity with saracatinib (4).
Fig. 2.
A: Representative immunoblots demonstrating the effects of the superoxide dismutase (SOD) mimic manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP; 50 μM, 60 min; SOD lanes) compared with untreated controls (C) on the expression of vascular-endothelial (VE-)cadherin, endothelial nitric oxide synthase (eNOS), Tyr416 phosphorylated Src, and total Src in aging rat tail arteries. Arteries were processed using sequential lysis in Triton X-100 (TTX)-containing buffer (TTX-soluble fraction) followed by SDS-containing buffer (TTX-insoluble or SDS fraction) (4). Immunoblots were obtained using the same lysate. MnTMPyP increased the level of VE-cadherin in the TTX-insoluble component, which was associated with decreased phosphorylation of Src relative to total Src. MnTMPyP did not significantly affect eNOS expression (from 100.0 + 9.8% in controls to 85.2 + 11.3% in MnTMPyP-treated arteries, respectively, n = 10, P = not significant). B: representative immunoblots demonstrating the expression of VE-cadherin, eNOS, Tyr416 phosphorylated Src, and total Src in aging (Age) compared with young (Yng) rat tail arteries. As previously described (4), the level of VE-cadherin in the TTX-insoluble component was reduced in aging compared with young arteries, which was associated with increased phosphorylation of Src relative to total Src. Expression of eNOS was significantly increased in aging compared with young rat tail arteries (150.9 + 17.2% and 100.0 + 8.7%, respectively, n = 13, P < 0.01).
Fig. 3.
Immunofluorescent analysis of adherens junctions in the endothelium of rat tail arteries, as assessed by staining for vascular-endothelial (VE-)cadherin (VE-cadherin: red; Draq5 nuclear staining: light blue). These representative images are maximal projections, which were calculated from Z-sections comprising the entire endothelial layer, and are presented in their original unprocessed state. Arterial samples in each row were processed at the same time using the same instrument settings. Scale bar = 10 μm. A: treatment of aging arteries with the superoxide dismutase (SOD) mimic manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP; 50 μM, 60 min; SOD images) increased the intensity of VE-cadherin staining at adherens junctions and junctional width. B: as previously described (4), the intensity and width of VE-cadherin staining at adherens junctions was reduced in aging compared with young arteries. C: treatment of young arteries with a function-blocking antibody (FBA) to VE-cadherin (50 μg/ml, 2.5 h) compared with a control antibody (50 μg/ml, 2.5 h) reduced the intensity of staining for the protein at adherens junctions (4).
DISCUSSION
The present study indicates a new role for ROS in the endothelial dysfunction of aging, namely, in the pathological disruption of endothelial adherens junctions. VE-cadherin-dependent signaling at adherens junctions ensures normal barrier function, promotes NO activity, inhibits apoptosis, maintains endothelial differentiation, and inhibits inflammatory activation (8). In contrast, disruption of endothelial adherens junctions and of VE-cadherin-dependent signaling is likely to be a major driving force behind the process of vascular aging and the development of vascular diseases (4, 8). A role of ROS in junctional disruption confirms the pathological contribution of oxidant stress to vascular dysfunction and disease.
ROS production is markedly increased in the arterial wall and endothelium of aging compared with young arteries, including rat tail arteries (3, 6, 9, 18). Previous studies have demonstrated that the impaired endothelium-dependent dilatation of aging arteries can be restored to a youthful level by inhibiting superoxide (3, 6, 18). This was confirmed in the present study for aging rat tail arteries. SOD mimics did not affect dilatation to acetylcholine in young arteries but increased the dilatation in aging arteries. Indeed, after treatment with SOD mimics, there was no longer any significant difference in endothelial dilatation to acetylcholine in young and aging rat tail arteries, indicating complete restoration of dilatation and suggesting that the aging-induced impairment in endothelium-dependent dilatation could be accounted for entirely by the increased ROS activity. The endothelium-dependent dilatation of aging and young rat tail arteries to acetylcholine is mediated predominantly by NO (4, 9). After SOD mimic treatment, the restored dilatation of aging arteries to acetylcholine was markedly inhibited by l-NAME, indicating that the restored response was also mediated predominantly by eNOS. By converting superoxide to H2O2, SOD mimics can increase levels of the dilator H2O2 and could therefore cause NO-independent dilatation (13, 24, 27). However, the restored dilatation of aging arteries to acetylcholine was not affected by PEG-catalase, which degrades H2O2, suggesting that the augmented response is mediated by NO. Such protection by SOD mimics is generally assumed to reflect direct inactivation of NO by superoxide (11, 21, 22).
Impairment of endothelium-dependent dilatation to acetylcholine in aging rat tail arteries also reflects disruption of endothelial adherens junctions and a selective loss in the contribution of adherens junctions to endothelium-dependent dilatation (4). The overlapping involvement of ROS and of junctional disruption in the endothelial dilator dysfunction of aging arteries suggested a functional link between these two processes. The disruption of endothelial adherens junctions in aging arteries results from increased Src activity and Src-dependent internalization and degradation of VE-cadherin (4). Src activity in endothelial cells is redox sensitive and can be increased by ROS, including superoxide (7, 14, 28). Indeed, in cultured endothelial cells, ROS play a key role in the disruption of adherens junctions in response to inflammatory mediators (1, 19, 20, 23), which can reflect ROS-dependent increases in Src activity and Src-dependent tyrosine phosphorylation and internalization of VE-cadherin (1, 19, 23).
In the present study, the SOD mimic MnTMPyP significantly reduced Src activity in aging arteries, assessed by immunoblot analysis of Tyr416 phosphorylated compared with total Src. MnTMPyP increased the intensity of VE-cadherin staining at adherens junctions and increased the width of endothelial adherens junctions in aging arteries. Increased junctional width is indicative of increased clustering of VE-cadherin and increased stability and strength of the junctions (2, 10, 17, 25). Indeed, MnTMPyP also increased the levels of a 140-kDa VE-cadherin species associated with the TTX-insoluble fraction, which represents VE-cadherin at endothelial adherens junctions (4). Therefore, in addition to restoring endothelium-dependent dilatation to acetylcholine in aging arteries, MnTMPyP also reversed the inhibitory effect of aging on endothelial adherens junctions, increasing the junctional levels of VE-cadherin and increasing the width and intensity of the junctions. The effects of MnTMPyP in rat tail arteries are remarkably similar to those observed after the pharmacological reduction in Src activity by the selective Src inhibitor saracatinib (4). In aging rat tail arteries, saracatinib increased the levels of the 140-kDa VE-cadherin in the TTX-insoluble fraction, increased the intensity of VE-cadherin staining at adherens junctions, increased junctional width, and normalized endothelial dilatation to acetylcholine (4). The results therefore suggest that the protective effects of the SOD mimic are likely mediated by its action to decrease Src activity in aging arteries. A limitation of these experiments is that Src activity was assessed by immunoblot analysis of arterial lysates and the cellular source of the activity has not been identified. However, the observed effects of modulating Src activity on adherens junctions is consistent with its role in cultured endothelial cells, suggesting that endothelial Src is involved in the pathological dysfunction of aging endothelial cells (4).
To determine whether the restoration of endothelial dilator function by the SOD mimics was in fact mediated by increased clustering of VE-cadherin at adherens junctions, the influence of the SOD mimics was reevaluated in the presence of a function-blocking antibody to VE-cadherin. The function-blocking antibody disrupts existing endothelial adherens junctions and prevents formation of new junctions (Fig. 3) (4, 5, 10). In young arteries, inhibition of adherens junctions by the antibody disrupts protective endothelial signaling and reduces endothelium-dependent dilatation to acetylcholine (4, 10). Adherens junctions are already impaired in aging arteries; therefore, the antibody does not significantly affect acetylcholine-induced dilatation in aging arteries (4). However, in the presence of the function blocking antibody to VE-cadherin, the SOD mimics no longer increased endothelium-dependent dilatation to acetylcholine in aging arteries. Therefore, the restoration of endothelium-dependent dilatation by SOD mimics in aging arteries is dependent on their effects to improve adherens junctions and increase the junctional clustering of VE-cadherin.
In addition to contributing to endothelial dilator dysfunction in aging arteries, the impaired activity of endothelial adherens junctions likely contributes to multiple aspects of endothelial dysfunction and arterial deterioration in the aging vascular system (4, 8). VE-cadherin activity at adherens junctions is essential for maintaining the protective structure and function of the endothelium, including amplifying NO activity, reducing apoptosis, inhibiting inflammatory activity, and reducing endothelial permeability and leukocyte extravasation (4, 8). Aging endothelial cells are severely compromised, and, in addition to diminished activity of NO, they display increased permeability, increased susceptibility to apoptosis, and a prominent proinflammatory phenotype (4, 8). Therefore, reversing the impairment of endothelial adherens junctions in aging arteries, including inhibiting ROS, would be expected to not only restore endothelium-dependent dilatation but also reverse other pathological effects of endothelial dysfunction in the aging vasculature.
In conclusion, the present study demonstrates, using biochemical, morphological and functional analyses of aging rat tail arteries, that inhibition of superoxide using SOD mimics restores endothelium-dependent dilatation to acetylcholine, decreases the heightened activity of Src, and reverses the Src-dependent loss of VE-cadherin from endothelial adherens junctions and the resulting disruption of the junctions. Indeed, the ability of SOD mimics to restore endothelium-dependent dilatation in aging arteries is dependent on their protective activity at endothelial adherens junctions and the increased junctional clustering of VE-cadherin. Endothelial dysfunction, including impaired endothelial dilatation, is considered a key contributor to the pathological process of vascular aging (12, 16, 26). The results of the present study confirm an important role for ROS in the endothelial dysfunction of the aging vasculature, and highlight a novel mechanism whereby ROS can inhibit NO-mediated endothelium-dependent dilatation, namely, by disrupting endothelial adherens junctions.
GRANTS
This work was support by National Institute of Child Health and Human Development Grant HD-078639 (to N. A. Nicholas A. Flavahan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.C., S.F., and N.A.F. conceived and designed research; F.C., S.F., and N.A.F. performed experiments; F.C., S.F., and N.A.F. analyzed data; F.C., S.F., and N.A.F. interpreted results of experiments; F.C. and N.A.F. prepared figures; F.C., S.F., and N.A.F. edited and revised manuscript; F.C., S.F., and N.A.F. approved final version of manuscript; N.A.F. drafted manuscript.
ACKNOWLEDGMENTS
We are grateful to the Biological Resources Branch of the National Institute on Aging for allowing temporary access to the Aged Rodent Colony. We are also grateful to Dr. Sarabdeep Singh (Senior Biostatistician, Department of Anesthesiology and Critical Care Medicine) for performing the linear mixed model with added random effect statistical analysis.
REFERENCES
- 1.Birukova AA, Starosta V, Tian X, Higginbotham K, Koroniak L, Berliner JA, Birukov KG. Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl Res 161: 495–504, 2013. doi: 10.1016/j.trsl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breslin JW, Zhang XE, Worthylake RA, Souza-Smith FM. Involvement of local lamellipodia in endothelial barrier function. PLoS One 10: e0117970, 2015. doi: 10.1371/journal.pone.0117970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KA, Chu Y, Lund DD, Heistad DD, Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 290: H2600–H2605, 2006. doi: 10.1152/ajpheart.00676.2005. [DOI] [PubMed] [Google Scholar]
- 4.Chang F, Flavahan S, Flavahan NA. Impaired activity of adherens junctions contributes to endothelial dilator dysfunction in ageing rat arteries. J Physiol 595: 5143–5158, 2017. doi: 10.1113/JP274189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood 97: 1679–1684, 2001. doi: 10.1182/blood.V97.6.1679. [DOI] [PubMed] [Google Scholar]
- 6.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- 7.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassègue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal 20: 281–294, 2014. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flavahan NA. In development−a new paradigm for understanding vascular Disease. J Cardiovasc Pharmacol 69: 248–263, 2017. doi: 10.1097/FJC.0000000000000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flavahan S, Chang F, Flavahan NA. Local renin-angiotensin system mediates endothelial dilator dysfunction in aging arteries. Am J Physiol Heart Circ Physiol 311: H849–H854, 2016. doi: 10.1152/ajpheart.00422.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flavahan S, Mozayan MM, Lindgren I, Flavahan NA. Pressure-induced maturation of endothelial cells on newborn mouse carotid arteries. Am J Physiol Heart Circ Physiol 305: H321–H329, 2013. doi: 10.1152/ajpheart.00099.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa Y, Saito T, Ogihara T, Ishigaki Y, Yamada T, Imai J, Uno K, Gao J, Kaneko K, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation 125: 1122–1133, 2012. doi: 10.1161/CIRCULATIONAHA.111.054346. [DOI] [PubMed] [Google Scholar]
- 13.Itoh T, Kajikuri J, Hattori T, Kusama N, Yamamoto T. Involvement of H2O2 in superoxide-dismutase-induced enhancement of endothelium-dependent relaxation in rabbit mesenteric resistance artery. Br J Pharmacol 139: 444–456, 2003. doi: 10.1038/sj.bjp.0705255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knock GA, Ward JP. Redox regulation of protein kinases as a modulator of vascular function. Antioxid Redox Signal 15: 1531–1547, 2011. doi: 10.1089/ars.2010.3614. [DOI] [PubMed] [Google Scholar]
- 15.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 38: 963–974, 1982. doi: 10.2307/2529876. [DOI] [PubMed] [Google Scholar]
- 16.Laurent S. Defining vascular aging and cardiovascular risk. J Hypertens 30, Suppl: S3–S8, 2012. doi: 10.1097/HJH.0b013e328353e501. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci USA 107: 9944–9949, 2010. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 296: H1914–H1919, 2009. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem 284: 25602–25611, 2009. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nwariaku FE, Liu Z, Zhu X, Nahari D, Ingle C, Wu RF, Gu Y, Sarosi G, Terada LS. NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood 104: 3214–3220, 2004. doi: 10.1182/blood-2004-05-1868. [DOI] [PubMed] [Google Scholar]
- 21.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol 250: H822–H827, 1986. [DOI] [PubMed] [Google Scholar]
- 22.Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med 25: 392–403, 1998. doi: 10.1016/S0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 23.Starosta V, Wu T, Zimman A, Pham D, Tian X, Oskolkova O, Bochkov V, Berliner JA, Birukova AA, Birukov KG. Differential regulation of endothelial cell permeability by high and low doses of oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine. Am J Respir Cell Mol Biol 46: 331–341, 2012. doi: 10.1165/rcmb.2011-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su B, Mitra S, Gregg H, Flavahan S, Chotani MA, Clark KR, Goldschmidt-Clermont PJ, Flavahan NA. Redox regulation of vascular smooth muscle cell differentiation. Circ Res 89: 39–46, 2001. doi: 10.1161/hh1301.093615. [DOI] [PubMed] [Google Scholar]
- 25.Sun X, Shikata Y, Wang L, Ohmori K, Watanabe N, Wada J, Shikata K, Birukov KG, Makino H, Jacobson JR, Dudek SM, Garcia JG. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc Res 77: 304–313, 2009. doi: 10.1016/j.mvr.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 65: 1028–1041, 2010. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yada T, Shimokawa H, Morikawa K, Takaki A, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Role of Cu,Zn-SOD in the synthesis of endogenous vasodilator hydrogen peroxide during reactive hyperemia in mouse mesenteric microcirculation in vivo. Am J Physiol Heart Circ Physiol 294: H441–H448, 2008. doi: 10.1152/ajpheart.01021.2007. [DOI] [PubMed] [Google Scholar]
- 28.Zgheel F, Alhosin M, Rashid S, Burban M, Auger C, Schini-Kerth VB. Redox-sensitive induction of Src/PI3-kinase/Akt and MAPKs pathways activate eNOS in response to EPA:DHA 6:1. PLoS One 9: e105102, 2014. doi: 10.1371/journal.pone.0105102. [DOI] [PMC free article] [PubMed] [Google Scholar]