Significance
Alzheimer’s disease is the most common cause of dementia in the elderly. Most cases occur sporadically, with 40–65% of patients carrying at least one copy of the E4 allele of Apolipoprotein E. Because no drug exists that can halt disease progress, there is strong interest in understanding the presymptomatic role of endosomes. We show that excessive endosomal acidification in ApoE4 astrocytes is caused by downregulation of the Na+/H+ exchanger NHE6 and results in defective clearance of amyloid beta (Aβ) peptide by intracellular sequestration of the LRP1 receptor. Epigenetic modifiers restore NHE6 expression to alkalinize endosomal pH, increase surface expression of LRP1, and correct Aβ clearance in astrocytes. Thus, endosomal pH emerges as a target for the correction of amyloid disorders.
Keywords: trichostatin A, amyloid beta, ApoE4, Na+/H+ exchanger, histone deacetylase
Abstract
Endosomes have emerged as a central hub and pathogenic driver of Alzheimer’s disease (AD). The earliest brain cytopathology in neurodegeneration, occurring decades before amyloid plaques and cognitive decline, is an expansion in the size and number of endosomal compartments. The strongest genetic risk factor for sporadic AD is the ε4 allele of Apolipoprotein E (ApoE4). Previous studies have shown that ApoE4 potentiates presymptomatic endosomal dysfunction and defective endocytic clearance of amyloid beta (Aβ), although how these two pathways are linked at a cellular and mechanistic level has been unclear. Here, we show that aberrant endosomal acidification in ApoE4 astrocytes traps the low-density lipoprotein receptor-related protein (LRP1) within intracellular compartments, leading to loss of surface expression and Aβ clearance. Pathological endosome acidification is caused by ε4 risk allele-selective down-regulation of the Na+/H+ exchanger isoform NHE6, which functions as a critical leak pathway for endosomal protons. In vivo, the NHE6 knockout (NHE6KO) mouse model showed elevated Aβ in the brain, consistent with a causal effect. Increased nuclear translocation of histone deacetylase 4 (HDAC4) in ApoE4 astrocytes, compared with the nonpathogenic ApoE3 allele, suggested a mechanistic basis for transcriptional down-regulation of NHE6. HDAC inhibitors that restored NHE6 expression normalized ApoE4-specific defects in endosomal pH, LRP1 trafficking, and amyloid clearance. Thus, NHE6 is a downstream effector of ApoE4 and emerges as a promising therapeutic target in AD. These observations have prognostic implications for patients who have Christianson syndrome with loss of function mutations in NHE6 and exhibit prominent glial pathology and progressive hallmarks of neurodegeneration.
The endosome is a central hub for incoming and outgoing traffic and a key recycling/degradation sorting station. Transit through the endolysosomal system is accompanied by an increasingly acidic pH gradient that controls receptor–ligand uncoupling, vesicle budding, exosome formation, membrane turnover, enzyme activation, nutrient uptake, and cellular signaling (1). As a defining feature of compartmental identity and function, the pH of the endolysosomal lumen is precisely set by a balance between proton pump and leak pathways (2). The discovery of endosomal Na+/H+ exchangers (eNHEs) first in yeast and soon after in plants and metazoans, including mammals, established their evolutionarily conserved role as a leak pathway for protons in compartmental pH homeostasis (2–4). Na+/H+ exchangers are estimated to have exceptionally high transport rates of ∼1,500 ions per second (5), so that even small perturbations in expression or activity result in dramatic changes in the ionic milieu within the limited confines of the endosomal lumen.
As a testament to the central role of the endosome at the crossroads of cellular traffic, mutations in eNHEs have been linked to a host of neurodevelopmental and neurodegenerative disorders (2, 6). Loss-of-function mutations in the Na+/H+ exchanger NHE6 (SLC9A6) cause Christianson syndrome (CS), an X-linked disorder characterized by autism and intellectual disability (7–9). Patients who have CS also show striking age-dependent neurodegeneration, with prominent glial pathology and phosphorylated tau deposits (7). Female carriers have learning difficulties and behavioral issues, and some present with low Mini-Mental State Examination scores suggestive of early cognitive decline (10). Interestingly, NHE6 was among the most highly down-regulated genes (up to sixfold) in the elderly (70 y) brain, compared with the adult (40 y) brain (11). These observations led us to consider a broader role for NHE6 in neurodegenerative disorders, including Alzheimer’s disease (AD), a major cause of dementia in the elderly. Consistent with this hypothesis, a coexpression analysis of quantitative trait loci in AD brains revealed NHE6 as a top hub transcript, with 202 network connections and a plethora of potential downstream effects (12). A knowledge-based approach for predicting gene–disease associations also identified a link between NHE6 and early-stage AD (13). Stronger evidence emerged from a recent analysis of the metastable aggregation-prone proteome in AD brains that identified NHE6 as a key component of the proteostasis machinery associated with amyloid plaques and neurofibrillary tangles containing amyloid beta (Aβ) peptide and tau protein, respectively (14).
Petsko and coworkers (15) recently proposed that endosomal “traffic jams” are the unifying mediators of downstream pathology in AD and interventions designed to “unjam” the endosome have high therapeutic promise. Endosomal aberrations, evidenced by enlarged and more numerous endosomes, are the earliest detectable brain cytopathology, emerging several decades before cognitive dysfunction is apparent in a subset of neurodegenerative disorders, including AD, Niemann–Pick type C, and Down syndrome (16–19). Genes associated with endosomal trafficking have also been implicated as major risk factors in AD (20). Indeed, the strongest genetic risk factor in sporadic AD is the ε4 allele of Apolipoprotein E (ApoE4), which potentiates both presymptomatic endosomopathy and defective clearance of Aβ (21–25), although how these two pathways are linked at a cellular level has been unclear.
Here, we show that a pathological acidification of endosomal pH in humanized mouse ApoE4 astrocytes is caused by the selective down-regulation of NHE6. This leads to endosomal sequestration and cell surface loss of the Aβ receptor, low-density lipoprotein receptor-related protein (LRP1). Increased nuclear translocation of the histone deacetylase 4 (HDAC4) in ApoE4 astrocytes can be abrogated by HDAC inhibitors that restore NHE6 expression, reroute LRP1 to the cell surface, and effectively restore defective amyloid clearance to nonpathological ApoE3 levels. Consistent with a proposed role in amyloid pathology, we found that in vivo Aβ levels were significantly higher in the brains of NHE6 knockout (NHE6KO) mice. Our findings could have prognostic implications for patients with CS and suggest therapeutic strategies for the treatment of amyloid disorders (7, 26).
Results
ApoE4 Astrocytes Have Cargo-Specific Defects in Endocytosis.
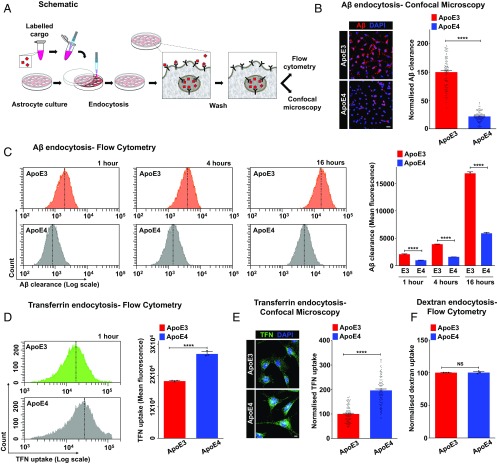
Studies in humans, mouse models, and cell cultures have revealed the importance of ApoE isotype-specific differences in Aβ uptake and clearance in AD pathogenesis, although the underlying mechanism remains to be determined (22, 23, 27, 28). The ApoE3 variant (Cys112) predominates in the human population at a frequency of 77.9%, whereas the relatively rare ApoE4 variant (Arg112) is dramatically increased from 13.7 to ∼40% in patients with AD (29). The therapeutic focus is on defective Aβ clearance in ApoE4 relative to ApoE3 because ApoE knockout (ApoEKO) mice clear Aβ faster than controls (30), and a rare case of human ApoE knockout showed no evidence of neurodegenerative disease (31). To this end, we developed a sensitive and quantitative fluorescent-based assay to monitor cell-associated Aβ peptide (Fig. 1A) in astrocytes from ApoEKO mice with isogenic knock-in of human ApoE3 and ApoE4 variants (23). Consistent with evidence in the literature (32–34), internalized Aβ is sorted to the lysosomal degradation pathway, as shown by high colocalization with late endosomal-lysosomal markers and low colocalization with the recycling compartment marker transferrin (TFN) (SI Appendix, Fig. S1 A–D). Strikingly, cell-associated Aβ was reduced by 78% in ApoE4 astrocytes, relative to ApoE3 (Fig. 1B). To distinguish between Aβ uptake and turnover, we monitored the time course of Aβ internalization by flow cytometry analysis (Fig. 1C) and confocal microscopy (SI Appendix, Fig. S1E). Consistent with defective uptake, there was significantly lower cell-associated Aβ in ApoE4 cells relative to ApoE3 at all time points, including very early time points between 1 and 30 min and as long as 12 h (Fig. 1C and SI Appendix, Fig. S1 F and G). In contrast, cell-associated TFN was 1.5–twofold higher in ApoE4 cells relative to ApoE3 as measured by flow cytometry (Fig. 1D) and confocal microscopy (Fig. 1E). Uptake of dextran (10 kDa) by fluid-phase endocytosis was not different between ApoE genotypes (Fig. 1F). Similar findings were observed in AD patient-derived fibroblasts with the ApoE4/4 genotype compared with an age-matched ApoE3/3 control (SI Appendix, Fig. S2 A–C). These observations reveal cargo-selective effects of ApoE isotype in astrocytes and point to alterations in specific receptor pathways.
Fig. 1.
ApoE isotype-specific differences in Aβ clearance and specific receptor pathways. (A) Fluorescent-based assay to monitor clearance of Aβ peptides by astrocytes. (B) Representative micrographs (Left) and quantification (Right) of ApoE3 and ApoE4 astrocytes subjected to 24 h of Aβ uptake. The fluorescence intensity and exposure settings were kept constant. Following background subtraction, fluorescence signal from cell-associated Aβ was reduced by 78% in ApoE4 astrocytes, relative to ApoE3 (****P = 9.6 × 10−71, Student’s t test; n = 100 per condition). (Scale bar, 50 μm.) (C, Left) Representative fluorescence-activated cell sorting (FACS) histograms demonstrating Aβ internalization by ApoE3 (Top, orange) and ApoE4 astrocytes (Bottom, gray) at 1 h, 4 h, and 16 h (n = 10,000 cells per experimental condition). The x axis depicts Aβ clearance in logarithmic scale, and the vertical dashed line represents median fluorescence intensity. (C, Right) Quantification of biological triplicate measurements of Aβ clearance from FACS analysis of ApoE3 and ApoE4 cells. Note the significantly lower cell-associated Aβ in ApoE4 relative to ApoE3 at all time points (53% lower at 1 h, 59% lower at 4 h, and 65% lower at 16 h; ****P < 0.0001, Student’s t test; n = 3). (D) Representative FACS histograms (Left) and quantification of mean fluorescence intensity of biological triplicates (Right) demonstrating TFN uptake following 60 min of endocytosis by ApoE3 (green) and ApoE4 (gray) astrocytes (∼1.5-fold higher; ****P = 6.8 × 10−5, Student’s t test; n = 3). The x axis of the FACS histograms depicts TFN uptake in logarithmic scale, and the vertical dashed line represents median fluorescence intensity. (E) ApoE3 and ApoE4 astrocytes were incubated with fluorescent TFN for 1 h to compare steady-state TFN uptake by confocal microscopy. Fluorescence intensity and exposure settings were kept constant. Representative images are shown (Left), and mean fluorescence ± SE was plotted (Right). Following background subtraction, fluorescence signal was increased by approximately twofold in ApoE4 astrocytes, relative to ApoE3 (****P = 3.5 × 10−32, Student’s t test; n = 100 per condition). (Scale bar, 10 μm.) (F) Quantification of mean fluorescence intensity of biological triplicates demonstrating dextran uptake by ApoE3 and ApoE4 astrocytes (P = 0.870, Student’s t test; n = 3). NS, not significant. (SI Appendix, Figs. S1 and S2).
Surface Expression of LRP1 Receptor Is Severely Reduced in ApoE4 Astrocytes.
Transcriptional down-regulation of the LRP1 receptor has been suggested as an underlying mechanism for defective Aβ clearance in patients with AD (35). However, we found no difference in brain LRP1 gene expression at different stages of AD (incipient, moderate, and severe), compared with normal controls, in publicly available microarray data (36) (SI Appendix, Fig. S3 A and B). Meta-analysis of nine independent gene expression studies from anatomically and functionally distinct brain regions, comprising a total of 103 AD and 87 control postmortem brains, also showed no significant changes in LRP1 gene expression in AD (SI Appendix, Fig. S3 C and D). Consistent with these findings, we observed no differences in LRP1 transcript (SI Appendix, Fig. S3E) and total LRP1 protein expression between ApoE3 and ApoE4 astrocytes (SI Appendix, Fig. S3 F and G).
LRP1 undergoes constitutive endocytosis from the membrane and recycling back to the cell surface (37). Therefore, we considered the possibility that alterations in LRP1 receptor recycling could result in differences in plasma membrane expression. ApoE isotype-specific surface expression of LRP1 was evaluated using four independent approaches (Fig. 2A). First, surface biotinylation revealed that plasma membrane expression of LRP1 receptor in ApoE4 astrocytes was lower by ∼50% (Fig. 2B). Second, an antibody directed against an external epitope of LRP1 to quantify surface expression in live cells by flow cytometry analysis showed a reduction of LRP1-positive cells by ∼55% in ApoE4 astrocytes (Fig. 2C) and by ∼51% in patient-derived ApoE4/4 fibroblasts (SI Appendix, Fig. S2D). Third, this was confirmed by confocal microscopy showing ∼49% lower LRP1 surface labeling by antibody in ApoE4 (Fig. 2D). In a fourth approach, surface-bound ligand (fluorescent Aβ) measured by confocal microscopy was 66% lower in ApoE4 astrocytes (Fig. 2E). Notably, the greater attenuation in Aβ binding compared with the ∼50% reduction in surface LRP1 levels suggests additional isotype-specific mechanisms that contribute to Aβ clearance, such as reduced ligand-receptor affinity in ApoE4 cells or reductions in other Aβ receptors. Confocal microscopy revealed a striking loss of LRP1 from astrocyte processes and increased perinuclear accumulation in ApoE4 cells, relative to ApoE3 (Fig. 2F). The rate of LRP1 endocytosis (at 37 °C for 10 min) in ApoE3 and ApoE4 astrocytes was similar (SI Appendix, Fig. S4), suggesting that LRP1 delivery by exocytosis or recycling may contribute to the differences in surface expression. Consistent with this possibility, we find significant colocalization of LRP1 with TFN in ApoE4 astrocytes (Fig. 2G). Thus, ApoE isotype-specific alterations in receptor recycling determine LRP1 surface expression and cellular Aβ uptake, revealing a pharmacological target for amyloid clearance defects in the pathological ApoE4 genotype.
Fig. 2.
Reduced surface expression of LRP1 receptor in ApoE4 astrocytes. (A) Four independent approaches to evaluate ApoE isotype-specific surface expression of LRP1. (B) Surface biotinylation (Left) and quantification (Right) of biological triplicates showing that plasma membrane levels of LRP1 are depressed by ∼50% in ApoE4 astrocytes, relative to ApoE3 (**P = 0.0022, Student’s t test; n = 3). Plasma membrane protein Na+/K+ ATPase is used as a loading control. (C) Fraction of LRP1-positive cells quantified following surface antibody labeling and fluorescence-activated cell sorting (FACS) analysis of 10,000 live, nonpermeabilized cells in biological triplicates. Unstained cells were used as a control. Note the ∼55% lower surface LRP1 positivity in ApoE4 relative to ApoE3 (****P = 4.8 × 10−−5, Student’s t test; n = 3). (D) Representative surface immunofluorescence micrographs (Left) and quantification (Right) showing prominent LRP1 staining on the cell surface and processes and faint, ∼49% lower, labeling on ApoE4 cells (****P = 2.4 × 10−16, Student’s t test; n = 75 per condition). (E) Plasma membrane level of LRP1 receptor was monitored by a ligand (fluorescent Aβ)-binding assay performed on ice that only allows Aβ to bind surface receptors. Representative images are shown (Left), and mean fluorescence ± SD was plotted (Right). Surface-bound Aβ was 66% lower in ApoE4 astrocytes, relative to ApoE3 (****P = 2.2 × 10−24, Student’s t test; n = 75 per condition). (F, Left) Confocal micrographs revealing a striking loss of LRP1 from astrocyte processes (white arrow) and increased perinuclear accumulation in ApoE4 cells, relative to ApoE3. (F, Right) Zoomed-in images of the boxed regions. Increased perinuclear accumulation of LRP1 in ApoE4 cells is also evident in orthogonal slices (Z) (black arrows). (G) Confocal micrographs revealing prominent colocalization of LRP1 (green) with TFN (red) in DAPI (blue)-stained ApoE4 astrocytes. Colocalization is evident in the merge and orthogonal slices (Z) as yellow puncta (SI Appendix, Figs. S2–S4). DIC, differential interference contrast. (Scale bars, 10 μm.)
Endolysosomal pH Is Defective in ApoE4 Astrocytes.
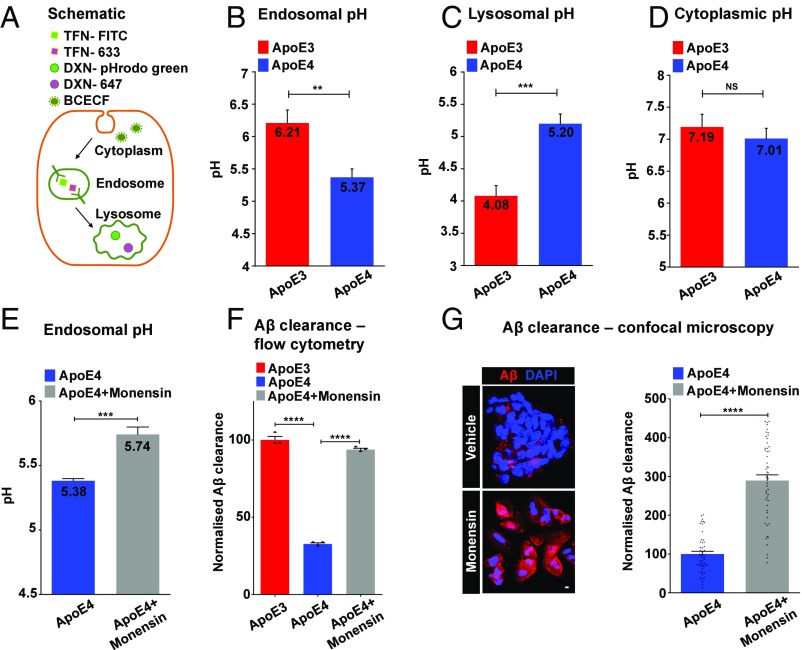
The pH within the endolysosomal system plays a critical role in receptor-mediated endocytosis and recycling (1). We used compartment-specific, pH-sensitive fluorescence reporters to probe ApoE isotype-dependent differences in endosomal, lysosomal, and cytoplasmic pH (Fig. 3A). Endosomal pH in ApoE4 astrocytes was strongly reduced by ∼0.84 pH unit, relative to ApoE3 (Fig. 3B). A similar acidification was observed in endosomes of ApoE4/4 patient fibroblasts, compared with an ApoE3/3 control (SI Appendix, Fig. S2E). In contrast, we observed >1 pH unit elevation of lysosomal pH in ApoE4 astrocytes (Fig. 3C). Previously, elevated lysosomal pH was observed in presenilin 1 (PS1)-deficient cell culture models and neurons, another genetic model of AD (38). Cytoplasmic pH showed no significant differences between the two ApoE isotypes (Fig. 3D).
Fig. 3.
Endolysosomal pH is defective in ApoE4 astrocytes. (A) Compartment-specific, ratiometric, pH-sensitive fluorescence reporters used to probe ApoE isotype-dependent differences in endosomal, lysosomal, and cytoplasmic pH. Endosomal pH was measured by incubations with pH-sensitive TFN-FITC, together with pH-nonsensitive Alexa Fluor 633-TFN (TFN-633). Lysosomal pH was measured by incubations with pH-sensitive pHrodo-green-Dextran (DXN-pHrodo green), together with pH-nonsensitive Alexa Fluor 647-Dextran (DXN-647). Cytoplasmic pH was measured ratiometrically using pH-sensitive green and pH-nonsensitive red fluorescence of 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF) dye. (B) Endosomal pH in ApoE4 astrocytes was strongly reduced by ∼0.84 pH unit, relative to ApoE3 (**P = 0.0037, Student’s t test; n = 3). (C) Lysosomal pH was elevated by >1 pH unit in ApoE4 astrocytes (***P = 0.0009, Student’s t test; n = 3). (D) Cytoplasmic pH showed no significant (NS) differences between ApoE3 and ApoE4 astrocytes (P = 0.2904, Student’s t test; n = 3). (E) Monensin treatment (50 μM for 1 h) corrected hyperacidic endosomal pH in ApoE4 astrocytes, relative to vehicle treatment (***P = 0.0005, Student’s t test; n = 3). (F) Quantitation of Aβ clearance from fluorescence-activated cell sorting (FACS) analysis of 10,000 cells in biological triplicates confirmed restoration of Aβ clearance (1 h) in ApoE4 astrocytes to ApoE3 levels with monensin treatment (****P = 6.5 × 10−7, Student’s t test; n = 3). (G) Representative micrographs (Left) and quantification (Right) showing an ∼2.9-fold increase in cell-associated Aβ following 1 h of uptake in ApoE4 astrocytes with monensin treatment (****P = 2.4 × 10−20, Student’s t test; n = 50) (SI Appendix, Figs. S2 and S5). (Scale bar, 10 μm.)
To determine if there was a causal link between endolysosomal pH and defective Aβ clearance in ApoE4 astrocytes, we treated ApoE4 cells with the ionophore monensin, which mediates Na+/H+ exchange across acidic compartments (39). Thus, monensin treatment (50 μM for 1 h) elevated endosomal pH in ApoE4 knock-in astrocytes from 5.38 ± 0.01–5.74 ± 0.03, relative to the vehicle-treated control (Fig. 3E), without altering cell viability (SI Appendix, Fig. S5A). Concomitantly, monensin treatment restored Aβ clearance in ApoE4 astrocytes to ApoE3 levels, as shown by flow cytometry analysis (Fig. 3F). Lower concentrations (1 μM) of monensin and shorter Aβ uptake times (1 and 5 min) gave similar results (SI Appendix, Fig. S5B). These observations were independently confirmed by confocal microscopy (Fig. 3G), suggesting that defective pH regulation could underlie the observed Aβ clearance defects.
NHE6 Restores Defective Aβ Clearance in ApoE4 Astrocytes.
Luminal pH in the endolysosomal network is set by the precise balance of proton pump and leak pathways, mediated by V-type H+-ATPase (V-ATPase) and endosomal NHE6, respectively (Fig. 4A) (2, 6, 8, 40). Changes in expression and activity of the pump and leak pathways could lead to significant dysregulation of endosomal pH in AD brains. Consistent with this possibility, analysis of a publicly available microarray dataset (GSE5281) comprising a total of 15 sporadic, late-onset AD and 12 matched control postmortem brains from the middle temporal gyrus (41) revealed that genes involved in hydrogen ion transmembrane transport, including the endosomal NHE6 and V-ATPase subunits, comprised 10% of the top 100 down-regulated genes, exhibiting the highest enrichment scores (>15-fold; SI Appendix, Fig. S5 C and D). In patients who have AD with the ApoE4/4 genotype, NHE6 was among the transcripts differentially down-regulated in the hippocampus, compared with ApoE3/3 (42). We validated these findings using an independent, large human brain transcriptome dataset (n = 363; GSE15222) to show ApoE4 isotype-specific differential gene expression of NHE6 in the aging brain (12) (Fig. 4B). Although the NHE6 transcript was similar in ApoEKO mouse astrocytes and ApoEKO astrocytes with knock-in of human ApoE3, it was ∼56% reduced in ApoE4 knock-in cells (SI Appendix, Fig. S5E). NHE6 protein was concomitantly decreased by ∼45% in ApoE4 astrocytes, compared with ApoE3 (Fig. 4C). There was also an ApoE4-specific reduction in the transcript for the related endosomal isoform NHE9 (∼70% lower) and the lysosomal V-ATPase V0a1 subunit (∼67%), but not for the plasma membrane NHE1 isoform (SI Appendix, Fig. S5F). Similar changes in gene expression were observed in AD patient-derived ApoE4/4 fibroblasts compared with ApoE3/3 fibroblasts from age-matched control (SI Appendix, Fig. S2 F–H). These large expression changes could account for the observed ApoE isotype-specific shifts in endolysosomal pH.
Fig. 4.
NHE6 restores defective Aβ clearance in ApoE4 astrocytes. (A) Endosomal pH is precisely tuned by a balance of proton pumping (acidification) through the V-ATPase and proton leak (alkalization) via NHE6. (B) Box plots of NHE6 transcript levels in postmortem brains extracted from a large microarray dataset (GSE15222; n = 363) showing significant down-regulation of NHE6 expression in ApoE4+ AD brains (****P = 1.2 × 10−10, Student’s t test). (C, Top) Western blot analysis of NHE6 protein revealed that it was ∼45% lower in ApoE4 astrocytes, relative to ApoE3. (C, Bottom) Densitometric quantification of the monomeric and dimeric forms of NHE6 relative to β-actin (***P = 0.0009, Student’s t test; n = 4). (D) Aβ levels were significantly higher in the brains of the NHE6-null mouse model (NHE6KO), relative to WT (**P = 0.0013, Student’s t test; n = 5 per condition), consistent with our hypothesis. Aβ was measured using ELISA and normalized to total protein concentration in the brain homogenate. (E) Fluorescence-activated cell sorting (FACS) scatter plots (Left) and quantification (Right) demonstrating Aβ internalization by an empty vector expressing ApoE3 (orange) and ApoE4 (gray) astrocytes and ApoE4 astrocytes with restored NHE6 expression (green). Note the remarkable correction of Aβ clearance in ApoE4 astrocytes with NHE6 expression to ApoE3 levels (****P = 4.9 × 10−6, Student’s t test; n = 3). (F) Representative surface immunofluorescence images (Left) and quantification (Right) of nonpermeabilized cells showing an ∼2.5-fold increase in plasma membrane LRP1 expression in ApoE4 cells expressing exogenous NHE6 (****P = 6.02 × 10−12, Student’s t test; n = 40 per condition) (SI Appendix, Figs. S2 and S5–S7). DIC, differential interference contrast. (Scale bars, 2.5 μm.)
Taken together, these data suggest an important, hitherto underappreciated role of proton transport and endosomal pH regulation in AD. We hypothesized that NHE6 is a potential ApoE effector, and that down-regulation of NHE6 in disease-associated ApoE4 variants is causal to a subset of AD phenotypes. We show that amyloid Aβ levels are elevated in brains from NHE6KO mice (Fig. 4D), together with diminished brain weight (SI Appendix, Fig. S5G), suggesting an underlying neurodegenerative pathology consistent with microcephaly and extensive gliosis in patients with CS (2). Furthermore, NHE6KO brains show ∼22% lower levels of neurofilament light chain (SI Appendix, Fig. S5H), characteristic of AD brains (43) (SI Appendix, Fig. S5D). In contrast, mRNA levels of the neuronal protein TUBB3 remained unchanged in NHE6KO brains (SI Appendix, Fig. S5H).
Next, we tested if ectopic expression of GFP-tagged NHE6 (SI Appendix, Fig. S6 A and B) could correct defective Aβ uptake in ApoE4 astrocytes. For comparison, we also evaluated the NHE6 variants L188P and G383D found in patients with CS (2, 44, 45), which localize to highly conserved sequences predicted to be within the membrane-embedded transporter domain (SI Appendix, Fig. S6 C–E). Wild-type NHE6 and CS variants were expressed at similar levels and localized to TFN-positive endosomes (SI Appendix, Fig. S6 F and G). Like monensin, NHE6 alkalinized the endosomal lumen in ApoE4 astrocytes, but the CS patient mutations did not (SI Appendix, Fig. S6H). Ectopic NHE6 expression does not alter lysosomal pH, suggesting that compartmental pH regulation is specific and localized (SI Appendix, Fig. S6I). Remarkably, Aβ clearance was restored to ApoE3 levels in ApoE4 astrocytes transfected with NHE6 (Fig. 4E). However, CS variants failed to correct Aβ clearance deficits in ApoE4 astrocytes, consistent with loss of function (SI Appendix, Fig. S6J). Intriguingly, ectopic delivery of NHE9 resulted in robust expression in TFN-positive endosomal compartments but failed to restore defective Aβ clearance in ApoE4 astrocytes (SI Appendix, Fig. S6 K and L), pointing to an isoform-specific role for NHE6 in Aβ clearance.
Colocalization of NHE6 with EEA1 and LRP1 (SI Appendix, Fig. S7 A and B) suggested a potential role for NHE6 in endosomal recycling of LRP1 receptors. Compared with the weak surface LRP1 staining in vector-transfected ApoE4 astrocytes, we observed prominent, ∼2.5-fold higher LRP1 staining in ApoE4 cells expressing ectopic NHE6 (Fig. 4F). Similar results were obtained in surface biotinylation experiments that showed robust, ∼5.7-fold higher surface LRP1 levels in ApoE4 cells with restored NHE6 expression, compared with transfection with empty vector (SI Appendix, Fig. S7C). We confirmed that there were no concomitant changes in LRP1 transcript (SI Appendix, Fig. S7D) or total protein expression (SI Appendix, Fig. S7C) levels, suggesting that increased surface LRP1 was due to posttranslational redistribution of the existing cellular LRP1 pool. Consistent with these findings, confocal microscopy revealed a reduction in perinuclear accumulation of LRP1 and partial restoration of staining on astrocyte processes in ApoE4 cells with NHE6 expression (SI Appendix, Fig. S7E). Taken together, our data point to diminished NHE6 expression as a major underlying cause for defective Aβ clearance in ApoE4 astrocytes. Furthermore, since LRP1 is a receptor for multiple ligands, loss of NHE6 may contribute to other ApoE4 defects, including defective synaptosome uptake and synapse pruning (37, 46).
HDAC Inhibitors Rescue NHE6-Mediated Aβ Clearance Deficits.
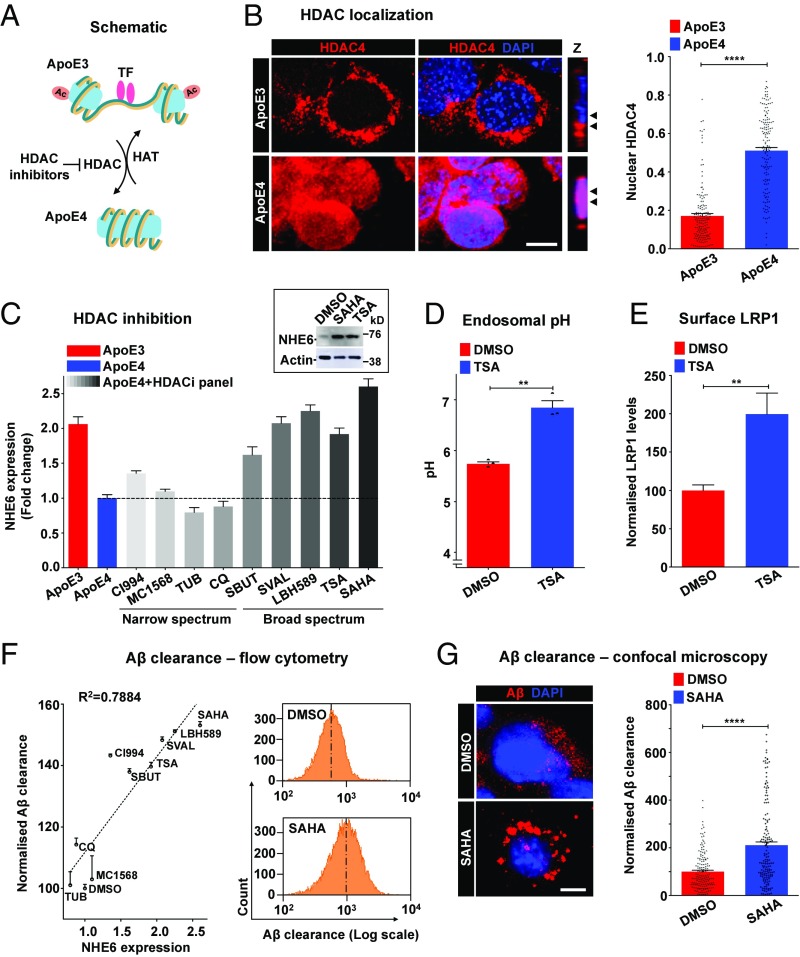
Reports of increased nuclear translocation of multiple HDACs in the ApoE4 isotype, relative to ApoE3 (Fig. 5A), in postmortem brains and neurons suggested a mechanistic basis for our observations (47). Fractional colocalization of HDAC4 with DAPI revealed prominent overlap, consistent with increased nuclear translocation in ApoE4 astrocytes (Fig. 5B). This was independently verified in Western blots of nuclear fractions, which showed higher nuclear HDAC4 in ApoE4 astrocytes relative to ApoE3 (SI Appendix, Fig. S8A). We recently discovered an evolutionarily conserved mechanism for nutrient and HDAC-dependent regulation of NHE6 gene expression (48). HDAC inhibitors could therefore potentially correct human pathologies resulting from NHE6 down-regulation and aberrant endosomal hyperacidification. To extend and translate these observations, we screened a panel of nine HDAC inhibitors comprising several different chemical classes for their potential to augment the expression of NHE6 in ApoE4 astrocytes. Whereas inhibitors of class I (CI994) or class II (MC1568) HDACs resulted in minimal changes in NHE6 expression, structurally distinct, broad-spectrum drugs inhibiting both classes, including sodium butyrate, sodium valproate, LBH589, trichostatin A (TSA), and suberoylanilide hydroxamic acid (SAHA) (vorinostat), resulted in significant restoration of NHE6 expression levels in ApoE4 astrocytes to levels comparable to ApoE3 astrocytes (Fig. 5C). Other narrow-spectrum HDAC inhibitors studied here (tubacin and clioquinol) had no significant effect. We confirmed a robust increase in NHE6 protein in ApoE4 astrocytes following HDAC inhibition (Fig. 5C and SI Appendix, Fig. S8B). Both TSA and SAHA elicited dose-dependent NHE6 increases with a half-maximal response of 6.50 ± 0.36 μM and 6.81 ± 0.53 μM (SI Appendix, Fig. S8 C and D), respectively, comparable to their therapeutic plasma concentrations (49). Neither TSA nor SAHA significantly altered NHE9 levels (SI Appendix, Fig. S8E). Next, we sought to determine if enhanced NHE6 expression resulting from inhibition of HDACs was physiologically effective in correcting hyperacidic endosomal pH in ApoE4 astrocytes. TSA treatment (5 μM for 12 h) exhibited a compartment-specific effect of significantly elevating endosomal pH (Fig. 5D) without effect on lysosomal pH (SI Appendix, Fig. S8F). Importantly, we observed prominent, approximately twofold higher surface LRP1 expression in ApoE4 cells with TSA treatment (Fig. 5E and SI Appendix, Fig. S8G). We confirmed that both TSA and SAHA stimulated acetylation of histones H3 and H4 in ApoE4 astrocytes following 60 min of treatment (SI Appendix, Fig. S8 H and I). Of note, TSA or SAHA treatment in ApoE4 astrocytes did not significantly affect cell viability measured using trypan blue exclusion (SI Appendix, Fig. S8J).
Fig. 5.
HDAC inhibitors rescue NHE6-mediated Aβ clearance deficits. (A) Schematic showing HDAC activation in ApoE4, compared with increased histone acetylase (HAT) activity and transcription factor (TF) binding in ApoE3. HDAC inhibitors could potentially exert therapeutic effects by reducing ApoE4-induced nuclear translocation of HDACs. (B) Representative micrographs (Left) and quantification using the Pearson correlation coefficient (Right) of colocalization of HDAC4 (red) with nuclear DAPI (blue) in ApoE3 and ApoE4 astrocytes. Colocalization is evident in the merge and orthogonal slices (Z) as magenta puncta. Note the prominent overlap between HDAC4 and DAPI, consistent with increased nuclear translocation, in ApoE4 astrocytes (Pearson’s correlation coefficient for ApoE3: 0.17 ± 0.01 vs. ApoE4: 0.51 ± 0.02; ****P = 4.6 × 10−44, Student’s t test; n = 150 per condition). (C) Quantitative PCR analysis to determine the efficacy of HDAC inhibitors to augment the expression of NHE6 in ApoE4 astrocytes following 12 h of treatment. Broad-spectrum drugs, including sodium butyrate, sodium valproate, LBH589, TSA, and SAHA, restored NHE6 expression in ApoE4 astrocytes to levels comparable to ApoE3 astrocytes. (Inset) Robust increase of NHE6 protein with HDAC inhibition by SAHA and TSA treatment. The complete Western blot is shown in SI Appendix, Fig. S8B. (D) HDAC inhibition by TSA treatment (5 μM for 12 h) corrected hyperacidic endosomal pH in ApoE4 astrocytes, relative to DMSO treatment (**P = 0.0014, Student’s t test; n = 3). (E) HDAC inhibition by TSA treatment significantly increased surface levels of LRP1, as determined by surface labeling at 4 °C and flow cytometry, in ApoE4 astrocytes (**P = 0.0037, Student’s t test; n = 3). The fluorescence-activated cell sorting (FACS) histogram is shown in SI Appendix, Fig. S8G. (F, Left) Quantitation of Aβ clearance by FACS analysis (10,000 cells in biological triplicates) to determine the efficacy of HDAC inhibitors to rescue Aβ clearance deficits in ApoE4 astrocytes. Note the prominent linear relationship between Aβ clearance and the fold change in NHE6 expression (R2 = 0.7884, P < 0.0001) elicited by DMSO and nine HDAC inhibitors. CQ, clioquinol; SBUT, sodium butyrate; SVAL, sodium valproate; TUB, tubacin. (Right) Representative FACS histograms demonstrating the increase in Aβ internalization by ApoE4 with SAHA treatment. (G) Representative micrographs (Left) and quantification (Right) demonstrating internalized Aβ following SAHA treatment. Note the prominent, vesicular Aβ staining in SAHA-treated ApoE4 cells (****P = 9.9 × 10−13, Student’s t test; n = 160 per condition) (SI Appendix, Fig. S8). (Scale bars, 10 μm.)
Key to the potential efficacy of HDAC inhibitors in AD therapy is their ability to rescue Aβ clearance deficits in ApoE4 astrocytes. We observed a prominent linear relationship between Aβ clearance and the fold change in NHE6 expression (R2 = 0.7884; Fig. 5F) elicited by the panel of nine HDAC inhibitors. HDAC inhibitors with lower induction of NHE6 expression (e.g., MC1568, tubacin) conferred minimal changes in Aβ clearance. Notably, broad-spectrum HDAC inhibitors (e.g., TSA, SAHA) that significantly restored NHE6 expression also elicited proportionally complete correction of defective Aβ clearance in ApoE4 astrocytes to levels similar (up to 92.4%) to ApoE3 cells (Fig. 5F and SI Appendix, Fig. S8K). ApoE4 cells treated with SAHA showed prominent, vesicular Aβ staining relative to vehicle control (Fig. 5G). Taken together, we show distinct effects of ApoE3 and ApoE4 genotypes on nucleocytoplasmic shuttling of HDAC4. These findings lead to a molecular mechanism, with clinical implications, for ApoE4-associated down-regulation of NHE6 in postmortem brain and astrocyte models.
Discussion
AD is characterized by a pathological increase of amyloid Aβ in the brain, resulting from an imbalance between its production and clearance. Recent studies suggest that accumulation of Aβ in the brain begins at least 20 y before symptoms appear (50). Although several promising drugs targeting the amyloid cascade have been developed, their astoundingly high failure rates (99.6%) in the clinic suggest that by the time amyloid plaques, neurofibrillary tangles, and neuronal death are detected, it is unlikely that disease progression can be halted and reversed (51). In this context, understanding and targeting preclinical endosomal pathologies may be critical for an effective cure.
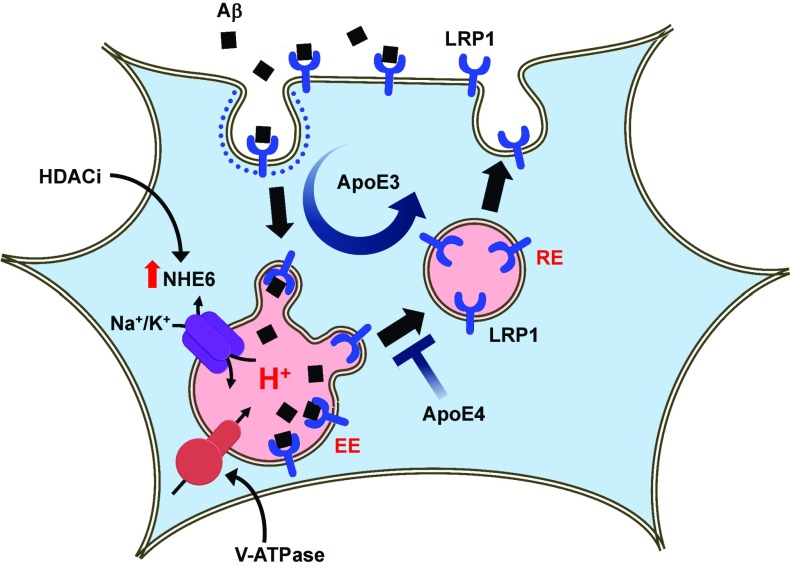
Endosomes take the center stage in an emerging model of AD. Dysfunction of endosomes is proposed to be a pathogenic hub and driver of the disease (15). Previously, we showed that NHE6 limits the acidification of early endosomes to regulate trafficking and BACE1-mediated processing of the amyloid precursor protein (APP), effectively limiting production of Aβ peptide (52). In this study, we demonstrate a critical role for NHE6 in the uptake and clearance of Aβ in astrocytes. Aβ clearance in cell culture is defined as the endocytic uptake of Aβ from the extracellular milieu and is quantified by measuring internalized Aβ (23, 34, 53, 54). We used mouse astrocytes expressing human ApoE isoforms that produce, lipidate, package, and secrete ApoE in a brain-relevant physiological fashion (23). The well-documented pathogenic deficiency of ApoE4 astrocytes to clear Aβ is mediated by decreased expression of NHE6, which results in endosomal overacidification and reduced surface levels of the Aβ receptor LRP1. Thus, NHE6 is an important ApoE4 effector in astrocytes. The precise pH-sensitive perturbation in trafficking remains to be determined. We suggest that hyperacidification of early and recycling endosomes blocks receptor recycling to the plasma membrane, trapping the endosomes in a nonproductive intracellular pool (Fig. 6). Recent observations also point to a role for ApoE4 in binding and trapping insulin receptor within intracellular compartments (55), which could result from acid pH-mediated ApoE4 aggregation within the endosomes (56). Taken together, we propose that loss of NHE6 function contributes to the endosomal pathology observed in presymptomatic AD brains both by accelerating Aβ production and by inhibiting Aβ clearance, promoting the development of amyloid plaques and culminating in neurodegeneration. Finally, given our previous studies suggesting a profound panspecific effect of eNHE activity on membrane persistence of multiple cell surface proteins (2, 40, 57), we propose that pathogenic endosomal acidification can occur as an upstream event and impair endocytic recycling of multiple cell surface receptors and transporters, including glutamate and insulin receptors previously reported to be reduced in brains of ApoE4 carriers (55, 58).
Fig. 6.
Proposed role for NHE6 in Aβ clearance in astrocytes. Aβ receptor LRP1 is constitutively recycled to the cell surface through early (EE) and recycling (RE) endosomes in ApoE3 astrocytes. Loss of NHE6 expression in ApoE4 astrocytes hyperacidifies endosomes and impairs trafficking of LRP1 receptor, resulting in defective Aβ clearance. HDAC inhibitors (HDACi) restore expression of NHE6 and Aβ clearance in ApoE4 cells.
Abnormalities in histone acetylation have been linked to neurodegenerative diseases, including AD, and HDAC inhibitors appear to show a neuroprotective effect, improving memory and cognition in mouse models (59, 60). Here, we link increased nuclear translocation of HDAC4 in ApoE4 astrocytes to down-regulation of NHE6 expression. We show that broad-spectrum HDAC inhibitors restore NHE6 expression, normalize endosomal pH, and correct Aβ clearance defects in ApoE4 astrocytes. Thus, the amelioration of AD pathogenesis observed in vitro and in vivo by small-molecule inhibitors of HDACs may be mediated, in part, by NHE6. Future work could test the efficacy of these pharmacological agents on amyloid pathology in well-defined animal models. Given the well-known link between NHE6 dysfunction and epilepsy (2, 9), we suggest that increased NHE6 expression could potentially contribute to antiepileptic mechanisms of the HDAC inhibitor drug sodium valproate. Importantly, our data demonstrate a hitherto unrecognized ability of HDAC inhibitors to specifically enhance endosomal pH, which could potentially correct human pathologies resulting from aberrant endosomal hyperacidification. Our recent observations showing that NHE6 is a target of the transcription factor cAMP-response element-binding protein (CREB), known to be negatively regulated by HDACs, provide a molecular mechanism for HDAC inhibitor-mediated activation of NHE6 expression in ApoE4 astrocytes (48). Of note, reduced phosphorylation of CREB has been described in brains of ApoE4 carriers (58, 61, 62).
Dysfunction in endolysosomal pH is an emerging theme in AD with clear potential for intervention to exploit the disease-modifying effects of endosomal pH (19). Amphipathic drugs, such as bepridil and amiodarone, partition into acidic compartments, alkalinize endosomes, and correct Aβ pathology in cell culture and animal models (63). Our study supports a rational, mechanistic basis for such repurposing of existing US Food and Drug Administration-approved drugs with well-established safety and pharmacokinetic profiles, known to have off-label activity of endosomal alkalization, to target the cellular microenvironment in AD. Similar to our observations in AD, down-regulation of NHE6 gene expression has been reported in autism brains (64). We suggest that endosomal pH may be a critical mechanistic link between neurodevelopmental and neurodegenerative disorders. Thus, a subset of patients with autism who have dysregulated NHE6 activity, either from loss-of-function mutations or by down-regulated gene expression, is likely to have a high risk of developing neurodegenerative disorders, thereby providing a rational basis to stratify patients for targeted therapies.
Methods
Animals.
All procedures were carried out with the approval of the Institutional Animal Care and Use Committee of the University of California, San Francisco and the Johns Hopkins University School of Medicine, Baltimore. The Slc9a6 knockout mice (no. 005843, strain name B6.129P2-Slc9a6<tm1Dgen) were obtained from The Jackson Laboratory. The model was engineered by inserting the LacZ reporter gene, which encodes β-galactosidase into the Slc9a6 genomic locus (Deltagen). In all experiments, male Slc9a6−/Y mice were used as mutants and wild-type male Slc9a6+/Y mice were used as controls. On average, five mice of each genotype were used in each experiment.
Aβ Clearance Assays.
Human ApoE isoform-expressing (ApoE3 and ApoE4) astrocyte cells were plated in six-well plates and grown to confluence. To measure Aβ uptake, cells were washed with serum-free medium, followed by incubation with 100 nM fluorescently labeled HiLyte Fluor 647-Aβ (no. AS-64161; AnaSpec) for various time points. Cells were washed with PBS and fixed for confocal imaging using an LSM 700 confocal microscope (Zeiss) or trypsinized for flow cytometry analysis of ∼10,000 cells in biological triplicates using a FACSAria instrument (BD Biosciences). Cells were gated on a forward scatter and side scatter. Live single cells were further gated to obtain a normally distributed Aβ+ cell population. Unstained cells without any exposure to fluorescently labeled Aβ were used as a control for background fluorescence.
Aβ Assay on Mouse Brain.
A human/rat/mouse β-amyloid ELISA kit from Wako (no. 294-64701) was used for the estimation of Aβ40 levels in brain homogenates, as per the manufacturer’s instructions. Briefly, brains of mice were dissected on ice, weighed, and homogenized in ice-cold radioimmunoprecipitation assay buffer (PBS + 1% Triton + 0.1% SDS + 0.5% deoxycholate) containing protease inhibitor (Roche). Lysate was centrifuged for 8–10 min at 6,000–7,000 × g, and supernatant was collected and used for ELISA. The bicinchoninic acid method was used to measure the total protein concentrations. Aβ40 was normalized to total protein concentration in the lysate.
Endosomal, Lysosomal, and Cytoplasmic pH Measurement.
Detailed protocols are provided in SI Appendix, Extended Experimental Procedures.
Statistical Analysis.
All data were analyzed statistically by Student’s t test, ANOVA, and linear regression test using GraphPad Prism. All data are presented as the mean ± SD.
Supplementary Material
Acknowledgments
We thank Drs. Robert Edwards and Julie Ullman (University of California, San Francisco) for providing mouse brains and Dr. David M. Holtzman (Washington University in St. Louis) for the gift of ApoE astrocytes. We thank Dr. Seth S. Margolis for helpful discussions and Richard L. Blosser for assistance with the flow cytometry analysis. This work was made possible by support from the Johns Hopkins Medicine Discovery Fund (R.R.). Additional support came from NIH grant DK054214 (to R.R.). H.P. is a Fulbright Fellow supported by the International Fulbright Science and Technology Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801612115/-/DCSupplemental.
References
- 1.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 2.Kondapalli KC, Prasad H, Rao R. An inside job: How endosomal Na(+)/H(+) exchangers link to autism and neurological disease. Front Cell Neurosci. 2014;8:172. doi: 10.3389/fncel.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- 4.Fuster DG, Alexander RT. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch. 2014;466:61–76. doi: 10.1007/s00424-013-1408-8. [DOI] [PubMed] [Google Scholar]
- 5.Lee C, et al. A two-domain elevator mechanism for sodium/proton antiport. Nature. 2013;501:573–577. doi: 10.1038/nature12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondapalli KC, et al. Functional evaluation of autism-associated mutations in NHE9. Nat Commun. 2013;4:2510. doi: 10.1038/ncomms3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbern JY, et al. A mutation affecting the sodium/proton exchanger, SLC9A6, causes mental retardation with tau deposition. Brain. 2010;133:1391–1402. doi: 10.1093/brain/awq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouyang Q, et al. Christianson syndrome protein NHE6 modulates TrkB endosomal signaling required for neuronal circuit development. Neuron. 2013;80:97–112. doi: 10.1016/j.neuron.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanni G, et al. A novel mutation in the endosomal Na+/H+ exchanger NHE6 (SLC9A6) causes Christianson syndrome with electrical status epilepticus during slow-wave sleep (ESES) Epilepsy Res. 2014;108:811–815. doi: 10.1016/j.eplepsyres.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Sinajon P, Verbaan D, So J. The expanding phenotypic spectrum of female SLC9A6 mutation carriers: A case series and review of the literature. Hum Genet. 2016;135:841–850. doi: 10.1007/s00439-016-1675-5. [DOI] [PubMed] [Google Scholar]
- 11.Naumova OY, et al. Age-related changes of gene expression in the neocortex: Preliminary data on RNA-seq of the transcriptome in three functionally distinct cortical areas. Dev Psychopathol. 2012;24:1427–1442. doi: 10.1017/S0954579412000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster JA, et al. NACC-Neuropathology Group Genetic control of human brain transcript expression in Alzheimer disease. Am J Hum Genet. 2009;84:445–458. doi: 10.1016/j.ajhg.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayburd A, Baranova A. Knowledge-based compact disease models identify new molecular players contributing to early-stage Alzheimer’s disease. BMC Syst Biol. 2013;7:121. doi: 10.1186/1752-0509-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kundra R, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M. Protein homeostasis of a metastable subproteome associated with Alzheimer’s disease. Proc Natl Acad Sci USA. 2017;114:E5703–E5711. doi: 10.1073/pnas.1618417114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Small SA, Simoes-Spassov S, Mayeux R, Petsko GA. Endosomal traffic jams represent a pathogenic hub and therapeutic target in Alzheimer’s disease. Trends Neurosci. 2017;40:592–602. doi: 10.1016/j.tins.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troncoso JC, et al. Neuropathology of preclinical and clinical late-onset Alzheimer’s disease. Ann Neurol. 1998;43:673–676. doi: 10.1002/ana.410430519. [DOI] [PubMed] [Google Scholar]
- 17.Nixon RA. Endosome function and dysfunction in Alzheimer’s disease and other neurodegenerative diseases. Neurobiol Aging. 2005;26:373–382. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, et al. Alzheimer’s-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci USA. 2010;107:1630–1635. doi: 10.1073/pnas.0908953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nixon RA. Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: Inseparable partners in a multifactorial disease. FASEB J. 2017;31:2729–2743. doi: 10.1096/fj.201700359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cataldo AM, et al. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: Differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu CC, et al. ApoE4 accelerates early seeding of amyloid pathology. Neuron. 2017;96:1024–1032.e3. doi: 10.1016/j.neuron.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verghese PB, et al. ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc Natl Acad Sci USA. 2013;110:E1807–E1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanekiyo T, Xu H, Bu G. ApoE and Aβ in Alzheimer’s disease: Accidental encounters or partners? Neuron. 2014;81:740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmukler E, Michaelson DM, Pinkas-Kramarski R. The interplay between Apolipoprotein E4 and the autophagic-endocytic-lysosomal axis. Mol Neurobiol. January 20, 2018 doi: 10.1007/s12035-018-0892-4. [DOI] [PubMed] [Google Scholar]
- 26.Strømme P, et al. X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction. Brain. 2011;134:3369–3383. doi: 10.1093/brain/awr250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mawuenyega KG, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castellano JM, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118, and erratum (2013). doi: 10.1038/nmeurol.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeMattos RB, et al. ApoE and clusterin cooperatively suppress Abeta levels and deposition: Evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- 31.Mak AC, et al. Effects of the absence of Apolipoprotein E on lipoproteins, neurocognitive function, and retinal function. JAMA Neurol. 2014;71:1228–1236. doi: 10.1001/jamaneurol.2014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, et al. Mechanisms of U87 astrocytoma cell uptake and trafficking of monomeric versus protofibril Alzheimer’s disease amyloid-β proteins. PLoS One. 2014;9:e99939. doi: 10.1371/journal.pone.0099939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chafekar SM, Baas F, Scheper W. Oligomer-specific Abeta toxicity in cell models is mediated by selective uptake. Biochim Biophys Acta. 2008;1782:523–531. doi: 10.1016/j.bbadis.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Mandrekar S, et al. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang DE, et al. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blalock EM, et al. Incipient Alzheimer’s disease: Microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci USA. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost. 2005;3:1884–1893. doi: 10.1111/j.1538-7836.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muro S, et al. Control of intracellular trafficking of ICAM-1-targeted nanocarriers by endothelial Na+/H+ exchanger proteins. Am J Physiol Lung Cell Mol Physiol. 2006;290:L809–L817. doi: 10.1152/ajplung.00311.2005. [DOI] [PubMed] [Google Scholar]
- 40.Kondapalli KC, et al. A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nat Commun. 2015;6:6289. doi: 10.1038/ncomms7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang WS, et al. Gene expression profiles in anatomically and functionally distinct regions of the normal aged human brain. Physiol Genomics. 2007;28:311–322. doi: 10.1152/physiolgenomics.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu PT, et al. Differences in apolipoprotein E3/3 and E4/4 allele-specific gene expression in hippocampus in Alzheimer disease. Neurobiol Dis. 2006;21:256–275. doi: 10.1016/j.nbd.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Kittur S, et al. Cytoskeletal neurofilament gene expression in brain tissue from Alzheimer’s disease patients. I. Decrease in NF-L and NF-M message. J Geriatr Psychiatry Neurol. 1994;7:153–158. doi: 10.1177/089198879400700305. [DOI] [PubMed] [Google Scholar]
- 44.Pescosolido MF, et al. Genetic and phenotypic diversity of NHE6 mutations in Christianson syndrome. Ann Neurol. 2014;76:581–593. doi: 10.1002/ana.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu H, et al. Mutation screening in 86 known X-linked mental retardation genes by droplet-based multiplex PCR and massive parallel sequencing. HUGO J. 2009;3:41–49. doi: 10.1007/s11568-010-9137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung WS, et al. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci USA. 2016;113:10186–10191. doi: 10.1073/pnas.1609896113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sen A, Nelson TJ, Alkon DL. ApoE4 and Aβ oligomers reduce BDNF expression via HDAC nuclear translocation. J Neurosci. 2015;35:7538–7551. doi: 10.1523/JNEUROSCI.0260-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prasad H, Rao R. Histone deacetylase-mediated regulation of endolysosomal pH. J Biol Chem. 2018;293:6721–6735. doi: 10.1074/jbc.RA118.002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alam MS, Getz M, Haldar K. Chronic administration of an HDAC inhibitor treats both neurological and systemic Niemann-Pick type C disease in a mouse model. Sci Transl Med. 2016;8:326ra23. doi: 10.1126/scitranslmed.aad9407. [DOI] [PubMed] [Google Scholar]
- 50.Villemagne VL, et al. Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 51.Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prasad H, Rao R. The Na+/H+ exchanger NHE6 modulates endosomal pH to control processing of amyloid precursor protein in a cell culture model of Alzheimer disease. J Biol Chem. 2015;290:5311–5327. doi: 10.1074/jbc.M114.602219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Q, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nazer B, Hong S, Selkoe DJ. LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiol Dis. 2008;30:94–102. doi: 10.1016/j.nbd.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao N, et al. Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron. 2017;96:115–129.e5. doi: 10.1016/j.neuron.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garai K, Baban B, Frieden C. Self-association and stability of the ApoE isoforms at low pH: Implications for ApoE-lipid interactions. Biochemistry. 2011;50:6356–6364. doi: 10.1021/bi2006702. [DOI] [PubMed] [Google Scholar]
- 57.Prasad H, Rao R. Applying knowledge of autism to brain cancer management: What do we know? Future Oncol. 2015;11:1847–1850. doi: 10.2217/fon.15.93. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci USA. 2010;107:12011–12016. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu K, Dai XL, Huang HC, Jiang ZF. Targeting HDACs: A promising therapy for Alzheimer’s disease. Oxid Med Cell Longev. 2011;2011:143269. doi: 10.1155/2011/143269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gräff J, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu DS, et al. APOE4 enhances age-dependent decline in cognitive function by down-regulating an NMDA receptor pathway in EFAD-Tg mice. Mol Neurodegener. 2015;10:7. doi: 10.1186/s13024-015-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiao F, Gao XP, Yuan L, Cai HY, Qi JS. Apolipoprotein E4 impairs in vivo hippocampal long-term synaptic plasticity by reducing the phosphorylation of CaMKIIα and CREB. J Alzheimers Dis. 2014;41:1165–1176. doi: 10.3233/JAD-140375. [DOI] [PubMed] [Google Scholar]
- 63.Mitterreiter S, et al. Bepridil and amiodarone simultaneously target the Alzheimer’s disease beta- and gamma-secretase via distinct mechanisms. J Neurosci. 2010;30:8974–8983. doi: 10.1523/JNEUROSCI.1199-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwede M, Garbett K, Mirnics K, Geschwind DH, Morrow EM. Genes for endosomal NHE6 and NHE9 are misregulated in autism brains. Mol Psychiatry. 2014;19:277–279. doi: 10.1038/mp.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.