Significance
The number of inmates imprisoned for violent aggression is increasing, as are the penitentiaries, but still our understanding of mechanisms underlying criminality is limited. Our analysis of violent aggressor inmates reveals unique properties of IgG reactive with adrenocorticotropic hormone (ACTH). We show that these IgGs can regulate ACTH-induced cortisol secretion in the adrenal gland, and they exhibit a clear-cut difference in ACTH epitope binding in violent aggressors vs. controls. Additionally, IgG from a subset of aggressive subjects selectively bind to hypothalamic vasopressin neurons. Thus, using several in vitro and in vivo approaches, the study reveals a molecular mechanism involved in the variability of stress response relevant to the neurobiology of aggression and possibly other stress-related conditions.
Keywords: psychoendocrinology, neuroimmunology, autoantibodies, HPA axis, corticotropin
Abstract
Violent aggression in humans may involve a modified response to stress, but the underlying mechanisms are not well understood. Here we show that naturally present autoantibodies reactive to adrenocorticotropic hormone (ACTH) exhibit distinct epitope-binding profiles to ACTH peptide in subjects with a history of violent aggression compared with controls. Namely, while nonaggressive male controls displayed a preferential IgG binding to the ACTH central part (amino acids 11–24), subjects who had committed violent acts of aggression had IgG with increased affinity to ACTH, preferentially binding to its N terminus (amino acids 1–13). Purified IgGs from approximately half of the examined sera were able to block ACTH-induced cortisol secretion of human adrenal cells in vitro, irrespective of the source of sample (from a control subject or a violent aggressor). Nevertheless, in the resident–intruder test in mice, i.p. injection of residents with ACTH and IgG from aggressive subjects, but not from control subjects, shortened latency for the first attack against intruders. Immunohistochemical screening of violent aggressors’ sera on rat brain and pituitary sections did not show IgG binding to ACTH-producing cells, but 4 of 16 sera revealed selective binding to a nonidentified antigen in vasopressinergic neurons of the hypothalamic paraventricular and supraoptic nuclei. Thus, the data show that ACTH-reactive plasmatic IgGs exhibit differential epitope preference in control and violently aggressive subjects. These IgGs can modulate ACTH-induced cortisol secretion and, hence, are involved in the regulation of the stress response. However, the possible role of ACTH-reactive autoantibodies in aggressive behavior needs further investigation.
It is currently accepted that aggressive behavior can be viewed as a strategy by humans and animals to cope with stress, implying that neurobiological mechanisms involved in stress responses should underlie both physiological and pathological aggression (1–3). The hypothalamic–pituitary–adrenal (HPA) axis is a key system in the stress response, linking the brain with cortisol secretion via pituitary release of the adrenocorticotropic hormone (corticotropin; ACTH) (4). Cortisol suppresses the activity of the HPA axis at all its levels, modulates behavioral modalities including anxiety and distress (5), and diminishes the production of testosterone (6). Both deficient and increased activation of the HPA axis have been associated with aggressive behavior. Hypo-arousal–associated aggressiveness is characteristic of antisocial personality disorder and glucocorticoid deficiency (7). In contrast, hyper-arousal–driven aggressiveness, which can be related to an acute exaggerated glucocorticoid response to stress, is seen in conditions such as posttraumatic stress disorder and intermittent explosive disorder (7, 8). The molecular mechanisms underlying altered activation of the HPA axis that may predispose to aggressive behavior, including proactive violent aggression typical of murder, are currently unknown (9–13).
In the present study, we tested the hypothesis that altered activation of the HPA axis in aggressive humans may involve ACTH-reactive Igs. Indeed, humans naturally and ubiquitously display IgG and other classes of Ig nonspecifically reactive with ACTH and other peptide hormones, supporting their constitutive contribution to peptidergic signaling (14–18). Increased plasma levels of ACTH-reactive IgG have been found in male prisoners and adolescents with conduct disorder (15). However, it is unknown whether ACTH-reactive IgG may influence ACTH-induced cortisol secretion and whether such an influence can be different in aggressive subjects. In fact, functional activities of some peptide hormones, such as ghrelin, can be regulated by plasmatic IgG, depending on their affinities (19, 20).
To address these questions, we analyzed plasma levels and affinity kinetics of ACTH-reactive IgG in prisoners who had committed violent acts of aggression, including murder, and compared the results with those from healthy nonaggressive controls [prisoners in whom violence was not a major feature and bodybuilders who were on active treatment with performance-enhancing substances (PES) and who previously had been characterized by increased physical aggressiveness but not hostility and anger (21)]. We then studied the functional relevance of the observed differences in IgG affinity and epitope binding for ACTH with regard to IgG’s ability to modulate ACTH-induced cortisol secretion in vitro. We also studied aggressive behavior in mice after peripheral injections of ACTH and IgG from aggressive and control subjects. Furthermore, to determine the presence of other autoantibodies potentially interfering with the stress axis in aggressive subjects, we performed an immunohistochemical analysis of IgG binding to the rat brain and pituitary as well as guinea pig adrenal cortex sections.
Results
ACTH-Reactive IgG and ACTH.
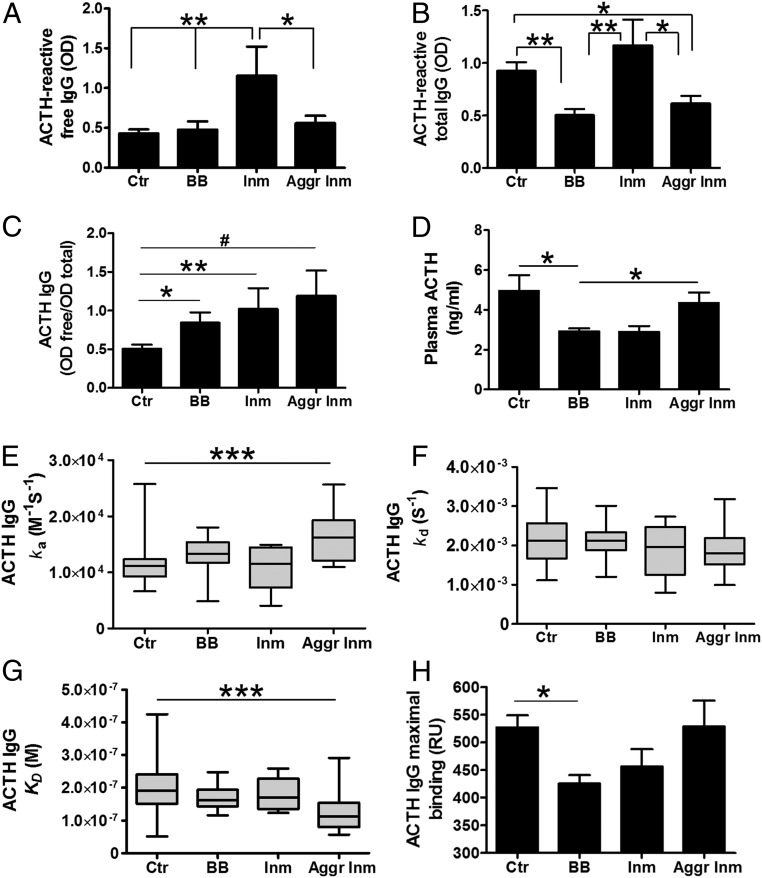
Free and total levels of ACTH-reactive IgG were measured in normal or dissociative buffers. Mean plasma levels of ACTH-reactive free IgG were similar among groups of violent aggressors, bodybuilders, and healthy controls but were significantly higher in nonaggressive inmates than in the other three groups (Fig. 1A). However, ACTH-reactive total IgG levels were lower in violent aggressors and bodybuilders than in healthy controls and nonaggressive inmates (Fig. 1B). The free/total ratios of ACTH-reactive IgG were higher in all study groups than in the healthy controls (Fig. 1C), but the intergroup differences were not statistically significant (P = 0.06, Kruskal–Wallis test). Plasma concentrations of the ACTH peptide were lower in bodybuilders than in healthy controls and violent aggressors but were not significantly different between healthy controls and violent aggressors (Fig. 1D).
Fig. 1.
ACTH-reactive IgG and ACTH in violent aggressive inmates (Aggr Inm), healthy controls (Ctr), bodybuilders (BB), and nonaggressive inmates (Inm). (A–C) Plasma ACTH-reactive free (A) and total (B) IgG levels and their ratios (C). (D) Plasma concentrations of ACTH. (E–H) Affinity kinetics: SPR analysis of IgG binding to ACTH showing the association (E) and dissociation (F) rates, the dissociation equilibrium constants (G), and maximum binding capacity in resonance units (RU) (H) between IgG and ACTH. (A) ANOVA, P = 0.004, Tukey’s posttests, *P < 0.05, **P < 0.01. (B) ANOVA, P = 0.0003, Tukey’s posttests; *P < 0.05, **P < 0.01. (C) Student’s t tests, *P < 0.05, **P < 0.01; Mann–Whitney test, #P < 0.05. (D) Kruskal–Wallis test, P = 0.005; Dunn’s post hoc tests, *P < 0.05. (E) Kruskal–Wallis test, P = 0.001; Dunn’s post hoc tests, ***P < 0.001. (F) Kruskal–Wallis test, P = 0.45. (G) Kruskal–Wallis test, P = 0.002; Dunn’s post hoc tests, ***P < 0.001. (H) Kruskal–Wallis test, P = 0.02; Dunn’s post hoc test; *P < 0.05. Data are shown as mean ± SE. n = 21 control subjects; n = 13, body builders; n = 6 nonaggressive inmates; and n = 16 aggressive inmates.
Analysis of the affinity kinetics of ACTH IgG using surface plasmon resonance (SPR) showed significant differences between violent aggressors and healthy controls. These differences included an increase in the association rate (Fig. 1E) and a decrease (by a factor of 1.7) in the dissociation equilibrium constant in aggressive subjects (Fig. 1G). The mean values of the dissociation rate were not significantly different among the groups (Fig. 1F). The maximum binding capacity of IgG to ACTH was measured by SPR at the end of the association and was lower in the bodybuilders than in healthy controls (Fig. 1H). Since no significant differences in affinity kinetics for the groups of bodybuilders and nonaggressive inmates were found, in subsequent experiments we compared IgG only from violent aggressors and healthy controls.
To see whether ACTH levels may be functionally related to ACTH-reactive IgG, we analyzed correlations between plasma ACTH peptide concentrations and ACTH-reactive IgG levels and properties. The ACTH concentrations correlated negatively with ratios of free/total ACTH IgG levels (Spearman’s r = −0.25, P < 0.05) and positively with the IgG maximum binding capacity for ACTH (Spearman’s r = 0.26, P < 0.05). No significant correlations were found between ACTH and plasma levels of either free or total IgG or their affinity kinetics.
In Vitro Cortisol Assay.
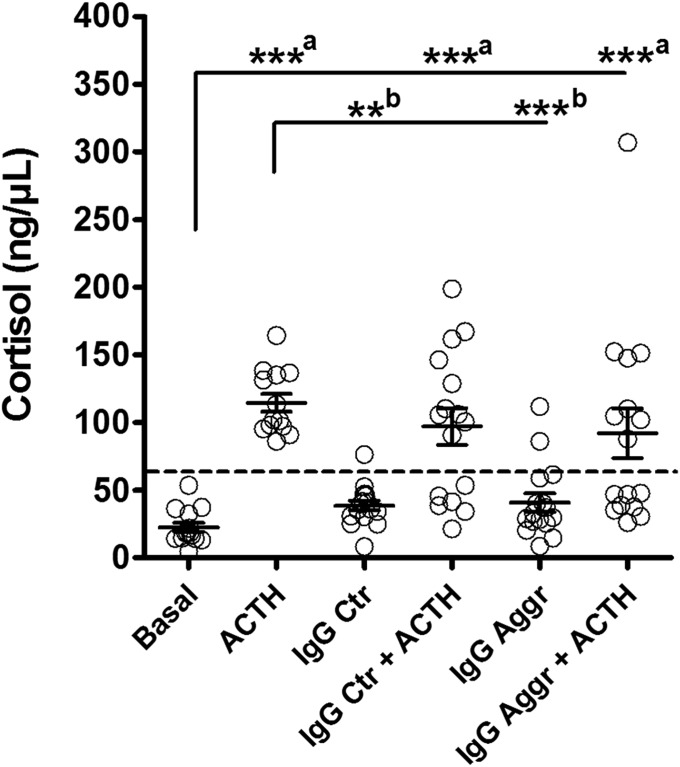
In cultured human adrenocortical cells the medium mean cortisol levels were 22.6 ± 3.4 ng/μL. As expected ACTH (amino acids 1–24) peptide, known to have full ACTH (amino acids 1–39) cortisol-stimulating activity, stimulated cortisol release in all samples (mean levels 114.7 ± 6.5 ng/µL) (Fig. 2), i.e., a fivefold increase (P < 0.0001, Mann–Whitney U test,). To see whether IgG alone might influence cortisol release from the adrenocortical cells, cells were incubated with IgG from healthy controls and violent aggressors. We found a small but significant increase in cortisol induced by IgG from both the control and the aggressive groups, to mean levels of 38.6 ± 3.7 ng/µL (P = 0.005, Mann–Whitney U test) and 40.8 ± 6.7 ng/µL (P = 0.017, Mann–Whitney U test), i.e., 1.7- and 1.8-fold increases, respectively. To see whether IgG might influence ACTH-induced cortisol release, adrenocortical cells were incubated with ACTH (1–24) and IgG from healthy controls or violent aggressors. We found that ACTH significantly elevated mean levels of cortisol after the addition of IgG from either control or aggressive groups, compared with the basal cortisol levels, corresponding to 97.03 ± 13.5 ng/µL and 92.07 ± 18.3 ng/µL, respectively (P < 0.0001, Kruskal–Wallis test; P < 0.001, Dunn’s post-hoc test), i.e., 4.3- and 4.1-fold increases, respectively. The results of the multiple comparisons of cortisol release between all experimental conditions are shown in Fig. 2.
Fig. 2.
Effects of IgG from violent aggressors (Aggr) and from healthy controls (Ctr) on basal and ACTH (amino acids 1–24)-stimulated cortisol release from human adrenocortical cells in vitro. Dashed threshold line divides responders vs. nonresponders in the IgG + ACTH groups and it was established just above the maximum basal cortisol values at 60 ng/ml. Mean ±SE; n = 14, Basal; n = 13, ACTH; n = 16, other groups. ANOVA, P < 0.0001; Dunn’s post hoc tests, vs. Basal, ***aP < 0.001, and vs. ACTH, **bP < 0.01, ***bP < 0.001.
The mean levels of ACTH-induced cortisol release were not significantly affected by IgG (P = 0.24, Kruskal–Wallis test), but the two types of IgG-related cortisol response showing either stimulation or inhibition were distinguishable in both control and aggressive groups (Fig. 2). Accordingly, by drawing a threshold line for activation just above the maximum basal cortisol values at 60 ng/μL (Fig. 2), 37.5% of controls and 50% of aggressive subjects showed no ACTH-induced increase in cortisol in the presence of their IgG.
To see whether the ability of IgG to either inhibit (nonresponders) or to preserve (responders) ACTH-induced cortisol release can be associated with certain ACTH IgG properties and/or with aggressiveness and other behavioral characteristics of controls and violent aggressors, we compared these characteristics between responders and nonresponders (SI Appendix, Table S4). For the ACTH IgG properties, only the free/total ratios tended to be higher in both aggressive and control groups, resulting in an increase in these ratios in all nonresponders vs. responders (P < 0.01, Student’s t test). Among the behavioral characteristics, aggressive responders scored higher for urgency in the UPPS [Urgency, Premeditation (lack of), Perseverance (lack of), and Sensation seeking] impulsivity scale; no other significant differences between responders and nonresponders were found (SI Appendix, Table S4).
Epitope Mapping of ACTH-Reactive IgG.
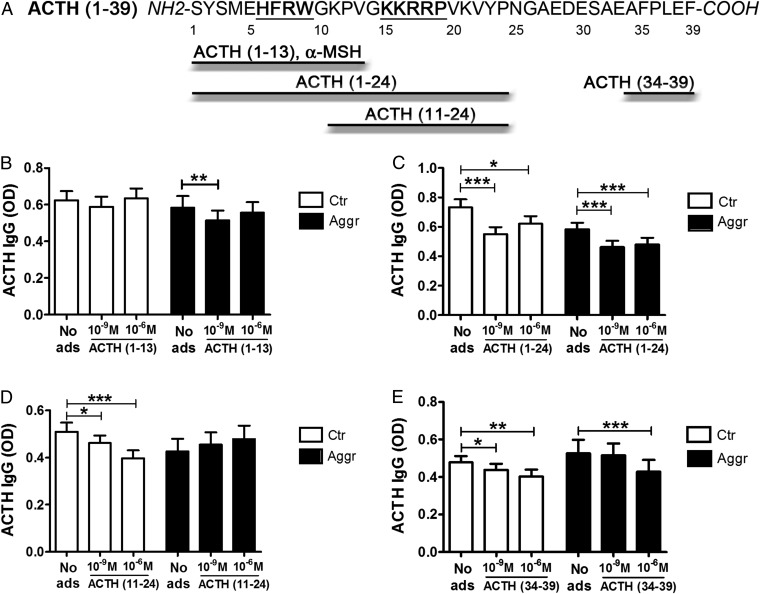
To determine the preferential binding site(s) of ACTH-reactive IgG, plasma samples from violent aggressors and healthy controls were preincubated with one of four ACTH fragments covering different parts of the peptide, including its core pharmacological sequences necessary for cortisol release via the melanocortin-2 receptor (MC2R) (Fig. 3A). Adsorption with the N-terminal ACTH fragment (amino acids 1–13), i.e., with α-MSH, significantly reduced IgG binding only in the aggressive group (Fig. 3B). A larger ACTH N-terminal fragment, (amino acids 1–24), reduced IgG binding in both groups (Fig. 3C). However, the central ACTH fragment (amino acids 11–24) reduced IgG binding only in controls (Fig. 3D). Finally, the C-terminal ACTH fragment (amino acids 34–39) reduced binding in both groups (Fig. 3E). Thus, while in controls epitopes for ACTH-reactive IgG were detected in all parts of ACTH, in violent aggressors they were absent in the central part of ACTH, which includes the MC2R pharmacophore, and were more pronounced in the α-MSH part, which includes the pharmacophore for other melanocortin receptors.
Fig. 3.
Epitope mapping of ACTH-reactive IgG in violent aggressors (Aggr) and healthy controls (Ctr). (A) Amino acid sequence of human ACTH (amino acids 1–39). The MC4R- and MC2R-binding sites HFRW and KKRRP, respectively, are underlined. Four different ACTH fragments used for plasma adsorption are shown. (B–E) Plasma levels in OD of IgG reactive with ACTH (amino acids 1–39) were measured before and after adsorption (ads) with 10−9 M and 10−6 M of each of the peptide fragments: (B) ACTH (amino acids 1–13); (C) ACTH (amino acids 1–24); (D) ACTH (amino acids 11–24); and (E) ACTH (amino acids 34–39). *P < 0.05, **P < 0.01, ***P < 0.001, paired t tests. Data are shown as mean ± SE; n = 20 control subjects and n = 16 violent aggressors.
The adsorption results were also analyzed with regard to the ability of IgG to block or preserve ACTH-induced cortisol release in our in vitro study. The responders showed a preferential binding site of their IgG in the central ACTH fragment (amino acids 11–24), which was reduced in nonresponders (SI Appendix, Fig. S1).
Resident–Intruder Test.
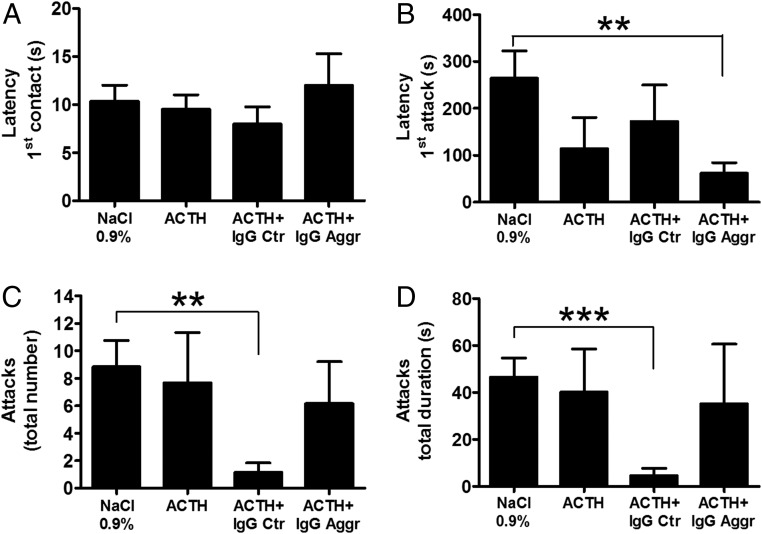
The possible effects of IgG on aggressive behavior in mice have been studied using the resident–intruder test. In this test, the resident mouse, which has been kept in isolation for several weeks, typically displays aggressive attacks toward an intruder mouse placed in the resident mouse’s cage for 10 min. We found that ACTH (1.0 µg) injected alone to the resident mouse 30 min before the presentation of the intruder did not significantly affect aggressive behavior (Fig. 4). Injections of ACTH alone or together with human IgG (from either control or violent aggression subjects) did not change the latency of nonaggressive first physical contact (Fig. 4A). However, coadministration of ACTH together with IgG from violent aggressors, but not from healthy controls, reduced the latency for the first attack (Fig. 4B). Furthermore, the total number of attacks and their duration were reduced in mice that received IgG from controls, while no significant effects were seen after treatment with IgG from violent aggressors (Fig. 4 C and D).
Fig. 4.
Results of the resident–intruder behavioral test in mice after i.p. injection to the resident of 0.9% NaCl or ACTH (1 μg) alone or together with IgG (100 μg) pooled from individual IgG samples from healthy controls (Ctr) or violent aggressors (Aggr). (A) Latency for the first contact. (B) Latency for the first attack. (C) Total number of attacks. (D) Total duration of all attacks. **P < 0.01; ***P < 0.001, Student’s t test. Data are shown as mean ± SE; n = 6 in each group.
Immunohistochemical IgG Analysis.
Sera from aggressive (n = 16) and control (n = 22) subjects were applied for IgG immunohistochemical analysis on colchicine-treated rat brain sections, including hypothalamus, and on sections from rat pituitary and guinea pig adrenal glands. No immunostaining was detected in the rat arcuate nucleus and the pituitary, both tissues containing pro-opiomelanocortin–expressing cells (SI Appendix, Fig. S2 B, D, and F). In contrast, immunostaining of adjacent sections using a commercial anti-ACTH antibody revealed typical staining of pro-opiomelanocortin–expressing neurons in the arcuate nucleus (SI Appendix, Fig. S2 A and C) and of corticotropes in the anterior pituitary lobe (SI Appendix, Fig. S2E). No immunostaining was found in the guinea pig adrenal gland using either human IgG or anti-ACTH antibodies (SI Appendix, Fig. S2 G and H).
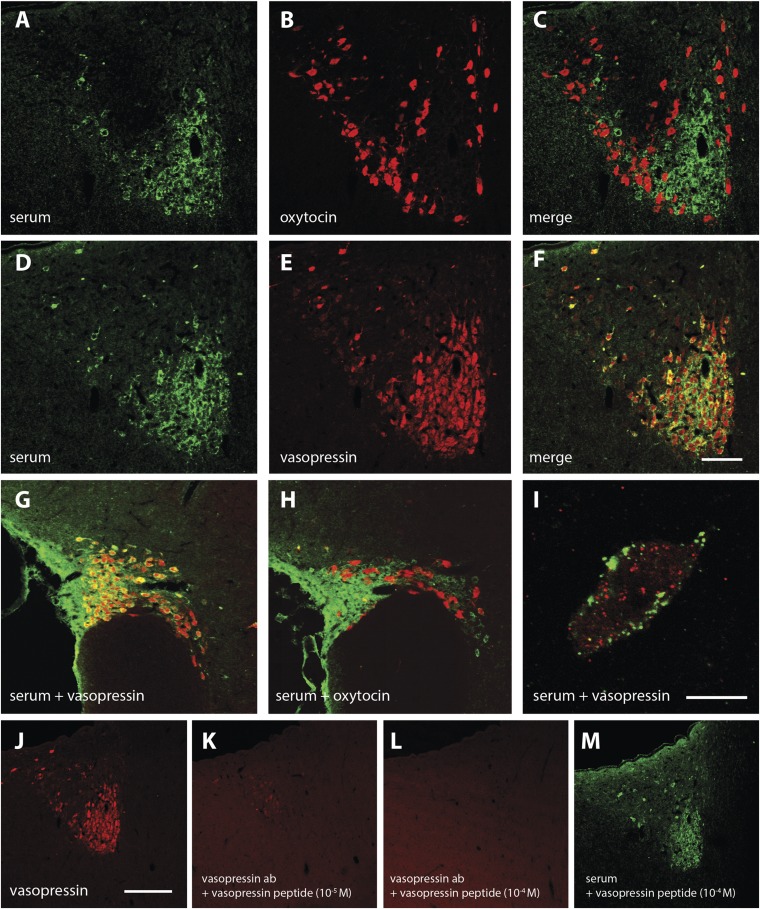
Sera from four aggressive subjects, but not sera from any of the controls, produced distinct immunostaining in hypothalamic paraventricular nucleus (PVN) rat brain sections (Fig. 5 A and D). Using costaining with anti-oxytocin and anti-vasopressin antibodies (Fig. 5 B and E), the human IgG immunostaining was colocalized selectively in vasopressin but not oxytocin neurons (Fig. 5 C and F). A similar pattern was observed in the supraoptic nucleus (SON) (Fig. 5 G and H). High-power magnification revealed that the staining by human IgG was mainly localized in association with the cell membrane of vasopressinergic neurons but apparently not with vasopressin-immunopositive storage vesicles (Fig. 5I). In agreement, adsorption of the serum with vasopressin peptide did not prevent the PVN immunostaining (Fig. 5M). As a control, the same adsorption protocol for an anti-vasopressin antibody abolished the staining of PVN neurons (Fig. 5 J–L).
Fig. 5.
Immunohistochemical identification of hypothalamic neurons bound by IgG from aggressive subjects’ sera. (A–C) Human serum and oxytocin antibody label different neuronal populations in the PVN. (D–F) Human serum and vasopressin antibody label the same neuronal population with complete overlap in the PVN. (G and H) Human serum labels the same neuronal population as vasopressin antibody (G), but no cellular overlap with oxytocin immunostaining (H) is seen in the SON. (I) High-resolution confocal analysis (100× objective, 0.8 μm optical layer, PVN) shows that human serum and vasopressin antibody label the same neurons but with different subcellular localization. (J–M) Vasopressin immunostaining (J) can be abolished by preincubation of the antibody solution with 10−5 M (K) or 10−4 M (L) vasopressin peptide (absorption control), but staining with the human serum from an aggressive subject cannot be abolished by preincubation with 10−4 M vasopressin peptide (M). (Scale bars: 100 μm in F, applied to A–H; 10 μm in I; 200 μm in J, applied to J–M.)
Discussion
We show that plasma IgG in humans binds ACTH with different affinity kinetics and epitopes and is associated with differential activation of ACTH-induced cortisol secretion. These results may at least partly explain the mismatch between plasma levels of ACTH and cortisol that can be observed in various pathological conditions and in response to stress (22). Moreover, the data also show that the role of ACTH-binding IgGs in the variability of the activation of the HPA axis activity is unlikely causal for aggressiveness. However, the results and interpretations are limited by the small groups of subjects studied, who, in addition to aggressiveness, may differ by other biological, psychological, and environmental factors potentially influencing the activity of their HPA stress axis.
If ACTH-reactive IgG can differentially influence ACTH-mediated signaling and behavior in aggressive subjects, such IgG should display different levels or ACTH-binding properties. We found that plasma levels of ACTH-reactive free IgG were unaffected in violent aggressors but were elevated in nonaggressive inmates, which is in agreement with previous data (15). However, violent aggressors had increased ratios of free/total ACTH-reactive IgG levels associated with low plasma ACTH, indicating a functional significance in ACTH signaling. These ratios were also increased in bodybuilders and in nonviolent inmates, but an increased affinity of IgG for ACTH was detected only in the violent aggression group. In all studied subjects, the affinity values of plasmatic IgG for ACTH were found to be in the micromolar range, which a priori should not compete with the nanomolar binding affinity of ACTH to MC2R. Thus, a slightly increased affinity of ACTH IgG in violent aggressors should not, in theory, neutralize ACTH. Instead, the positive correlation between plasma ACTH concentrations and IgG maximum ACTH-binding capacities supports a role of plasmatic IgG as an ACTH carrier.
We show here in several subjects that IgG can prevent ACTH-induced cortisol secretion, i.e., can block ACTH activation of the MC2R. The ability of plasmatic IgG to interfere with ACTH-stimulated cortisol production may contribute to mechanisms underlying individual variability of the stress-induced cortisol release, relevant not only to aggression but also to several stress-related disorders, such as depression (23, 24). Since we detected two types of IgG effects on the ACTH-induced release of cortisol (blocking or not blocking) among both aggressive and nonaggressive subjects, an abnormal IgG modulation of the ACTH-induced cortisol response cannot be directly causative of violent aggression. Nevertheless, it is likely that the IgG-mediated type of stress-induced cortisol response, i.e., its preservation and even potentiation, or its absence, may be important for the emotional context during the act of violent aggression. This act can be classified, as previously proposed, as impulsive (reactive) or instrumental (proactive) aggressive behavior (3, 25, 26). Such a possibility is supported by observations that either high or low glucocorticoids may be associated with aggression (27–29), whereby a low cortisol response to stress has been combined with psychopathic features (30). Of relevance, a study of biological and criminal profiles of 545 inmates showed distinct clustering, with lowest plasma cortisol levels present in a group characterized by violent aggression (31).
Our finding of an increased urgency score in the impulsivity scale in aggressive subjects, whose IgG did not block ACTH-induced cortisol release, and vice versa, suggests that ACTH-reactive IgG may modulate impulsivity in response to stress via cortisol release. Impulsivity requires the mobilization of several neuronal circuits controlling motor and autonomic reactions (11), including the mesolimbic dopaminergic system (32, 33). Moreover, glucocorticoids have been shown to regulate the responsiveness of dopaminoceptive neurons in the basal ganglia (34, 35) and may also provide positive feedback on centrally induced aggression (36).
What changes in IgG properties may convey their blocking effects on ACTH activation of MC2R? Although we did not find significant differences in IgG affinity for ACTH between cortisol responders and nonresponders, increased ratios of free:total ACTH IgG levels were seen in cortisol nonresponders. Such ratios were inversely associated with plasma ACTH levels. This suggests that ACTH-reactive IgG may carry more or less ACTH, regardless of differences in ACTH IgG affinity and that activation of MC2R involves different binding epitopes. In fact, epitope mapping showed that in cortisol responders preferential binding of IgG occurs to the ACTH sequence containing the MC2R pharmacophore KKRRP (amino acids 11–24) (37–39). This binding was slightly lower in cortisol nonresponders. Notably, aggressive subjects did not show binding to ACTH amino acids 11–24 but instead bound to ACTH amino acids 1–13, the section that contains the melanocortin pharmacophore HFRW (40) and hence may underlie the increased prevalence of nonresponders (50% vs. 37.5%) among aggressive subjects.
Melanocortin peptides have been associated with aggressive behavior. For example, simple peripheral injection of α-MSH or ACTH amino acids 4–10, but not the complete ACTH molecule (41), induced aggressive behavior in mice (42, 43), suggesting that the melanocortin peptide pharmacophore is necessary for ACTH proaggressive effects. What are the melanocortin receptive sites in the brain that may mediate such ACTH functions? The hypothalamic ventromedial nucleus (VMN) has been established as a key central structure for attack initiation in rats (44). Moreover, a recent study using optogenetic activation of neurons in the ventrolateral part of the VMN showed that they were responsible for triggering aggressive behavior in mice (45). Altered hypothalamic oscillations have also been detected in an aggressive patient (46). We found that IgG sera from 4 of 16 aggressive subjects were bound to vasopressinergic neurons of hypothalamic PVN and SON. This finding is of interest, because hypothalamic vasopressin potentiates corticotropin-releasing hormone-induced ACTH secretion during the stress response (47, 48) and is implicated in sex-dependent regulation of aggressive behavior (49). The present study did not identify the autoantigen. However, its preferential location in the cell membrane of vasopressinergic neurons indicates that it can be involved in the regulation of vasopressin secretion relevant to aggressive behavior (50). By screening sera of aggressive subjects on the pituitary and adrenal cortex, no binding to ACTH-expressing cells was found, indicating that naturally present IgGs are not indicative of an autoimmune reaction against these tissues (51).
Increased plasma ACTH in rats in a resident–intruder paradigm was previously associated with lower aggressiveness in resident rats (52). In our study, peripheral administration of ACTH alone in resident mice did not significantly change their aggressive behavior, but ACTH coinjected with IgG from violent aggressors reduced the latency of the first attack without affecting the total number of attacks. Interestingly, aggressive behavior was inhibited in resident mice who received ACTH together with IgG from nonaggressive controls. Such behavioral responses support a role for peripheral IgG in the regulation of both impulsive and defensive aggressive behavior. This also suggests that plasmatic IgG in healthy controls may suppress natural aggressiveness, in agreement with IgG having a putative role in depression (53).
The origin of differences in ACTH IgG-binding properties in aggressive and nonaggressive subjects is presently unclear. Posttranslational modification of IgG by physicochemical factors may induce their polyreactivity (54, 55). It may also be related to homologous antigenic stimulation from bacteria or viruses. For instance, ClpB protein produced by Enterobacteriaceae was recently identified as an α-MSH conformational mimetic, responsible for production of α-MSH cross-reactive autoantibodies (56). Immunization of mice with ClpB stimulated α-MSH– but not ACTH-reactive IgG (56). Instead, chronic supplementation of rats with Escherichia coli resulted in an increased affinity of ACTH-reactive IgG, suggesting that such bacteria may contain ACTH-like antigens (57). Therefore, it will be of interest to determine what factors may induce IgG cross-reactivity to different parts of the ACTH molecule associated with modulation of its potential physiological functions, including aggressive behavior.
Materials and Methods
Ethics Statement.
The entire project was approved by the Norwegian Regional Ethics Committee East (NRECE) as Project 2010/792. No approaches to the study subjects were allowed before the receipt of such approval. Thus, subjects who gave their written informed consent did so only after the recruitment form, consent form, project information, sampling procedures, collaboration, storing of samples with attached procedures, and all planned tests had been approved by the NRECE. All study subjects including controls were presented the same NRECE-approved detailed information, and their written consent was received before their inclusion in the study.
Subjects.
Violent aggressors.
Sixteen violent aggressor inmates were included. Eleven of these had committed at least one murder or had attempted to commit murder, and one inmate had participated in gang-related activity resulting in murder. Four inmates had committed brutal physical violence with violent sex-related components, such as rape, molestation, or grievous bodily harm. Inmates who had committed pedophilic acts were excluded from this study.
All violent male inmates except one were recruited from a high-security prison outside Oslo. The inmates were serving long-term sentences, the majority in preventive detention. One of the study subjects had been released and was tested between commissions of violent crimes. He was later rearrested and charged following violent behavior and is currently serving a sentence in a different prison. In Norway, the imposition of preventive detention indicates that the court considers the defendant at high risk for reoffending and therefore is an imminent threat to society. According to Norwegian law, after having served a minimum term not exceeding 10 y, a prisoner in preventive detention may make an appeal to the court to reconsider his case. None of the 16 prisoners had serious mental illness.
Controls.
Healthy male volunteers from various walks of life in Norwegian society were included. Healthy controls had no history of psychiatric disorders or ongoing psychiatric symptoms at the time of inclusion. They also had to have a clean criminal record and a regular job.
Two groups of healthy male volunteers were recruited. Group 1 had 21 subjects who underwent plasma sampling. Group 2 had 37 subjects. Group 2 was recruited to compare the results from the UPPS impulsive behavior scale with those of the inmates. The UPPS was not applied in the first control group (SI Appendix, Tables S1–S3).
As a separate control group we also included bodybuilders actively using PES at the time of examination and who had previously achieved scores on the Bryant and Smith’s 12-item refined version of the Buss–Perry Aggression Questionnaire (BS-rAQ) (21, 58) indicating increased physical aggression but not hostility and anger.
Another control group was comprised of prisoners in whom violence was not the main issue. These men were serving sentences of less than 9 mo for crimes such as car theft, fraud, and burglary. Two inmates, not native Norwegian speakers in this control group, had language problems; thus, the BS-rAQ scores for this group are incomplete.
Clinical Psychiatric Examinations.
The inmates and controls underwent clinical psychiatric screening interviews to exclude past and present serious psychiatric (e.g., psychotic or bipolar disorders) and somatic (e.g., serious head trauma and conditions of the nervous system) conditions. A majority of the inmates had a history of alcohol and drug abuse, and some reported that they had experienced related transient hallucinations. Some inmates described problems with adjustment to prison life as reflected in the Hospital Anxiety and Depression (HAD) scale scores.
Scales.
BS-rAQ.
The original aggression questionnaire (AQ) published by Buss and Perry (59) had four scales: Physical Aggression, Verbal Aggression, Anger, and Hostility, which correlated differently with various personality traits. The scale scores were found to correlate with peer nominations of the various kinds of aggression, suggesting the need to assess individual aggressiveness components. Bryant and Smith (58) later found that the four scales did not show adequate common variance (i.e., about 80%), and they consequently omitted items with low loadings or multiple loadings and excluded items with reverse-scored wording. This yielded a 12-item, refined four-factor measurement model which contains fewer than half as many items as the original and is also psychometrically superior. This refined 12-item aggression questionnaire was used in the present study to evaluate aggressiveness. Baseline data show significant differences between violent aggressors and controls on all BS-rAQ subscales except verbal aggression (SI Appendix, Table S2). Data from BS-rAQ scores for bodybuilders (21) showing their significantly increased physical aggressiveness compared with healthy controls (P = 0.002) have previously been reported. The violent inmates scored significantly higher for anger (P = 0.006) and verbal aggression (P = 0.006) than the bodybuilders but not for hostility and physical aggression (21).
HAD scale.
To screen for anxiety and depression, the HAD scale was used (SI Appendix, Table S1) (60). This scale is a reliable self-assessment scale developed to detect states of depression and anxiety in the setting of a hospital medical outpatient clinic. It contains an anxiety and a depression subscale, each consisting of seven items. In this study the cutoff point was ≥8.
Impulsivity.
Fifteen aggressive inmates and all group 2 nonaggressive controls completed the UPPS (SI Appendix, Table S3). The UPPS was originally designed to measure impulsivity across dimensions of the Five Factor Model of personality. It contains 45 items using a four-point Likert-type scale and has four subscales: Premeditation (lack of), Urgency, Sensation Seeking, and Perseverance (lack of) (61–63).
Blood Samples.
After the screening interview, venous blood samples were collected from a cubital vein of eligible subjects. Samples were collected in EDTA tubes and were stored on ice before centrifugation, after which plasma was drawn off, and the sample was stored at −80 °C until transported on dry ice and then thawed for ACTH and IgG analyses as described below. All inmates and bodybuilders and some healthy controls gave blood.
ACTH Autoantibody and Peptide Assays.
Plasma levels of ACTH-reactive IgG were measured using ELISA according to a published protocol (64). Briefly, human ACTH (Bachem AG) was coated onto 96-well MaxiSorp plates (Nunc) using 100 µL and a concentration of 2 µg/mL in 100 mM NaHCO3 buffer (pH 9.6) for 72 h at 4 °C. The plates were washed three times (5 min each washing) in PBS [10 mmol/L Tris (pH 8), 150 mM/L NaCl] with 0.05% Tween 200 (pH 7.4), and then were incubated overnight at 4 °C with 100 μL of human plasma diluted 1:400 in PBS to determine free autoantibody levels or in a dissociative 3 M NaCl, 1.5 M glycine buffer (pH 8.9) to determine total autoantibody levels. The plates were washed three times and were incubated with 100 μL of alkaline phosphatase (AP)-conjugated antibodies (1:2,000; Jackson ImmunoResearch Laboratories, Inc.). Following washing, 100 μL of p-nitrophenyl phosphate solution (Sigma) was added as the AP substrate. After 40 min of incubation at room temperature, the reaction was stopped by adding 3 M NaOH. The OD was determined at 405 nm using a Metertech 960 microplate reader (Metertech Inc.). Blank OD values resulting from the reading of plates without the addition of plasma samples were subtracted from the sample OD values. Each determination was done in duplicate. The variation between duplicate values was less than 5%. Plasma ACTH concentrations were measured using an ELISA kit according to the manufacturer’s instructions (Phoenix Pharmaceuticals).
ACTH Autoantibody Epitope Mapping.
To determine preferential binding epitopes of human IgG to ACTH, plasma samples from violent aggressors and controls were diluted 1:200 in PBS and preincubated with 10−6 M and 10−9 M of several ACTH peptide fragments (Bachem) overnight at 4 °C before the samples were added to 96-well MaxiSorp plates (Nunc) coated with human ACTH amino acids 1–39 (Bachem). ACTH peptide fragments included ACTH amino acids 1–13, ACTH amino acids 1–24, ACTH amino acids 11–24, and ACTH amino acids 34–39 (Bachem). The choice of the peptide fragments was based on their complementarity with the ACTH-binding sites on melanocortin receptors (Fig. 4).
IgG Purification from Plasma.
IgG purification from plasma was done by plasma acidification and separation of plasma globulins on a C-18 SEP column (Phoenix Pharmaceuticals) followed by IgG extraction using the Melon Gel Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions and a published protocol (65). Lyophilized IgG was reconstituted in the HBS-EP buffer (GE Healthcare).
Affinity Kinetics Assay.
The affinity kinetics of IgG for ACTH was determined by SPR using a BIAcore 1000 instrument (GE Healthcare). Human ACTH (Bachem) was diluted at 0.5 mg/mL in 10 mM sodium acetate buffer, pH 5.0 (GE Healthcare), and was covalently coupled on the sensor chips CM5 (GE Healthcare) using the amine coupling kit (GE Healthcare). All measures were performed on the same ACTH-coated chip. For the affinity kinetics analysis, a multicycle method was run with five serial dilutions of each IgG sample: 3,360, 1,680, 840, 420, and 210 nmol, including a duplicate of 840 nmol and a blank sample (HBS-EP buffer only). Each cycle included 2 min of analyte injection and 5 min of dissociation with a flow speed of 30 µL/min at 25 °C. Between injections of each sample, the binding surface was regenerated with 10 mM NaOH, resulting in the same baseline level of the sensorgram. The affinity kinetics data were analyzed using the BIAevaluation 4.1.1 program (GE Healthcare). Langmuir’s 1:1 model was used to fit the kinetics data, and the sample values were corrected by blank subtractions.
Human Adrenocortical Cell Culture and Cortisol Assay.
Adrenal glands were collected from a brain-dead renal transplant donor. The protocol for tissue collection and the experimental procedures were approved by the regional ethics committees and the French Agence Nationale de Biomédecine. Written consent from the donor’s relatives was also obtained. After dissection from adherent fat, the adrenals were immersed in DMEM supplemented with 1% antibiotic–antimycotic solution (Fisher Scientific) until cell dispersion. Tissue samples were stirred for 45 min at 37 °C in culture medium containing collagenase type IA (60 mg/mL; Sigma) and deoxyribonuclease I type IV (4 mg/mL; Sigma) in a 5% CO2, 95% air atmosphere. Dispersed adrenocortical cells were cultured at a density of 106 cells/mL at 37 °C in a 5% CO2, 95% air atmosphere with 100% relative humidity in culture medium (50% DMEM, 50% HamF12; Life Technologies, Inc.) supplemented with 1% insulin-transferrin-selenium solution (Thermo Fisher Scientific) and 5% FCS (Bio-Whittaker). Cell incubation experiments were conducted for 24 h after 2 d in culture. Cultured cells were incubated with fresh DMEM (basal cortisol secretion) with 10−10 M ACTH amino acids 1–24 (Sigma-Tau Pharmaceuticals) or with DMEM containing IgG purified from the plasma of violent aggressors or controls (500 ng/mL) in the presence or absence of ACTH amino acids 1–24. Then, aliquots of cell-culture supernatants were immediately frozen at −20 °C. Cortisol concentrations in medium were measured using an RIA procedure. Cross-reactivity of cortisol antibodies (Sigma) with corticosterone was less than 0.01%.
Resident–Intruder Test.
Two-month-old BALB/c male mice were purchased from Janvier Labs and acclimated to the animal facility for 1 wk with a 12-h light/dark cycle with lights on at 7:00 AM. Animal experiments were performed in accordance with the French and European Directives and the recommendation for care of laboratory animals (2007/526/EC). Mice were fed ad libitum with standard pelleted rodent chow (RM1 diet; SDS) with drinking water always available. The resident–intruder test for evaluation of aggression was performed by introducing an intruder mouse into the home cage of the resident mouse (66). Resident mice (n = 32) were housed in isolation for 21 d without bedding change before testing. Intruder mice (n = 8) were housed as a group of four mice per cage for 21 d. Resident mice were distributed into four groups (n = 8 per group) and 30 min before the test received an i.p. injection of 0.1 mL of 0.9% NaCl (control) or the same volume of 0.9% NaCl with ACTH (1.0 µg) or ACTH (1.0 µg) and IgG (100 µg) pooled from the IgG of individual control subjects or ACTH (1.0 µg) and IgG (100 µg) pooled from the IgG of individual violent aggressors. The aggressive behavior of the resident mouse was assessed by measuring the latency before the first attack, the total number of bite attacks, and the duration of attacks during 10 min. The latency before the first nonaggressive contact was also recorded.
Immunohistochemical Screening for Autoantibodies.
The experiments were performed on male Wistar rats and guinea pigs as described previously (67). All procedures used in animals were approved by the local Ethical Committee (Stockholms norra djurförsöksetiska nämnd; N100-101/14). Some rats received intracerebroventricular injections of colchicine and were killed after 24 h. All animals were deeply anesthetized and were perfused via the ascending aorta with a picric acid–formalin solution. After removal, the brains were cryoprotected, snap-frozen, and coronally sectioned at 20-μm thickness in a cryostat. Sections were incubated with human sera (1:1,000) and with antibodies against ACTH (1:10,000; T-5024; Peninsula Laboratories), vasopressin (1:5,000; T5048; Peninsula Laboratories), and oxytocin (1:5,000; MAB5296; Millipore) and were visualized using a commercial kit based on tyramide signal amplification (TSA) (PerkinElmer Life Science). Sections were examined using a Nikon Eclipse E600 fluorescence microscope. Digital images from the microscopy were slightly modified to optimize for brightness and contrast. For immunohistochemical absorption control experiments, diluted sera (1:1,000) were incubated overnight at 4 °C with 10−5 M–10−4 M peptides (vasopressin, 065-09; Phoenix Pharmaceuticals; or oxytocin, 051-01; Phoenix Pharmaceuticals), and these mixtures were applied as primary antibodies in the subsequent immunostainings.
Statistical Analysis.
Data were analyzed and graphs were plotted using GraphPad Prism 5.02 software (GraphPad Software Inc.). Normal distribution was evaluated by the Kolmogorov–Smirnov test. Group differences were analyzed by ANOVA or the nonparametric Kruskal–Wallis test with Tukey’s or Dunn’s posttests, depending on the normal distribution results. Where appropriate, individual groups were compared using the Student’s t test or the Mann–Whitney test, depending on the normal distribution results. Adsorption results were analyzed using the paired t test. Correlations were analyzed using the Pearson’s and Spearman’s tests.
Supplementary Material
Acknowledgments
We thank the staff at Ila high security prison and Kongsvinger prison for valuable assistance; bioengineers Mrs. Elin Hareton and Mrs. Homa Riga for help at the study sites; Dr. Anna Frengen for good advice, as always; Mrs. Solveig Lundsvoll for help with practical issues; and Mr. Alistair Reaves for help with language. H.V. received financial support from the Alvhilde Eliassens Research Foundation.
Footnotes
Conflict of interest statement: P.D. has received research grants from Nestlé and Fresenius Kabi and honoraria for speeches and consulting from Nestlé, Fresenius-Kabi, and Aguettant, is a cofounder of TargEDys SA, and is a member of its advisory board. S.O.F. is a cofounder of and serves as a consultant to TargEDys SA. N.L. and R.L. are currently employees of TargEDys SA. T.H. owns shares in AstraZeneca.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720008115/-/DCSupplemental.
References
- 1.de Boer SF, Olivier B, Veening J, Koolhaas JM. The neurobiology of offensive aggression: Revealing a modular view. Physiol Behav. 2015;146:111–127. doi: 10.1016/j.physbeh.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 2.Haller J, Kruk MR. Normal and abnormal aggression: Human disorders and novel laboratory models. Neurosci Biobehav Rev. 2006;30:292–303. doi: 10.1016/j.neubiorev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 4.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 5.Vedhara K, et al. An investigation into the relationship between salivary cortisol, stress, anxiety and depression. Biol Psychol. 2003;62:89–96. doi: 10.1016/s0301-0511(02)00128-x. [DOI] [PubMed] [Google Scholar]
- 6.van Honk J, Peper JS, Schutter DJLG. Testosterone reduces unconscious fear but not consciously experienced anxiety: Implications for the disorders of fear and anxiety. Biol Psychiatry. 2005;58:218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Haller J, Mikics E, Halász J, Tóth M. Mechanisms differentiating normal from abnormal aggression: Glucocorticoids and serotonin. Eur J Pharmacol. 2005;526:89–100. doi: 10.1016/j.ejphar.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 8.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Gronek P, Wieliński D, Gronek J. Genetic and non-genetic determinants of aggression in combat sports. Open Life Sci. 2015;10 [Google Scholar]
- 10.Sohrabi S. The criminal gene: The link between MAOA and aggression. BMC Proc. 2015;9(Suppl 1):A49. [Google Scholar]
- 11.de Almeida RMM, Cabral JCC, Narvaes R. Behavioural, hormonal and neurobiological mechanisms of aggressive behaviour in human and nonhuman primates. Physiol Behav. 2015;143:121–135. doi: 10.1016/j.physbeh.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 12.Summers CH, Winberg S. Interactions between the neural regulation of stress and aggression. J Exp Biol. 2006;209:4581–4589. doi: 10.1242/jeb.02565. [DOI] [PubMed] [Google Scholar]
- 13.Wrangham RW. Two types of aggression in human evolution. Proc Natl Acad Sci USA. 2018;115:245–253. doi: 10.1073/pnas.1713611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fetissov SO, et al. Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci USA. 2005;102:14865–14870. doi: 10.1073/pnas.0507204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fetissov SO, et al. Aggressive behavior linked to corticotropin-reactive autoantibodies. Biol Psychiatry. 2006;60:799–802. doi: 10.1016/j.biopsych.2006.03.081. [DOI] [PubMed] [Google Scholar]
- 16.Fetissov SO, et al. Autoantibodies against appetite-regulating peptide hormones and neuropeptides: Putative modulation by gut microflora. Nutrition. 2008;24:348–359. doi: 10.1016/j.nut.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karaiskos D, et al. Psychopathological and personality features in primary Sjogren’s syndrome–Associations with autoantibodies to neuropeptides. Rheumatology (Oxford) 2010;49:1762–1769. doi: 10.1093/rheumatology/keq158. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer JM, et al. Corticotropin (ACTH)-reactive immunoglobulins in adolescents in relation to antisocial behavior and stress-induced cortisol response. The TRAILS study. Psychoneuroendocrinology. 2013;38:3039–3047. doi: 10.1016/j.psyneuen.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Takagi K, et al. Anti-ghrelin immunoglobulins modulate ghrelin stability and its orexigenic effect in obese mice and humans. Nat Commun. 2013;4:2685. doi: 10.1038/ncomms3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fetissov SO, Lucas N, Legrand R. Ghrelin-reactive immunoglobulins in conditions of altered appetite and energy balance. Front Endocrinol (Lausanne) 2017;8:10. doi: 10.3389/fendo.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaeroy H. Aggression questionnaire scores in extremely violent male prisoners, male bodybuilders, and healthy non-violent men. Open J Psychiatr. 2013;3:293–300. [Google Scholar]
- 22.Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19:175–180. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 24.Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 25.Poulin F, Boivin M. Reactive and proactive aggression: Evidence of a two-factor model. Psychol Assess. 2000;12:115–122. doi: 10.1037//1040-3590.12.2.115. [DOI] [PubMed] [Google Scholar]
- 26.Siegel A, Victoroff J. Understanding human aggression: New insights from neuroscience. Int J Law Psychiatry. 2009;32:209–215. doi: 10.1016/j.ijlp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Cima M, Smeets T, Jelicic M. Self-reported trauma, cortisol levels, and aggression in psychopathic and non-psychopathic prison inmates. Biol Psychol. 2008;78:75–86. doi: 10.1016/j.biopsycho.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Cavigelli SA, Dubovick T, Levash W, Jolly A, Pitts A. Female dominance status and fecal corticoids in a cooperative breeder with low reproductive skew: Ring-tailed lemurs (Lemur catta) Horm Behav. 2003;43:166–179. doi: 10.1016/s0018-506x(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 29.McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Arch Gen Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- 30.O’Leary MM, Taylor J, Eckel L. Psychopathic personality traits and cortisol response to stress: The role of sex, type of stressor, and menstrual phase. Horm Behav. 2010;58:250–256. doi: 10.1016/j.yhbeh.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Horn M, et al. Male inmate profiles and their biological correlates. Can J Psychiatry. 2014;59:441–449. doi: 10.1177/070674371405900807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise RA. Dual roles of dopamine in food and drug seeking: The drive-reward paradox. Biol Psychiatry. 2013;73:819–826. doi: 10.1016/j.biopsych.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckholtz JW, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barik J, et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- 35.Schoffelmeer AN, et al. Intermittent morphine administration induces a long-lasting synergistic effect of corticosterone on dopamine D1 receptor functioning in rat striatal GABA neurons. Synapse. 1997;25:381–388. doi: 10.1002/(SICI)1098-2396(199704)25:4<381::AID-SYN9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Kruk MR, Halász J, Meelis W, Haller J. Fast positive feedback between the adrenocortical stress response and a brain mechanism involved in aggressive behavior. Behav Neurosci. 2004;118:1062–1070. doi: 10.1037/0735-7044.118.5.1062. [DOI] [PubMed] [Google Scholar]
- 37.Gallo-Payet N, Payet MD. Mechanism of action of ACTH: Beyond cAMP. Microsc Res Tech. 2003;61:275–287. doi: 10.1002/jemt.10337. [DOI] [PubMed] [Google Scholar]
- 38.Kovalitskaia IuA, et al. [Synthetic peptide KKRR corresponding to the human ACTH fragment 15-18 is an antagonist of the ACTH receptor] Bioorg Khim. 2008;34:29–35. Russian. [PubMed] [Google Scholar]
- 39.Fridmanis D, et al. Identification of domains responsible for specific membrane transport and ligand specificity of the ACTH receptor (MC2R) Mol Cell Endocrinol. 2010;321:175–183. doi: 10.1016/j.mce.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 40.Holder JR, Haskell-Luevano C. Melanocortin ligands: 30 years of structure-activity relationship (SAR) studies. Med Res Rev. 2004;24:325–356. doi: 10.1002/med.10064. [DOI] [PubMed] [Google Scholar]
- 41.Brain PF, Nowell NW, Wouters A. Some relationships between adrenal function and the effectiveness of a period of isolation in inducing intermale aggression in albino mice. Physiol Behav. 1971;6:27–29. doi: 10.1016/0031-9384(71)90008-4. [DOI] [PubMed] [Google Scholar]
- 42.Plotnikoff NP, Kastin AJ. Neuropharmacological tests with alpha-melanocyte stimulating hormone. Life Sci. 1976;18:1217–1222. doi: 10.1016/0024-3205(76)90197-1. [DOI] [PubMed] [Google Scholar]
- 43.Paterson AT, Vickers C. Stimulation of aggression in male mice by alpha-MSH and its relation to light phase and to saline intake effects. Behav Brain Res. 1985;15:183–189. doi: 10.1016/0166-4328(85)90173-1. [DOI] [PubMed] [Google Scholar]
- 44.Kruk MR, et al. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain Res. 1983;260:61–79. doi: 10.1016/0006-8993(83)90764-3. [DOI] [PubMed] [Google Scholar]
- 45.Lin D, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosa M, et al. Hypothalamic oscillations in human pathological aggressiveness. Biol Psychiatry. 2012;72:e33–e35. doi: 10.1016/j.biopsych.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- 48.Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature. 1983;305:325–327. doi: 10.1038/305325a0. [DOI] [PubMed] [Google Scholar]
- 49.Steinman MQ, et al. Hypothalamic vasopressin systems are more sensitive to the long term effects of social defeat in males versus females. Psychoneuroendocrinology. 2015;51:122–134. doi: 10.1016/j.psyneuen.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veenema AH, Neumann ID, Inga DN, Rainer L. Progress in Brain Research. Vol 170. Elsevier; Amsterdam: 2008. Central vasopressin and oxytocin release: Regulation of complex social behaviours; pp. 261–276. [DOI] [PubMed] [Google Scholar]
- 51.Bensing S, Kasperlik-Zaluska AA, Czarnocka B, Crock PA, Hulting A. Autoantibodies against pituitary proteins in patients with adrenocorticotropin-deficiency. Eur J Clin Invest. 2005;35:126–132. doi: 10.1111/j.1365-2362.2005.01459.x. [DOI] [PubMed] [Google Scholar]
- 52.Ebner K, Wotjak CT, Landgraf R, Engelmann M. Neuroendocrine and behavioral response to social confrontation: Residents versus intruders, active versus passive coping styles. Horm Behav. 2005;47:14–21. doi: 10.1016/j.yhbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Iseme RA, et al. Autoantibodies and depression: Evidence for a causal link? Neurosci Biobehav Rev. 2014;40:62–79. doi: 10.1016/j.neubiorev.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Lecerf M, Jarossay A, Kaveri SV, Lacroix-Desmazes S, Dimitrov JD. Methods for posttranslational induction of polyreactivity of antibodies. In: Kaveri SV, Bayry J, editors. Natural Antibodies: Methods and Protocols. Springer; New York: 2017. pp. 135–145. [DOI] [PubMed] [Google Scholar]
- 55.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25:174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Tennoune N, et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl Psychiatry. 2014;4:e458. doi: 10.1038/tp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tennoune N, et al. Sex-related effects of nutritional supplementation of Escherichia coli: Relevance to eating disorders. Nutrition. 2015;31:498–507. doi: 10.1016/j.nut.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Bryant FB, Smith BD. Refining the architecture of aggression: A measurement model for the Buss–Perry aggression questionnaire. J Res Pers. 2001;35:138–167. [Google Scholar]
- 59.Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 60.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 61.Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30:669–689. [Google Scholar]
- 62.Miller JD, Zeichner A, Wilson LF. Personality correlates of aggression: Evidence from measures of the five-factor model, UPPS model of impulsivity, and BIS/BAS. J Interpers Violence. 2012;27:2903–2919. doi: 10.1177/0886260512438279. [DOI] [PubMed] [Google Scholar]
- 63.Derefinko KJ, et al. Relations between trait impulsivity, behavioral impulsivity, physiological arousal, and risky sexual behavior among young men. Arch Sex Behav. 2014;43:1149–1158. doi: 10.1007/s10508-014-0327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fetissov SO. Neuropeptide autoantibodies assay. Methods Mol Biol. 2011;789:295–302. doi: 10.1007/978-1-61779-310-3_19. [DOI] [PubMed] [Google Scholar]
- 65.Legrand R, Takagi K, Fetissov SO. Immunoglobulin G preparation from plasma samples and analysis of its affinity kinetic binding to peptide hormones. Protoc Exch. 2014 doi: 10.1038/protex.2014.004. [DOI] [Google Scholar]
- 66.Koolhaas JM, et al. The resident-intruder paradigm: A standardized test for aggression, violence and social stress. J Vis Exp. 2013:e4367. doi: 10.3791/4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergman P, et al. Narcolepsy patients have antibodies that stain distinct cell populations in rat brain and influence sleep patterns. Proc Natl Acad Sci USA. 2014;111:E3735–E3744. doi: 10.1073/pnas.1412189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.