Significance
We provide evidence that our intentions can be fragmented in various components subserved by partially dissociable neural circuits. We found that the decision of what to do, when to do it, and whether to do it depends on separable systems that go beyond the mesial prefrontal wall of previous proposals, involving cortical and subcortical brain regions in a component-specific manner. In addition, we found that deciding whether to act or not requires strong interhemispheric interactions of the frontal lobes. This explicit evidence of dissociable neural foundations of intentional actions will guide the exploration of brain disorders of specific components of intentionality.
Keywords: intentional action, motor control, fMRI, DTI, www-model
Abstract
Here we challenge and present evidence that expands the what, when, and whether anatomical model of intentional action, which states that internally driven decisions about the content and timing of our actions and about whether to act at all depend on separable neural systems, anatomically segregated along the medial wall of the frontal lobe. In our fMRI event-related paradigm, subjects acted following conditional cues or following their intentions. The content of the actions, their timing, or their very occurrence were the variables investigated, together with the modulating factor of intentionality. Besides a shared activation of the pre-supplementary motor area (pre-SMA) and anterior cingulate cortex (ACC) for all components and the SMA proper for the when component, we found specific activations beyond the mesial prefrontal wall involving the parietal cortex for the what component or subcortical gray structures for the when component. Moreover, we found behavioral, functional, anatomical, and brain connectivity evidence that the self-driven decisions on whether to act require a higher interhemispheric cooperation: This was indexed by a specific activation of the corpus callosum whereby the less the callosal activation, the greater was the decision cost at the time of the action in the whether trials. Furthermore, tractography confirmed that the fibers passing through the callosal focus of activation connect the two sides of the frontal lobes involved in intentional trials. This is evidence of non-unitary neural foundations for the processes involved in intentional actions with the pre-SMA/ACC operating as an intentional hub. These findings may guide the exploration of specific instances of disturbed intentionality.
Every day we face the need to make conscious decisions on intentional acts: We may see two highly desirable foods, e.g., ice cream or a cake of our liking, and we might have to decide which one to eat and when would be the right time to do so. We may eventually decide to impose a veto on our desires and have neither for a while because of worries about our body weight. This everyday life situation portrays the several possible phases behind purposeful, goal-directed behaviors (1).
The question that we address here is whether their intentional component is under a unitary or a multidimensional controller or indeed multiple controllers. Even when seen from the reductionist perspective of cognitive neuroscience and its minimalistic paradigms (such as the one adopted here), this remains a question in search of an answer, while fairly articulated models of motor control (2) remain underspecified in this respect. Other models do not postulate an orthogonal organization of these components of self-generated behavior (3).
In an early attempt, Jahanshahi and Frith (1) discussed that, in principle, intentional actions could be characterized for three different aspects: their content (the what component), their timing (the when component), or their possibility of being executed (the whether component). In reviewing the then-available imaging literature, they concluded that there was not sufficient evidence that such components are represented in discrete brain circuits.
Ten years later, Brass and Haggard (4) reevaluated the same concepts to propose the so-called “What, When and Whether” model (henceforth, the “www-model”) of intentional actions. Contrary to Jahanshahi and Frith (1), and taking advantage of a larger set of functional anatomical datasets published to that date, they claimed that the model is also justified on anatomical grounds. In particular, they proposed a segregation of different components of intention for the medial wall of the frontal lobe (see SI Appendix for a full list of imaging articles on intention). However, this proposal was not based on a formal anatomical assessment of the data: As illustrated in SI Appendix, Fig. S1, their segregated distribution can hardly be assessed by mere exploration.
To overcome this limitation, we recently published a quantitative meta-analysis which, by its nature, avoids a comparison of different results based only on nominal descriptions of the regions involved [e.g., the supplementary motor area (SMA), pre-SMA, and so forth]; rather, it uses triplets of stereotactic coordinates to assess their anatomical consistency and their assignations to functional dimensions (5). We first used hierarchical clustering (HC) on intention-specific experiments and tested the hypothesis that the anatomical segregations identified using HC were replicable in a coactivation clustering analysis made on the www.brainmap.org database. In short, the meta-analysis replicated the overall proposal of the www-model about the medial wall of the frontal lobe whereby the most rostral part is associated with the whether component, the intermediate region with the what component, and the SMA proper with the when component. Yet, we found that the dissociations related to intentionality also involve brain regions outside the frontal lobe in a component-specific way: the supramarginal gyrus for the what component, the pallidum and the thalamus for the when component, and the putamen and the insula for the whether component.
However, the analyses of the BrainMap.org data also showed that similar segregations were generated by paradigms in which subjects acted in response to conditional stimuli rather than while following their own intentions, suggesting that perhaps “intentionality manifests itself in discrete components through the boosting of general purpose action-related regions specialized for different aspects of action selection and inhibition” (5).
This is not the only problem of the www-model as it stands. In several of the relevant experiments, the when and whether components were studied using a metacognitive paradigm, the Libet task (6). In this paradigm, subjects make voluntary finger movements while watching a dot moving around a clock face; they then have to report the time (i.e., the position of the dot) when they have felt their “intention to move.” This paradigm has been criticized (7) due to the detection of bias in the perception of the timing of actions or of the intention to act. (The Libet’s paradigm, when used to study the whether component, is problematic because the timing of action inhibition remains ambiguous as the inhibition timings used to fit the hemodynamic response on fMRI data are based on guessed estimates.)
Also, there are only a few replications for the when and the whether components of the www-model (SI Appendix, Table S1). More importantly, in none of those studies were the three components evaluated in the same subjects with a unitary procedure, a potential source of diverging results not necessarily representing genuine intention-specific dissociations.
Although our meta-analysis makes the anatomical assignations less prone to nominal confusions, it cannot be considered conclusive, either. All the suggestions about specialized neural responses were based only on the concept of regional segregation of the activation foci; these, however, did not come from statistical effects describing the comparative magnitude of the response for the different intentional components. In other words, the regional effects submitted to meta-analysis did not come from experiments in which, for example, the what component was compared with the whether and when components.
Aims of the Study
The aim of our study was to assess the functional anatomical foundations of the www-model of intentionality, overcoming the methodological limitations of previous attempts. To this end, we adopted an event-related fMRI experimental design in which the subcomponents of intentionality postulated by current models were assessed with a uniform procedure in the same group of subjects. The advantages of a uniform procedure are obvious: Significant differences between the intentional tasks in the different conditions should not be confounded by factors such as different populations, tasks, or scanning protocols.
One aspect of our design deserves an immediate comment: Contrary to intentional inhibition paradigms (8, 9), our design implied a minimal difference between the three components at the stage of the decisions, the hypothesis tested being that different intentional stances, focused on what to do or on when to act or on whether to act at all, would have been sufficient to reveal specific functional anatomical patterns. Furthermore, subjects were instructed to actively keep their intentions into motor working memory to maximize the chances that what could have been similar and transient nominal decisions (right/left; early/late; yes/no) would rather translate into a sustained specific blood oxygenation level-dependent (BOLD) response pattern associated with the specific aspects of intentionality under investigation.
Results
Behavioral Results.
Choices.
Subjects balanced the number of intentional responses between the two alternatives in all three tasks (average balance index: what condition = 0.15; when condition = 0.16; whether condition = 0.17).
Moreover, the distribution of sequences in each task of the trials in which the subjects decided to press the same button before switching response reflected an exponential distribution (fitted with the dashed line in the SI Appendix, Fig. S3A), suggesting that subjects responded randomly from trial to trial (10).
Reaction times.
The paired comparisons showed a significant difference between reaction times (RTs) recorded during stimulus-driven and intention-driven conditions only for the whether component (W = 115; Z = −0.49; P = 0.03), with RTs significantly longer for intention driven trials. For the other two components, the RTs were not significantly different (NS): what component: W = 173, Z = −0.27, P = 0.09, NS; when component: W = 246, Z = 0.33, P = 0.18, NS (SI Appendix, Fig. S3B).
fMRI Results.
Decision phase.
Main effects: Intention-driven actions and stimulus-driven actions.
The comparison between trials in which the decision was triggered by subjects’ own intentions and trials in which the decision was dependent on an external stimulus showed a specific activation of a large fronto-mesial region involving the pre-SMA and the anterior cingulate cortex (ACC). Further foci were seen in the middle frontal gyrus and the cerebellum bilaterally, in the right inferior parietal lobule (IPL), in the left inferior frontal gyrus, the middle temporal gyrus, and the insula (SI Appendix, Table S2) and on the midline at the junction between the pons and the brainstem just in front of the fourth ventricle.
The opposite comparison (stimulus-driven decisions > intention-driven decisions) was associated with significant activity in occipital regions at the level of the left superior occipital gyrus, the left cuneus, the right lingual gyrus, and the right calcarine fissure (SI Appendix, Table S2).
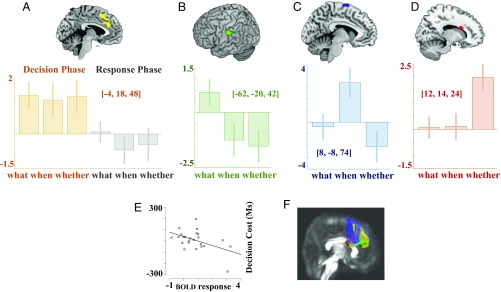
A conjunction analysis (intermediate) of these effects confirmed that the maximal shared activation was in the pre-SMA: x = −4; y = +18; z = 48; z score = 5.3; P < 0.05 familywise error rate (FWER) corrected. A further FWER-corrected effect was in the ACC: x = −6; y = +32; z = 28; z score = 5.2 (Fig. 1A).
Fig. 1.
fMRI results. Decision phase: fMRI activations recorded during trials in which the decision was triggered by subjects’ own intentions. (A) fMRI activations shared by the three components (conjunction analysis). (B) What component (what component > when and whether components). (C) When component (when component > what and whether components). (D) Whether component (whether component > what and when components). The y axes of the histograms display the contrast estimates and 90% CIs. The direct comparison of each condition with another single condition reached at least a P = 0.00017 uncorrected significance down to P = 0.0000011 (see SI Appendix, Table S3). (E) Negative correlation between the fMRI BOLD response signal in the corpus callosum and the decision cost for the whether condition. (F) DTI analysis. The DTI data show that the tracts passing through the callosal activation specific for the whether component connect the two sides of the region identified by the main effect of intentional action (depicted in yellow). The bulk of the left-to-right fibers (in red) are in the corpus callosum; antero–posterior directions are shown in green; fibers going from inferior to superior are shown in blue. See also SI Appendix, Fig. S7.
What component > when and whether component.
The intentional decision about the content of an action was associated with additional specific activations of the left supramarginal gyrus (Fig. 1B and Table 1). A trend for a similar activation was present in a mirror focus of the supramarginal gyrus in the right hemisphere (x = 60; y = −26; z = 42; z score = 3.75; P = 0.0002 uncorrected).
Table 1.
Component-specific fMRI activations
| Brain region | x | y | z | z score |
| What > when and whether | ||||
| Supramarginal gyrus L | −62 | −20 | 42 | 4.66*,† |
| When > what and whether | ||||
| SMA L | −4 | 4 | 70 | 3.47† |
| SMA L | −10 | −10 | 70 | 3.26† |
| SMA L | 8 | −8 | 74 | 4.44† |
| Inferior frontal gyrus pars triangularis L | −36 | 14 | 26 | 4.28† |
| Inf. frontal gyrus pars opercularis L | −44 | 10 | 14 | 3.70† |
| Insula L | −32 | 14 | 16 | 3.66† |
| Putamen/thalamus L | −28 | −16 | 16 | 4.13† |
| Putamen L | −32 | −4 | 6 | 3.38† |
| Whether > what and when | ||||
| Corpus callosum R | 12 | 14 | 24 | 4.91* |
Stereotactic coordinates are in MNI space. L, left; R, right.
FWER 0.05 correction (voxel level).
FWER 0.05 correction (cluster level).
When component > what and whether component.
The intentional decision about the timing of a movement was associated with the additional activation of a network of both cortical and subcortical structures, such as the SMA proper, the thalamus, the putamen, and the insula (Fig. 1C and Table 1). The when-specific activation of the SMA was more dorsal and posterior to the signal of the conjunction effect, which was, in fact, in the pre-SMA.
Whether component > what and when component.
On average, the intentional decision about whether to produce or inhibit an action was associated with a highly significant activation of the corpus callosum in its body segment (Fig. 1D and Table 1).
The signal in the corpus callosum was negatively correlated with the decision cost (the difference in RTs between the intentional and the stimulus-driven responses subtracted from the average of the same measure for the what and whether conditions), whereby the higher the signal, the lower was the behavioral cost of deciding whether or not to act (Spearman’ r = −0.49; P = 0.018). This suggests that large traffic across the corpus callosum is crucial to efficiently resolve the decision about whether to act (Fig. 1E).
No-go versus go trials in the whether condition.
The search for the fMRI equivalents of veto decisions, i.e, making and holding the decision not to act, was not successful: We only observed trends for a significantly stronger activity in the ventral prefrontal and orbitofrontal cortex (P < 0.001 or less, uncorrected).
Brain connectivity analyses.
Prompted by the result showing a highly significant effect for the whether condition in the corpus callosum, we performed a diffusion tensor imaging (DTI) tractography study of the callosal fibers passing through the area of maximal fMRI signal change to test the hypothesis that this connects the brain regions involved in intentional action.
The results of the DTI analyses are self-evident and are presented in Fig. 1F. It can be seen that the tracts passing through the callosal activation specific for the whether component connect the two sides of the region identified by the fMRI main effect of intentional action. A detailed topographic analysis of the intersection between the fibers identified with DTI and the fMRI patterns and a functional connectivity analysis on resting-state fMRI data confirm this statement (SI Appendix, Fig. S7 and Table S2).
Response phase.
The explicit production of a movement after a decision triggered by a subject’s own intention activated regions located mainly in the right hemisphere at the level of the inferior frontal gyrus (opercular portion; see SI Appendix, Fig. S6 and Table S2).
In contrast, the execution of an action triggered by an external stimulus resulted in specific activations of the posterior portion of the cingulum and the bilateral hippocampus (SI Appendix, Table S2). On the other hand, there were no component-specific effects (e.g., what > when and whether) at the response phase.
Discussion
None of the experiments previously undertaken in support of the www-model had explored all three components with the same procedure in the same sample of subjects, an approach that we argue is highly desirable to minimize the chance that claims in favor of a multicomponent model are based on group/paradigm-driven differences rather than on specific differences in processing demands.
Our results, unbiased by the aforementioned confounds, reveal a partial neurofunctional dissociation between the three different components of the www-model; however, they also expand Brass and Haggard’s model (4) and our previous meta-analysis in a substantial way: (i) they show that the three components converge in the pre-SMA/ACC; (ii) the dissociable components also depend on brain regions outside the territory of the medial prefrontal wall; and (iii) they show that such differences exist not only in spatial distribution but in component-specific neuronal firing boosted by intentionality.
Internally and Externally Driven Actions: General.
Decision phase.
The idea that externally and internally driven actions depend on separable neural circuits is now at least 30 y old with the proposal that two distinct premotor neural networks, a medial system (involving the SMA, the pre-SMA, and the ACC) and a lateral system (e.g., the lateral premotor cortex) (11, 12) are specifically associated with internally and externally driven behaviors, respectively (for a recent review, see ref. 13). In its extreme formulation, the model was not confirmed by the earliest imaging studies, which instead pointed to a role of the dorsolateral prefrontal cortex in willed actions (14–16).
Our data confirm the existence of different brain networks associated with externally and internally driven actions: Internally driven decisions were associated with greater activation of a large neural system involving premotor and prefrontal areas, such as the pre-SMA, the SMA, the ACC, and the most anterior parts of the superior and inferior frontal gyri. However, regions outside the frontal lobe were also involved. Among these, it worth noting the highly significant activation of the insula, a structure strongly involved in interoception and body awareness (17).
Response phase.
Brain activity at the response phase for the intentional trials, compared with the externally driven trials, showed a pattern strikingly different from the one seen at the decision stage, with significant activations in the lateral ventral premotor cortex. One possible explanation for this differential pattern might be that activity of lateral premotor cortex represents a later stage of the motor outflow process when an already internally decided motor act is kept in active motor working memory.
Decision phase versus response phase.
A comparison of the decision and implementation stages confirmed the specificity of the aforementioned general observations. The medial wall activations and the insular activations were again highly significant compared with the implementation-phase patterns, similar to the lateral right premotor activity in the reversed comparison.
Such distinctions could not be drawn in earlier PET studies (e.g., ref. 15) when decision and response generation were lumped together in regional cerebral blood flow data collection and analyses.
The What, the When, and the Whether of Intentional Actions.
Our analyses of the different intentional conditions revealed that the pre-SMA and ACC are activated in the three components at the decision stage, confirming their general core role in intentional actions, in line with most of the literature (18).
However, we also found some interesting significant differences between the investigated components, which were characterized by means of specific interaction effects. The following discussion focuses on the comparative magnitude of the activation of the condition-specific comparisons. Yet, when looking at these comparative differences, it should be borne in mind that there is a shared activation of the pre-SMA/ACC. Therefore, each specific pattern discussed below should be seen as an addition to the shared pattern. Also, it is worth noting that all these differences were seen at the decision stage.
What should I do?
The intentional decision of what to do, in our case the free selection of moving the index or middle finger of the right hand, recruited the left supramarginal gyrus (a substantial trend, P = 0.0002, was seen in the right mirror supramarginal gyrus); the IPL, particularly the angular gyrus, is a critical node for the representation of actions and intentions to act (19). Gallivan et al. (20) showed that intentions for specific movements could be predicted by the spatial activity patterns in these areas. Moreover, direct electrical stimulation of the right IPL evoked a strong intention to move a body part and, with increased stimulation intensity, an illusory sense of movement (21). However, the area associated with the intentional what component was located more anteriorly, in the supramarginal gyrus, a region whose equivalent in monkeys, area 7b, is somatotopically organized (reviewed in ref. 22). Thus, in the context of intentional action, the supramarginal gyrus may inform motor intention and awareness of a somatotopic template of specific body parts.
When should I do it?
Studies investigating internally timed actions usually compare a condition in which subjects press a button at a moment of their own choice with a condition in which subjects are instructed to press the button by a visual or acoustic cue (23). In our results, there was a specific activation of the SMA proper, dorsally to the shared medial wall system; the SMA has been previously linked to the timing or to the intentional initiation of a movement (24). Supporting the association between the SMA and the timing of intentional action is evidence from Parkinson disease (PD). PD is characterized by difficulties in implementing intentional behaviors, but this impairment is reduced in the presence of an external salient cue, such as a fire (for examples, see ref. 1); for this reason, it has been widely hypothesized that PD patients have a malfunctioning of the internal timing of action (4). In PD patients the SMA has abnormal connectivity with the thalamus (another region specifically associated with the when component in our study), particularly during the off-medication phase (25).
Should I do it at all?
The whether component of intention is by far the most cognitively demanding, as it implies aspects of both free will and free veto. It is the aspect of intentionality that, if dysfunctional, manifests with higher-order behavioral deficits such as the pathological disinhibition typical of frontal lobe dementias (26).
Overall, in comparison with the what and the when conditions, the whether task at the decision stage was mainly associated with increased activity in the corpus callosum, a thought-provoking finding. Of course, activation of the corpus callosum carries one and only one meaning: augmented interhemispheric traffic. As regards as the genuineness of the signal, the fact that activation of the corpus callosum can be measured in specific paradigms is beyond question, as it has been documented by several fMRI studies (e.g., refs. 27–29) in which subjects performed tasks implying interhemispheric transmission of information (reviewed in ref. 30). In our case, the signal survived strict volumewise statistical corrections for multiple comparisons. Furthermore, the analysis of DTI data and of resting-state fMRI data documents that the particular region of the callosum identified connects the intention-specific regions of both sides of the medial wall of the frontal lobe. Hence, its involvement in our paradigm should have a biological meaning. This conclusion is supported by two lines of evidence. (i) In our data, the higher the callosal activity in the whether condition at the decision stage, the lower were the temporal costs in our subjects for the implementation of their decisions, as if two separate hemispheric deciders were reconciled at the decision stage of the task or as if a bilaterally distributed and interacting cell assembly were more efficiently engaged in the task, thanks to high callosal connectivity, to generate more efficient behavior (31). (ii) Patients with damage to the body of the corpus callosum without direct cortical involvement can display uncontrolled motor behavior only during intentional actions and not in response to the experimenter’s instructions (reviewed in ref. 32). The callosal variant of the anarchic hand syndrome may also be characterized by symptoms of competition by the two halves of the body: In diagonistic dyspraxia (33), the two hands behave as if two competing wills are in action. However, the anarchic hand syndrome is often revealed in bimanual behaviors. In our experiment the behavior under examination was unimanual; this, combined with the callosal activation, suggests that a mechanism of interhemispheric inhibition seems to regulate decisions to act/withhold action, even in unimanual behavior.
Finally, the comparison of the intentional no-go trials with the go trials, always at the decision stage, did not reveal a corrected significant difference among conditions. However, there were substantial trends for a stronger activation in the ventral orbital prefrontal cortex for the no-go trials, a finding that is consistent with neuropsychological cases of disordered behavioral control (34).
Comparison with Our Previous Meta-Analysis on the www-Model.
The present findings expand the results of our previous meta-analyses (5). In a nutshell, those analyses suggested a www-anatomical segregation along the medial wall of the frontal lobe, but when tested on the large BrainMap.org database, they showed that the aforementioned segregations were generated by paradigms in which subjects acted in response to conditional stimuli rather than while driven by their own intentions. We suggested that the model was still tenable, providing that one assumed that intentionality manifests itself in discrete components through the specific boosting of general-purpose action-related regions specialized for different aspects of action selection and inhibition. This particular aspect of our proposal is now explicitly demonstrated, at least in part, by the present data. The when component also involves the SMA proper in a specific manner. The evidence of the considerable interhemispheric traffic, testified by the callosal activation for the whether component, calls attention to the fact that our unitary mind is, in fact, the product of two interacting hemispheres. It should be pointed out, however, that the system does not show a fully orthogonal organization for the three what/when/whether components along the medial wall of the frontal lobe, as there was a core shared activity in the pre-SMA/ACC.
Conclusions and Future Directions
As much as we perceive ourselves as unitary and aware mental entities, studies on intentionality surprise us with findings such as the observation that our brain decides to act even before we become aware of these decisions, as shown by the original work of Libet et al. (6), or with the evidence presented here that our intentions can be fragmented in various components subserved by partially separable neural circuits. Clearly, we are only scratching the surface of these complex phenomena, as a partially fragmented intentionality system must work through intense interactions of the different components, particularly when the content of a decision has much greater value than just lifting one finger or another, when the timing or the very opportunity to act is affected by the content of the act itself, or when the act is relevant for others. Other aspects that remain to be addressed are the temporal scale in which different components may operate or the case of nested intentional motor plans and their relationship with internal motivations or moral constraints—all issues whose neural underpinnings remain largely unaddressed under the simple www-model framework. In addition, we should remain aware of the limitations of laboratory studies in which intentionality is studied in a less-than-ecological setting with time constraints that are not necessarily the same as in daily life. In this respect, neuropsychological observations of brain-damaged patients remain vital, as they permit the correlation of specific brain lesions with more natural behaviors. However, our template of normal functional brain patterns of the discrete components of intentional actions may help in the study of specific instances of disturbed intentionality, particularly in disorders not associated with macroscopic brain damage.
Materials and Methods
Participants.
Thirty-two subjects (mean age: 25.9 ± 4.8 y; mean education level 15.1 ± 2.3 y; 16 male and 16 female) with no cognitive, neurological, or psychiatric illness participated in the fMRI activation study. They were all right-handed as assessed by the Edinburgh handedness inventory (35). The study protocol was approved by the Ethics Committee ASL Città di Milano, and informed written consent was obtained from all subjects.
Experimental Design and Tasks.
This fMRI experiment conforms to a 3 × 2 × 2 within subject factorial design with the first factor being the component (three levels: 1, what; 2, when; and 3, whether), the second factor being the cognitive process involved (two levels: 1, decision and maintenance of motor plan; 2, response generation), and the third factor being the presence or absence of intentional demands in the task (two levels: 1, conditional trials; 2, internally driven trials).
The fMRI scans were performed during the execution of three different experimental tasks (one run for each task, for a total of three fMRI runs, each made of 280 scans) based on a unitary procedure; in all the experimental conditions, each trial started with the appearance of a traffic light image (SI Appendix, Fig. S2).
Formation and maintenance of the motor plans.
The color of the traffic light instructed the participants to program different motor plans: intentionally driven plans for a yellow light or stimulus-driven plans for a green or a red light. The association of the green or the red light with one of the two possible instructions (e.g., move sooner for the green light; move later for the red light) was reversed in half of the subjects.
For the intention-driven trials, subjects were instructed to make their decision and hold it actively in mind until the appearance of a go-signal. For the conditional trials, subjects were trained similarly to keep the received instruction in their mind.
In the what condition, subjects freely decided or were conditionally instructed whether to move the right index finger or the middle finger. In the when condition, subjects freely decided or were conditionally instructed on the delay in executing a particular movement (key press with the index finger; short delay: immediate response; long delay: about 2 s). In the whether condition, subjects freely decided or were instructed on whether to act or not.
Implementations of the motor plans.
Implementations of the motor plans occurred after the appearance of a go-signal. All movements were produced with the right hand. The go-signal, a cross in the center of the display, appeared at irregular intervals (ranging from 3 to 4.5 s) to further condition the participants to make their decision and hold it immediately after the appearance of the preceding traffic light. [This variable delay allowed us to achieve a beneficial jittering in the relationship between the timing of the events and the repetition time (TR) of the scanning protocol.] There were 100 trials for each task, equally distributed between intention-driven and stimulus-driven trials. Before scanning, subjects were made familiar with the task demands during a short session with the same stimuli later used during the fMRI scans.
Statistical Analyses of the Behavioral Data.
Behavioral performance during the fMRI experiment was analyzed using SPSS software (IBM). Errors and outliers (threshold = mean RTs ± 2 SD) were excluded from the analyses. To estimate whether subjects spontaneously balanced their choices between the two given alternatives during intention-driven trials, we followed the approach of Soon et al. (10) and measured the ratio between the two possible choices (choice 1 and choice 2) using the formula (choice 1 − choice 2)/(choice 1 + choice 2). Absolute values below 0.30 were considered an index of balanced distributions between the two choices.
Moreover, to make sure that (i) there was not a carry-over effect between trials and (ii) subjects chose between alternative responses randomly from trial to trial (an indication of intentional control on actions), we analyzed the distribution of the response sequences for each task assuming that a negative exponential distribution of such sequences would be an indication of randomness (see ref. 10). RTs of intention- and stimulus-driven trials were compared, task by task, using paired t tests or the Wilcoxon signed-rank tests, depending on the data distribution. In Results, we reported the observed statistics (W), the standardized statistics (Z), and the correspondent P values (P). (It was impossible to perform a factorial ANOVA on such data since we did not have an explicit response in the no-go trials of the whether condition.)
fMRI Data Acquisition and Analysis.
Echo-planar imaging gradient-echo fMRI scans [flip angle 90°, echo time (TE) = 60 ms, TR = 3,000 ms, field of view = 280 × 210 mm, and matrix = 96 × 64] were collected using a 1.5-T Siemens Avanto scanner. We collected 280 volumes for each condition (what, when, or whether). The first 10 volumes of each sequence were discarded from the analyses.
Preprocessing.
fMRI data were reconstructed, realigned, and stereotactically normalized to the Montreal Neurological Institute (MNI) space and smoothed (10 × 10 × 10 mm) using SPM12 (SI Appendix).
Statistical Analyses of the fMRI Data.
The BOLD signal was first convolved with a canonical hemodynamic response function, proportionally scaled and high-pass filtered (128 s) to remove fluctuations of global signal and physiological noise due to cardiac or respiratory cycles.
First-level fixed-effect analyses.
We then performed a fixed-effect analysis for each subject to measure the BOLD response associated with the experimental conditions. For each task, we characterized (i) the activity during the formation of the intentional or the conditional motor plan, that is, the activity occurring between the disappearance of the traffic light and the go-signal and (ii) the activity occurring during the implementation of the free or conditional motor responses. For each subject and for each experimental run, we generated two different t-contrasts (decision phase: intention-driven > stimulus-driven; response phase: intention-driven > stimulus-driven).
Second-level random-effect analyses.
The contrast images generated for each subject (what, whether, and when contrast images at the decision phase and the similar images for the response phase) were brought to a group second-level full factorial design conforming to a random-effect analysis.
The full factorial ANOVA generated F-contrasts for the main effect of the event (decision and response phase), the component (what, when and whether components), and the interaction effects. We then performed post hoc analyses to examine the direction of the aforementioned effects using linear contrasts to generate SPM[t] maps.
In particular, we assessed the component-specific patterns by comparing the data of a given component with the other two: for example, for the what component, the comparison was in the form of what > (when and whether).
Furthermore, in separate ANOVAs we assessed whether the different levels of each component (e.g., early versus late responses for the when condition) were associated with specific patterns at the decision phase. In the corrections for multiple comparisons the results are reported using the nested-taxonomy strategy recommended by Friston et al. (36), that is, regional effects meeting either a clusterwise or voxelwise FWER correction for multiple comparisons. The voxelwise threshold applied to the statistical maps before the clusterwise correction was P < 0.001 uncorrected, as recommended by Flandin and Friston (37). For clusters significant at the P < 0.05 FWER-corrected level, we also report the other peaks at P < 0.001.
Brain Tractography Analysis.
To identify the callosal fibers passing through the area of maximal fMRI signal change for the whether condition in the corpus callosum, we generated a normal-subject fiber atlas by normalizing 15 normal subjects’ DTI datasets (acquisition parameters: 35 directions of diffusion, b = 1,000 s/mm2, on a 3-Tesla Siemens Trio scanner) to MNI space using DTI-TK, calculating the mean DTI for each voxel and performing fiber tracking on the mean diffusion tensor image. Demographic data of the subjects were comparable to those of the participants in the fMRI study: nine males, six females, mean age of the group = 25 y, age range = 21–32 y. Fiber tracking was performed using Trackvis (trackvis.org/dtk/) using the activated fMRI regions of interest as starting areas. Minimum anisotropy of 0.2 and a maximum angle of 35° were used as stop criteria.
Supplementary Material
Acknowledgments
We thank the technical staff of the Istituto di Ricovero e Cura a Carattere Scientifico Galeazzi for making this study possible. This work was funded by an Italian Ministry of Education, Universities and Research grant (Progetti di Ricerca di Interesse Nazionale 2010-2011; protocol no. 2010 ENPRYE) and by an Italian Ministry of Health (Ricerca Corrente; Project L3008) grant (to E.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718891115/-/DCSupplemental.
References
- 1.Jahanshahi M, Frith CD. Willed action and its impairments. Cogn Neuropsychol. 1998;15:483–533. doi: 10.1080/026432998381005. [DOI] [PubMed] [Google Scholar]
- 2.Frith CD, Blakemore SJ, Wolpert DM. Abnormalities in the awareness and control of action. Philos Trans R Soc Lond B Biol Sci. 2000;355:1771–1788. doi: 10.1098/rstb.2000.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shallice T. From Neuropsychology to Mental Structure. Cambridge Univ Press; New York: 1988. [Google Scholar]
- 4.Brass M, Haggard P. The what, when, whether model of intentional action. Neuroscientist. 2008;14:319–325. doi: 10.1177/1073858408317417. [DOI] [PubMed] [Google Scholar]
- 5.Zapparoli L, Seghezzi S, Paulesu E. The what, the when, and the whether of intentional action in the brain: A meta-analytical review. Front Hum Neurosci. 2017;11:238. doi: 10.3389/fnhum.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106:623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- 7.Lau HC, Rogers RD, Passingham RE. On measuring the perceived onsets of spontaneous actions. J Neurosci. 2006;26:7265–7271. doi: 10.1523/JNEUROSCI.1138-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynn MT, Demanet J, Krebs RM, Van Dessel P, Brass M. Voluntary inhibition of pain avoidance behavior: An fMRI study. Brain Struct Funct. 2016;221:1309–1320. doi: 10.1007/s00429-014-0972-9. [DOI] [PubMed] [Google Scholar]
- 9.Schel MA, et al. Neural correlates of intentional and stimulus-driven inhibition: A comparison. Front Hum Neurosci. 2014;8:27. doi: 10.3389/fnhum.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soon CS, Brass M, Heinze HJ, Haynes JD. Unconscious determinants of free decisions in the human brain. Nat Neurosci. 2008;11:543–545. doi: 10.1038/nn.2112. [DOI] [PubMed] [Google Scholar]
- 11.Passingham RE. Two cortical systems for directing movement. Ciba Found Symp. 1987;132:151–164. doi: 10.1002/9780470513545.ch10. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg G. Supplementary motor area structure and function: Review and hypotheses. Behav Brain Sci. 1985;8:567–588. [Google Scholar]
- 13.Passingham RE, Lau HC. Acting, seeing, and conscious awareness. Neuropsychologia. June 13, 2017 doi: 10.1016/j.neuropsychologia.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Deiber MP, et al. Cortical areas and the selection of movement: A study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- 15.Frith CD, Friston K, Liddle PF, Frackowiak RS. Willed action and the prefrontal cortex in man: A study with PET. Proc Biol Sci. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- 16.Playford ED, et al. Impaired mesial frontal and putamen activation in Parkinson’s disease: A positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- 17.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB. The “what” and “when” of self-initiated movements. Cereb Cortex. 2013;23:520–530. doi: 10.1093/cercor/bhr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tunik E, Rice NJ, Hamilton A, Grafton ST. Beyond grasping: Representation of action in human anterior intraparietal sulcus. Neuroimage. 2007;36:T77–T86. doi: 10.1016/j.neuroimage.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallivan JP, McLean DA, Valyear KF, Pettypiece CE, Culham JC. Decoding action intentions from preparatory brain activity in human parieto-frontal networks. J Neurosci. 2011;31:9599–9610. doi: 10.1523/JNEUROSCI.0080-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desmurget M, et al. Movement intention after parietal cortex stimulation in humans. Science. 2009;324:811–813. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- 22.Paulesu E, Frackowiak RS, Bottini G. Maps of somatosensory systems. In: Frackowiak RS, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human Brain Function. Academic; San Diego: 1997. pp. 183–242. [Google Scholar]
- 23.Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and readiness for voluntary movement: A high-field event-related fMRI study of the Bereitschafts-BOLD response. Neuroimage. 2003;20:404–412. doi: 10.1016/s1053-8119(03)00291-x. [DOI] [PubMed] [Google Scholar]
- 24.Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs external generation of movements: Differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage. 2003;19:764–776. doi: 10.1016/s1053-8119(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 25.Michely J, et al. Dopaminergic modulation of motor network dynamics in Parkinson’s disease. Brain. 2015;138:664–678. doi: 10.1093/brain/awu381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley RE, El-Khoury R. Frontotemporal dementia. Neurol Clin. 2016;34:171–181. doi: 10.1016/j.ncl.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tettamanti M, et al. Interhemispheric transmission of visuomotor information in humans: fMRI evidence. J Neurophysiol. 2002;88:1051–1058. doi: 10.1152/jn.2002.88.2.1051. [DOI] [PubMed] [Google Scholar]
- 28.Weber B, et al. Attention and interhemispheric transfer: A behavioral and fMRI study. J Cogn Neurosci. 2005;17:113–123. doi: 10.1162/0898929052880002. [DOI] [PubMed] [Google Scholar]
- 29.Courtemanche MJ, Sparrey CJ, Song X, MacKay A, D’Arcy RCN. Detecting white matter activity using conventional 3 Tesla fMRI: An evaluation of standard field strength and hemodynamic response function. Neuroimage. 2018;169:145–150. doi: 10.1016/j.neuroimage.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Innocenti GM. Network causality, axonal computations, and Poffenberger. Exp Brain Res. 2017;235:2349–2357. doi: 10.1007/s00221-017-4948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulvermüller F, Mohr B. The concept of transcortical cell assemblies: A key to the understanding of cortical lateralization and interhemispheric interaction. Neurosci Biobehav Rev. 1996;20:557–566. doi: 10.1016/0149-7634(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa T, et al. Conflict of intentions due to callosal disconnection. J Neurol Neurosurg Psychiatry. 2001;71:462–471. doi: 10.1136/jnnp.71.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akelaitis AJ. Studies on the corpus callosum. IV. Diagonistic dyspraxia in epileptics following partial and complete section of the corpus callosum. Am J Psychiatry. 1945;101:594–599. [Google Scholar]
- 34.Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: Clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- 35.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 36.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: Levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 37.Flandin G, Friston KJ. Analysis of family-wise error rates in statistical parametric mapping using random field theory. Hum Brain Mapp. November 1, 2017 doi: 10.1002/hbm.23839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.