Significance
Insulin is currently available as an injectable formulation, but an oral product would enjoy higher patient compliance and would significantly improve the quality of life of diabetic patients worldwide. However, oral delivery of proteins such as insulin is challenging due to various gastrointestinal barriers to oral absorption of macromolecules. We have developed a safe and highly effective ionic liquid-based oral insulin formulation that significantly enhanced oral insulin absorption by efficiently circumventing the gastrointestinal barriers. Besides, the formulation demonstrated good stability at room temperature and under refrigeration. Evidence from cell and animal studies supports a promising prospect of development of the formulation into a clinical product.
Keywords: ionic liquid, oral insulin delivery, bioavailability, stability, peroral
Abstract
With the rise in diabetes mellitus cases worldwide and lack of patient adherence to glycemia management using injectable insulin, there is an urgent need for the development of efficient oral insulin formulations. However, the gastrointestinal tract presents a formidable barrier to oral delivery of biologics. Here we report the development of a highly effective oral insulin formulation using choline and geranate (CAGE) ionic liquid. CAGE significantly enhanced paracellular transport of insulin, while protecting it from enzymatic degradation and by interacting with the mucus layer resulting in its thinning. In vivo, insulin-CAGE demonstrated exceptional pharmacokinetic and pharmacodynamic outcome after jejunal administration in rats. Low insulin doses (3–10 U/kg) brought about a significant decrease in blood glucose levels, which were sustained for longer periods (up to 12 hours), unlike s.c. injected insulin. When 10 U/kg insulin-CAGE was orally delivered in enterically coated capsules using an oral gavage, a sustained decrease in blood glucose of up to 45% was observed. The formulation exhibited high biocompatibility and was stable for 2 months at room temperature and for at least 4 months under refrigeration. Taken together, the results indicate that CAGE is a promising oral delivery vehicle and should be further explored for oral delivery of insulin and other biologics that are currently marketed as injectables.
The oral route of drug administration is preferred over injections due to its ease of administration, high patient compliance, and low manufacturing costs. However, due to various gastrointestinal barriers to drug absorption, it is unsuitable for the delivery of biologics. For example, insulin is an indispensable medication for type 1 diabetes management. It is currently administered as an s.c. injection but is associated with lack of patient adherence due to pain and needle phobia associated with injections (1). Orally delivered insulin could significantly enhance patient compliance. In addition, it would closely mimic the physiological path of pancreatic insulin (2, 3). Oral/pancreatic insulin is transported to the liver via the portal vein where 80% is retained and the rest reaches systemic circulation, creating up to threefold higher insulin concentration in the portal vein compared with systemic circulation (3). This portal-peripheral insulin gradient is disrupted when insulin is injected s.c. due to a higher systemic insulin concentration compared with that in the portal vein (only ∼20%) which disrupts the liver’s fine balance between glycogen storage and glucose output. This often results in hyperglycemia, which, when treated with higher insulin dose, can lead to hypoglycemia (3, 4).
The pursuit of an oral insulin product began many decades ago. Several strategies have been developed to overcome the gastrointestinal barriers to oral absorption of biologics. However, no formulation has successfully cleared all clinical hurdles, and therefore, no oral insulin products are commercially available. Products that have completed or are currently in phase II clinical trials include enterically coated capsules with additives to improve oral insulin uptake (Capsulin by Diabetology Ltd and ORMD-0801 by Oramed Ltd), hepatic directed liposomal insulin (HDV-Insulin by Diasome Pharmaceuticals Inc.), polyethylene glycol (PEG) conjugated insulin (IN-105 by Biocon Ltd.), insulin-proinsulin-c-peptide in Oshadi carrier (Oshadli Icp by Oshadi Drug Administration Ltd), and long-acting insulin analog tablets using gastrointestinal permeation enhancer technology (GIPET 1 by Novo Nordisk) (5–7). Active recruitment of participants for safety and efficacy studies is currently underway only for ORMD-0801 and Oshadi Icp, according to the clinicaltrials.gov list. In addition, many products require multistep formulation procedures, various additives, or chemical modification of the protein, which have their own shortcomings. With the emerging global diabetes epidemic, there is a sense of urgency to develop safe, effective, and easily scalable oral insulin products.
We have developed an ionic liquid (IL)-based oral formulation of insulin and determined its safety, efficacy, and long-term stability. Ionic liquids consist of organic/inorganic salts with melting points below 100 °C and have been widely used in various novel chemical and pharmaceutical technologies (8–10). In the present work, we utilized a room temperature stable choline and geranate (CAGE) deep eutectic solvent that had earlier demonstrated remarkable efficacy in transdermal delivery of antibiotics and insulin (11, 12). Insulin was dispersed in CAGE in a single-step process, and its safety and efficacy was evaluated both in vitro and in vivo as well as its storage stability. The study demonstrates an unprecedented improvement in oral bioavailability of insulin with excellent oral efficacy, biocompatibility, and long-term stability.
Results
Insulin Was Stable in CAGE for Long Periods of Time.
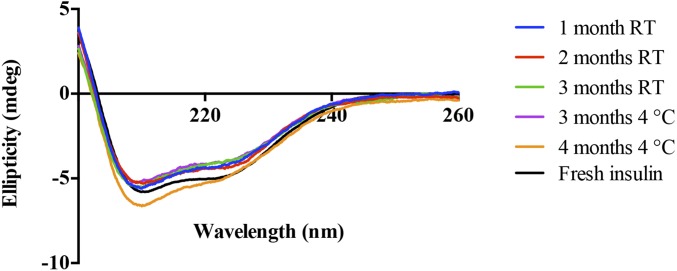
Insulin has an inherent alpha-helical conformation, which is essential for its receptor interaction and hence its bioactivity (13). Before performing functional tests of oral delivery with CAGE, we assessed whether insulin is stable in CAGE for extended periods of time. We stored insulin-CAGE at room temperature (RT, away from direct sunlight) or under refrigeration at 4 °C and evaluated the secondary structure of insulin isolated from CAGE every month for a period of 4 mo using circular dichroism (CD). Results showed the presence of double negative troughs at around 207 and 222 nm, which is a typical representation of an alpha-helix in a CD graph (Fig. 1). No difference in the shape or degree of ellipticity was noticed between freshly prepared insulin solution and insulin stored in CAGE at RT or 4 °C for up to 3 and 4 mo, respectively. The result suggests that CAGE preserves the secondary structure of insulin for long periods of time.
Fig. 1.
Circular dichroism spectra of insulin isolated from CAGE at different months. Insulin was dispersed in CAGE and stored at RT (25 °C) or under refrigeration at 4 °C for up to 4 mo. The alpha-helical secondary conformation of insulin was retained in CAGE for extended periods of time.
To validate the CD stability data, we evaluated the biological activity of insulin isolated from CAGE at different times in nondiabetic rats. For this purpose, rats that were fasted overnight were s.c. injected with insulin (isolated from CAGE and resuspended in sterile saline), and blood glucose was monitored for a period of 8 h. No significant difference in biological activity was seen between fresh insulin and insulin-CAGE stored at RT for 1–2 mo or at 4 °C for 1–4 mo (SI Appendix, Fig. S1). An attenuated efficacy was observed for insulin stored at RT for 3 mo, whereas insulin stored at 4 °C with CAGE did not show any loss in bioactivity even after 4 mo of storage. This clearly illustrates that CAGE is an excellent solvent for long-term storage of insulin.
Insulin-CAGE Caused Profound Hypoglycemia and Demonstrated Exemplary Pharmacokinetics upon Intrajejunal Administration.
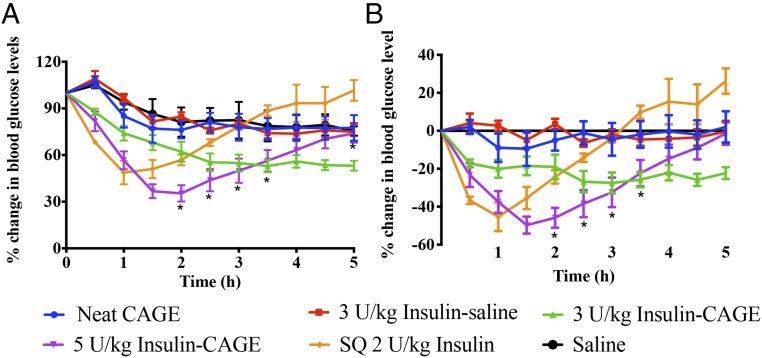
To gauge the efficacy of insulin-CAGE in lowering blood glucose levels, 3–5 U/kg insulin was dispersed in CAGE and administered to anesthetized nondiabetic rats intrajejunally, followed by blood glucose monitoring every 0.5 h for a total of 5 h (Fig. 2). Rats administered with 3 U/kg insulin-CAGE showed a steady drop in blood glucose levels from 0.5 h till it dropped by 45% of its initial value (55 ± 6%) in 2.5 h. Beyond this point, the blood glucose levels plateaued, ending at 47% in 5 h. The group treated with 5 U/kg insulin-CAGE showed a sharp decrease in blood glucose levels that culminated in an ∼65% drop within 1.5 and 2 h (37 ± 5 and 35 ± 5% of initial levels, respectively). Blood glucose increased thereafter, which is a typical homeostatic response in nondiabetic rats that are subjected to a sudden and rapid decrease in blood glucose levels. At the end of 5 h, the blood glucose level was 74% of initial levels. The rats that were s.c. injected with 2 U/kg insulin also showed a similar pattern of blood glucose drop but to a lesser extent compared with 5 U/kg insulin-CAGE. A maximum drop of 51% was observed in 1 h (49 ± 8% of initial level), which rapidly recovered to 100% at the end of the study at 5 h (101 ± 7%). Controls such as neat CAGE, saline, or 3 U/kg insulin–saline intrajejunal administration did not bring about a significant drop in blood glucose. In all these control groups, blood glucose decreased slowly (most likely due to continued fasting) by about 25% of initial levels in 5 h.
Fig. 2.
Efficacy of insulin-CAGE in lowering blood glucose levels upon intrajejunal administration in nondiabetic rats. (A) Blood glucose lowering efficacy of various formulations compared with initial levels. (B) Blood glucose changes after normalizing for fasting effect related blood glucose changes. Animals injected with saline alone were considered as the fasting group, and data were plotted after subtracting the blood glucose obtained from the saline group. All data are represented as mean ± SE (n = 6). A significantly higher (P < 0.05) efficacy was noted in the efficacy of 5 U/kg insulin-CAGE–treated rats at various time points of the study compared with s.c. administered 2 U/kg insulin (represented by asterisks).
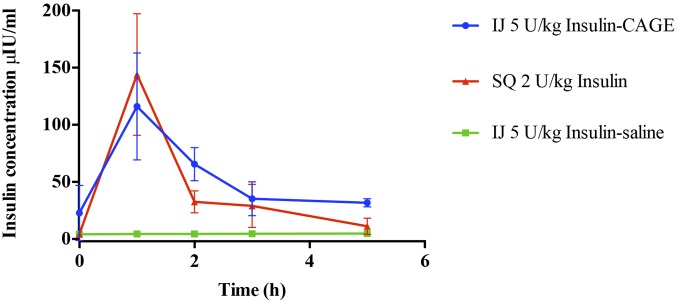
The pharmacokinetics of insulin absorption and elimination was determined by measuring serum insulin levels at different time points (Fig. 3). Insulin concentration rapidly increased within an hour after s.c. (SQ in figures) injection of 2 U/kg insulin and intrajejunal (IJ) administration of 5 U/kg insulin-CAGE and subsequently dropped to follow a similar pattern in elimination. The pharmacokinetic parameters calculated using serum insulin concentrations showed that the elimination half-life of intrajejunally administered insulin-CAGE was almost twofold higher than s.c. insulin (Table 1). The oral bioavailability of 5 U/kg IJ Insulin-CAGE thus calculated was found to be 51% relative to 2 U/kg s.c. injection. The pharmacodynamic bioavailability of the formulation as calculated from efficacy plots was 66% relative to 2 U/kg s.c. injection. On the contrary, 5 U/kg insulin-saline administered IJ did not show any increase in insulin level with time.
Fig. 3.
Efficacy of CAGE in enhancing the oral bioavailability of insulin. Data are represented as mean ± SE (n = 4).
Table 1.
Pharmacokinetic parameters of insulin-CAGE given intrajejunally and insulin solution administered s.c
| Formulation | Kel, h−1 | t1/2, h | AUCtotal, μIU h/mL | % F |
| Insulin-CAGE IJ | 0.32 | 2.2 | 300.6 | 51 |
| Insulin s.c. | 0.58 | 1.2 | 237.6 |
Elimination rate constant (Kel), half-life (t1/2), area under curve (AUC), and bioavailability relative to s.c. injection (F).
Insulin-CAGE Administered Orally in Capsules Demonstrated Notable Efficacy in Lowering Blood Glucose Levels.
The remarkable efficacy of CAGE in enhancing oral bioavailability of insulin upon intrajejunal administration prompted us to investigate its efficacy upon oral delivery using capsules. To this end, we placed 10 U/kg insulin-CAGE or its controls in enterically coated elongated size 9 capsules as illustrated in SI Appendix, Fig. S2, and administered them to nondiabetic rats using an oral gavage.
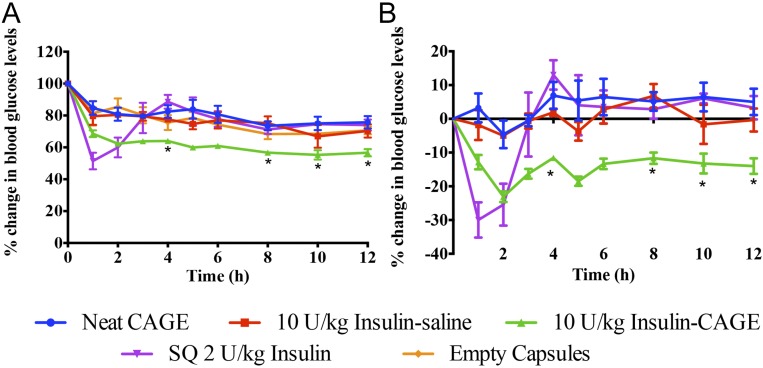
The group treated with 10 U/kg insulin-CAGE demonstrated a rapid 38% drop in blood glucose levels within 2 h post-capsule administration (62 ± 2% of initial level) (Fig. 4). Beyond this point, blood glucose dropped slowly but steadily by around 45% at 10 h (55 ± 3%). In comparison, s.c. administration of 2 U/kg insulin led to a sharp 49% drop in blood glucose levels (51 ± 5%) in 1 h, which rose steadily and subsequently peaked at 88% of the initial value in 4 h. Thereafter, blood glucose levels dropped in a pattern similar to formulation controls of neat CAGE, empty capsules, and insulin–saline. As earlier observed with IJ administration, insulin-CAGE in capsules demonstrated significantly sustained efficacy till the end of study, unlike s.c. insulin. To assess whether CAGE is acting solely as a permeation enhancer, we administered rats with neat CAGE in capsules followed by 10 U/kg insulin powder in capsules after a 0.5-h delay (SI Appendix, Fig. S3). A significant difference in efficacy between insulin-CAGE coadministration and consecutive administration was observed in the first few hours of the study (compare SI Appendix, Fig. S3, and Fig. 4), whereas no significant difference between insulin solution (no CAGE) and consecutive dosing of insulin and CAGE was observed through all time points (SI Appendix, Fig. S3). The study further suggests that insulin and CAGE need to be delivered together to obtain significant efficacy.
Fig. 4.
In vivo efficacy of insulin-CAGE orally administered in capsules. (A) Blood glucose lowering efficacy of various formulations compared with initial levels. (B) Blood glucose changes after normalizing for fasting effect related blood glucose changes. Animals administered with empty capsules were considered as the fasting group, and data were plotted after subtracting blood glucose changes obtained from the empty capsule group. All data are represented as mean ± SE (n = 6). A significantly higher (P < 0.05) efficacy of CAGE-insulin was observed at various time points (represented as asterisks) compared with s.c. administered insulin.
CAGE Demonstrated Good Oral Biocompatibility in Vivo.
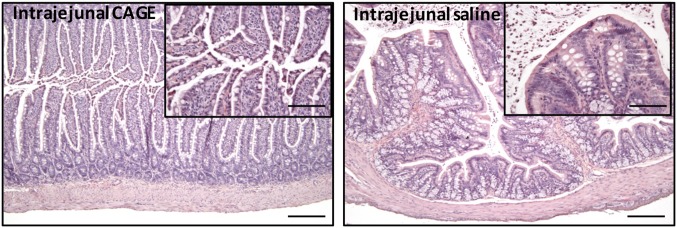
Histological examination of small intestine samples collected 5 h after intrajejunal administration, 12 h after oral capsule dosing, or after 7 d of once-a-day repeat oral dosing showed no remarkable difference in morphology between CAGE and saline-treated animals/insulin-treated animals (Fig. 5 and SI Appendix, Fig. S4). Additionally, no significant structural damage to the small intestine tissues was noted. Particularly, fingerlike villi were found in all of the tissues. The results clearly showcase the excellent biocompatibility of CAGE with intestinal tissue, thereby validating the suitability of the formulation for oral administration.
Fig. 5.
Photomicrographs of Hematoxylin and Eosin staining of small intestine tissue sections after intrajejunal administration of (Left) neat CAGE or (Right) saline. (Scale bar: 200 μm.) (Insets) Sections at higher magnification. (Scale bar: 50 μm.)
Discussion
Over the last few decades, the prevalence of diabetes mellitus has grown so steadily that it is now being referred to as the “epidemic of the century” (14). Current recommendations for management of the disease include insulin therapy alone or in combination with oral hypoglycemics such as metformin (15). Individuals on insulin therapy alone often require either administration of intermediate-acting insulin two times a day or long-acting insulin once a day (15). Insulin is not available in the clinic as an oral pill and is almost exclusively administered as an s.c. injection. However, despite its effectiveness in the management of hyperglycemia and mitigating risks of neuropathy, nephropathy, and retinopathy, injectable insulin has lower patient compliance due to pain, interference with daily activities, and reluctance of use in public, resulting in intentional omission and poor long-term glycemic control in as much as 60% of patients (5, 16). This leads to higher hemoglobin A1C levels and increased hospitalization due to diabetes-associated complications (16). Given the rapid growth and magnitude of diabetes, it is imperative to develop an insulin therapy that would appeal to patients and avoid formulation-based adverse effects.
Oral delivery enjoys a high patient compliance but is not suitable for delivery of biologics. This is due to the fact that orally delivered drugs need to traverse through the acidic environment of the stomach which can degrade protein/peptide drugs. This can be avoided by encapsulating them in enteric or other protective coated systems. However, upon release in the intestine from their protective casings, peptide/protein drugs are exposed to the proteolytic milieu of the intestine where they are easily cleaved into smaller amino acid units by resident enzymes. If a proportion of the drug escapes proteolysis, its absorption through the intestinal mucus layer and enterocytes into the systemic circulation as an intact molecule is challenging. Oral insulin absorption is further impeded due to erratic GI transit time and lack of specific insulin uptake mechanisms in the intestine (17). A perturbation in insulin structure during GI transit may lead to significant denaturation and loss in biological activity. Therefore, not surprisingly, protein and peptide drugs have a negligible oral bioavailability of less than 1%, a stark divergence from injectable formulations where 100% of the dose is available for pharmacological activity (18). Several researchers have attempted to resolve the perennial problem of low oral bioavailability of insulin by modifying insulin; encapsulating it in novel carriers; or employing enteric coatings, absorption enhancers, or proteolytic inhibitors. Some examples include use of poly[lactic-coglycolic acid] (PLGA) or chitosan-based nanoparticles for oral insulin delivery. Pan et al. obtained a pharmacological bioavailability of 10.3% using 10 U/kg insulin loaded in PLGA nanoparticles and 15.3% using 21 U/kg insulin in chitosan nanoparticles, whereas Cui et al. obtained an oral bioavailability of 3.7 and 6.3% using 20 U/kg insulin placed in PLGA and PLGA-55 nanoparticles, respectively (19–21). Sarmento et al. (22) used chitosan–dextran nanoparticles to encapsulate insulin and observed a pharmacological bioavailability of 5.6 and 3.4% after placing 50 and 100 U/kg insulin, respectively, in the particles. Zhang et al. (23) encapsulated 50 U/kg insulin in solid lipid nanoparticles (SLN) and wheat germ agglutinin-modified SLN and obtained pharmacological bioavailabilities of 4.5 and 6.1%, respectively. Other strategies of improving oral delivery of proteins involve using proteolytic enzyme inhibitors such as sodium glycocholate, aprotinin, soybean trypsin inhibitor, bacitracin, and camostat mesilitate (24). Chemical modifications of insulin such as attaching a targeting ligand like transferrin or cell-penetrating peptides like TAT peptide have shown to assist transcytosis of insulin across enterocytes (25, 26). Absorption enhancers include bile salts, surfactants, fatty acids, calcium ion chelating agents, certain polymers such as chitosan/thiolated chitosan, and zonula occluden toxins that operate by either modulation of the cell membrane structure of intestinal epithelium for transcellular uptake or tight junction permeability for paracellular transport (24).
Recently, ionic liquids have garnered immense interest as a class of polar organic solvents for delivery of small- and large-molecule therapeutics. ILs constitute a group of salts with an organic cation and organic/inorganic anion that are typically liquid below 100 °C (27). By pairing different ions, ILs can be tailored to have different physicochemical properties such as viscosity, hydrophobicity, solubility, and biodegradability for a wide range of pharmaceutical applications in the fields of biocatalysis, enzymatic processes, protein stability, permeation enhancement, and solubilization, among others (11, 12, 27–31). In oral drug delivery, ILs have been utilized for the delivery of poorly water-soluble small molecules such as danazol, itraconazole, cinnarizine, and halofantrine (32, 33). When administered to rats orally, the plasma concentrations of the drugs in IL systems were severalfold higher compared with same dose physical mixtures or suspensions and demonstrated prolonged exposure.
Both choline and geranic acid are recognized by the Food and Drug Administration as generally regarded as safe (GRAS) ingredients. Choline, an important constituent of lecithin that is present in both plants and animals and required for various physiological functions, has an oral lethal dose 50% (LD50) of 3,400 mg/kg, whereas geranic acid, commonly used as a flavoring agent in foods, has an oral LD50 of 3,700 mg/kg in rats (34, 35). In this study, the oral capsule CAGE dose after single administration was 80 mg (80 μL), which comprises ∼27 mg choline and 53 mg geranic acid. Therefore, we administered a much lower dose than the oral LD50 of individual constituents. An adequate choline intake is considered to be 550 mg in men and 425 mg in women per day (36). On the other hand, geranic acid is a common food additive and found in cardamom, lemongrass, petitgrain, and other essential oils (37).
Interactions of CAGE with Caco-2 monolayers were studied in vitro. CAGE exhibited concentration-dependent cellular viability in Caco-2 cells (SI Appendix, Fig. S5). CAGE also exhibited concentration-dependent enhancement of insulin transport, with the enhancement of greater than 10-fold for 50 mM CAGE (SI Appendix, Figs. S6 and S7). The enhancement mechanism is likely mediated by the paracellular route. This was further evaluated using paracellular permeability markers Lucifer yellow and fluorescein isothiocyanate (FITC)-dextran (38). The transport of Lucifer yellow also exhibited a concentration-dependent enhancement with CAGE (SI Appendix, Fig. S8). Furthermore, transport of 4 kDa FITC-dextran (comparable size to insulin) was also increased in a concentration-dependent manner, reaching an enhancement of 13-fold (SI Appendix, Fig. S9). This was corroborated by measuring the intestinal tight junction integrity through determination of transepithelial electrical resistance (TEER). As observed earlier, a concentration-dependent decrease in TEER was observed upon exposure to CAGE for 5 h, followed by concentration-dependent recovery (SI Appendix, Fig. S10). Treatment of cells with 10 mM CAGE resulted in significant decrease in TEER at 1 and 5 h, whereas treatment with 25 and 50 mM CAGE led to a significant (40–50%) decrease in TEER at all time points and TEER recovery to 90 and 58% of initial levels within 24 h, respectively. For reference, drop in TEER after exposure to a well-known permeation enhancer drug sodium caprate was also measured (39). The transcellular permeability of coumarin-6 (a fluorescent marker for passive transcytosis) in the presence of CAGE was thereafter assessed (40). Surprisingly, CAGE reduced the transcellular uptake of coumarin-6 even at low concentrations, thus suggesting that transcytosis of drugs across intestinal epithelia is unlikely to explain the observed transport enhancement for insulin (SI Appendix, Fig. S11). In addition to enhancement in paracellular permeability, CAGE significantly hindered tryptic digestion of insulin from 1 h onward till the end of study at 24 h compared with buffer control (SI Appendix, Fig. S12). CAGE was also found to significantly decrease viscosity of mucin hydrogel at 1 and 5% wt/vol concentrations (SI Appendix, Fig. S13), suggesting that CAGE likely assists in mucus penetration in vivo, a key barrier in oral uptake of macromolecules.
In vivo, CAGE demonstrated outstanding efficacy in enhancing oral uptake of insulin when administered either intrajejunally or in capsules. Intrajejunally injected 3 U/kg insulin-CAGE led to a 47% drop in blood glucose levels in 5 h, which was comparable to the drop observed with s.c. injected 2 U/kg insulin. Using 5 U/kg insulin-CAGE, an even more drastic 65% drop in blood glucose levels was observed in 2 h, and levels remained significantly lower compared with s.c. injected insulin for the remaining period of study. This is one of the lowest oral insulin doses that we have seen in the literature demonstrating such remarkable efficacy. Among other notable work, Pan et al. (20) demonstrated a 52% drop in blood glucose levels in 4 h upon oral administration of 10 U/kg insulin in PLGA particles. Using vitamin B12-conjugated dextran nanoparticles loaded with 20 U/kg insulin, Chalasani et al. (41) obtained a 70–75% drop in blood glucose and an oral bioavailability of 29.4%. Intrajejunal administration of 10 U/kg insulin in dogs using bile salt mixed micelles exhibited an absolute bioavailability of 1.8% (42). Intrajejunal placement of rectangular mucoadhesive patches loaded with 50 U/kg insulin in rats led to a relative bioavailability of 3.9%. In the presence of permeation enhancer drug dimethyl palmitoyl ammonio propanesulfonate, the relative bioavailability increased to 7.7% (43). Yin et al. (44) showed that intestinal injection of trimethyl chitosan-cysteine conjugate nanoparticles encapsulating 50 U/kg insulin decreased blood glucose levels in rats by 70%. Using Labrasol, Takada and coworkers observed bioavailabilities of 0.25 and 0.2% for intraileum and intracolonic administrations, respectively (45). An intragastric delivery of lecithin-based microemulsion containing 200 IU/kg resulted in a bioavailability of 0.148 (without aprotinin) and 0.159 (with aprotinin) in normal rats (46).
In our study, a high jejunal bioavailability of insulin was obtained. It is also to be noted that elimination half-life of the insulin-CAGE was almost twofold higher than s.c. injected insulin, indicating a more sustained efficacy. To validate the significant efficacy observed thus far, we further encapsulated insulin-CAGE in enterically coated capsules and orally administered it to rats. Enteric coating prevents breakdown of capsules in the acidic environment of the stomach and releases encapsulated material only in the more alkaline environment of the small intestine (47). Bypassing the stomach using enteric coating technology therefore prevents degradation of biologics by gastric acids. To account for dose dilution upon release of insulin-CAGE in the intestine, we used a higher insulin dose (10 U/kg) than that used in the IJ delivery. Again, insulin-CAGE produced a similar extent of blood glucose drop compared with s.c. injected insulin. However, unlike s.c. insulin, the effectiveness of insulin-CAGE was sustained till the end of study at 12 h, demonstrating the potential for its development into a long-acting oral insulin formulation. Administration of neat CAGE followed by insulin after 0.5 h did not result in any significant blood glucose-lowering efficacy (SI Appendix, Fig. S3). This indicates that coadministration of insulin and CAGE is required for achieving significant in vivo efficacy.
Ionic liquids interact with various hydrophilic and hydrophobic amino acids of a protein through an intricate balance of hydrogen bonds, disulfide bridges, hydrophobic effects, and ionic interactions (48). When mixed with other liquids such as water, a more complex interplay between ions occurs that, depending on the nature of ions, can result in formation of micelles or microemulsions (48). A prolonged and higher oral plasma concentration of poorly water-soluble drugs dispersed in ILs was also reported by Williams et al. (32). This was described to be due to improved interaction of IL-based formulations with endogenous bile salt micelles that resulted in generation of dispersed systems with better access to the intestinal absorptive surface (32). In addition, IL-based systems were found to have high drug loading and were “insensitive to gastrointestinal digestive processes” (27). CAGE also prevents enzymatic degradation, likely by limiting the access of intestinal enzymes to loaded insulin by forming a physical barrier and tempering their proteolytic activity. Enzyme activity is also modulated by its surrounding environment, and molecular dynamics simulation studies have shown that ILs can remove water from the surface of enzymes to the same extent as polar organic solvents like acetonitrile (49). Based on this information and our mechanistic studies, we believe that CAGE protects the insulin from enzymatic degradation, assists in its transport across the mucus layer, and mediates paracellular uptake through the opening of tight junctions. The high oral delivery efficacy of CAGE may be attributed to synergism between its ability to enhance permeation through the paracellular route, inhibit proteolytic enzymes, and penetrate through the mucus layer.
In addition to high efficacy, CAGE exhibited biocompatibility and long-term stability for potential application in the clinic (Fig. 5 and SI Appendix, Fig. S4). For safety determination in vivo, we isolated small intestine sections of rats treated with neat CAGE or insulin-CAGE either after single dose or once-a-day repeat dose administration for a week and found that there was no toxicity at the morphological level after intrajejunal or oral administration, demonstrating excellent oral tolerability of CAGE in vivo. However, chronic or subchronic toxicity of oral CAGE needs to be thoroughly examined.
Insulin-CAGE was stable at RT for 2 mo and at 4 °C for at least 4 mo as assessed through secondary structure and in vivo bioactivity evaluation (Fig. 1 and SI Appendix, Fig. S1). Physicochemical degradation of insulin occurs primarily due to hydrolysis, aggregation, and intermolecular transformation reactions leading to loss of potency (50). Ionic liquids can prevent interaction of proteins with water molecules, whereas protic ionic liquids have been reported to stabilize several amino acids as well as insulin’s native conformation and mitigate its self-aggregation propensity (49, 51). We believe that storage of insulin with neat CAGE prevented interaction of the protein with water, mitigating hydrolytic interaction and stabilizing its alpha-helical secondary structure. Given that the monomeric form of insulin is the bioactive form, the enhanced oral bioactivity of insulin in CAGE may also be partly attributed to presentation of insulin molecules to intestinal cells and systemic circulation as monomers.
Overall, this study outlines the development of a highly effective oral insulin formulation that has tremendous potential for clinical translation in the future. Insulin-CAGE can be prepared effortlessly in a single-step process and therefore can be easily scaled up for industrial production. The product does not require modification in insulin structure or development of complex nanostructures and therefore precludes generation of immunological reactions to modified protein or loss of active ingredient during multistep formulation development. Moreover, it is a simple and robust formulation comprising only insulin and an ionic liquid made of GRAS ingredients that eliminates the necessity to use further additives to enhance efficacy. The formulation demonstrated profound efficacy in vivo at very low insulin doses. By delivering insulin-CAGE in enterically coated capsules, we evade gastric degradation of insulin and enhance its intestinal permeability, thereby overcoming the barriers of oral delivery of biologics. Furthermore, the formulation is biocompatible and has good long-term stability. Outcomes of this study may facilitate realization of oral insulin delivery in the clinic. To this end, additional preclinical studies for chronic and subchronic tolerability, effect of food in the intestine, and efficacy in diabetic rats as well as in larger animals should be performed in the future. In addition, studies to further understand insulin-CAGE–intestinal fluid interactions should also be conducted.
Methods
Materials used for the study and preparation of insulin-CAGE are provided in SI Appendix.
Assessment of Insulin Stability in CAGE.
Samples containing human insulin (100 U, 3.5 mg) were suspended in either 1 mL CAGE or PBS in 2 mL microcentrifuge tubes and incubated at room temperature (25 °C) or under refrigeration (4 °C). After 1 mo, and approximately each month thereafter for 4 mo total, samples were centrifuged for 10 min at 10,000 × g, CAGE was removed via pipette, and the soft insulin pellet was washed with 1 mL PBS and centrifuged again. PBS-CAGE was removed, and the washing/centrifugation steps were repeated until the insulin did not form a pellet during centrifugation. The collected insulin was analyzed for its stability using CD and bioactivity in vivo (described in SI Appendix). To collect spectra in the far-UV region (190–250 nm) indicating protein secondary structures, CD spectrophotometry (Jasco J-1500) was performed with rectangular quartz cells (1-mm path length, Starna Cells, 1-Q-q) loaded with 400 μL of sample.
Determination of Efficacy of Insulin-CAGE upon Intrajejunal Administration and Evaluation of Pharmacokinetic Parameters.
The efficacy of insulin-CAGE injected intrajejunally was determined in adult nondiabetic male Wistar rats fasted overnight but given free access to water. All animal experiments were performed in accordance with the University of California Santa Barbara animal care committee guidelines and the Guide for the Care and Use of Animals of the Institute of Laboratory Animal Resources, National Research Council. For the study, the rats were injected with 100 μL of either 3 or 5 U/kg insulin-CAGE or 100 μL of controls and blood glucose monitored for 0.5 h till the end of study at 5 h. A separate group of three rats were s.c. injected with 2 U/kg insulin in saline for comparison of efficacy. The results were plotted as percentage change in blood glucose levels with respect to initial reading vs. time. In addition, the saline group was considered as fasting control, and graphs were plotted after subtracting blood glucose levels of the saline group. About 250 μL of blood was collected at hourly intervals from various test groups and serum insulin levels determined using ELISA. Pharmacokinetic parameters were calculated from serum insulin concentrations vs. time plots. Pharmacodynamic bioavailability was calculated using area under curve obtained from efficacy plots. More details about the surgical procedure and pharmacokinetics study are given in the SI Appendix.
In Vivo Oral Efficacy.
To determine the efficacy of insulin-CAGE administered through the oral route, elongated size 9 capsules were used. These capsules were filled with 80 μL of either 10 U/kg insulin-CAGE or neat CAGE or left empty. Following this, the capsules were enterically coated three times with 12.5% wt/vol Eudragit L-100 dissolved in isopropanol. For the oral efficacy study, adult nondiabetic male Wistar rats were fasted overnight but given free access to water. Next day, the capsules were administered to the rats using an oral gavage followed by s.c. administration of 5 mg/kg metoclopramide HCl to promote gastric emptying. Blood glucose was thereafter measured using a commercial glucose meter and subsequently every hour till 12 h. Rats were fasted throughout the period of study. A control of 10 U/kg insulin in saline was also tested by orally administrating the formulation as a solution (sans capsule). In addition, the efficacy of s.c. injected 2 U/kg insulin–saline was evaluated. For 10 U/kg insulin-CAGE and neat CAGE, a group of six rats per formulation was used, whereas three rats per group were utilized for studying the efficacy of empty capsules, 10 U/kg insulin solution, and 2 U/kg insulin–saline administered s.c. The results were plotted as percent change in blood glucose level vs. time. In addition, the empty capsule group was considered as fasting control, and graphs were plotted after subtracting blood glucose levels of the empty capsule group. After 12 h of study, rats were euthanized, and small intestinal sections were collected for tissue histology.
Tissue Histology.
Small intestinal tissue was fixed in 10% buffered formalin, dehydrated in ethanol, and embedded in paraffin. Five-μm cross-sections of intestine tissue were deparaffinzed, rehydrated, and stained with Hematoxylin and Eosin. Histological morphology was examined using a light microscope at 10× and 40× magnification (Olympus BX60 Upright Compound Microscope).
Data Analysis.
All data are presented as mean ± SE. For statistical analysis, Student’s t test was utilized. Significant difference was considered at P < 0.05. All experiments were conducted in at least triplicates.
Supplementary Material
Acknowledgments
This work was supported by funds from National Institutes of Health Grant R01DK097379. In addition, funds from National Science Foundation Graduate Research Fellowship under Grant DGE-1144085 (to K.I.) and under Grant DGE-1745303 (to T.B.) were also used. The authors thank the Biological Nanostructures Laboratory within the California NanoSystems Institute, supported by the University of California, Santa Barbara, and the University of California, Office of the President. C.A. is grateful to the Natural Sciences and Engineering Research Council of Canada for a postdoctoral fellowship. The authors acknowledge funding from Blavatnik Biomedical Accelerator at Harvard University.
Footnotes
Conflict of interest statement: S.M. is an inventor on patents on ionic liquids for oral delivery and a shareholder of Liquideon.
This article is a PNAS Direct Submission. J.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722338115/-/DCSupplemental.
References
- 1.Fu AZ, Qiu Y, Radican L. Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes. Curr Med Res Opin. 2009;25:1413–1420. doi: 10.1185/03007990902905724. [DOI] [PubMed] [Google Scholar]
- 2.Matteucci E, et al. Insulin administration: Present strategies and future directions for a noninvasive (possibly more physiological) delivery. Drug Des Devel Ther. 2015;9:3109–3118. doi: 10.2147/DDDT.S79322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbit E, Kidron M. Oral insulin delivery in a physiologic context. J Diabetes Sci Technol. 2017;11:825–832. doi: 10.1177/1932296817691303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonte P, et al. Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnol Adv. 2015;33:1342–1354. doi: 10.1016/j.biotechadv.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Fonte P, Araújo F, Reis S, Sarmento B. Oral insulin delivery: How far are we? J Diabetes Sci Technol. 2013;7:520–531. doi: 10.1177/193229681300700228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zijlstra E, Heinemann L, Plum-Mörschel L. Oral insulin reloaded: A structured approach. J Diabetes Sci Technol. 2014;8:458–465. doi: 10.1177/1932296814529988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguirre TAS, et al. Current status of selected oral peptide technologies in advanced preclinical development and in clinical trials. Adv Drug Deliv Rev. 2016;106:223–241. doi: 10.1016/j.addr.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Rogers RD, Seddon KR. Chemistry. Ionic liquids–Solvents of the future? Science. 2003;302:792–793. doi: 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- 9.Shamshina JL, Barber PS, Rogers RD. Ionic liquids in drug delivery. Expert Opin Drug Deliv. 2013;10:1367–1381. doi: 10.1517/17425247.2013.808185. [DOI] [PubMed] [Google Scholar]
- 10.Lei Z, Chen B, Koo YM, MacFarlane DR. Introduction: Ionic liquids. Chem Rev. 2017;117:6633–6635. doi: 10.1021/acs.chemrev.7b00246. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee A, Ibsen K, Iwao Y, Zakrewsky M, Mitragotri S. Transdermal protein delivery using choline and geranate (CAGE) deep eutectic solvent. Adv Healthc Mater. 2017;6:1601411. doi: 10.1002/adhm.201601411. [DOI] [PubMed] [Google Scholar]
- 12.Zakrewsky M, et al. Choline and geranate deep eutectic solvent as a broad-spectrum antiseptic agent for preventive and therapeutic applications. Adv Healthc Mater. 2016;5:1282–1289. doi: 10.1002/adhm.201600086. [DOI] [PubMed] [Google Scholar]
- 13.Huang K, et al. How insulin binds: The B-chain alpha-helix contacts the L1 beta-helix of the insulin receptor. J Mol Biol. 2004;341:529–550. doi: 10.1016/j.jmb.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Kharroubi AT, Darwish HM. Diabetes mellitus: The epidemic of the century. World J Diabetes. 2015;6:850–867. doi: 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCulloch DK. 2017 Patient education: Diabetes mellitus type 2: Insulin treatment (beyond the basics). Available at https://www.uptodate.com/contents/diabetes-mellitus-type-2-insulin-treatment-beyond-the-basics. Accessed November 8, 2017.
- 16.Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33:240–245. doi: 10.2337/dc09-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soares S, Costa A, Fonte P, Sarmento B. In: Drug Delivery: An Integrated Clinical and Engineering Approach. Rosen Y, Gurman P, Elman N, editors. Taylor and Francis; Boca Raton, FL: 2017. [Google Scholar]
- 18.Shaji J, Patole V. Protein and peptide drug delivery: Oral approaches. Indian J Pharm Sci. 2008;70:269–277. doi: 10.4103/0250-474X.42967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui FD, Tao AJ, Cun DM, Zhang LQ, Shi K. Preparation of insulin loaded PLGA-Hp55 nanoparticles for oral delivery. J Pharm Sci. 2007;96:421–427. doi: 10.1002/jps.20750. [DOI] [PubMed] [Google Scholar]
- 20.Pan Y, Xu H, Zhao HY, Wei G, Zheng JM. Study on preparation and oral efficacy of insulin-loaded poly(lactic-co-glycolic acid) nanoparticles. Yao Xue Xue Bao. 2002;37:374–377. [PubMed] [Google Scholar]
- 21.Pan Y, et al. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm. 2002;249:139–147. doi: 10.1016/s0378-5173(02)00486-6. [DOI] [PubMed] [Google Scholar]
- 22.Sarmento B, Ribeiro A, Veiga F, Ferreira D, Neufeld R. Oral bioavailability of insulin contained in polysaccharide nanoparticles. Biomacromolecules. 2007;8:3054–3060. doi: 10.1021/bm0703923. [DOI] [PubMed] [Google Scholar]
- 23.Zhang N, et al. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int J Pharm. 2006;327:153–159. doi: 10.1016/j.ijpharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Wong CY, Martinez J, Dass CR. Oral delivery of insulin for treatment of diabetes: Status quo, challenges and opportunities. J Pharm Pharmacol. 2016;68:1093–1108. doi: 10.1111/jphp.12607. [DOI] [PubMed] [Google Scholar]
- 25.Shah D, Shen WC. Transcellular delivery of an insulin-transferrin conjugate in enterocyte-like Caco-2 cells. J Pharm Sci. 1996;85:1306–1311. doi: 10.1021/js9601400. [DOI] [PubMed] [Google Scholar]
- 26.Liang JF, Yang VC. Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem Biophys Res Commun. 2005;335:734–738. doi: 10.1016/j.bbrc.2005.07.142. [DOI] [PubMed] [Google Scholar]
- 27.Adawiyah N, Moniruzzaman M, Hawatulaila S, Goto M. Ionic liquids as a potential tool for drug delivery systems. MedChemComm. 2016;7:1881–1897. [Google Scholar]
- 28.Yang M, et al. Using ionic liquids in whole-cell biocatalysis for the nucleoside acylation. Microb Cell Fact. 2014;13:143. doi: 10.1186/s12934-014-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zakrewsky M, et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc Natl Acad Sci USA. 2014;111:13313–13318. doi: 10.1073/pnas.1403995111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivapragasam M, Moniruzzaman M, Goto M. Recent advances in exploiting ionic liquids for biomolecules: Solubility, stability and applications. Biotechnol J. 2016;11:1000–1013. doi: 10.1002/biot.201500603. [DOI] [PubMed] [Google Scholar]
- 31.Patel R, Kumari M, Khan AB. Recent advances in the applications of ionic liquids in protein stability and activity: A review. Appl Biochem Biotechnol. 2014;172:3701–3720. doi: 10.1007/s12010-014-0813-6. [DOI] [PubMed] [Google Scholar]
- 32.Williams HD, et al. Ionic liquids provide unique opportunities for oral drug delivery: Structure optimization and in vivo evidence of utility. Chem Commun (Camb) 2014;50:1688–1690. doi: 10.1039/c3cc48650h. [DOI] [PubMed] [Google Scholar]
- 33.Sahbaz Y, et al. Transformation of poorly water-soluble drugs into lipophilic ionic liquids enhances oral drug exposure from lipid based formulations. Mol Pharm. 2015;12:1980–1991. doi: 10.1021/mp500790t. [DOI] [PubMed] [Google Scholar]
- 34.Davis KL, Hollister LE, Vento AL, Simonton S. Choline chloride in methylphenidate- and apomorphine-induced stereotypy. Life Sci. 1978;22:2171–2177. doi: 10.1016/0024-3205(78)90568-4. [DOI] [PubMed] [Google Scholar]
- 35. Anonymous (1979) Geranic acid. Food Cosmet Toxicol 17:785–786.
- 36.Zeisel SH, da Costa KA. Choline: An essential nutrient for public health. Nutr Rev. 2009;67:615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Center for Biotechnology Information 2018 PubChem Compound Database 3,7-dimethylocta-2,6-dienoic acid. Available at https://pubchem.ncbi.nlm.nih.gov/compound/9989. Accessed April 8, 2018.
- 38.Konsoula R, Barile FA. Correlation of in vitro cytotoxicity with paracellular permeability in Caco-2 cells. Toxicol In Vitro. 2005;19:675–684. doi: 10.1016/j.tiv.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Krug SM, et al. Sodium caprate as an enhancer of macromolecule permeation across tricellular tight junctions of intestinal cells. Biomaterials. 2013;34:275–282. doi: 10.1016/j.biomaterials.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 40.Simovic S, Song Y, Nann T, Desai TA. Intestinal absorption of fluorescently labeled nanoparticles. Nanomedicine (Lond) 2015;11:1169–1178. doi: 10.1016/j.nano.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalasani KB, Russell-Jones GJ, Jain AK, Diwan PV, Jain SK. Effective oral delivery of insulin in animal models using vitamin B12-coated dextran nanoparticles. J Control Release. 2007;122:141–150. doi: 10.1016/j.jconrel.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Scott-Moncrieff JC, Shao Z, Mitra AK. Enhancement of intestinal insulin absorption by bile salt-fatty acid mixed micelles in dogs. J Pharm Sci. 1994;83:1465–1469. doi: 10.1002/jps.2600831020. [DOI] [PubMed] [Google Scholar]
- 43.Gupta V, et al. Oral delivery of exenatide and insulin using mucoadhesive intestinal devices. Ann Biomed Eng. 2016;44:1993–2007. doi: 10.1007/s10439-016-1558-x. [DOI] [PubMed] [Google Scholar]
- 44.Yin L, et al. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials. 2009;30:5691–5700. doi: 10.1016/j.biomaterials.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 45.Eaimtrakarn S, et al. Absorption enhancing effect of labrasol on the intestinal absorption of insulin in rats. J Drug Target. 2002;10:255–260. doi: 10.1080/10611860290022688. [DOI] [PubMed] [Google Scholar]
- 46.Cilek A, Celebi N, Tirnaksiz F, Tay A. A lecithin-based microemulsion of rh-insulin with aprotinin for oral administration: Investigation of hypoglycemic effects in non-diabetic and STZ-induced diabetic rats. Int J Pharm. 2005;298:176–185. doi: 10.1016/j.ijpharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 47.Thakral S, Thakral NK, Majumdar DK. Eudragit: A technology evaluation. Expert Opin Drug Deliv. 2013;10:131–149. doi: 10.1517/17425247.2013.736962. [DOI] [PubMed] [Google Scholar]
- 48.Schroder C. Proteins in ionic liquids: Current status of experiments, simulations. Top Curr Chem (Cham) 2017;375:25. doi: 10.1007/s41061-017-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Micaêlo NM, Soares CM. Protein structure and dynamics in ionic liquids. Insights from molecular dynamics simulation studies. J Phys Chem B. 2008;112:2566–2572. doi: 10.1021/jp0766050. [DOI] [PubMed] [Google Scholar]
- 50.Brange J, Langkjoer L. Insulin structure and stability. Pharm Biotechnol. 1993;5:315–350. doi: 10.1007/978-1-4899-1236-7_11. [DOI] [PubMed] [Google Scholar]
- 51.Kumar A, Venkatesu P. Prevention of insulin self-aggregation by a protic ionic liquid. R Soc Chem Adv. 2013;3:362–367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.