Significance
Methicillin-resistant Staphylococcus aureus (MRSA) infections are responsible for approximately 11,285 deaths per year in the United States. With the ability to acquire antibiotic resistance and counteract host immunity, there is an emergent need for novel therapeutic agents for these infections. Biofilms persist in chronic infections and can tolerate significantly higher antimicrobial concentrations than single cells, greatly complicating the prognosis of MRSA infections. Our knowledge of the persistence mechanisms associated with these infections is limited. This report describes some major strategies used by MRSA biofilms to evade the host innate immune response and shows that S. aureus utilizes the combined activity of leukocidins to persist during chronic infections. Host cells efficiently cleared biofilms that were unable to express leukocidins, presenting an exciting avenue for antiinfection therapeutic agents.
Keywords: biofilms, MRSA, neutrophil extracellular traps, leukocidins
Abstract
Bacterial biofilms efficiently evade immune defenses, greatly complicating the prognosis of chronic infections. How methicillin-resistant Staphylococcus aureus (MRSA) biofilms evade host immune defenses is largely unknown. This study describes some of the major mechanisms required for S. aureus biofilms to evade the innate immune response and provides evidence of key virulence factors required for survival and persistence of bacteria during chronic infections. Neutrophils are the most abundant white blood cells in circulation, playing crucial roles in the control and elimination of bacterial pathogens. Specifically, here we show that, unlike single-celled populations, S. aureus biofilms rapidly skew neutrophils toward neutrophil extracellular trap (NET) formation through the combined activity of leukocidins Panton–Valentine leukocidin and γ-hemolysin AB. By eliciting this response, S. aureus was able to persist, as the antimicrobial activity of released NETs was ineffective at clearing biofilm bacteria. Indeed, these studies suggest that NETs could inadvertently potentiate biofilm infections. Last, chronic infection in a porcine burn wound model clearly demonstrated that leukocidins are required for “NETosis” and facilitate bacterial survival in vivo.
The gram-positive bacterium Staphylococcus aureus is an opportunistic pathogen that causes numerous debilitating infections (1). In addition to secreting multiple virulence factors, S. aureus is successful at persisting as robust biofilms, commonly found in chronic infections (2). Biofilms and single-celled/planktonic populations often have distinct virulomes and differentially interact with the host (3, 4). With the continuing rise in antibiotic-resistant S. aureus, biofilm formation therefore presents an additional hurdle to treating these infections (2, 5).
Biofilms often evade the host immune response by concealing their existence rather than using active defense mechanisms typically associated with planktonic S. aureus. Biofilms accomplish this by masking pathogen-associated molecular patterns, impeding the entry and function of phagocytes, as well as the expression and secretion of proteins that skew the immune system toward antiinflammatory responses (6, 7). Neutrophils play a crucial role in eliminating bacterial pathogens. They do so via phagocytosis, release of antimicrobial proteins, reactive oxygen/nitrogen species, and formation of neutrophil extracellular traps (NETs) (8). Histopathological analyses of various animal models of infection reveal that, whereas host immune cells, including neutrophils, are able to migrate to the site of biofilms, they are unable to clear these infections (9–11). Coupled with tissue necrosis and immune cell death, this suggests active, biofilm-mediated host cell killing (12–14). Here we describe some of the major mechanisms used by biofilms to combat neutrophil-mediated killing and provide some explanations for their persistence during chronic infections.
S. aureus strains can produce as many as five different bicomponent, pore-forming leukocidins, namely Panton–Valentine leukocidin (PVL), LukAB (also known as LukGH), HlgAB, HlgCB, and LukED (15, 16). Although these toxins contribute to virulence during acute infections, their role during chronic infections remains largely unknown (17). In this study, we show that, as biofilms, community-acquired methicillin-resistant S. aureus (CA-MRSA) USA300, the predominant CA-MRSA in the United States (18), releases PVL and HlgAB to elicit NET formation. “NETosis” is a controlled release of the neutrophil chromatin, laced with modified histones and granular proteins, often occurring in response to infections with bacteria or large objects unable to be phagocytosed, such as a biofilm (19, 20).
Specifically, the activity of either toxin resulted in NET-associated neutrophil death by USA300 biofilms, a response that was unique to biofilms and not elicited by planktonic populations. This response did not require physical contact with bacteria. Upon direct contact, although neutrophils were unable to clear WT biofilm bacteria, they were more effective at eliminating S. aureus strains lacking PVL and HlgAB. Studies with a porcine chronic burn wound model clearly demonstrate that leukocidins are required for the induction of NETosis in the wound bed and significantly contribute to bacterial survival in wounds. Thus, induction of NETosis by PVL and HlgAB prevents clearance of biofilms by neutrophils, facilitating the persistence of S. aureus during chronic infections.
Results
CA-MRSA Biofilms Release Proteins That Kill Human Neutrophils.
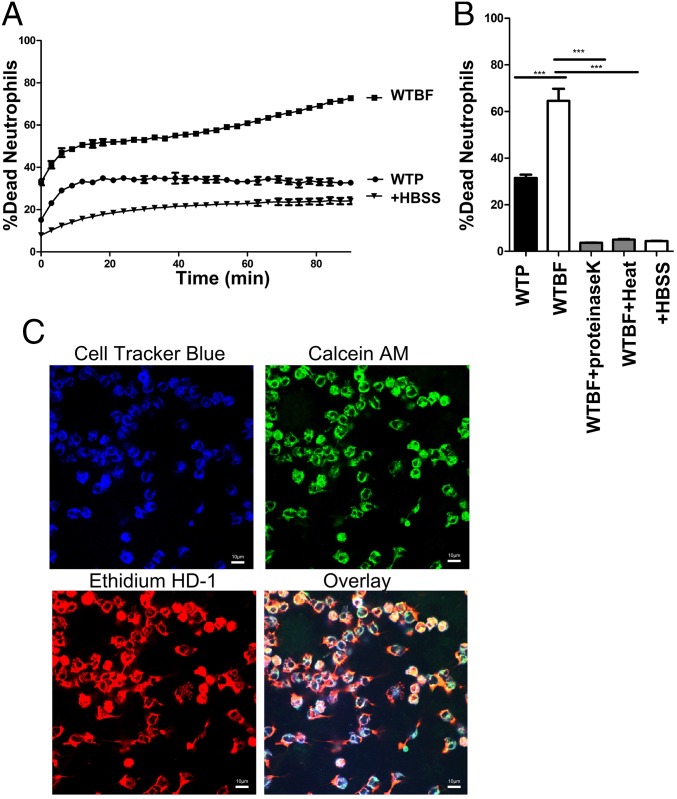
To assess the effects of CA-MRSA biofilm-derived factors on neutrophils, we treated primary blood-derived human neutrophils with spent media from USA300 strain LAC (hereafter USA300) biofilms and monitored neutrophil viability over 90 min by using a LIVE-DEAD assay. Results indicate a significant reduction in neutrophil viability compared with protein concentration-matched planktonic spent media (Fig. 1A).
Fig. 1.
Proteins in S. aureus biofilm-spent media kill neutrophils. Primary human neutrophils incubated with planktonic (WTP) or biofilm (WTBF) spent media from WT USA300LAC S. aureus. Neutrophil viability was monitored for 90 min by using a LIVE-DEAD assay (A). LIVE-DEAD measurements for neutrophils incubated with proteinase K- or heat-treated (100 °C) biofilm-spent media for 30 min (B). CLSM images of neutrophils (CellTracker Blue) treated with WT biofilm-spent media for 30 min (600×) and stained with Calcein AM/EthHD-1 (C). Percentage cell death was calculated in comparison with neutrophils treated with 0.1% SDS. Results represent an average of six independent experiments performed in triplicate ± SEM (***P < 0.001. one-way ANOVA and Tukey’s post hoc analysis). (Scale bars: 10 μm.)
Biofilm-spent media treated with proteinase K or heat (Fig. 1B) had significantly lower neutrophil killing activity in comparison with an untreated control, suggesting a role for biofilm-released proteins. To assess whether proteins secreted during planktonic growth were being degraded by proteases, indirectly reducing the cytotoxicity of these media, we tested the neutrophil killing activity of planktonic and biofilm spent media in an isogenic deletion mutant of the major proteases, namely aureolysin, SspAB, ScpA, and Spl (Δprotease) (21–23). These media showed results similar to WT spent media (SI Appendix, Fig. S1A). To test for protease-independent factors that might be inhibiting the activity of planktonic toxins, we preincubated planktonic and biofilm-spent media together (1:1) for 60 min before neutrophil treatment (SI Appendix, Fig. S1B). This mixture retained neutrophil killing activity, further supporting the presence of neutrophil killing proteins unique to biofilms.
By using confocal laser scanning microscopy (CLSM), we then visualized the fate of neutrophils treated with WT biofilm-spent media. DNA of membrane damaged/dead cells was stained with Ethidium homodimer-1 (EthHD-1/red). Esterase activity was used as an output for enzymatic activity in cells (Calcein AM/green). Neutrophils treated with biofilm-spent media showed a large proportion of dying cells, with membrane damage and DNA release that stained with EthHD-1 and Calcein AM (Fig. 1C) (24). This was in contrast to treatment with planktonic spent media (SI Appendix, Fig. S1C).
Neutrophil Killing Is Regulated by Agr/saeRS and Requires Leukocidins.
Approximately 90% of the exoproteome, including most S. aureus toxins, are regulated by the accessory gene regulator (agr) network (25, 26). We observed a significant decrease in neutrophil killing activity from biofilm-spent media of an isogenic Δagr strain, without a loss in biofilm biomass (SI Appendix, Fig. S2 A and B). This was concomitant with a loss of activity in mutants of the associated saeRS two-component system, indicating a role for Agr/SaeRS-regulated toxins (SI Appendix, Fig. S2C) (26, 27).
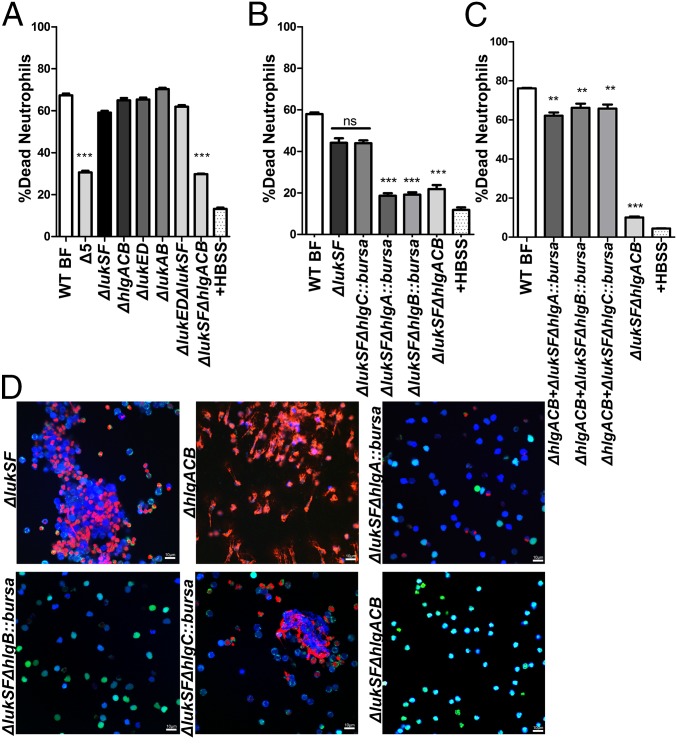
Among the proteins regulated by the Agr/SaeRS network are five bicomponent leukocidins that can puncture phagocyte membranes (16). The S-subunits of PVL (LukSF-PV), LukED, LukAB, and γ-hemolysins HlgAB and HlgCB bind to cell specific receptors and recruit their corresponding F-subunit, assembling into octameric pore-forming complexes within the target cell membrane (16, 28). We found that biofilm-spent media from an isogenic USA300 deletion mutant lacking all bicomponent leukocidin genes (Δ5) showed a significant decrease in neutrophil killing activity, strongly indicating a role for leukocidins in this process. Single leukocidin deletion strains, however, had no such effect, suggesting the involvement of multiple biofilm-released leukocidin proteins in neutrophil killing (Fig. 2A).
Fig. 2.
PVL and HlgAB are required for biofilm-mediated neutrophil-killing activity. LIVE-DEAD assays measuring viability of neutrophils treated with biofilm-spent media from isogenic deletion mutants of respective leukocidins (A). LIVE-DEAD analysis of neutrophils treated with spent media from strains in a ΔlukSF background with deletion of each of three γ-hemolysin subunits (ΔlukSFΔhlgA, ΔlukSFΔhlgC, ΔlukSFΔhlgB). WT and ΔlukSFΔhlgACB-treated neutrophils are shown for comparison (B). Spent media shown in B, mixed 1:1 (volume) with those from biofilms of a ΔhlgACB strain. LIVE-DEAD measurements were made as described earlier (C). CLSM of neutrophils treated with spent media from indicated strains. Cells were stained as described for Fig. 1D (D). Percentage death was calculated in comparison with neutrophils treated with 0.1% SDS for all assays. Neutrophils in HBSS were used as a negative control. Statistical significance shown in all panels is in comparison with neutrophils treated with WT biofilm-spent media. Results represent an average of six independent experiments performed in triplicate ± SEM (**P < 0.01 and ***P < 0.001, one-way ANOVA and Tukey’s post hoc analysis; ns, not significant). (Scale bars: 10 μm.)
PVL and HlgAB Are Necessary for Biofilm-Mediated Neutrophil Killing.
MS and Western blot analyses identified PVL subunits in biofilm-spent media (SI Appendix, Fig. S2D and Table S1). However, as single leukocidin deletions did not reduce neutrophil killing activity (Fig. 2A), we reasoned that PVL might be functioning with other leukocidins. This notion is supported by previous observations that bicomponent leukocidins can form noncognate active toxins and can also potentiate each other (28–30). Accordingly, we tested neutrophil killing activity in biofilm-spent media derived from a series of isogenic strains that lacked lukSF in combination with genes for the other leukocidins. We found no significant loss in killing activity in the ΔlukSFΔlukED strain, suggesting that LukED was not playing a role. In contrast, spent media from ΔlukSFΔhlgACB biofilms showed a significant loss in killing activity (Fig. 2A). This abrogation was comparable to an isogenic deletion of all five leukocidins, indicating that PVL and HlgACB mediate neutrophil killing. Although none of the single-deletion mutants showed a loss in killing activity after 30 min, a LIVE-DEAD assay carried out to 60 min showed a partial loss of killing activity with ΔlukSF biofilm-spent media, suggesting a more substantial role for PVL vs. HlgACB (SI Appendix, Fig. S2E). These results indicate that, although the expression of PVL or HlgACB may kill neutrophils, the release of both toxins under biofilm growth conditions results in much more rapid killing activity.
To understand whether HlgAB or HlgCB was essential for killing activity, we created double mutants of lukSF combined with hlgA, hlgC, or hlgB mutations. As HlgB is the common F subunit for HlgA and HlgC (31), the ΔlukSFΔhlgB::bursa strain showed a substantial loss of killing activity, comparable to the Δ5 strain (Fig. 2B). In contrast to the ΔlukSFΔhlgC::bursa strain, deletion of hlgA (hlgA::bursa) in the ΔlukSF background resulted in a loss of activity comparable to the ΔlukSFΔhlgB::bursa strain (Fig. 2B). Addition of ΔhlgACB biofilm-spent media (containing PVL) to those from the strains ΔlukSFΔhlgA::bursa,ΔlukSFΔhlgC::bursa or ΔlukSFΔhlgB::bursa significantly restores killing activity, comparable to WT (Fig. 2C). Last, when genes encoding each leukocidin were overexpressed in the ΔlukSFΔhlgACB strain, this resulted in a restoration of neutrophil killing to WT levels (SI Appendix, Fig. S2F). Altogether, these results implicate a role for PVL and HlgAB in biofilm-mediated neutrophil killing.
PVL and HlgAB Induce Formation of NETs.
Calcein AM and EthHD-1 were used to stain neutrophils treated with biofilm-spent media from USA300 strains ΔlukSF and ΔhlgACB. Similar to LIVE-DEAD assays, biofilm-spent media from both strains killed neutrophils. Deletion of hlgA or hlgB in the ΔlukSF mutant resulted in a loss of cell damage, whereas a deletion of hlgC had no such effect. In stark comparison, the ΔlukSFΔhlgACB mutant lost neutrophil killing activity, comparable to a negative control (Fig. 2D). A striking feature observed in neutrophils incubated with WT biofilm-spent media was the ability of media containing PVL or HlgAB to induce the release of nuclear DNA. This was evident from staining with EthHD-1, specific for DNA of damaged or dying mammalian cells. Staining for DNA with EthHD-1 was concomitant with enzymatic activity as seen by staining with Calcein AM, suggesting a controlled mechanism for neutrophil death associated with DNA release. We therefore hypothesized the induction of neutrophil death associated with the release of NETs, or NETosis (19).
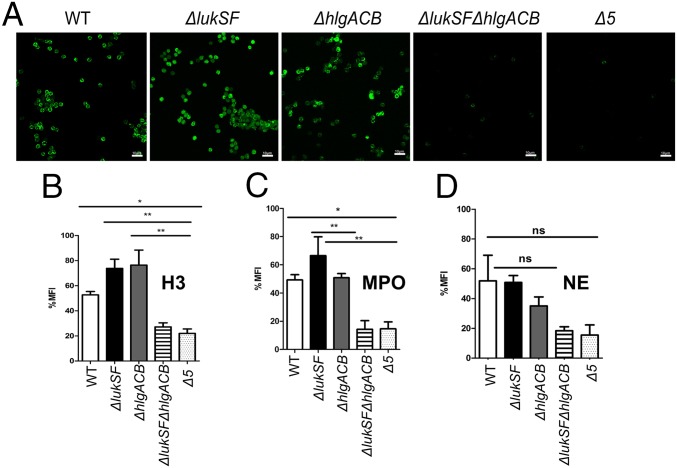
A hallmark of NETosis is the modification of arginines by peptidyl arginine deiminases in chromatin histones to the nonconventional amino acid citrulline (32). To verify NET formation, neutrophils were incubated with biofilm-spent media from WT USA300 and isogenic mutants ΔlukSF, ΔhlgACB, ΔlukSFΔhlgACB, or Δ5 and stained with antibodies against citrullinated H3 histone proteins. In comparison with WT and single-deletion strains, incubation with ΔlukSFΔhlgACB or Δ5 biofilm-spent media resulted in a loss of staining with the anti-citrullinated H3 antibody, comparable to a negative control (Fig. 3A). Either LukSF or HlgACB was sufficient to show significant levels of histone citrullination staining. To understand the contribution of HlgAB or HlgCB to NET formation, we again used mutants in each of the three genes hlgA, hlgB, or hlgC in the ΔlukSF background. Indeed, as seen by staining with antibodies against histone citrullination, deletion of hlgA and hlgB but not hlgC significantly reduced the induction of NETs (SI Appendix, Fig. S3).
Fig. 3.
Biofilm-spent media containing PVL or HlgACB is sufficient for release of NETs. CLSM of neutrophils treated with biofilm-spent media of indicated strains, labeled with an anti-histone H3 (citrulline R2) antibody and Alexa Fluor 488 as a secondary antibody (green, A). Quantification of neutrophil staining with anti-citrullinated histone (B), anti-MPO (C), and anti-NE (D) antibodies after incubation with biofilm-spent media of indicated strains. ImageJ software 5.3 was used to calculate MFI per 100 cells for 10 fields from six independent experiments. Images were captured at a 600× total magnification and represent the majority population phenotype of six independent experiments performed in triplicate ± SEM (*P < 0.1, **P < 0.01, and ***P < 0.001, one-way ANOVA and Tukey’s post hoc analysis; ns, not significant). Neutrophils incubated with HBSS were used as a negative control. (Scale bars: 10 μm.)
Release of myeloperoxidase (MPO) and neutrophil elastase (NE) is also associated with NET formation (33). In support of the results in Fig. 3, neutrophils treated with biofilm-spent media stained with antibodies against MPO (SI Appendix, Fig. S4) and NE (SI Appendix, Fig. S5). Quantification of mean fluorescence intensities from 10 independent fields showed that strains producing PVL or HlgACB had significantly higher staining with antibodies against citrullinated histones (Fig. 3B) and MPO (Fig. 3C) as well as higher levels of staining with antibodies against NE (Fig. 3D). In contrast, mutants lacking both toxins showed negligible levels of fluorescence. We therefore concluded that PVL and HlgAB were required for biofilm-mediated NET formation.
Leukocidins Prevent Bacterial Killing and Biofilm Clearance by Neutrophils.
Our studies indicate that either PVL or HlgAB released from USA300 biofilms is sufficient to kill neutrophils via NETosis. However, neutrophils adhere and penetrate S. aureus biofilms under conditions mimicking physiological shear stress (34). We therefore sought to understand what the consequence of direct neutrophil–biofilm contact would be to bacteria in a biofilm.
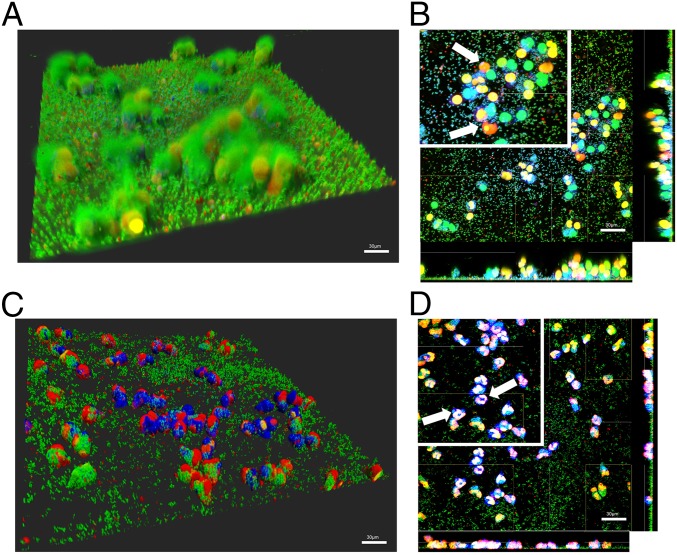
WT (24 h) USA300 biofilms were incubated with CellTracker Blue-labeled neutrophils for 2 h. Bacterial viability was assessed by using Syto 9 (green), and neutrophil death and DNA release was visualized with EthHD-1 (red). After a 1-h incubation with neutrophils, levels of Syto-9 staining suggested that bacteria remain viable while neutrophils release DNA (Fig. 4 C and D, white arrows; Insets show DNA). Remarkably, at 2 h post incubation with neutrophils, biomass levels are comparable to an untreated control (SI Appendix, Figs. S6C and S7D). This was concomitant with EthHD-1–stained neutrophils that penetrate the depth of the biofilm, suggesting that USA300 biofilms kill neutrophils and persist (SI Appendix, Fig. S6D). Similar experiments performed with a USA300LAC13C:PftsAZ:gfp strain constructed with a chromosomal gfp fusion showed GFP activity up to 2 h after incubation with neutrophils, confirming retention of biofilm viability (SI Appendix, Fig. S7 A–C).
Fig. 4.
NET formation does not affect biofilm biomass and viability. Neutrophils (CellTracker Blue) incubated with 24-h WT S. aureus biofilms for 1 h. Viable bacteria were stained with Syto-9 (green), and DNA of membrane-damaged or dead cells were stained with ethidium homodimer-1 (red). Images were collected as a z-stack, representing the thickness of the biofilm (A). Sections through the biofilm were imaged to assess penetration of neutrophils into the biofilm biomass (B). Similar experiments performed with the isogenic Δ5 strain lacking all five leukocidins (C). Section through the biofilm shown in C (D). White arrows are used to highlight nuclear DNA. Images are representative of six independent experiments performed in triplicate. (Scale bars: 30 μm.) (Magnification: B and D, Insets digital zoom, 1.5×.)
To understand the contribution of leukocidins to bacterial survival upon direct contact with neutrophils, we performed similar experiments by using biofilms of the Δ5 strain, lacking all leukocidin genes. In contrast to observations with WT USA300, the Δ5 strain showed reduced levels of bacterial biomass at 30 min (SI Appendix, Fig. S6E) and 60 min (Fig. 4 C and D) post incubation with neutrophils, in comparison with an untreated control (SI Appendix, Fig. S7E). In stark contrast to DNA release observed with WT biofilms, neutrophils treated with Δ5 biofilms retained nuclear structure (Fig. 4D and SI Appendix, Fig. S6F, Inset, white arrows). Biomass of Δ5 biofilms after a 2-h incubation with neutrophils (SI Appendix, Fig. S6 G and H) was comparable to similarly treated ΔlukSFΔhlgAB biofilms (SI Appendix, Fig. S7F) and was significantly lower than the WT (SI Appendix, Fig. S7G).
Leukocidins Released During Chronic Infections Induce NET Formation and Aid Bacterial Survival.
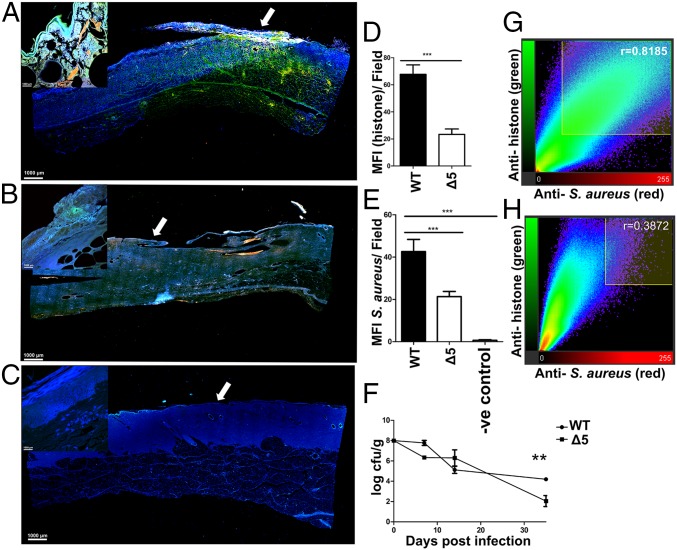
Although our studies suggest that PVL or HlgAB is sufficient to cause NETosis, it is likely that other leukocidins might be playing a role during chronic infections in vivo. Previous studies using a porcine model of chronic burn wound infection have described the establishment of mixed bacterial biofilm communities in the wound bed for as long as 35 d without consequent dissemination of bacteria or development of a systemic infection (11). This model was also found to recapitulate some of the key immunological responses that occur during chronic biofilm S. aureus wound infections in humans (14). To understand the relevance of NET induction by S. aureus biofilms in vivo, we therefore compared infections caused by USA300LAC and Δ5 strains in this previously established porcine full-thickness burn wound model of chronic infection (11). Indeed, by using this model, bacterial biofilm aggregates were observed in the wound bed at 7, 14, and 35 d post infection (SI Appendix, Fig. S8A). Additionally, an anti-S. aureus antibody (red) was used to stain longitudinal sections collected from wounds at 7 d after infection. Longitudinal sections were also stained with the anti-citrullinated histone antibody described here earlier (green) to determine whether host cells released extracellular traps in a leukocidin-dependent manner. In comparison with animals infected with the WT strain (Fig. 5A), those infected with the Δ5 strain (Fig. 5B) showed significantly lower levels of staining with both antibodies. This was comparable to pigs infected with a ΔlukSFΔhlgACB strain, attenuated in the ability to induce NETs (SI Appendix, Fig. S8 B–E), as well as an uninfected control section (Fig. 5 C and D).
Fig. 5.
Leukocidins induce NETs and aid bacterial survival in vivo. Longitudinal sections taken across the wound bed of pigs infected with WT S. aureus USA300LAC (A) and the isogenic Δ5 strain lacking all five leukocidins (B) at day 7 post inoculation. Similar section taken from an uninfected control wound shown for comparison (C). Sections were stained with an anti-citrullinated histone antibody (green) and an anti-S. aureus antibody (red). Tissue cells were stained with DAPI and visualized at 100× total magnification. Images taken at 600× magnification from surface (white arrows) of corresponding wounds A–C (Inset). Mean fluorescence intensities of cells stained with antibodies against citrullinated histone and anti-S. aureus antibodies calculated for 10 independent fields taken across WT (D) and Δ5-infected (E) wounds. Counts of cfu per gram of tissue obtained by biopsy from WT and isogenic Δ5-infected animals (F). Pearson’s coefficients quantifying the degree of colocalization for anti-histone and anti-S. aureus antibodies for 10 fields of view, taken across WT (G) and Δ5-infected wounds (H). Results represent an average of two independent infections per strain performed in triplicate ± SEM (**P < 0.01 and ***P < 0.001, one-way ANOVA and Tukey’s post hoc analysis).
Concurrently, pigs infected with the Δ5 strain showed significantly lower bacterial burdens as assessed by cfu enumeration (Fig. 5 E and F). Last, staining with the anti-histone antibody colocalized with S. aureus and was higher in pigs infected with WT bacteria (Fig. 5G) compared with pigs infected with the Δ5 strain (Fig. 5H). These results demonstrate that the leukocidins PVL and HlgAB induce NETosis and that leukocidins play a role in allowing the persistence of S. aureus during chronic infections in vivo.
Discussion
S. aureus tightly controls the expression of >100 virulence factors, tailored to the host environment and mode of growth (planktonic vs. biofilm), with simultaneous expression of multiple virulence factors allowing for rapid evasion of immune defenses (4, 35). Here we show that biofilms release PVL and HlgAB to induce NETosis. Although low concentrations of PVL induce NETs similar to results described here, high concentrations of the toxin cause necrosis, which might explain the differences between planktonic and biofilm-mediated cell death (36).
There are very few studies that clearly ascribe roles to γ-hemolysins HlgAB and HlgCB (37). The lack of evidence so far might be a result of the low expression levels of the toxin under the planktonic conditions used to perform these studies (35). We show that, when grown as a biofilm, USA300 produces sufficient HlgAB to kill neutrophils. Unlike USA300 that produces PVL and HlgACB, most S. aureus clinical strains are PVL-negative, with 99.5% encoding HlgACB (38). Our studies show that either toxin is sufficient to induce death, which might account for the ability of PVL-negative strains to persist.
When placed in contact with a biofilm, although neutrophils can penetrate and engulf bacteria, large bacterial populations remain viable, with intact neutrophils found close to the biofilm base. This is reminiscent of studies describing “nonlytic” NET formation, whereby release of chromatin leaves anuclear cells still capable of phagocytosis (39). To restrict the spread of large numbers of bacteria, nonlytic NET formation becomes an effective means for neutrophils to engulf and contain/kill microbes while NETs eliminate extracellular bacteria.
Our study describes an important role for released leukocidins in neutrophil-mediated S. aureus biofilm clearance. Specifically, our data support the idea that S. aureus expresses the leukocidins PVL and HlgAB, either of which can induce the release of NETs independent of neutrophil–biofilm contact. When placed in contact with bacterial biofilms, we show that NETosis is inefficient at clearing biofilms in vitro. Together with recently published work by us and others, it is clear that these toxins need to be evaluated as a whole, as they may interact to synergize or antagonize their effects toward host cells (29, 30, 40). Moreover, these toxins also work in concert with other virulence factors. For instance, studies with murine macrophages describe a role for LukAB and α-toxin in killing and preventing phagocytosis of 6-d-old S. aureus biofilms. Similar to our work, these toxins were found exclusively in spent media of biofilms, with planktonic cultures remaining noncytotoxic (17). Similarly, phenol-soluble modulin-α peptides, surfactant molecules released during later stages of biofilm maturation, lyse neutrophils during planktonic growth and could therefore contribute to virulence in vivo (41–43). Although our results indicate that PVL or HlgAB can induce NETosis, the nature of the interaction between these leukocidins, as well the expression patterns of each leukocidin during biofilm formation, likely influences neutrophil killing activity and is therefore important for future studies.
Our results indicate that biofilms retain viable cells and biomass even after exposure to neutrophils. This suggests that S. aureus can circumvent the bactericidal activity of NET antimicrobial proteins. The staphylococcal thermonuclease NucA digests NET DNA, which is converted to nonantimicrobial deoxyadenosine via an S. aureus adenosine synthase (44, 45). Indeed, our MS findings identified NucA in biofilm-spent media, suggesting a role in NET DNA breakdown (SI Appendix, Table S1). The activity of this nuclease, however, can promote biofilm dispersal and might be causing dissemination, leaving viable, phagocytosed bacteria to eventually perpetuate the biofilm (46). Therefore, whether this nuclease plays a role in the dissemination of S. aureus biofilms via the breakdown of NETs in vivo remains to be evaluated.
Last, our in vivo studies corroborate in vitro findings, wherein we show that the leukocidins PVL and HlgAB play an important role in the induction of extracellular trap formation. Of note, these in vivo analyses do not differentiate between neutrophils and other immune cells such as macrophages, monocytes, or dendritic cells. Given the ability of these cell types to release extracellular traps, further studies are required to understand whether leukocidins released from biofilms in vivo can cause trap formation in other host cells (47). Additionally, although these results indicate that PVL and HlgAB play major roles in the induction of extracellular traps in vivo, the contribution of other leukocidins (LukAB, LukED, and HlgCB) as well as other toxins, independent of NETosis, remain to be evaluated.
All together, this study provides evidence of an active mechanism for biofilm-mediated neutrophil killing dependent on leukocidins PVL and HlgAB. More importantly, regardless of physical contact with bacteria, both toxins induce NET formation, a controlled, noninflammatory response. The drastic reduction in biofilm biomass after neutrophil treatment of the Δ5 strain in comparison with WT, as well as the reductions in cell numbers in vivo, suggest that the use of a leukocidin antibody mixture with antibiotic agents could provide a viable therapeutic option to boost the host’s ability to eliminate biofilms.
Materials and Methods
Ethics Statement.
All experiments with animals were reviewed and approved by the institutional animal care and use committee at The Ohio State University (2008A0012-R3). Primary human neutrophils were obtained from healthy adult donors according to the protocol approved by The Ohio State University Biomedical Sciences Institutional Review Board (2009H0314), whereby informed, written consent was obtained from all donors.
Bacterial Strains and Growth Conditions.
Unless otherwise specified, all studies were performed in USA300LAC. Details are provided in SI Appendix.
Spent Media Collection.
To generate spent media for neutrophil-killing assays, isolated colonies were used to inoculate 5 mL trypticase soy broth and grown for 16–18 h at 37 °C under shaking (200 rpm) conditions. These were then allowed to grow to an optical density (OD at 600 nm) of 0.5–0.7. Planktonic (shaking/200 RPM) and biofilm (static) cultures were grown for 24 h, centrifuged and filter sterilized (0.22 µm) (48). Details are provided in SI Appendix.
Neutrophil Isolation.
Neutrophils were isolated from whole blood using previously published methods (49). For details refer to SI Appendix.
LIVE- DEAD Killing Assays.
Neutrophils were incubated with bacterial spent media for 30 min and stained with a 1:1 mixture (by volume) of Syto-9 (3.34 mM) and propidium iodide (20 mM) for 15 min (Thermo Fisher), after which the ratio of fluorescence generated by Syto-9 (excitation, 485 nm/emission, 525 nm) and propidium iodide (excitation, 525 nm/emission, 630 nm) was measured by using a standard fluorometer. For microscopy, neutrophils were stained with 100 µM CellTracker Blue (30 min; Thermo Fisher) followed by a 15-min incubation with spent media and 15 min staining with 2 µM Calcein AM (Invitrogen) and 4 µM ethidium homodimer-1 (Invitrogen). More information is provided in SI Appendix.
Porcine Full-Thickness Burn Wound Model.
Porcine full-thickness burn wound studies were performed per established techniques (11). Details are provided in SI Appendix.
Citrullinated Histone Staining.
For imaging of neutrophils with antibodies against citrullinated histone proteins, 4 × 106 cells per milliliter were stained by using the LIVE-DEAD protocol described earlier and stained with rabbit polyclonal primary antibody against histone H3 (citrulline R2; ab174992; Abcam). Alexa Fluor 488 goat anti-rabbit protein was used as a fluorescent secondary antibody. Details are provided in SI Appendix.
Quantification of Antibody Staining.
Neutrophils were stained with respective antibodies as described earlier. ImageJ software version 5.3 was used to set the same threshold for all conditions, and mean fluorescence intensity (MFI) was calculated per 100 cells for 10 fields from six independent experiments.
Neutrophil–Biofilm Interaction Studies.
Cultures were grown as described for spent media collection, seeded in chambers of a six-channel µ-slide (ibidi), and incubated for 16–18 h (37 °C/static) and treated with neutrophils as described for microscopy analyses.
Quantification and Statistical Analysis.
All statistical analysis was carried out by using GraphPad Prism version 5.0b. One-way ANOVA with Tukey’s least significant difference was used as a post hoc test where appropriate. Details are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Dominique H. Limoli for critique of the manuscript. This work was funded by National Institute of Nursing Research/National Institute of Health (NIH) Award R01NR013898, Cystic Fibrosis Foundation WOZNIA16GO (to D.J.W.), National Institutes of Allergy and Infectious Diseases Awards AI099394 and AI105129 (to V.J.T.), and a Rubicon fellowship from the Netherlands Organization for Scientific Research (to E.T.M.B.). V.J.T. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases. Microscopy was performed at the Campus Microscopy and Imaging Facility at The Ohio State University. Mass spectrometry was performed at the Mass Spectrometry and Proteomics facility at The Ohio State University and is supported by NIH Grant P30 CA16058.
Footnotes
Conflict of interest statement: V.J.T. is an inventor on patents and patent applications filed by New York University School of Medicine, currently under commercial license to Janssen Biotech. The Editor, R.P.N., is also affiliated with New York University School of Medicine.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721949115/-/DCSupplemental.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 3.Ammons MCB, et al. Quantitative NMR metabolite profiling of methicillin-resistant and methicillin-susceptible Staphylococcus aureus discriminates between biofilm and planktonic phenotypes. J Proteome Res. 2014;13:2973–2985. doi: 10.1021/pr500120c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resch A, et al. Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics. 2006;6:1867–1877. doi: 10.1002/pmic.200500531. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti Infect Ther. 2015;13:1499–1516. doi: 10.1586/14787210.2015.1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu Khweek A, et al. Biofilm-derived Legionella pneumophila evades the innate immune response in macrophages. Front Cell Infect Microbiol. 2013;3:18. doi: 10.3389/fcimb.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherr TD, Heim CE, Morrison JM, Kielian T. Hiding in plain sight: Interplay between staphylococcal biofilms and host immunity. Front Immunol. 2014;5:37. doi: 10.3389/fimmu.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: From mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 9.Fazli M, et al. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol. 2009;47:4084–4089. doi: 10.1128/JCM.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjarnsholt T, et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 11.Roy S, et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J Pathol. 2014;233:331–343. doi: 10.1002/path.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho JS, et al. Neutrophil-derived IL-1βis sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voyich JM, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 14.Chaney SB, et al. Histopathological comparisons of Staphylococcus aureus and Pseudomonas aeruginosa experimental infected porcine burn wounds. Wound Repair Regen. 2017;25:541–549. doi: 10.1111/wrr.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonzo F, Torres VJ. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev. 2014;78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spaan AN, van Strijp JAG, Torres VJ. Leukocidins: Staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol. 2017;15:435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherr TD, et al. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. MBio. 2015;6:e01021-15. doi: 10.1128/mBio.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrel M, Perencevich EN, David MZ. USA300 methicillin-resistant Staphylococcus aureus, United States, 2000-2013. Emerg Infect Dis. 2015;21:1973–1980. doi: 10.3201/eid2111.150452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 20.Branzk N, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loughran AJ, et al. Impact of individual extracellular proteases on Staphylococcus aureus biofilm formation in diverse clinical isolates and their isogenic sarA mutants. Microbiology Open. 2014;3:897–909. doi: 10.1002/mbo3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolar SL, et al. Extracellular proteases are key mediators of Staphylococcus aureus virulence via the global modulation of virulence-determinant stability. Microbiology Open. 2013;2:18–34. doi: 10.1002/mbo3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wörmann ME, Reichmann NT, Malone CL, Horswill AR, Gründling A. Proteolytic cleavage inactivates the Staphylococcus aureus lipoteichoic acid synthase. J Bacteriol. 2011;193:5279–5291. doi: 10.1128/JB.00369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 26.Chapman JR, et al. Using quantitative spectrometry to understand the influence of genetics and nutritional perturbations on the virulence potential of Staphylococcus aureus. Mol Cell Proteomics. 2017;16(suppl S1):S15–S28. doi: 10.1074/mcp.O116.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flack CE, et al. Differential regulation of staphylococcal virulence by the sensor kinase SaeS in response to neutrophil-derived stimuli. Proc Natl Acad Sci USA. 2014;111:E2037–E2045. doi: 10.1073/pnas.1322125111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DuMont AL, Torres VJ. Cell targeting by the Staphylococcus aureus pore-forming toxins: It’s not just about lipids. Trends Microbiol. 2014;22:21–27. doi: 10.1016/j.tim.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoong P, Torres VJ. Counter inhibition between leukotoxins attenuates Staphylococcus aureus virulence. Nat Commun. 2015;6:8125. doi: 10.1038/ncomms9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes-Robles T, Lubkin A, Alonzo F, 3rd, Lacy DB, Torres VJ. Exploiting dominant-negative toxins to combat Staphylococcus aureus pathogenesis. EMBO Rep. 2016;17:428–440. doi: 10.15252/embr.201540994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spaan AN, et al. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun. 2014;5:5438. doi: 10.1038/ncomms6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 33.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70:6339–6345. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balasubramanian D, et al. Staphylococcus aureus coordinates leukocidin expression and pathogenesis by sensing metabolic fluxes via RpiRc. MBio. 2016;7:e00818-16. doi: 10.1128/mBio.00818-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilsczek FH, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 37.Spaan AN, et al. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun. 2014;5:5438. doi: 10.1038/ncomms6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Eiff C, Friedrich AW, Peters G, Becker K. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 2004;49:157–162. doi: 10.1016/j.diagmicrobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Yipp BG, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Münzenmayer L, et al. Influence of Sae-regulated and Agr-regulated factors on the escape of Staphylococcus aureus from human macrophages. Cell Microbiol. 2016;18:1172–1183. doi: 10.1111/cmi.12577. [DOI] [PubMed] [Google Scholar]
- 41.Surewaard BGJ, et al. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol. 2013;15:1427–1437. doi: 10.1111/cmi.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DuMont AL, et al. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun. 2013;81:1830–1841. doi: 10.1128/IAI.00095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Periasamy S, et al. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci USA. 2012;109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berends ETM, et al. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342:863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moormeier DE, Bose JL, Horswill AR, Bayles KW. Temporal and stochastic control of Staphylococcus aureus biofilm development. MBio. 2014;5:e01341-14. doi: 10.1128/mBio.01341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Köckritz-Blickwede M, Blodkamp S, Nizet V. Interaction of bacterial exotoxins with neutrophil extracellular traps: Impact for the infected host. Front Microbiol. 2016;7:402. doi: 10.3389/fmicb.2016.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shanks RMQ, et al. Heparin stimulates Staphylococcus aureus biofilm formation. Infect Immun. 2005;73:4596–4606. doi: 10.1128/IAI.73.8.4596-4606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nauseef WM. Isolation of human neutrophils from venous blood. Methods Mol Biol. 2007;412:15–20. doi: 10.1007/978-1-59745-467-4_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.