Significance
A common feature of all domestic animals is their tame behavior and lack of fear for humans. Consistent with this, we have previously demonstrated that genes with a role in brain or neural development have been particularly targeted during rabbit domestication. Here we show, using high-resolution magnetic resonance imaging, that domestic rabbits have an altered brain architecture consistent with reduced emotional processing, including attention to behaviorally relevant stimulation, such as fear detection, learning, expression, and control, as well as compromised information processing. The results, here based on rabbits, are significant for understanding both domestication-induced reorganization of brain architecture and how adaptions in brain territories and networks supporting emotion, cognition, and behavior coincide with an altered behavioral repertoire.
Keywords: rabbit, domestication, brain morphology, magnetic resonance imaging, fear
Abstract
The most characteristic feature of domestic animals is their change in behavior associated with selection for tameness. Here we show, using high-resolution brain magnetic resonance imaging in wild and domestic rabbits, that domestication reduced amygdala volume and enlarged medial prefrontal cortex volume, supporting that areas driving fear have lost volume while areas modulating negative affect have gained volume during domestication. In contrast to the localized gray matter alterations, white matter anisotropy was reduced in the corona radiata, corpus callosum, and the subcortical white matter. This suggests a compromised white matter structural integrity in projection and association fibers affecting both afferent and efferent neural flow, consistent with reduced neural processing. We propose that compared with their wild ancestors, domestic rabbits are less fearful and have an attenuated flight response because of these changes in brain architecture.
Domestic animals show striking changes in behavior compared with their wild ancestors (1). This transformation evolved during the domestication process, and as a result, domestic animals tolerate close contact with humans and can be handled with a reduced risk of triggering flight responses or aggressive behavior. The domestic rabbit is particularly well suited for exploring the relationship between phenotypic and genetic changes associated with domestication because (i) domestication is relatively recent compared with most domesticated animals; (ii) wild rabbits are still abundant in southern France, where domestication took place, enabling comparative studies of wild and domestic rabbits; and (iii) the rabbit is both a domestic animal and an experimental organism well suited for in-depth phenotypic studies. The drastic change in the behavior of the domestic rabbit was well phrased by Charles Darwin, who stated that “no animal is more difficult to tame than the young of the wild rabbit; scarcely any animal is tamer than the young of the tame rabbit” (2).
We previously investigated the genetic basis for domestication using whole-genome sequencing of multiple population samples of wild rabbits from southern France and the Iberian Peninsula, as well as samples representing multiple breeds of domestic rabbits (3). The results demonstrated that phenotypic changes during rabbit domestication evolved as a result of highly polygenic selection, since we observed shifts in allele frequencies at many loci rather than fixed differences at a few domestication loci. Furthermore, genetic changes at evolutionary conserved, noncoding sequences in the vicinity of genes with a role in brain and/or neural development have been particularly important, implying alterations in brain architecture. Whether the brains of domestic and wild rabbits differ substantially, and whether any potential differences are generalized or localized to specific brain areas, remain unknown, however.
Results
Experimental Design.
To investigate the impact of domestication and selection for tameness on brain architecture, we evaluated brain morphology by analyzing gray matter (GM) volume and white matter (WM) microstructure in eight domestic rabbits and eight wild rabbits. GM volume was explored using high-resolution postmortem structural magnetic resonance imaging (MRI) and a region of interest (ROI) approach, together with voxel-based morphometry to assess between-group changes in GM density. WM analysis involved diffusion tensor imaging, used to extract fractional anisotropy maps, reflecting the anisotropicity of water diffusion along and across neural fibers. Tract-based spatial statistics was used to explore differences in fractional anisotropy, associated with alterations in axonal structural integrity and level of myelination (SI Appendix, SI Text and Fig. S1).
Domestic Rabbits Have Reduced Amygdala Size and Increased Medial Prefrontal Cortex Volume.
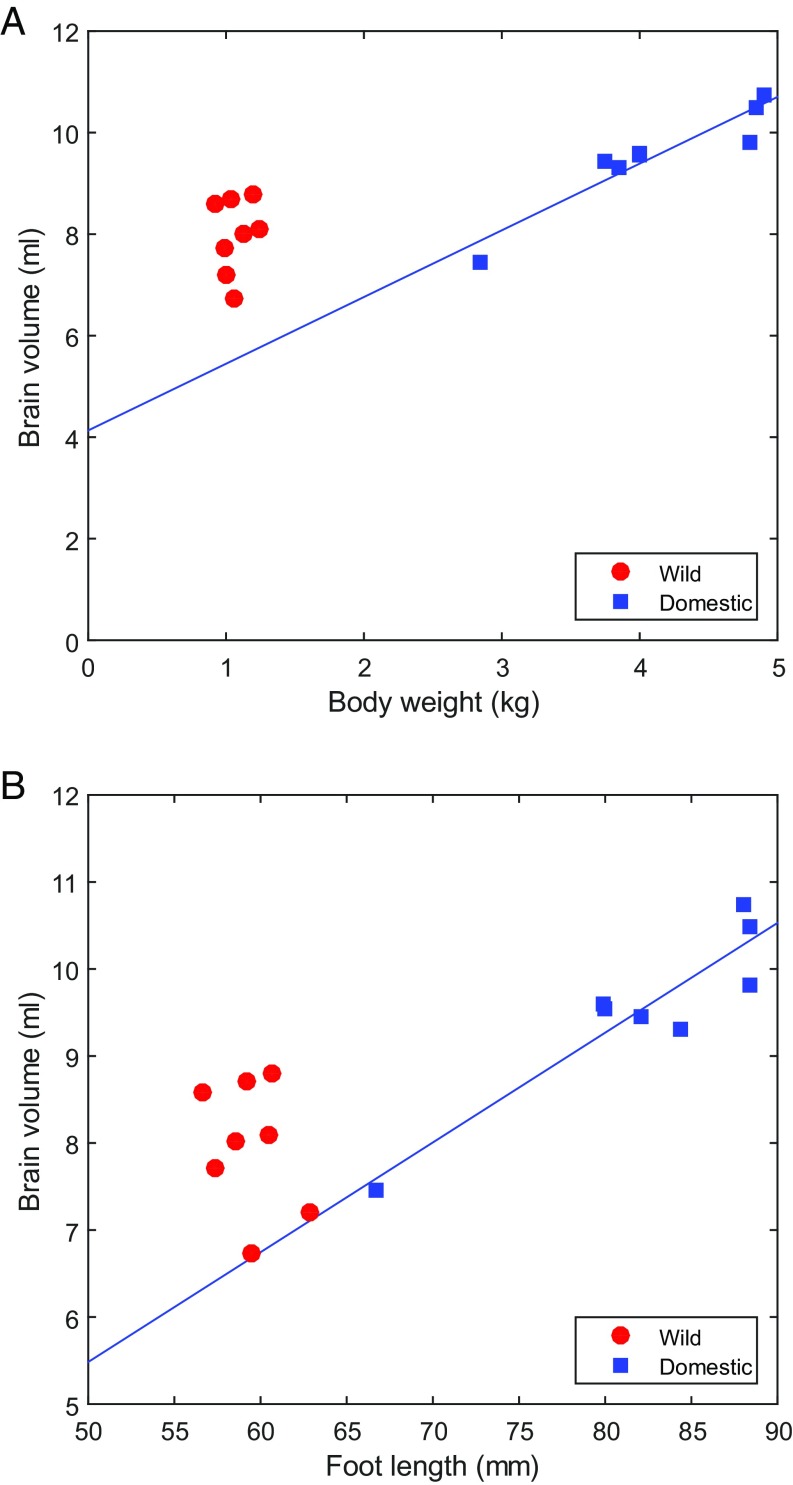
The domestic rabbits were approximately fourfold heavier than the wild rabbits (x ± SEM = 4.12 ± 0.25 kg and 1.07 ± 0.04 kg, respectively), yet had only a slightly larger absolute brain size (total brain volume, 9.55 ± 0.35 mL vs. 7.98 ± 0.26 mL) (Fig. 1A). This resulted in encephalization quotients (the ratio between observed and expected brain volume for a mammal corrected for body weight) of 0.22 ± 0.003 in the domestic rabbits and 0.46 ± 0.02 in the wild rabbits (SI Appendix, SI Text). An altered brain-to-body size ratio was also supported by an analysis comparing brain volume with foot length as an indicator of body size, showing that the altered ratio could not be explained by a difference in body composition (Fig. 1B). This result is consistent with the general observation of a reduced brain-to-body ratio in domestic animals compared with their wild ancestors (4).
Fig. 1.
Relationship between body size and brain volume in wild (red) and domestic (blue) rabbits. Highly significant correlations between body weight and brain volume (A), as well as between brain volume and foot length (B) were noted in domestic rabbits (body weight: r = 0.94, P < 0.001; foot length: r = 0.92, P = 0.001), but not in wild rabbits (body weight: r = 0.18, P = 0.66; foot length: r = −0.30, P = 0.48).
To highlight changes associated with domestication and minimize within-group variability, all brain regions were normalized to the total cerebrum volume (SI Appendix, Supplementary Text). We first performed volumetric analysis for the GM ROIs included in the rabbit brain atlas (5) after visual checks and manual refinements (SI Appendix, SI Text and Fig. S1). For each ROI, we assessed the probability to obtain the observed data by chance, by performing t tests using 5,000 permutations (SI Appendix, SI Text). To protect against false-positive results, we performed Bonferroni corrections taking the number of ROIs considered into account.
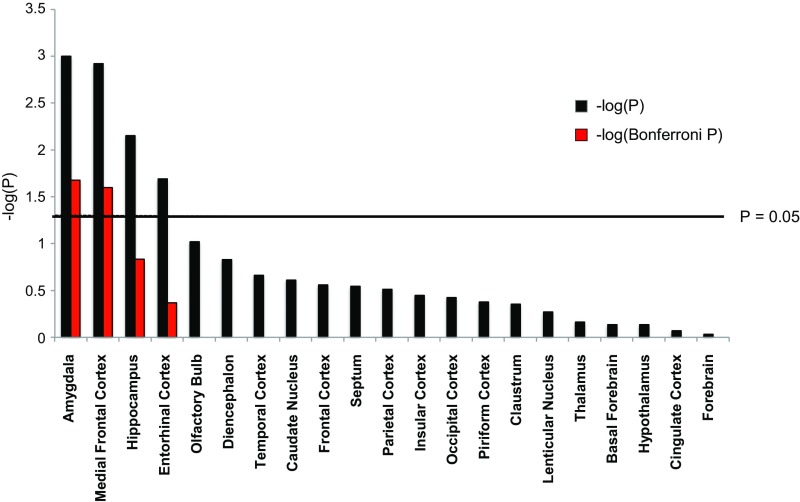
We observed specific and localized, rather than uniform and generalized, GM changes associated with domestication. We noted domestication-induced volume changes, with a bilateral reduction in the size of the amygdala and enlargement of the medial prefrontal cortex the most salient (Fig. 2). The right and left amygdala reductions amounted to −10.1% and −8.7%, respectively, whereas the right and left medial frontal cortex volume increased by 12.1% and 11.1%, respectively (SI Appendix, Fig. S2 and Table S1). Thus, our data show that brain structures that generate and consolidate a negative affect (6), in concert with those that modulate and control fear and anxiety (7, 8), have been primarily targeted during rabbit domestication. Furthermore, suggestive but not statistically significant volume changes were noted in the hippocampus and the entorhinal cortex (Fig. 2 and SI Appendix, SI Text).
Fig. 2.
Summary of volumetric analysis of ROIs in wild and domestic rabbits. The black bars represent significance levels based on 5,000 permutations for each ROI, and the red bars represent Bonferroni-corrected P values taking the total number of independent statistical tests into account. Here results are merged over hemispheres; the results for the left and right hemispheres are given separately in SI Appendix, Fig. S2.
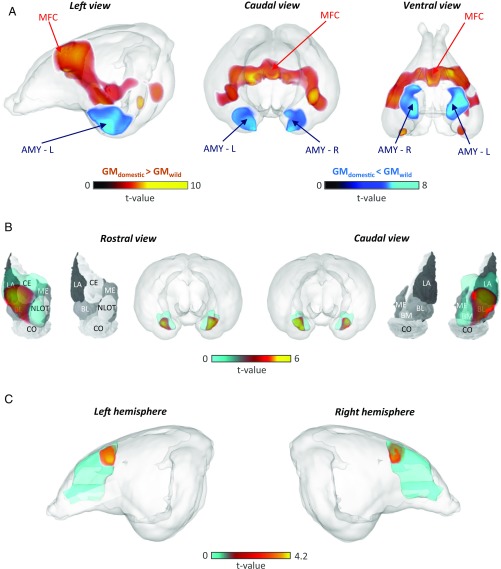
In contrast to ROI analyses, which reveal total GM volume within a ROI, voxel-based morphometry estimates GM density in subregions voxel by voxel, enabling more precise localization (9, 10). In agreement with the aforementioned ROI-based analysis, our voxel-based morphometry analysis also suggested two clusters of structures affected by domestication, one centered around the amygdala showing GM loss and one centered around the frontal cortex showing GM gain (SI Appendix, Table S2). The amygdala-centered clusters extended bilaterally into nearby entorhinal and piriform cortices, as well as into the hippocampus (SI Appendix, Table S2). The cluster of frontal GM increases included the medial frontal cortex and encompassed temporal and parietal areas (Fig. 3 A and C and SI Appendix, Table S2).
Fig. 3.
Specific changes in the size of the amygdala and prefrontal cortex between wild and domestic rabbits. (A) Amygdala (AMY-L and AMY-R, in blue) volume was smaller and medial prefrontal cortex (MFC, in red) volume was larger in domestic rabbits compared with wild rabbits. The two small areas with enhanced volume in domestic rabbits visible in the ventral view are not located entirely inside the cerebral region, but mainly intersect superficial vessel traces, and do not reflect meaningful GM changes. (B) The reduced amygdala volume in domestic rabbits compared with wild rabbits primarily concerns the basolateral (BL), lateral (LA), and central (CE) nuclei; these regions are denoted in red and superimposed on the rabbit nuclei map (11). (Modified from ref. 11.) (C) The medial frontal cortex ROI was enlarged bilaterally in domestic rabbits, with the maximum located dorsally as determined from VBM. The t value statistics in A–C were derived using the threshold free cluster enhancement method (33). L, left; R, right.
To further test whether volume alterations occurred uniformly across the amygdala or were localized to specific subnuclei, we superimposed volume alterations onto rabbit amygdala anatomy (11). Voxel-based analyses demonstrated that reductions within the amygdala were not uniform, but rather were localized to the entire basolateral area and most parts of the central and lateral amygdala (Fig. 3B and SI Appendix, Table S2). These nuclei support efferent and afferent processing, respectively, and the basolateral amygdala is pivotal for forming associations between stimuli (6), supporting that amygdala subregions involved in fear detection, learning, and expression have been targeted during domestication.
WM Alterations in Domestic Rabbits Are Consistent with Reduced Neural Speed and Compromised Information Processing.
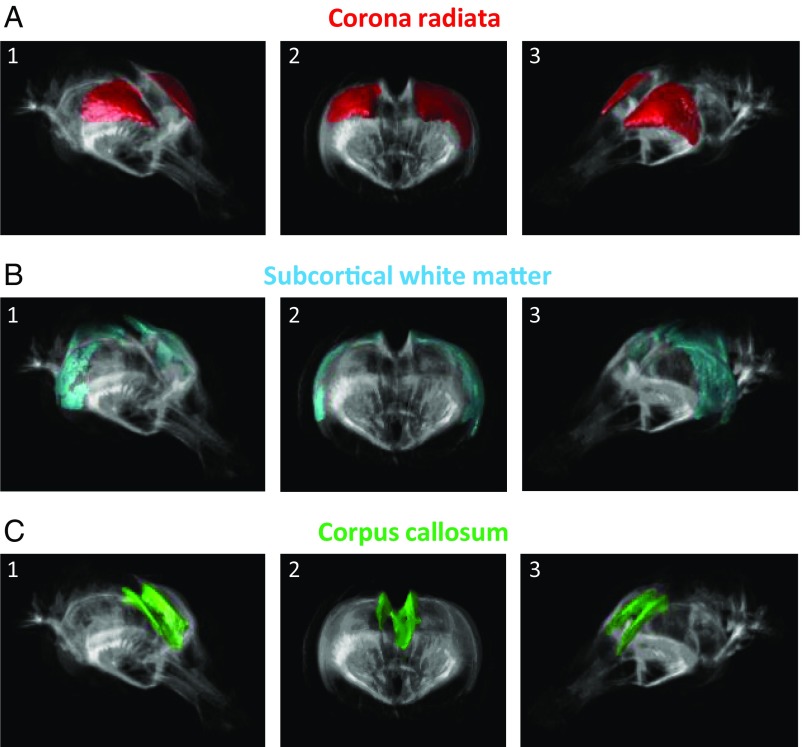
Next, to evaluate WM microstructure alterations, we used tract-based spatial statistics for fractional anisotropy, reflecting myelination, fiber diameter, and density. Permutation tests involving 5,000 runs and corrected for multiple comparisons demonstrated that in domestic rabbits, fractional anisotropy was lower in 86% of the voxels in the corona radiata, in 69% of all voxels in the subcortical WM, and in 71% of the corpus callosum (Fig. 4 and SI Appendix, SI Text). No region displayed significantly higher anisotropy in domestic rabbits compared with wild rabbits. Thus, in contrast to the localized GM reductions and enlargements, WM alterations were unidirectional, uniform, and generalized across multiple fiber tracts. The altered water diffusion profile in the WM is consistent with reduced neural speed and compromised information processing (12) and may mirror reduced fiber density, myelination, and/or axonal diameter. In essence, the basis for neural conduction is compromised in domestic rabbits. Such alterations may be developmentally important, given that dysgenesis of the corpus callosum in humans is associated with mental retardation (13). Because ascending projections and the descending projections between the cortex and corticopontine, corticobulbar, and corticospinal tracts involve the corona radiata (14), our results support that genetic changes during rabbit domestication compromises WM functions in both association and projection fibers. This implicates reduced information processing capacity both between and within hemispheres, affecting both afferent and efferent processes.
Fig. 4.
WM microstructure differs between wild and domestic rabbits. More than 50% of the voxels in the WM regions corona radiata (A), subcortical WM (B), and corpus callosum (C) displayed reduced fractional anisotropy in domestic rabbits. Areas within each principal WM structure are highlighted in color and shown as (1) tilted frontal side view (Right) of the rabbit brain, (2) rostral frontal view, and (3) tilted frontal side view (Left). These findings support that domestication compromises WM integrity in both association and projection fibers. This affects afferent and efferent neural flow of the rabbit brain, implicating reduced information processing capacity both between and within hemispheres.
Discussion
Previous studies have uncovered a generally reduced brain volume relative to body size in domestic animals compared with their wild ancestors (4). The present study based on high-resolution MRI confirmed a reduced brain-to-body size in domestic rabbits and allowed us to compare the relative sizes of 54 brain ROI. This revealed a striking reduction in the size of the amygdala but also a profound relative enlargement of the medial prefrontal cortex in domestic rabbits compared with wild rabbits. Fear conditioning, when an environmental threat is predicted by an external cue, is present in all mammals and processed in the amygdala. In humans, a relatively larger amygdala facilitates fear conditioning (15), and amygdala enlargement results in or from certain anxiety disorders (16). In contrast, enlargement of the medial frontal cortex facilitates extinction, the process of fear reduction through safety learning (16, 17), and is generally associated with enhanced emotional control (7, 15, 16). Interestingly, a previous study in rabbits showed that electrical activation of the area in the medial frontal cortex corresponding to the enlarged area in domestic rabbits inhibited expression of conditioned responses, supporting its functional role in attenuating the expression of learned behaviors (18). The amygdala prefrontal network also influences aggression (19), and amygdala activity is pivotal for detecting behaviorally relevant stimulation, reflecting its role in salience processing. Of note, automated reflexive behaviors supported by the amygdala, such as the defense response (20), are apparently attenuated in domestic rabbits adapted to a life in captivity and in close contact with humans, where strong flight responses are maladaptive and there is less need for predatory-mediated fight-or-flight behavior. Moreover, selective breeding for tame and tolerant animals is expected to favor nonaggressive and nonattentive individuals, in contrast to natural selection, which favors aggressive, avoidant, and attentive wild rabbits. Collectively, these behaviors reflect detection of behaviorally relevant stimulation, suggestive of attenuated salience processing as an effect of domestication. Because we used conservative Bonferroni corrections, the risk for committing type I errors is reduced, but at the same time the risk for Type II errors is increased. Thus, false-positive results are unlikely, but false-negative results might occur, meaning that we might have missed true alterations in some brain territories.
In conclusion, rabbit domestication has not affected the GM of the brain uniformly, but has led to circumscribed alterations, enlarging areas involved in control of emotional behavior and diminishing areas relevant for emotional memory and reflexive and learning-related fear processing. In contrast, WM alterations were more widespread, affected both projection and association fibers involved in neural afferent and efferent processes, and, we speculate, implicate reduced communication between hemispheres as well as hampered top-down control. Thus, our study based on high-resolution postmortem MRI demonstrates that the brain architecture is remodeled in domestic rabbits, showing that genetic changes underlying successful domestication reshape the structural foundation for brain function.
Materials and Methods
Animal Husbandry.
All of the animals used in this experiment were born and raised in the Research Center of Wild Lagomorphs (REGA-ES1402100962), a facility in Córdoba, Spain registered for the breeding and use of experimental animals accredited by the regional government (Junta de Andalucía) in accordance with the European Union guidelines for animal welfare. Female domestic rabbits were kept in 0.65 × 0.35-m metal cages under standard housing conditions. As female wild rabbits are more susceptible to stress, they were housed in 2 × 3-m enclosures with access to the ground and wood cages to function as shelters. Both wild and domestic rabbits had unrestricted access to food and water. The experimental procedures were reviewed and approved by the Ethical Committee for Animal Research of the Consejo Superior de Investigaciones Científicas (Register Project CGL2013-43197-R), in agreement with the guidelines and regulations concerning animal welfare and experimentation set forth by Spanish law.
Cardiovascular Perfusion Fixation.
Eight wild and eight domestic female rabbits were selected for the imaging studies. To avoid breed-specific effects, we sampled domestic rabbits of three different breeds: New Zealand white, Californian, and French lop. Rabbits were deeply anesthetized with an i.m. injection of a mixture of xylazine (Rompun, 8 mg/kg; Bayer) and ketamine (Imalgéne 1000, 40 mg/kg; Merial) and then euthanized with an intracardiac injection of thiopental (Tiopental 0.5 g, 100 mg/kg; B. Braun). Next, using a peristaltic pump (TPU2AD; Aalborg) we first used a PBS solution to flush the vascular system free of blood, followed by cardiovascular perfusion fixation using a 10% solution of neutral buffered formalin (NBF) containing 10% (50 mM) gadoteridol (ProHance; Bracco Diagnostics), a magnetic resonance imaging contrast agent (21, 22). After perfusion, heads were separated, and skin and muscle around the skulls were removed. Brains were maintained inside the cranium to preserve the natural shape, and were then transferred to 10% NBF containing 1% of gadoteridol at 4 °C until imaging analysis.
Image Acquisition.
Each formalin-fixed rabbit head was placed in a condom and immersed in Fomblin (Solvay Solexis), an inert perfluorinated oil, providing a black background for the MRI images with magnetic susceptibility similar to that of biological tissue. Each sample was imaged at 9.4 T in a horizontal bore scanner (Varian) equipped with a gradient system with an inner diameter of 120 mm, a maximal gradient strength of 60 G/cm, and a birdcage coil with an inner diameter of 72 mm (Rapid Biomedical). Mainly T1-weighted (T1W) 3D gradient echo MRI images were acquired to a voxel size of 100 μm using the following parameters: field of view, 64 × 51.2 × 51.2 mm3; matrix, 640 × 512 × 512; recovery time, 8.01 ms; echo time, 4.02 ms; flip angle, 20°; and four excitations. The scan time was 2 h and 20 min for each head. Diffusion-weighted MRI was performed using a 3D spin echo sequence with diffusion-coding gradients in 12 directions, and two reference scans without diffusion weighting were obtained. The following parameters were used: field-of-view, 80 × 43 × 43 mm3; matrix, 512 × 192 × 192; recovery time, 90 ms; echo time, 13.24 ms; b value, 1,000 s/mm2; 16 dummy scans, and one excitation. The scan time was 12 h and 54 min.
Image Registration and Segmentation.
Brain extraction (i.e., removal of the skull from the image) was performed on each anatomic image (T1W image) by an experienced human observer unaware of group designation, using an interactive level-set method (23). Subsequently, for each rabbit, all T1W data were manually registered to diffusion data. Fractional anisotropy (FA) maps were obtained for each rabbit using FMRIB’s Diffusion Toolbox, a software tool for analysis of diffusion MRI images that is part of FSL (FMRIB Software Library) (24, 25). Finally, to improve alignment to the T1W data, automatic rigid registrations were performed on each FA map using the ITK toolbox (https://itk.org/).
After image alignment, GM and WM parcellation were performed by applying an automatic atlas-based segmentation method using the rabbit brain atlas (5). The atlas-based segmentation, including a total of 60 ROIs, was carried out using NiftyReg (26, 27). Further manual refinements of the segmentation of some brain structures of interest (medial frontal cortex, caudate nucleus, amygdala, and hippocampus; SI Appendix, Fig. S1) were performed to improve the segmentation quality because of the resolution difference between the data acquired and the atlas used (0.16 × 0.17 × 0.17 mm vs. 0.15 × 0.15 × 7 mm). Refinements generally reduced the size of the ROIs, resulting in increased anatomic precision. Cerebrum volume was calculated by subtracting the following six volumes from the total brain volume: the pons, medulla, left and right cerebellar hemispheres, vermis, and mesencephalon. In this way, 54 ROIs in the cerebrum were obtained. Between-group differences in ROI volumes were evaluated using MATLAB release 2016b (MathWorks).
Voxel-Based Morphometry.
Between-group differences in GM volume were analyzed with FSL-VBM (28) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimized voxel-based morphometry (VBM) protocol (9) implemented within FSL. The protocol, tailored for human brain studies, was slightly modified and adapted to rabbit brain images. First, the GM segmentation of a reference rabbit (randomly chosen as one of the eight wild rabbits) was blurred with a discrete Gaussian filter of variance 0.1 mm2 to create an initial reference segmentation. Then, a study-specific GM template was created by nonlinearly registering the GM segmentations of each rabbit to the reference and averaging them. Subsequently, all native GM images were nonlinearly registered to this study-specific template to correct for local expansion (or contraction) due to the nonlinear component of the spatial transformation. The modulated GM images were smoothed with an isotropic Gaussian kernel with a sigma of 1 mm, and finally, statistical evaluations were performed using a voxelwise generalized linear model and permutation-based nonparametric testing with a total of 5,000 permutations, thus correcting for multiple comparisons. Two types of statistics were calculated, GMdomestic > GMwild and GMwild > GMdomestic, and voxels showing a permutation-corrected P value < 0.05 were considered significant.
FA Analysis.
To characterize the microstructural basis of WM and evaluate FA differences between domestic and wild rabbits in the major tracts of the brain, we used tract-based spatial statistics (TBSS) (29), also part of FSL. Similar to the analyses with FSL-VBM, the whole FSL-TBSS pipeline was adapted to the rabbit brain images. FA maps were aligned to a reference FA map of the same wild rabbit used to reference the FSL-VBM analyses by applying the nonlinear registration tool FNIRT (30, 31), which uses a b-spline representation of the registration warp field (32). Next, the mean FA image was created and thinned to create a conservative mean FA skeleton representing the centers of all major tracts in the rabbit brain atlas. The WM regions of the atlas include the left and right periventricular WM, internal capsule, corona radiata, fimbria of the hippocampus, fornix, subcortical WM external capsule, corpus callosum, and anterior commissure. Each animal’s aligned FA data were then projected onto this skeleton, and the resulting data were fed into voxelwise cross-subject statistics, again using permutation tests with 5,000 permutations. Two types of statistics were calculated, FAdomestic > FAwild and FAwild > FAdomestic. To define the location of significant group differences, we identified all WM areas with P < 0.05 in the rabbit brain atlas.
Statistical Analysis.
Between-group differences in ROI volumes were evaluated using t tests with 5,000 permutations. The statistical tests on VBM and TBSS results were carried out using the threshold free cluster enhancement method implemented in FSL (33), as specified in the online user guide. To protect from false positives, permutation tests using 5,000 permutations were performed for all analysis, both when evaluating GM volume and fractional anisotropy. Each hemisphere was analyzed separately. Variability between groups was evaluated and compared using Hartley’s Fmax test (34), while independent t tests were used for weight, hind foot, and body size, as well as total brain volume. In all analyses, P < 0.05 was the significance threshold.
Supplementary Material
Acknowledgments
The study was funded by the Knut and Alice Wallenberg foundation (L.A.), the Swedish Research Council (L.A. and M.F.), the Swedish Brain Foundation (M.F.), and POPH-QREN funds from the European Social Fund and Portuguese Ministry of Science, Technology and Higher Education (FCT Investigator Programme IF/00283/2014/CP1256/CT0012 and Postdoctoral Grant SFRH/BPD/65464/2009, to J.A.B.-A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801024115/-/DCSupplemental.
References
- 1.Jensen P. Behavior genetics and the domestication of animals. Annu Rev Anim Biosci. 2014;2:85–104. doi: 10.1146/annurev-animal-022513-114135. [DOI] [PubMed] [Google Scholar]
- 2.Darwin C. 1859. Instinct. On the Origins of Species by Means of Natural Selection or the Preservation of Favoured Races in the Struggle for Life (John Murray, London), Chap 7.
- 3.Carneiro M, et al. Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science. 2014;345:1074–1079. doi: 10.1126/science.1253714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruska DCT. On the evolutionary significance of encephalization in some eutherian mammals: Effects of adaptive radiation, domestication, and feralization. Brain Behav Evol. 2005;65:73–108. doi: 10.1159/000082979. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz-Moreno E, et al. A magnetic resonance image-based atlas of the rabbit brain for automatic parcellation. PLoS One. 2013;8:e67418. doi: 10.1371/journal.pone.0067418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agren T, et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012;337:1550–1552. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- 7.Urry HL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Good CD, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 10.Ashburner J, Friston KJ. Voxel-based morphometry: The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 11.Równiak M, et al. The morphometric study of the amygdala in the rabbit. Folia Morphol (Warsz) 2007;66:44–53. [PubMed] [Google Scholar]
- 12.Turken A, et al. Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42:1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul LK, et al. Agenesis of the corpus callosum: Genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- 14.Kandel E, Schwartz J, Jessell T, Siegelbaum S, Hudspeth A. Principles of Neural Science. 5th Ed McGraw-Hill Education; New York: 2012. [Google Scholar]
- 15.Winkelmann T, et al. Brain morphology correlates of interindividual differences in conditioned fear acquisition and extinction learning. Brain Struct Funct. 2016;221:1927–1937. doi: 10.1007/s00429-015-1013-z. [DOI] [PubMed] [Google Scholar]
- 16.Hilbert K, Lueken U, Beesdo-Baum K. Neural structures, functioning and connectivity in generalized anxiety disorder and interaction with neuroendocrine systems: A systematic review. J Affect Disord. 2014;158:114–126. doi: 10.1016/j.jad.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Leal-Campanario R, Fairén A, Delgado-García JM, Gruart A. Electrical stimulation of the rostral medial prefrontal cortex in rabbits inhibits the expression of conditioned eyelid responses but not their acquisition. Proc Natl Acad Sci USA. 2007;104:11459–11464. doi: 10.1073/pnas.0704548104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation: A possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 20.Björkstrand J, et al. Disrupting reconsolidation attenuates long-term fear memory in the human amygdala and facilitates approach behavior. Curr Biol. 2016;26:2690–2695. doi: 10.1016/j.cub.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Johnson GA, Cofer GP, Gewalt SL, Hedlund LW. Morphologic phenotyping with MR microscopy: The visible mouse. Radiology. 2002;222:789–793. doi: 10.1148/radiol.2223010531. [DOI] [PubMed] [Google Scholar]
- 22.Johnson GA, Calabrese E, Badea A, Paxinos G, Watson C. A multidimensional magnetic resonance histology atlas of the Wistar rat brain. Neuroimage. 2012;62:1848–1856. doi: 10.1016/j.neuroimage.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Frimmel H, Smedby Ö. Fast level-set based image segmentation using coherent propagation. Med Phys. 2014;41:073501. doi: 10.1118/1.4881315. [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 26.Modat M, et al. Fast free-form deformation using graphics processing units. Comput Methods Programs Biomed. 2010;98:278–284. doi: 10.1016/j.cmpb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Modat M, et al. Global image registration using a symmetric block-matching approach. J Med Imaging (Bellingham) 2014;1:024003. doi: 10.1117/1.JMI.1.2.024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douaud G, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Andersson J, Jenkinson M, Smith S. 2007 Non-linear registration, aka spatial normalization. FMRIB Technical Report TR07JA2. Available at: https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja2/tr07ja2.pdf. Accessed June 3, 2018.
- 31.Andersson J, Jenkinson M, Smith S. 2007 Non-linear optimization. FMRIB Technical Report TR07JA1. Available at: https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja1/tr07ja1.pdf. Accessed June 3, 2018.
- 32.Rueckert D, et al. Nonrigid registration using free-form deformations: Application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 34.Hartley HO. The maximum F-ratio as a short-cut test for heterogeneity of variance. Biometrika. 1950;37:308–312. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.