Abstract
Purpose
Little is known about how the structure of interdisciplinary groups of physicians affects the timeliness of breast cancer surgery their patients receive. We used social network methods to examine variation in surgical delay across physician peer groups and the association of this delay with group characteristics.
Methods
We used linked Surveillance, Epidemiology, and End Results-Medicare data to construct physician peer groups based on shared breast cancer patients. We used hierarchical generalized linear models to examine the association of three group characteristics, patient racial composition, provider density (the ratio of potential vs. actual connections between physicians), and provider transitivity (clustering of providers within groups), with delayed surgery.
Results
The study sample included 8338 women with breast cancer in 157 physician peer groups. Surgical delay varied widely across physician peer groups (interquartile range 28.2–50.0%). For every 10% increase in the percentage of black patients in a peer group, there was a 41% increase in the odds of delayed surgery for women in that peer group regardless of a patient’s own race [odds ratio (OR) 1.41, 95% confidence interval (CI) 1.15–1.73]. Women in physician peer groups with the highest provider density were less likely to receive delayed surgery than those in physician peer groups with the lowest provider density (OR 0.65, 95% CI 0.44–0.98). We did not find an association between provider transitivity and delayed surgery.
Conclusions
The likelihood of surgical delay varied substantially across physician peer groups and was associated with provider density and patient racial composition.
Keywords: Breast cancer care, Surgery delay, Network transitivity, Network density
Background
Despite considerable progress in breast cancer treatment, many women with breast cancer fail to receive the highest quality of care, including prompt surgical treatment [1–4]. Delays in surgical care for breast cancer have been linked to higher overall mortality as well as increased patient distress and anxiety [5, 6]. Prior work exploring factors associated with delayed cancer care has largely focused on patient factors [7–10]. Little is known, however, about whether or how provider factors, including the relationships between different providers, influence the risk of delayed surgery.
Breast cancer care is collaborative, involving interdisciplinary care delivered across multiple locations and in different settings. Delays between a woman’s diagnosis and her surgical treatment may be a result of several factors that involve not only individual physicians but also the relationship between different physicians in the larger network of physicians providing care in a given area. Physicians frequently seek advice from other providers with whom they work closely to guide their treatment decisions [11], and delays may result from different levels of coordination across physicians.
Increasingly, social network analysis has been used to study how the relationships between physicians impact the delivery of cancer care. For example, prior research using social networks has linked different groups of tightly connected physicians (often termed physician peer groups) with the uptake of imaging for breast cancer [12], and variation in the type of treatments provided for prostate cancer [13]. Moreover, the network structure and patient composition of physician peer groups have been linked to complications following prostate cancer surgery [13]. Because peer groups are built from collaborative relationships and represent the structure of health care delivery across different practices or hospital systems, it is plausible that peer group characteristics could be associated with timeliness of cancer care.
The racial composition of patients within physician peer groups is one factor that could plausibly influence surgical delay. Despite well-established evidence on racial disparities in healthcare [8], little is known about how the composition of patients within provider groups may be associated with care. Prior work has demonstrated that physicians who care for a higher proportion of black patients are more likely to be less clinically trained and have access to fewer high quality clinical resources [14]. Hence, regardless of their individual race, patients treated by physician peer groups that care for a large portion of minority patients may be more likely to experience surgical delays [15, 16].
We also seek to add to the literature by studying the association between the structure of physician peer groups and surgical delay. Physician peer groups with a higher proportion of connections between providers, also known as provider density, may provide more coordinated care [17]. This could result in fewer patients experiencing surgical delays. Similarly, physician peer groups with higher provider transitivity, or increased clustering of providers who work together in “cliques”, may work more collaboratively in tightly knit patient-sharing groups and provide more expedient care [18].
We sought to examine whether there are variations in delayed surgery after needle biopsy across physician peer groups and to assess whether specific characteristics of physician peer groups—the racial composition of patients, provider density, and provider transitivity—were associated with likelihood of breast cancer surgery delays.
Methods
Study design and data source
We conducted a retrospective cohort study of Medicare beneficiaries who received breast surgery, either lumpectomy or mastectomy, after breast cancer diagnosis. Our outcome variable was delay between needle biopsy and surgery. We obtained data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database, which contains Medicare claims data, patient demographic data, and tumor-specific cancer registry data. We used these data to identify patient-sharing physician peer groups and assessed how characteristics of these peer groups were associated with breast cancer surgical delays. The Yale Human Investigations Committee determined that this study did not directly involve human subjects. The data that support the findings of this study are available from the National Cancer Institute but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Study sample
Our study sample included 8338 women with breast cancer who met the following criteria: received a needle biopsy before breast cancer surgery, age 66–94 years old at diagnosis, known month of diagnosis, first or only diagnosis of stage I–III breast cancer of epithelial origin between 2007 and 2009, and continuously enrolled in Medicare Parts A and B from 1 year prior to diagnosis to 1 year after diagnosis or until death. We excluded women who received neoadjuvant chemotherapy since this would be a clinically justified reason for delaying surgery. Women were assigned to physician peer groups based on the surgeon who performed their breast cancer surgery.
Outcome variable
Consistent with prior literature and based on clinical experience, we defined delayed breast cancer surgery as a period of > 30 days between needle biopsy and surgery [5].
Patient characteristics
Patient sociodemographic characteristics included in the analysis were age at diagnosis, race/ethnicity, marital status, and median household income. Household income was based on area-level measures (census tract if available, otherwise zip code) from the 2000 Census. Tumor characteristics included hormone (estrogen or progesterone) receptor status, nodal status, grade, stage, and size. We measured patient comorbidity between 12 and 1 month prior to diagnosis using a modified list of comorbidity conditions suggested by Elixhauser et al. [19, 20]. Lastly, we included type of breast surgery and whether a patient had a visit with a primary care provider (PCP) in the year prior to diagnosis as an indicator for access to care.
Construction of physician peer groups
Our method for constructing physician peer groups has been previously described [12]. Briefly, for each hospital referral region, we created regionally based local networks of breast cancer providers using the validated Girvan–Newman algorithm [21, 22]. Using this algorithm, we divided networks into smaller, mutually exclusive physician peer groups by removing edges (i.e., physician ties) with high betweenness scores and determining the optimum number of local networks through modularity, or a goodness-of-fit test [21, 22]. We considered providers connected to one another if they billed care for ≥ 2 overlapping patients in the 3 months prior to diagnosis through 9 months after.
Building upon prior work, our construction of physician peer groups used a sample of women who received their first diagnosis of stage 0–III breast cancer from 2004 to 2006 and non-cancer patients from the Medicare 5% sample who resided in SEER regions during the same period [12]. Non-cancer patients were randomly assigned an index date, which was used analogously to the date of diagnosis [12]. Using Medicare claims, we identified all surgeons, radiologists, radiation oncologists, medical oncologists, and primary care providers who provided care for any of these women, and used the Girvan–Newman algorithm to construct subsets (“peer groups”) of these physicians who treated the same patients. We excluded physicians who had fewer than five cancer or non-cancer patients and peer groups with fewer than two surgeons or fewer than 20 patients receiving needle biopsy and surgery.
Peer group characteristics
We sought to examine whether the racial composition of women with breast cancer in a peer group was associated with an increased odds of surgery delay. To avoid collinearity between patient race and peer group racial composition in our regression models, we partitioned the patient level race variable into a within group and between group effect, following prior research [23]. Specifically, we first coded each patient as black (1) or white/other race (0). We then subtracted the percent of black patients in a patient’s peer group from her race dummy variable. Using this newly created patient-level race variable in our models along with our peer group-level percent black patients allowed us to separate the individual-level and peer group-level effect of race in our analysis.
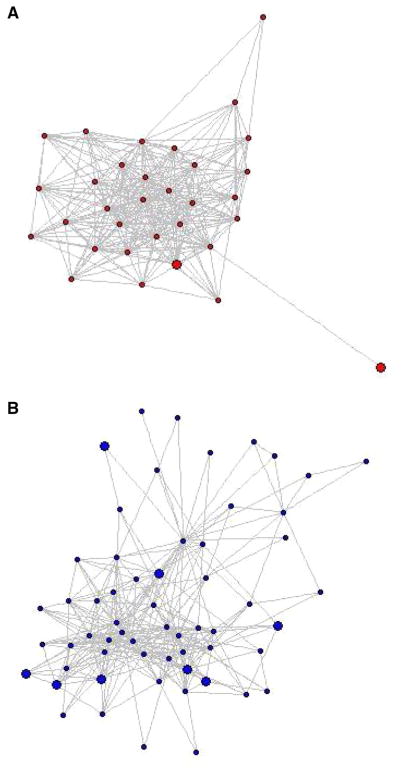
We also examined two structural characteristics of physician peer groups: provider density and transitivity. Density measures the likelihood that all physicians in a peer group are connected to one another. Formally, it is assessed by dividing the number of observed connections (via shared patients) by the total number of possible connections in a physician peer group. Figure 1a, b depict differences in provider density between two physician peer groups included in the analysis. The small black circles, which we refer to as nodes, represent providers and the lines between providers represent the patients who connect them. The model in Fig. 1a has greater network density compared to the model in Fig. 1b because there are more connections between the different nodes.
Fig. 1.
Models of physician peer groups depicting provider density. The colored circles are nodes that represent providers and the lines between the providers represent the shared patients. a Higher density (0.54) and more connections. b Lower density (0.18) and fewer connections
Transitivity is the probability that for every instance one physician is connected to two other physicians, those two other physicians are also connected. That is, if physician A and B share patients, and physician B and C share patients, then transitivity represents the probability that physician A and C also share patients. Hence, provider transitivity takes into account providers who work closely together in cliques or triads, in which all providers within the clique are more likely to work together. Transitivity was calculated for all the physicians and for different physician specialties (PCPs and surgeons), then averaged over all physicians in the group. Provider density and transitivity are continuous variables ranging from 0 to 1; we divided these structural measures into quintiles for analysis.
Statistical analysis
We used descriptive statistics to summarize characteristics of the patients and physician peer groups, and conducted Chi square tests to examine bivariate associations of sociodemographic and tumor characteristics with surgical delay. To assess the multivariable relationship between the physician peer group characteristics specified above and delayed surgery, we estimated hierarchical generalized linear models (HGLM) with a logit response and a random effect for peer group. Variables in the HGLM were selected by first estimating unadjusted models including one patient or peer group characteristic at a time and only considering those that were statistically significant at p < 0.10 for the final adjusted model. We then used a backward elimination strategy to determine the reduced or most parsimonious model. We iteratively removed variables that had a Wald’s p value > 0.05 or that did not have an odds ratio with a p value < 0.05 in any of their categories. For each peer group, we calculated the predicted probability of delayed surgery at the mean of all covariates included in the model. All analyses were conducted using SAS 9.4, R 3.2, igraph 0.9, and Stata SE 14.
Results
Study sample and patient characteristics
Our final study sample consisted of 8338 cancer patients assigned to 157 physician peer groups. More than 90% of the women were white, and the majority were hormone receptor positive (83.8%), had stage I breast cancer (62.0%), had a tumor smaller than 2 cm (67.4%), and had undergone breast-conserving surgery (63.4%) (Table 1).
Table 1.
Patient characteristics
| Characteristic | N (%)a |
|---|---|
| Age (years) | |
| 67–69 | 1981 (23.8) |
| 70–74 | 2137 (25.6) |
| 75–79 | 1900 (22.8) |
| 80–84 | 1457 (17.5) |
| 85–89 | 863 (10.4) |
| Race | |
| White/otherb | 7786 (93.4) |
| Black | 552 (6.6) |
| Income quintile ($) | |
| 0 ≤ to < 33,000 | 1345 (16.1) |
| 33,000 ≤ to < 40,000 | 1223 (14.7) |
| 40,000 ≤ to < 50,000 | 1863 (22.3) |
| 50,000 ≤ to < 63,000 | 1773 (21.3) |
| 63,000 ≤ | 2134 (25.6) |
| Marital status | |
| Married | 3895 (46.7) |
| Not married | 4176 (50.1) |
| Unknown | 267 (3.2) |
| Comorbid conditions | |
| 0 | 4595 (55.1) |
| 1–2 | 2961 (35.5) |
| ≥ 3 | 782 (9.4) |
| Breast surgery type | |
| Lumpectomy | 5288 (63.4) |
| Mastectomy | 3050 (36.6) |
| Hormone receptor status | |
| Negative | 1070 (12.8) |
| Positive | 6985 (83.8) |
| Unknown | 283 (3.4) |
| Tumor grade | |
| I | 2303 (27.6) |
| II | 3785 (45.4) |
| III/IV | 1987 (23.8) |
| Unknown/missing | 263 (3.2) |
| Tumor stage | |
| I | 5165 (62.0) |
| II | 2566 (30.8) |
| III | 607 (7.3) |
| Tumor size (cm) | |
| < 2.0 | 5604 (67.4) |
| 2.0–5.0 | 2455 (29.5) |
| > 5.0 | 254 (3.1) |
| Node status | |
| Positive | 1828 (21.9) |
| Negative/unknown | 6510 (78.1) |
| PCP visit in year before diagnosis | |
| Yes | 7908 (94.8) |
| No | 430 (5.2) |
All percentages are column percentages
Patients coded as other race are included in the white category
Peer group characteristics
Physician peer groups had a median of 81 physicians and < 11 surgeons (Table 2). The number of women with breast cancer in the physician peer groups ranged from 20 to 245, with a median of 42 (interquartile range (IQR) 39). The median proportion of women who experienced delayed breast cancer surgery after needle biopsy across the physician peer groups was 0.35 (IQR 0.25). The median proportion of black women with breast cancer across the physician peer groups was 0.03 (IQR 0.08). Of the 157 physician peer groups, 51 did not contain any black women with breast cancer. Provider density ranged from 0 to 0.76, with a median density of 0.24 (IQR 0.14). The transitivity of providers in a physician peer group ranged from 0.33 to 0.91, with a median of 0.50 (IQR 0.12).
Table 2.
Characteristics of physician peer groups (N = 157)
| Peer group characteristics | Mean (SD) | Median (IQR) | Min, Max |
|---|---|---|---|
| # of physicians | 103 (73) | 81 (69) | < 11, 477 |
| # of PCPs | 54 (43) | 44 (35) | 0, 286 |
| # of surgeons | 11 (< 11) | < 11 (< 11) | 0, 50 |
| # of patients | 53 (37) | 42 (39) | 20, 245 |
| # of blacks patients | < 11 (< 11) | < 11 (< 11) | 0, 35 |
| Proportion of black patients | 0.07 (0.13) | 0.03 (0.08) | 0, 0.95 |
| Proportion of patients with delayed surgery | 0.37 (0.16) | 0.35 (0.25) | 0.03, 0.82 |
| Provider density | 0.25 (0.11) | 0.24 (0.14) | 0, 0.76 |
| Provider transitivity | 0.51 (0.08) | 0.50 (0.12) | 0.33, 0.91 |
Some numbers are obscured due to the Centers for Medicare and Medicaid Services’ prohibition against displaying cell sizes < 11
Patient characteristics and association with surgical delay
Overall, 37.1% of women experienced delayed surgery. In bivariate analyses, black (47.3%) and unmarried (40.0%) women were significantly more likely to have delayed surgery compared to white (36.4%) and married (34.3%) women (p < 0.001 for both comparisons; Table 3). Women who received a mastectomy were more likely to have received delayed surgery (42.2%) compared to women who received breast-conserving surgery (34.2%, p< 0.001).
Table 3.
Patient characteristics and receipt of delayed surgery
| Characteristic | % Delayed surgery | p valuea |
|---|---|---|
| Age | < 0.001 | |
| 67–69 years old | 36.1 | |
| 70–74 years old | 36.1 | |
| 75–79 years old | 35.2 | |
| 80–84 years old | 39.1 | |
| 85–89 years old | 43.1 | |
| Race | < 0.001 | |
| White/other | 36.4 | |
| Black | 47.3 | |
| Median household income ($) | 0.12 | |
| 0 ≤ to < 33,000 | 36.8 | |
| 33,000 ≤ to < 40,000 | 34.0 | |
| 40,000 ≤ to < 50,000 | 37.1 | |
| 50,000 ≤ to < 63,000 | 38.1 | |
| 63,000 ≤ | 38.4 | |
| Marital status | < 0.001 | |
| Married | 34.3 | |
| Not married | 40.0 | |
| Unknown | 38.2 | |
| Comorbid conditions | 0.04 | |
| 0 conditions | 36.0 | |
| 1–2 conditions | 38.3 | |
| ≥ 3 conditions | 39.6 | |
| Breast surgery type | < 0.001 | |
| Lumpectomy | 34.2 | |
| Mastectomy | 42.2 | |
| Hormone receptor status | 0.10 | |
| Negative | 34.2 | |
| Positive | 37.6 | |
| Unknown | 37.8 | |
| Node status | 0.70 | |
| Negative/unknown | 37.3 | |
| Positive | 36.8 | |
| Tumor grade | 0.27 | |
| Grade I | 36.8 | |
| Grade II | 37.6 | |
| Grade III/IV | 36.0 | |
| Unknown/missing | 41.8 | |
| Tumor stage | 0.35 | |
| Stage I | 36.9 | |
| Stage II | 37.0 | |
| Stage III | 40.0 | |
| Tumor size (cm) | 0.05 | |
| < 2.0 | 36.8 | |
| 2.0–5.0 | 37.0 | |
| > 5.0 | 44.5 | |
| PCP visit in year before diagnosis | 0.74 | |
| Yes | 37.1 | |
| No | 37.9 |
p value corresponds to a Chi square test; Significant p values (< 0.05) are bolded
Peer group characteristics associated with delayed surgery
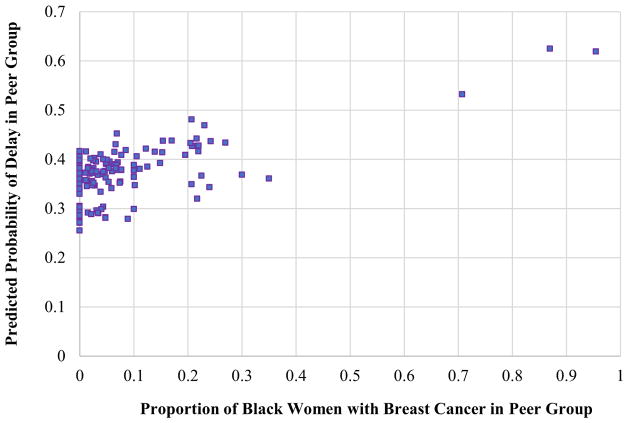
Women whose physician peer groups treated a greater proportion of black women with breast cancer were more likely to receive delayed surgery compared to women whose physician peer groups treated a lower proportion of black women. As the percentage of black women with breast cancer in a peer group increased, the adjusted predicted probability of surgery delay also increased (Spearman’s rank-order correlation: 0.46, p < 0.001, Fig. 2). Specifically, for every 10% increase in the percentage of black women in a peer group, there was a 11% increase in the odds of delayed surgery (adjusted OR 1.41, 95% CI 1.15–1.73; Table 4).
Fig. 2.
Comparing the adjusted probability of surgical delay with the percent of black women with breast cancer in each peer group
Table 4.
Factors associated with delayed breast cancer surgery after needle biopsy
| Unadjusted model | p value | Adjusted modela | p value | |
|---|---|---|---|---|
| Odds ratio (95% confidence interval) | Odds ratio (95% confidence interval) | |||
| Patient characteristics | ||||
| Age | < 0.001 | 0.006 | ||
| 67–69 years old | 1.00 | 1.00 | ||
| 70–74 years old | 0.98 (0.86–1.12) | 0.98 (0.86–1.12) | ||
| 75–79 years old | 0.94 (0.82–1.08) | 0.91 (0.79–1.04) | ||
| 80–84 years old | 1.13 (0.98–1.31) | 1.08 (0.93–1.25) | ||
| 85–89 years old | 1.35 (1.14–1.60) | 1.26 (1.05–1.50) | ||
| Race | 0.002 | 0.01 | ||
| White/other | 1.00 | 1.00 | ||
| Black | 1.38 (1.13–1.68) | 1.29 (1.06–1.59) | ||
| Median Household Income ($) | 0.19 | |||
| 0 ≤ to < 33,000 | 1.00 | |||
| 33,000 ≤ to < 40,000 | 0.93 (0.78–1.11) | |||
| 40,000 ≤ to < 50,000 | 0.95 (0.81–1.11) | |||
| 50,000 ≤ to < 63,000 | 0.91 (0.78–1.08) | |||
| 63,000 ≤ | 0.82 (0.70–0.97) | |||
| Marital status | < 0.001 | < 0.001 | ||
| Married | 1.00 | 1.00 | ||
| Not married | 1.14 (0.85–1.50) | 1.22 (1.10–1.34) | ||
| Unknown | 1.30 (1.18–1.43) | 1.10 (0.83–1.46) | ||
| Comorbid conditions | 0.080 | |||
| 0 conditions | 1.00 | |||
| 1–2 conditions | 1.10 (1.00–1.22) | |||
| ≥ 3 conditions | 1.14 (0.97–1.34) | |||
| Breast surgery type | < 0.001 | < 0.001 | ||
| Lumpectomy | 1.00 | 1.00 | ||
| Mastectomy | 1.59 (1.44–1.76) | 1.60 (1.44–1.78) | ||
| Hormone receptor status | 0.16 | |||
| Negative | 1.00 | |||
| Positive | 1.15 (1.00–1.33) | |||
| Unknown | 1.16 (0.87–1.55) | |||
| Node status | 0.83 | |||
| Negative/unknown | 1.00 | |||
| Positive | 1.01 (0.90–1.13) | |||
| Tumor grade | 0.25 | |||
| Grade I | 1.00 | |||
| Grade II | 1.04 (0.93–1.17) | |||
| Grade II/IV | 0.95 (0.83–1.09) | |||
| Unknown/missing | 1.21 (0.92–1.59) | |||
| Tumor stage | 0.22 | |||
| Stage I | 1.00 | |||
| Stage II | 1.01 (0.91–1.12) | |||
| Stage III | 1.17 (0.98–1.41) | |||
| Tumor size (cm) | 0.04 | 0.01 | ||
| < 2.0 | 1.00 | 1.00 | ||
| 2.0 to ≤ 5.0 | 1.02 (0.92–1.13) | 0.85 (0.76–0.95) | ||
| > 5.0 | 1.42 (1.09–1.85) | 1.02 (0.77–1.35) | ||
| PCP visit in year before diagnosis | 0.32 | |||
| Yes | 0.90 (0.73–1.11) | |||
| No | 1.00 | |||
| Number of surgeons | 1.01 (0.99–1.02) | 0.13 | ||
| Physician peer group characteristics | ||||
| Peer group % blackb | 1.14 (1.04–1.25) | 0.004 | 1.41 (1.15–1.73) | 0.001 |
| Provider density | 0.007 | 0.02 | ||
| Quintile 1 | 1.00 | 1.00 | ||
| Quintile 2 | 0.84 (0.55–1.29) | 0.90 (0.58–1.39) | ||
| Quintile 3 | 0.99 (0.66–1.48) | 0.99 (0.65–1.49) | ||
| Quintile 4 | 1.04 (0.70–1.53) | 1.10 (0.74–1.64) | ||
| Quintile 5 | 0.62 (0.42–0.91) | 0.65 (0.44–0.98) | ||
| Provider transitivity | 0.03 | |||
| Quintile 1 | 1.00 | |||
| Quintile 2 | 0.90 (0.60–1.36) | |||
| Quintile 3 | 1.07 (0.71–1.59) | |||
| Quintile 4 | 1.13 (0.77–1.67) | |||
| Quintile 5 | 0.69 (0.47–1.01) | |||
| Unknown | 0.40 (0.07–2.13) |
Significant odds ratios, with a p-value less than 0.05, are bolded
Odds ratio reflects the increase in odds of delayed surgery for every 10% increase in the proportion of the peer group that is black
Women in physician peer groups with the highest density were significantly less likely to receive delayed surgery compared to women in physician peer groups with the lowest density (adjusted OR 0.65, 95% CI 0.44–0.98; Table 4). There was no association between provider transitivity and patients’ likelihood of having delayed surgery. Similarly, we did not find any association between PCP transitivity or surgeon transitivity and delayed surgery (data not shown).
Discussion
We found that breast cancer surgical delays were common among Medicare beneficiaries and varied substantially across physician peer groups. In addition, two characteristics of physician peer groups, the racial composition of patients in a peer group and provider density, were significantly associated with surgery delays. Our results underscore the importance of mapping physician peer groups to better the impact of peer group characteristics on the quality of cancer care.
While prior work has identified patient-level racial disparities in the likelihood of experiencing surgical delay, the degree to which the racial composition of provider networks’ patients contributes to the quality of breast cancer care has not been explored. We found that not only do black patients have greater odds of experiencing delayed breast surgery after needle biopsy, but patients in physician peer groups that treat a greater proportion of black patients are also more likely to experience delayed surgery, regardless of individual patient race. This finding is consistent with a previous study showing that the proportion of non-white patients in a physician peer group was linked with increased complications following prostate cancer surgery [13]. Prior research has also shown that a high proportion of minority patients receive care from a small subgroup of providers and that these providers tend to have access to fewer high-quality clinical resources [4]. Hence, it is plausible that the racial composition of women with breast cancer in a peer group could be a marker for fewer resources among physician teams; future work should explore this potential mechanism.
Women in physician peer groups with the greatest provider density were significantly less likely to experience surgical delay. Patients belonging to physician peer groups with greater provider density may receive more expedient care because providers in those physician peer groups may tend to interact more frequently and have better care coordination, resulting in more timely care. This finding is consistent with prior work showing that patients in physician peer groups where providers are more interconnected and share a greater number of patients achieve better health outcomes [17].
Contrary to our hypothesis, however, provider transitivity did not significantly influence the odds of surgical delay. Prior work examining physician peer groups of Medicare beneficiaries undergoing coronary artery bypass grafting [3], found that patients in physician peer groups where physicians displayed greater transitivity during their surgical episodes achieved lower rates of 60-day emergency department visits, readmission, and mortality [3]. This finding was attributed to high levels of teamwork among physicians, both surgeons and non-surgeons. This discrepancy in the importance of transitivity on outcomes could be attributed to several mechanisms. First, we explored different outcomes. Additionally, because breast cancer care is inherently multidisciplinary, it may be possible that overall interconnectedness across all of the physicians in a peer group (provider density) is more conducive to prompt surgery than the degree to which smaller groups of surgeons are sharing patients within “closed loops” (provider transitivity).
There are several limitations to our study. First, we focused on Medicare fee-for-service beneficiaries ages 66 and older. This limits the generalizability of our study to patients with Medicare managed care, as well as to younger women with breast cancer [24, 25]. Another limitation is the accuracy of claims data in capturing real relationships between providers. However, previous research has shown there is a high correlation between actual provider relationships and billing information captured in claims data [6]. Lastly, we excluded women in the smallest physician peer groups (less than 20 women), which may also limit the generalizability of our results.
Women who receive delayed surgery after needle biopsy face significant health consequences. In order to provide high quality care to women with breast cancer, it is essential to understand the underlying mechanisms and factors that contribute to such delay. Our results indicate that breast cancer surgical delays vary across physician peer groups and two characteristics of physician peer groups, patient racial composition and provider density, were associated with the likelihood of delayed surgery. Further research investigating the relationship between these peer group characteristics and other aspects of breast cancer care may provide additional insight for improving quality of care for women with breast cancer and strategically allocating resources to better help those receiving suboptimal care.
Acknowledgments
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Funding This work was supported by the National Cancer Institute of the National Institutes of Health (R01CA190017).
Footnotes
Compliance with ethical standards
Conflict of interests Ms. Soulos and Dr. Gross receive research funding from twenty-first Century Oncology LLC. Dr. Pollack has stock ownership in Gilead Pharmaceuticals. Dr. Ma served as a consultant for Celgene and Incyte. Dr. Gross has also received research funding from NCCN-Pfizer and from Johnson & Johnson to help develop new approaches for sharing clinical trial data. The authors report no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Hassett MJ, Hughes ME, Niland JC, Ottesen R, Edge SB, Book-man MA, Carlson RW, Theriault RL, Weeks JC. Selecting high priority quality measures for breast cancer quality improvement. Med Care. 2008;46:762–770. doi: 10.1097/MLR.0b013e318178ead3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassett MJ, Schymura MJ, Chen K, Boscoe FP, Gesten FC, Schrag D. Variation in breast cancer care quality in New York and California based on race/ethnicity and Medicaid enrollment. Cancer. 2016;122:420–431. doi: 10.1002/cncr.29777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassett MJ, Weeks JC. Identifying high-priority quality measures for breast cancer quality improvement using data from a nationally representative sample. J Clin Oncol. 2009;27:6507. [Google Scholar]
- 4.Kiely D. Timeliness in breast cancer care as an indicator of quality. Clin J Oncol Nurs. 2014;18:82–88. doi: 10.1188/14.CJON.82-88. [DOI] [PubMed] [Google Scholar]
- 5.Bleicher RJ, Ruth K, Sigurdson ER, Beck JR, Ross E, Wong YN, Patel SA, Boraas M, Chang EI, Topham NS, Egleston BL. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2:330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA. 2000;283:2579–2584. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- 8.Gwyn K, Bondy ML, Cohen DS, Lund MJ, Liff JM, Flagg EW, Brinton LA, Eley JW, Coates RJ. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 9.Robertson R, Campbell NC, Smith S, Donnan PT, Sullivan F, Duffy R, Ritchie LD, Millar D, Cassidy J, Munro A. Factors influencing time from presentation to treatment of colorectal and breast cancer in urban and rural areas. Br J Cancer. 2004;90:1479–1485. doi: 10.1038/sj.bjc.6601753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thongsuksai P, Chongsuvivatwong V, Sriplung H. Delay in breast cancer care: a study in Thai women. Med Care. 2000;38:108–114. doi: 10.1097/00005650-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Kinchen KS, Cooper LA, Levine D, Wang NY, Powe NR. Referral of patients to specialists: factors affecting choice of specialist by primary care physicians. Ann Fam Med. 2004;2:245–252. doi: 10.1370/afm.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollack CE, et al. The impact of social contagion on physician adoption of advanced imaging tests in breast cancer. J Natl Cancer Inst. 2017;109:djw330. doi: 10.1093/jnci/djw330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack CE, Weissman G, Bekelman J, Liao K, Armstrong K. Physician social networks and variation in prostate cancer treatment in three cities. Health Serv Res. 2012;47:380–403. doi: 10.1111/j.1475-6773.2011.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Eng J Med. 2004;351:575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 15.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002;40:4–19. doi: 10.1097/01.MLR.0000020934.40692.C0. [DOI] [PubMed] [Google Scholar]
- 16.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166:2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 17.Pollack CE, Weissman GE, Lemke KW, Hussey PS, Weiner JP. Patient sharing among physicians and costs of care: a network analytic approach to care coordination using claims data. J Gen Intern Med. 2013;28:459–465. doi: 10.1007/s11606-012-2104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollingsworth JM, Funk RJ, Garrison SA, Owen-Smith J, Kaufman SA, Pagani FD, Nallamothu BK. Association between physician teamwork and health system outcomes after coronary artery bypass grafting. Circulation. 2016 doi: 10.1161/circoutcomes.116.002714. [DOI] [PMC free article] [PubMed]
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 21.Girvan M, Newman ME. Community structure in social and biological networks. Proc Natl Acad Sci USA. 2002;99:7821–7826. doi: 10.1073/pnas.122653799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci USA. 2006;103:8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22:2591–2602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- 24.Adami HO, Malker B, Holmberg L, Persson I, Stone B. The relation between survival and age at diagnosis in breast cancer. N Eng J Med. 1986;315:559–563. doi: 10.1056/nejm198608283150906. [DOI] [PubMed] [Google Scholar]
- 25.Arvold ND, Taghian AG, Niemierko A, Abi Raad RF, Sreedhara M, Nguyen PL, Bellon JR, Wong JS, Smith BL, Harris JR. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29:3885–3891. doi: 10.1200/jco.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]