Abstract
Within the intracranial vasculature, atherosclerosis occurs in two distinctive patterns: (1) in Western populations who have severe extracranial and systemic atherosclerosis, the severity of intracranial involvement is consistently less than that within extracranial arteries; and (2) in Asians, Africans, and Hispanics, who often have isolated intracranial arterial disease that is found to be more often accompanied by brain infarction than comparable extracranial atherosclerotic disease. Compared to coronary and extracranial carotid atherosclerosis, intracranial atherosclerosis has distinct pathological characteristics compared to that of extracranial arteries. Intracranial atherosclerosis (ICAS) had been understudied due to the relative inaccessibility of cerebral artery specimens under current treatment strategies. Acquiring post-mortem cerebral vessel specimens for histology processing is the most direct method to analyze the pathological characteristics of ICAS, in order to analyze both lumen stenosis and plaque components contributing to brain infarctions. The developments in high resolution magnetic resonance imaging (HRMRI) make it feasible to assess human ICAS in vivo. It is nevertheless challenging to understand vessel wall changes within brain vasculature demonstrated on HRMRI, as well as to identify biomarkers for stroke risk stratification and treatment strategy modification. Knowledge about intracranial atherosclerosis remains limited due to lack of human arterial specimens, and the development of proper animal models of human cerebral atherosclerosis is necessary to explore the pathogenesis of intracranial atherosclerosis and to assess various strategies preventing or treating ICAS-related stroke.
Atherosclerosis involves multiple arteries throughout the body, including the aorta, coronary arteries, and the limb arteries. Within the intracranial vasculature, atherosclerosis occurs in two separate patterns: (1) in Western populations who have severe extracranial and systemic atherosclerosis [1–6], the severity of which is consistently less than that of the extracranial arteries; and (2) in Asians, Africans, and Hispanics who often have isolated intracranial arterial disease with little evidence of extracranial, coronary or systemic disease. Similar to coronary or carotid artery atherosclerosis, conventional risk factors [7–12] such as age [13], gender, hypertension, diabetes mellitus, hyperlipidemia and cigarette smoking, may account for the occurrence of intracranial atherosclerosis. It remains unclear, whether race-ethnicity is an independent risk factor of intracranial atherosclerosis (ICAS) or is confounded by differences in stroke risk factors among different ethnic groups.
Atherosclerosis is a lifetime illness evolving slowly over many years [14], beginning with inflammatory reactions and endothelial injury. Platelets gather at sites of endothelial disruption, followed by smooth muscle proliferation and thickening of the arterial wall. Although cholesterol accumulation in the endothelial cells of the arterial wall influences the course and maturation of atherosclerosis [15], the process of atherosclerosis is a complex interaction of cellular events [16] involving intercellular messengers, shear stress, elevated or modified low-density lipoproteins (LDL), free radicals, and vascular risk factors [17]. The cellular contributors to plaque development include monocytes/macrophages, endothelial cells, smooth muscle cells, and, to a lesser degree, lymphocytes and platelets. Circulating monocytes and later macrophages precipitate the formation of early plaque with foam cells, which leads to plaque growth and maturation.
Hemodynamic factors also influence the atherosclerotic process in determining the location of early plaques and subsequently contributing to their eventual destabilization [18]. Within the cerebral vasculature, the origin of the internal carotid artery (ICA) is the most frequent site for severe atherosclerosis in subjects of European ancestry [19], whereas intracranial arteries, especially the first portion (M-1 segment) of middle cerebral artery (MCA), are most commonly affected among persons of African or Asian heritage [8, 20–24]. In the vertebrobasilar system, plaques are most commonly found at the origin of the vertebral artery (VA) and in the proximal portion of the basilar artery (BA).
Compared to extracranial carotid atherosclerosis, ICAS has been understudied due to the relative inaccessibility of specimens. Intracranial vessels have a thinner media, less abundant adventitia, and fewer elastic fibers compared to extracranial arteries of similar size [25] (fig. 1). Vessel wall metabolism of intracranial arteries is distinct from that of extracranial arteries [25], suggesting that ICAS may demonstrate distinct pathological characteristics from coronary or carotid artery atherosclerosis. To delineate the pathology of ICAS, acquiring post-mortem brain vessel specimens for histology processing is crucial [10, 26–28]. A complete examination of the brain vasculature should include the entire ICA, MCA, VA and the circle of Willis (fig. 2). Pathological examination should include gross (macroscopic) examination using the method of Baker et al. [26]. The narrowest parts of the individual arteries should then be taken for histological sectioning (microscopic examination). The presence of thrombotic or embolic occlusion and ulceration should also be recorded. During the process, caution should be taken to ensure perpendicular cutting and embedding. Careful use of correct terminology is a requirement [29].
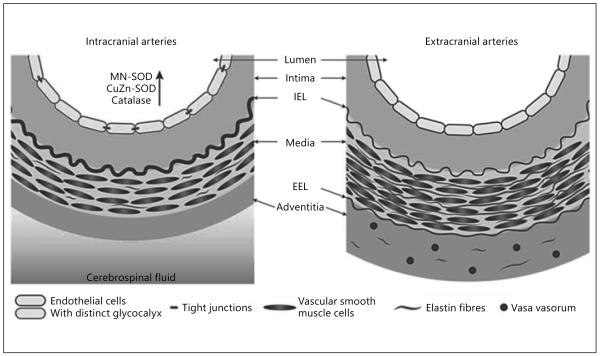
Fig. 1.
Major structural characteristics of intracranial and extracranial arteries. Intracranial arteries tend to be muscular arteries with a thin media and adventitia, few elastic medial fibers, thicker and denser internal elastic lamina (IEL), and lacking external elastic lamina (EEL) and vasa vasorum. With permission [91].
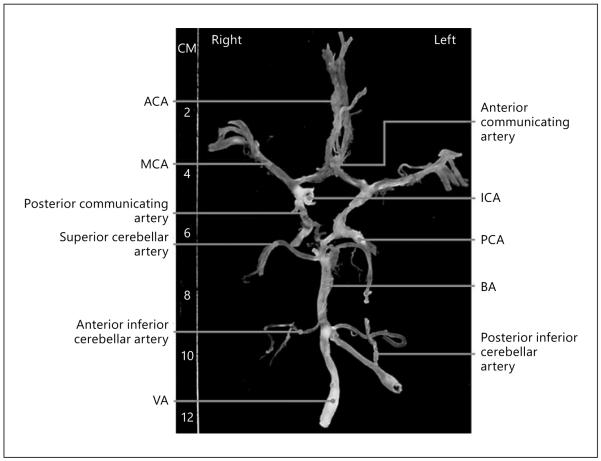
Fig. 2.
Circle of Willis from a 90-year-old subject. Atherosclerotic lesions are identified by white vessels, and non-diseased arteries appear transparent. This case shows prominent atherosclerosis in internal carotid, vertebral, basilar, middle cerebral, and posterior cerebral arteries. With permission [91].
During the 1960s, several autopsy studies were performed in exploring the distribution and natural history of ICAS [30–33]. A large-scale autopsy study of asymptomatic cohorts extending to patients in their 10th decade of life revealed intracranial atherosclerotic changes occurring from the first to second decade and progressing with age [30–32]. Compared to the relatively linear progression of aortic atherogenesis, ICAS increased more slowly initially and paralleled aortic lesions. Advanced atherosclerotic lesions were almost nonexistent up to the fourth decade [33]. Increased activity of antioxidant enzymes in intracranial arteries may contribute to their greater resistance to atherogenesis; with increasing age intracranial arteries respond with accelerated atherosclerosis probably because their antioxidant protection decreases relatively more than that of extracranial arteries [34]. In Japan, a nation-wide study [35] in 1988 examined atherosclerosis in autopsied patients (from 1 month to 39 years old). This study showed that ICAS occurred later and was less extensive than aortic or coronary artery disease. A later autopsy study among Chinese individuals showed that the extent of ICA was much more severe, while atherosclerotic narrowing of the extracranial ICA was less severe, than that shown in white and Japanese populations [10].
The distribution of atherosclerosis within the brain vasculature shown by early autopsy studies is also consistent with current knowledge from recent epidemiologic and clinical studies [8, 23, 36, 37]. Overall, American and European studies showed a similar pattern: The ICA and intracranial VA were most commonly affected, followed by the BA, MCA, posterior cerebral artery (PCA), and anterior cerebral artery (ACA) [30, 38, 39]. The MCA was most often involved in Asians, followed by the ICA, BA, VA, PCA, and ACA [36, 40]. In all cohorts, cerebellar and communicating arteries were rarely affected. Atherosclerosis of the intracranial ICA was observed mainly in the cavernous and supraclinoid segments [38, 41]. The BA was commonly affected in the upper and lower regions, and less affected in the mid-basilar region [26].
Atherosclerotic plaques consist of cells, connective tissue extracellular matrix, and intra- and extracellular lipid deposits. The proportion of these three components varies in different atherosclerotic plaques, which affects plaque stability (fig. 3) and give rise to a wide spectrum of lesions [42–44]: (1) The so-called fatty streaks are characterized by adhesion of monocytes to the endothelium and migration to the subendothelial potions of the arterial wall [17] in the aorta and the coronary arteries of adolescents and young adults; (2) With increasing age, fatty streaks are transformed into fibrous plaques consisting of a core of cellular debris, free extracellular lipid, and cholesterol crystals under a ‘cap’ of foam cells, transformed smooth muscle cells, lymphocytes, and connective tissue [17]; (3) The most advanced stage of atherosclerosis is the complicated lesion, which includes calcification, hemosiderin deposition, and lumen surface disruption. Coronary artery atherosclerosis has been well-studied and rupture-prone plaques, the so-called ‘vulnerable plaques’, are described as having a thin fibrous cap and a large lipid core, which may lead to acute coronary syndromes [44]. For extracranial carotid atherosclerosis, the procedure of carotid endarterectomy provides specimens to examine the pathology and components of carotid atherosclerotic plaques [45]. Conventional pathologic and imaging markers of plaque vulnerability include carotid artery intima/media thickness (IMT), status of fibrous caps, erosion or ulceration, status of the lipid core, intraplaque hemorrhage or neovascularization, thrombus, extent of plaque inflammation, and microembolic signals detected by transcranial Doppler [46–48].
Fig. 3.
Schematic overview of a stable atherosclerotic plaque (left) and an unstable atherosclerotic plaque (right). With permission [92].
To help define pathological characteristics of intracranial atherosclerosis, we searched the literature for relevant histology information. In Caucasians, Daemen et al. [25] screened 283 circles of Willis segments from 18 asymptomatic elderly individuals (mean age, 70.2 years) to investigate the basic structural features of intracranial arteries (ICA, MCA, VA, and BA) including the smaller arteries (ACA, PCA, and cerebellar and communicating arteries). This study reported an intermediate phenotype sharing structural features of both the larger extracranial and the smaller intracranial arteries, such as the lack of vasa vasorum and an external elastic lamina and only a few medial elastic fibers compared with extracranial arteries (table 1). The study also detected mainly early lesions (63%) and only a few advanced atherosclerotic lesions (15%), within which calcifications were rare and macrophage load was relatively low, consistent with a previous study [25]: 0.9 ± 0.7% CD68 positivity per plaque area compared with 1.8 ± 2.4% in coronary arteries [49]. In 1966, Moossy et al. [7] also reported that hemorrhage, ulceration, and calcification in intracranial plaques were much less frequent as compared to extracranial atherosclerotic disease. In a recent study, eight subjects with a history of stroke had atherosclerotic lesions present in seven basilar arteries, with a mean estimated stenosis of 34% [50]; lumenal thrombus with disrupted fibrous cap was seen in 1 lesion, neovascularity and calcification were seen in 1 lesion, mild to moderate inflammation was seen in 3 lesions, and necrotic core was present in 4/7 lesions (with one plaque rupture).
Table 1.
The morphological features of plaques associated and not associated with infarcts in the MCA territory. With permission [93]
| Plaques associated with infarct (n = 46) |
Plaques not associated with infarct (n = 65) |
p value | 95% confidence interval |
|
|---|---|---|---|---|
| Degree of area stenosis, % | 62.2±16.8 | 52.4±10.5 | 0.001 | −15.4 to −4.19 |
| Fibrous cap | 1.59±0.83 | 1.46±0.69 | 0.403 | −0.42 to 0.17 |
| Lipid core | 2.48±0.91 | 1.98±0.82 | 0.004 | −0.83 to −0.16 |
| Intraplaque hemorrhage | 14/46 | 10/65 | 0.066 | 0.958 to 6.045 |
| Intraplaque neovasculature | 13/46 | 5/65 | 0.008 | 1.549 to 14.423 |
| Intraplaque thrombus | 7/46 | 1/65 | 0.008 | 1.361 to 96.932 |
| Intraplaque calcification | 16/46 | 15/65 | 0.202 | 0.770 to 4.107 |
| CD45RO | 2.38±0.78 | 1.79±0.81 | 0.007 | −0.968 to 0.158 |
| CD68 | 3.07±0.64 | 2.58±0.70 | 0.009 | −0.849 to −0.130 |
Among Asian populations (based on a series of Chinese autopsy adults, mean age, 74.7 years; range, 46–99 years), Chen et al. [51] reported that 69 MCAs out of 152 had more than 40% cross-sectional area luminal narrowing, among which calcification was detected in nearly 30% of atherosclerotic lesions and intraplaque hemorrhage in 20% of lesions. They also found that in addition to lumen stenosis, the percentage of lipid core and intraplaque neovasculature played a key role in leading to clinical ischemic events [52] (table 2); this partly verified the previously hypothesized stroke mechanisms caused by intracranial atherosclerosis [53]. Narrowing of the involved vessel leads to reduced blood flow supplying brain tissue [14], a major mechanism of ischemic stroke related to ICAS [53]. Moreover, vulnerable plaques (those with a large lipid core, intraplaque hemorrhage, or a thin or ruptured fibrous cap) may be prone to rupture, exposing the thrombogenic core to tissue and clotting factors, resulting in thrombus that either occludes the artery locally or embolizes distally; note that artery-to-artery embolism has been verified in stroke patients by multiple clinical studies using TCD monitoring [54–58]. Thirdly, unique to ICAS, plaque extension over small penetrating artery ostia (also known as branch atheromatous disease) [53, 59] results in reduced blood flow in the penetrators, leading to single subcortical infarctions [60].
Table 2.
Data from this review on the basic characteristics of the large intracranial arteries of 18 asymptomatic patients. With permission [91]
| ICA | VA | MCA | BA | |
|---|---|---|---|---|
| Type of lesion, % (n/N) | ||||
| Early | 57 (17/30) | 54 (15/28) | 68 (19/28) | 75 (24/32) |
| Advanced | 33 (10/30) | 25 (7/28) | 25 (7/28) | 16 (5/32) |
| High content of elastin fibers, % (n/N) | 37 (11/30) | 43 (12/28) | 29 (8/28) | 13 (4/32) |
| Continuous EEL, % (n/N) | 17 (5/30) | 79 (22/28) | 21 (6/28) | 9 (3/32) |
| Calcification, % (n/N) | 20 (6/30) | 18 (5/28) | 14 (4/28) | 9 (3/32) |
| Vasa vasorum, % (n/N) | 53 (16/30) | 43 (12/28) | 11 (3/28) | 16 (5/32) |
| Macrophages (mean ± SD), % | 0.9±0.7 | 0.4±0.5 | 0.8±0.7 | 0.9±0.9 |
In clinical practice, current imaging techniques such as CT angiography, digital subtraction angiography, and magnetic resonance angiography (MRA) are of limited utility in the diagnosis of intracranial arterial disease, because they show only the arterial lumen status of intracranial vessels [61]. These methods may underestimate the presence of intracranial arterial pathology because of the presence of non-occlusive atherosclerotic disease (due to arterial remodeling). Detailed visualization of vessel wall changes and the ability to derive information about plaque composition may be clinically important, because rupture of a non-occlusive plaque can potentially cause ischemic stroke [53]. Compared to carotid artery disease, a key factor that complicates detection of intracranial arterial pathology is the smaller diameter of the cerebral arteries, ranging from 2 to 3 mm proximally to 1 mm more distally [25].
The advent of high resolution MRI (3.0T or 7.0T) has been a major advance in image resolution, making it potentially feasible to yield excellent visualization of vessel wall changes by modified scanning techniques [62–64]. Clinical HRMRI studies in recent years have demonstrated the ability of HRMRI vessel wall imaging for quantitative measurement of plaque burden or vessel wall area [65–67]. This technique is more accurate than lumen stenosis in reflecting the degree of intracranial arterial stenosis, especially in the case of non-stenotic atherosclerotic disease. In addition, evaluating thickness of the fibrous cap or identifying intraplaque hemorrhage or lipid component by HRMRI vessel wall imaging has the potential to indicate whether intracranial atherosclerotic plaques are vulnerable [28, 68]. This assumes that intracranial atherosclerosis pathophysiology parallels that in the carotid artery [25, 25]. Histologic validation of HRMRI findings on intracranial vessel walls is needed to confirm the validity of HRMRI as a useful tool for stroke risk stratification and treatment strategy medication.
Pathological data from post-mortem brain artery specimens provide the basis to compare plaque morphology to corresponding vessel wall changes on in-vivo HRMRI. In a histology-MRI comparative study, Chen et al. [27] adopted 1.5T MRI to scan the cross-sections of bilateral MCAs in a series of Chinese autopsy adults and showed agreement between ex vivo MRI and histopathology in identifying MCA stenosis (fig. 4), as well as the relationship between MCA stenosis identified by MRI and radiologically- or histopathologically-evident brain infarcts. For example, a hyperintense signal in T1 sequences was shown to be intraplaque hemorrhage by histology (fig. 5) [28]. Compared to 1.5T MRI, 3.0T or even 7.0T HRMRI is a particularly promising tool for studying intracranial arterial disease in-vivo, with enhanced resolution [64, 69]. In one patient with symptomatic left cavernous carotid stenosis, Turan et al. [70] showed a correlation between atherosclerotic plaque components visualized on in-vivo 3T HRMRI images and pathological findings in postmortem artery specimens. Majidi et al. [71] also performed an in vitro comparative study and showed that virtual histology-intravascular ultrasonography and HRMRI are reliable imaging tools to detect atherosclerotic plaques within the intracranial arterial wall, although both imaging modalities have some limitations in accurate characterization of plaque components (fig. 6). A more recent study attempted to adopt 7T HRMRI to establish the clinical indications of HRMRI sequences by scanning five specimens of the circle of Wills [72]. This analysis showed that 7.0T MRI has the capability to identify focal intracranial vessel wall thickening and distinguish areas of different signal intensities spatially corresponding to plaque components within more advanced atherosclerotic plaques (fig. 7). Considering that signal intensity changes on HRMRI vessel wall images have not been well-interpreted, additional studies that further validate signal characteristics will improve in vivo characterization of intracranial atherosclerotic plaques and determine the clinical utility of defining plaque morphology and composition.
Fig. 4.
Examples of the plaques leading to >30% (upper row) and >50% (lower row) stenosis in MCA, corresponding images in MRI T1 sequence (left) and in histopathology (right). With barium distending the artery lumen appears dark and vessel wall appears bright in T1 sequence. With permission [94].
Fig. 5.
1.5T magnetic resonance imaging (MRI) (T1 sequence) shows hyperintense signal; histological demonstration of the haemorrhage within the plaque (haematoxylin and eosin (H&E) staining, magnification 1.6 × 1.6×). With permission [95].
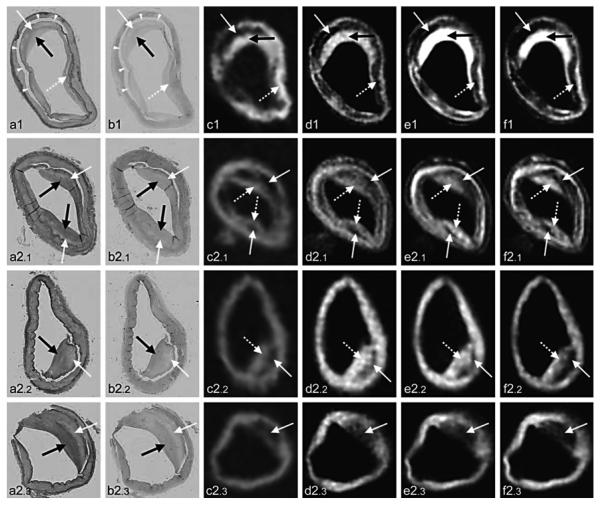
Fig. 6.
a–d Four different histopathologic sections of intracranial vessels with atherosclerotic plaque and their corresponding 3D SPACE MR imaging (in the middle) and VH-IVUS (on the right) images. With permission [96]. SPACE = Sampling perfection with application-optimized contrasts by use of different flip angle evolutions; VH-IVUS = virtual histology-intravascular ultrasonography.
Fig. 7.
Four examples of atherosclerotic plaques with corresponding signal heterogeneity on 7T MR images. With permission [97].
The development of proper animal models simulating human cerebral atherosclerosis is required to further explore the pathogenesis of ICAS and to assess various strategies to prevent or treat ICAS-related stroke [73–75]. Various species, including swine, chickens, dogs, rabbits, rats, and monkeys [73–80] have been used to evaluate atherosclerosis in intracranial arteries. Although systemic atherosclerosis can be produced in rabbits taking an atherogenic diet [81] or in Watanabe heritable hyperlipidemic (WHHL) rabbits [82], it is difficult to induce atherosclerosis in intracranial arteries. In homozygous WHHL rabbits that are known to have hypercholesterolemia and severe coronary atherosclerosis, Ito et al. [83] described spontaneously developing cerebral atherosclerosis beginning at 9 months of age. To produce cerebral artery atherosclerosis, additional application of hypertension was more effective than inducing hyperlipidemia alone [82–88], which was found to be successful in a cynomolgus monkey [85]. Aside from high blood pressure, hemodynamic derangement was also found to be effective for the development of fat deposition in cerebral arteries. Yamori et al. [89] successfully produced fat deposition in the posterior communicating arteries in normotensive rats by 10 weeks’ feeding with high-fat cholesterol, following ligation of one or both ICAs or the BA.
Successful animal models of cerebral atherosclerosis have shown morphological features of ICAS. In hypertensive WHHL rabbits [82], atherosclerotic lesions developed near the vertebral-basilar arterial confluence and the circle of Willis 6 months after hypertension-inducing surgery; the lesions remained less severe than in the aorta and coronary arteries and also had qualitative morphologic differences from extracranial atherosclerosis. Another study of experimental rabbits also produced early lesions of ICAS [84] with widespread thickening and contraction in almost all intracranial small arteries. Imai et al. [78] investigated diet-induced cerebral atherosclerosis in swine and observed the progression of lesions from proliferative to atheromatous stages. Animal models can also be used to study stroke mechanisms. For example, an animal model of cerebral athero-embolism was established by injecting human atheromatous material into the brain vasculature of rabbits via the common carotid artery [90]. Signs of neurologic deficit such as motor dysfunction were seen in some surviving animals; ischemic lesions were predominantly localized to ipsilateral cortical and subcortical areas within the territory of the MCA.
Acknowledgements
Supported by NIH R01 NS 20989 (Dr. Fisher).
References
- 1. Gorelick PB Mazzone T Plasma lipids and stroke J Cardiovasc Risk 1999. 6 217–221 [DOI] [PubMed] [Google Scholar]
- 2. Mathur KS Kashyap SK Kumar V Correlation of the extent and severity of atherosclerosis in the coronary and cerebral arteries Circulation 1963. 27 929–934 [DOI] [PubMed] [Google Scholar]
- 3. Sadoshima S Kurozumi T Tanaka K Ueda K Takeshita M Hirota Y et al. Cerebral and aortic atherosclerosis in hisayama, japan Atherosclerosis 1980. 36 117–126 [DOI] [PubMed] [Google Scholar]
- 4. McGarry P Solberg LA Guzman MA Strong JP Cerebral atherosclerosis in New Orleans Comparisons of lesions by age, sex, and race. Lab Invest 1985. 52 533–539 [PubMed] [Google Scholar]
- 5. Gorelick PB Distribution of atherosclerotic cerebrovascular lesions Effects of age, race, and sex. Stroke 1993. 24 I16–I19 discussion I20–I21 [PubMed] [Google Scholar]
- 6. Napoli C Witztum JL de Nigris F Palumbo G D’Armiento FP Palinski W Intracranial arteries of human fetuses are more resistant to hypercholesterolemia-induced fatty streak formation than extracranial arteries Circulation 1999. 99 2003–2010 [DOI] [PubMed] [Google Scholar]
- 7. Moossy J Cerebral infarcts and the lesions of intracranial and extracranial atherosclerosis Arch Neurol 1966. 14 124–128 [DOI] [PubMed] [Google Scholar]
- 8. Caplan LR Gorelick PB Hier DB Race, sex and occlusive cerebrovascular disease: a review Stroke 1986. 17 648–655 [DOI] [PubMed] [Google Scholar]
- 9. Moossy J Pathology of cerebral atherosclerosis. Influence of age, race, and gender Stroke 1993. 24 I22–I23 I31–I32 [PubMed] [Google Scholar]
- 10. Leung SY Ng TH Yuen ST Lauder IJ Ho FC Pattern of cerebral atherosclerosis in hong kong chinese. Severity in intracranial and extracranial vessels Stroke 1993. 24 779–786 [DOI] [PubMed] [Google Scholar]
- 11. Sacco RL Kargman DE Zamanillo MC Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the northern Manhattan stroke study Neurology 1995. 45 659–663 [DOI] [PubMed] [Google Scholar]
- 12. Ingall TJ Homer D Baker HL Jr Kottke BA O’Fallon WM Whisnant JP Predictors of intracranial carotid artery atherosclerosis. Duration of cigarette smoking and hypertension are more powerful than serum lipid levels Arch Neurol 1991. 48 687–691 [DOI] [PubMed] [Google Scholar]
- 13. Resch JA Okabe N Loewenson RB Kimoto K Katsuki S Baker AB Pattern of vessel involvement in cerebral atherosclerosis. A comparative study between a japanese and minnesota population J Atheroscler Res 1969. 9 239–250 [DOI] [PubMed] [Google Scholar]
- 14. Fuster V Badimon JJ Chesebro JH Atherothrombosis: Mechanisms and clinical therapeutic approaches Vasc Med 1998. 3 231–239 [DOI] [PubMed] [Google Scholar]
- 15. Yatsu FM Fisher M Atherosclerosis: Current concepts on pathogenesis and interventional therapies Ann Neurol 1989. 26 3–12 [DOI] [PubMed] [Google Scholar]
- 16. Ross R Atherosclerosis – an inflammatory disease N Engl J Med 1999. 340 115–126 [DOI] [PubMed] [Google Scholar]
- 17. Navab M Fogelman AM Berliner JA Territo MC Demer LL Frank JS et al. Pathogenesis of atherosclerosis Am J Cardiol 1995. 76 18C–23C [DOI] [PubMed] [Google Scholar]
- 18. Zarins CK Giddens DP Bharadvaj BK Sottiurai VS Mabon RF Glagov S Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress Circ Res 1983. 53 502–514 [DOI] [PubMed] [Google Scholar]
- 19. Fisher CM G I Okabe N White PD Atherosclerosis of the carotid and vertebral arteries-extracranial and intracranial J Neuropathol Exp Neurol 1965. 24 455–476 [Google Scholar]
- 20. Bogousslavsky J Barnett HJ Fox AJ Hachinski VC Taylor W Atherosclerotic disease of the middle cerebral artery Stroke 1986. 17 1112–1120 [DOI] [PubMed] [Google Scholar]
- 21. Feldmann E Daneault N Kwan E Ho KJ Pessin MS Langenberg P et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease Neurology 1990. 40 1541–1545 [DOI] [PubMed] [Google Scholar]
- 22. Wityk RJ Lehman D Klag M Coresh J Ahn H Litt B Race and sex differences in the distribution of cerebral atherosclerosis Stroke 1996. 27 1974–1980 [DOI] [PubMed] [Google Scholar]
- 23. Wong KS Li H Chan YL Ahuja A Lam WW Wong A et al. Use of transcranial doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease Stroke 2000. 31 2641–2647 [DOI] [PubMed] [Google Scholar]
- 24. Huang YN Gao S Li SW Huang Y Li JF Wong KS et al. Vascular lesions in chinese patients with transient ischemic attacks Neurology 1997. 48 524–525 [DOI] [PubMed] [Google Scholar]
- 25. Ritz K Denswil NP Stam OC van Lieshout JJ Daemen MJ Cause and mechanisms of intracranial atherosclerosis Circulation 2014. 130 1407–1414 [DOI] [PubMed] [Google Scholar]
- 26. Baker AB Iannone A Cerebrovascular disease. I. The large arteries of the circle of willis Neurology 1959. 9 321–332 [DOI] [PubMed] [Google Scholar]
- 27. Chen XY Lam WW Ng HK Zhao HL Wong KS Diagnostic accuracy of mri for middle cerebral artery stenosis: a postmortem study J Neuroimaging 2006. 16 318–322 [DOI] [PubMed] [Google Scholar]
- 28. Chen XY Wong KS Lam WW Ng HK High signal on t1 sequence of magnetic resonance imaging confirmed to be intraplaque haemorrhage by histology in middle cerebral artery Int J Stroke 2014. 9–E19 [DOI] [PubMed] [Google Scholar]
- 29. Velican D Anghelescu M Petrescu C Velican C Method dependent limits in a study on the natural history of coronary and cerebral atherosclerosis Med Interne 1982. 20 215–229 [PubMed] [Google Scholar]
- 30. Resch JA Baker AB Etiologic mechanisms in cerebral atherosclerosis. Preliminary study of 3,839 cases Arch Neurol 1964. 10 617–628 [DOI] [PubMed] [Google Scholar]
- 31. Lascelles RG Burrows EH Occlusion of the middle cerebral artery Brain 1965. 88 85–96 [DOI] [PubMed] [Google Scholar]
- 32. Silverstein A Hollin S Internal carotid vs middle cerebral artery occlusions; clinical differences Arch Neurol 1965. 12 468–471 [DOI] [PubMed] [Google Scholar]
- 33. Mathur KS Kashyap SK Mathur SC Distribution and severity of atherosclerosis of aorta, coronary and cerebral arteries in persons dying without morphologic evidence of atherosclerotic catastrophe in North India. A study of 900 autopsies J Assoc Physicians India 1968. 16 113–122 [PubMed] [Google Scholar]
- 34. D’Armiento FP Bianchi A de Nigris F Capuzzi DM D’Armiento MR Crimi G et al. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis Stroke 2001. 32 2472–2479 [DOI] [PubMed] [Google Scholar]
- 35. Tanaka K Masuda J Imamura T Sueishi K Nakashima T Sakurai I et al. A nation-wide study of atherosclerosis in infants, children and young adults in Japan Atherosclerosis 1988. 72 143–156 [DOI] [PubMed] [Google Scholar]
- 36. Gorelick PB Caplan LR Hier DB Parker SL Patel D Racial differences in the distribution of anterior circulation occlusive disease Neurology 1984. 34 54–59 [DOI] [PubMed] [Google Scholar]
- 37. Wong KS Ng PW Tang A Liu R Yeung V Tomlinson B Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients Neurology 2007. 68 2035–2038 [DOI] [PubMed] [Google Scholar]
- 38. Alkan O Kizilkilic O Yildirim T Atalay H Intracranial cerebral artery stenosis with associated coronary artery and extracranial carotid artery stenosis in Turkish patients Eur J Radiol 2009. 71 450–455 [DOI] [PubMed] [Google Scholar]
- 39. Moossy J Development of cerebral atherosclerosis in various age groups Neurology 1959. 9 569–574 [DOI] [PubMed] [Google Scholar]
- 40. Wong KS Huang YN Gao S Lam WW Chan YL Kay R Intracranial stenosis in chinese patients with acute stroke Neurology 1998. 50 812–813 [DOI] [PubMed] [Google Scholar]
- 41. Bae HJ Lee J Park JM Kwon O Koo JS Kim BK et al. Risk factors of intracranial cerebral atherosclerosis among asymptomatics Cerebrovasc Dis 2007. 24 355–360 [DOI] [PubMed] [Google Scholar]
- 42. Fuster V Fayad ZA Badimon JJ Acute coronary syndromes: biology Lancet 1999. 353 suppl 2 SII5–SII9 [DOI] [PubMed] [Google Scholar]
- 43. Falk E Shah PK Fuster V Coronary plaque disruption Circulation 1995. 92 657–671 [DOI] [PubMed] [Google Scholar]
- 44. Fuster V Lewis A Conner memorial lecture. Mechanisms leading to myocardial infarction: insights from studies of vascular biology Circulation 1994. 90 2126–2146 [DOI] [PubMed] [Google Scholar]
- 45. Yuan C Miller ZE Cai J Hatsukami T Carotid atherosclerotic wall imaging by MRI Neuroimaging Clin N Am 2002. 12 391–401 vi [DOI] [PubMed] [Google Scholar]
- 46. Nighoghossian N Derex L Douek P The vulnerable carotid artery plaque: current imaging methods and new perspectives Stroke 2005. 36 2764–2772 [DOI] [PubMed] [Google Scholar]
- 47. Bornstein NM Norris JW The unstable carotid plaque Stroke 1989. 20 1104–1106 [DOI] [PubMed] [Google Scholar]
- 48. Bornstein NM Krajewski A Lewis AJ Norris JW Clinical significance of carotid plaque hemorrhage Arch Neurol 1990. 47 958–959 [DOI] [PubMed] [Google Scholar]
- 49. Narula J Nakano M Virmani R Kolodgie FD Petersen R Newcomb R et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques J Am Coll Cardiol 2013. 61 1041–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Labadzhyan A Csiba L Narula N Zhou J Narula J Fisher M Histopathologic evaluation of basilar artery atherosclerosis J Neurol Sci 2011. 307 97–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen XY Wong KS Lam WW Zhao HL Ng HK Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study Cerebrovasc Dis 2008. 25 74–80 [DOI] [PubMed] [Google Scholar]
- 52. Chen XY Wong KS Lam WW Zhao HL Ng HK Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study Cerebrovasc Dis 2007. 25 74–80 [DOI] [PubMed] [Google Scholar]
- 53. Holmstedt CA Turan TN Chimowitz MI Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment Lancet Neurol 2013. 12 1106–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ogata J Masuda J Yutani C Yamaguchi T Mechanisms of cerebral artery thrombosis: a histopathological analysis on eight necropsy cases J Neurol Neurosurg Psychiatry 1994. 57 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Droste DW Junker K Hansberg T Dittrich R Ritter M Ringelstein EB Circulating microemboli in 33 patients with intracranial arterial stenosis Cerebrovasc Dis 2002. 13 26–30 [DOI] [PubMed] [Google Scholar]
- 56. Wong KS Gao S Chan YL Hansberg T Lam WW Droste DW et al. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study Ann Neurol 2002. 52 74–81 [DOI] [PubMed] [Google Scholar]
- 57. Gao S Wong KS Hansberg T Lam WW Droste DW Ringelstein EB Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis Stroke 2004. 35 2832–2836 [DOI] [PubMed] [Google Scholar]
- 58. Segura T Serena J Molins A Davalos A Clusters of microembolic signals: a new form of cerebral microembolism presentation in a patient with middle cerebral artery stenosis Stroke 1998. 29 722–724 [DOI] [PubMed] [Google Scholar]
- 59. Chung JW Kim BJ Sohn CH Yoon BW Lee SH Branch atheromatous plaque: a major cause of lacunar infarction (high-resolution mri study) Cerebrovasc Dis Extra 2012. 2 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim BJ Lee DH Kang DW Kwon SU Kim JS Branching patterns determine the size of single subcortical infarctions Stroke 2014. 45 1485–1487 [DOI] [PubMed] [Google Scholar]
- 61. Leng X Wong KS Liebeskind DS Evaluating intracranial atherosclerosis rather than intracranial stenosis Stroke 2014. 45 645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bodle JD Feldmann E Swartz RH Rumboldt Z Brown T Turan TN High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease Stroke 2013. 44 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mossa-Basha M Hwang WD De Have-non A Hippe D Balu N Becker KJ et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes Stroke 2015. 46 1567–1573 [DOI] [PubMed] [Google Scholar]
- 64. Dieleman N van der Kolk AG Zwanenburg JJ Harteveld AA Biessels GJ Luijten PR et al. Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions Circulation 2014. 130 192–201 [DOI] [PubMed] [Google Scholar]
- 65. Xu WH Li ML Gao S Ni J Zhou LX Yao M et al. Plaque distribution of stenotic middle cerebral artery and its clinical relevance Stroke 2011. 42 2957–2959 [DOI] [PubMed] [Google Scholar]
- 66. van der Kolk AG Zwanenburg JJ Brundel M Biessels GJ Visser F Luijten PR et al. Intracranial vessel wall imaging at 7.0-t mri Stroke 2011 42–2478 [DOI] [PubMed] [Google Scholar]
- 67. Li ML Xu WH Song L Feng F You H Ni J et al. Atherosclerosis of middle cerebral artery: evaluation with high-resolution mr imaging at 3t Atherosclerosis 2009. 204 447–452 [DOI] [PubMed] [Google Scholar]
- 68. Xu WH Li ML Gao S Ni J Yao M Zhou LX et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance Ann Neurol 2012. 71 195–198 [DOI] [PubMed] [Google Scholar]
- 69. Yuan C Hatsukami TS Obrien KD High-resolution magnetic resonance imaging of normal and atherosclerotic human coronary arteries ex vivo: discrimination of plaque tissue components J Investig Med 2001. 49 491–499 [DOI] [PubMed] [Google Scholar]
- 70. Turan TN Rumboldt Z Granholm AC Columbo L Welsh CT Lopes-Virella MF et al. Intracranial atherosclerosis: Correlation between in-vivo 3t high resolution mri and pathology Atherosclerosis 2014. 237 460–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Majidi S Sein J Watanabe M Hassan AE Van de Moortele PF Suri MF et al. Intracranial-derived atherosclerosis assessment: an in vitro comparison between virtual histology by intravascular ultrasonography, 7t mri, and histopathologic findings AJNR Am J Neuroradiol 2013. 34 2259–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van der Kolk AG Zwanenburg JJ Denswil NP Vink A Spliet WG Daemen MJ et al. Imaging the intracranial atherosclerotic vessel wall using 7t mri: initial comparison with histopathology AJNR Am J Neuroradiol 2015. 36 694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Robertson AL Jr Butkus A Ehrhart LA Lewis LA Experimental arteriosclerosis in dogs Evaluation of anatomopathological findings. Atherosclerosis 1972. 15 307–325 [DOI] [PubMed] [Google Scholar]
- 74. Suzuki M Experimental cerebral atherosclerosis in the dog I. A morphologic study. Am J Pathol 1972. 67 387–402 [PMC free article] [PubMed] [Google Scholar]
- 75. Bhardwaj JR Kukreja RS Banerjee AK Datta BN Chakravarti RN A morphological study of experimental cerebral atherosclerosis in rhesus monkeys Indian J Med Res 1984. 79 86–92 [PubMed] [Google Scholar]
- 76. Kahn SG Siller WG Absence of atherosclerosis in the cerebral arteries of chickens fed an atherogenic diet Nature 1967. 213 720–721 [DOI] [PubMed] [Google Scholar]
- 77. Ratcliffe HL Luginbuhl H Pivnik L Coronary, aortic and cerebral atherosclerosis in swine of 3 age-groups: Implications Bull World Health Organ 1970. 42 225–234 [PMC free article] [PubMed] [Google Scholar]
- 78. Imai H Thomas WA Cerebral atherosclerosis in swine: Role of necrosis in progression of diet-induced lesions from proliferative to atheromatous stage Exp Mol Pathol 1968. 8 330–357 [DOI] [PubMed] [Google Scholar]
- 79. Suzuki M Fukuuchi Y Shimazu K Kim HS Meyer JS Cerebral atherosclerosis in the dog. Ii. Cerebral circulation Arch Pathol 1973. 96 14–17 [PubMed] [Google Scholar]
- 80. Kato H [experimental cerebral atherosclerosis in the rabbit. Scanning electron microscopic observation of initial lesion sites] Fukuoka Igaku Zasshi 1987. 78 532–546 [PubMed] [Google Scholar]
- 81. Ooboshi H Rios CD Chu Y Christenson SD Faraci FM Davidson BL et al. Augmented adenovirus-mediated gene transfer to atherosclerotic vessels Arterioscler Thromb Vasc Biol 1997. 17 1786–1792 [DOI] [PubMed] [Google Scholar]
- 82. Kong J Tamaki N Asada M Early lesions of cerebral atherosclerosis from induced hypertension in watanabe heritable hyperlipidemic rabbits Kobe J Med Sci 2000. 46 87–101 [PubMed] [Google Scholar]
- 83. Ito T Shiomi M Cerebral atherosclerosis occurs spontaneously in homozygous whhl rabbits Atherosclerosis 2001. 156 57–66 [DOI] [PubMed] [Google Scholar]
- 84. Zhang T [an experimental study on cerebral arteriosclerosis] Zhonghua Shen Jing Jing Shen Ke Za Zhi 1991. 24 228–230 253 [PubMed] [Google Scholar]
- 85. Hollander W Prusty S Kemper T Rosene DL Moss MB The effects of hypertension on cerebral atherosclerosis in the cynomolgus monkey Stroke 1993. 24 1218–1226 discussion 1226–1227 [DOI] [PubMed] [Google Scholar]
- 86. Kato H Tokunaga O Watanabe T Sunaga T Experimental cerebral atherosclerosis in the rabbit Scanning electron microscopic study of the initial lesion site. Pathol Res Pract 1991. 187 797–805 [DOI] [PubMed] [Google Scholar]
- 87. Kurozumi T Tanaka K Yae Y Hypertension-induced cerebral atherosclerosis in the cholesterol-fed rabbit Atherosclerosis 1978. 30 137–145 [DOI] [PubMed] [Google Scholar]
- 88. Kurozumi T Imamura T Tanaka K Yae Y Koga S Permeation and deposition of fibrinogen and low-density lipoprotein in the aorta and cerebral artery of rabbits – immuno-electron microscopic study Br J Exp Pathol 1984. 65 355–364 [PMC free article] [PubMed] [Google Scholar]
- 89. Yamori Y Horie R Sato M Fukase M Hemodynamic derangement for the induction of cerebrovascular fat deposition in normotensive rats on a hypercholesterolemic diet Stroke 1976. 4 385–389 [DOI] [PubMed] [Google Scholar]
- 90. Jeynes BJ Warren BA Cerebral atheroembolism. An animal model Stroke 1982. 13 312–318 [DOI] [PubMed] [Google Scholar]
- 91. Ritz K Denswil NP Stam OC van Lieshout JJ Daemen MJ Cause and mechanisms of intracranial atherosclerosis Circulation 2014. 130 1407–1414 [DOI] [PubMed] [Google Scholar]
- 92. van Lammeren GW Moll FL De Borst GJ de Kleijn DPV de Vries JPPM Pasterkamp G Atherosclerotic plaque bio-markers: beyond the horizon of the vulnerable plaque Curr Cardiol Rev 2011. 7 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen XY Wong KS Lam WW Zhao HL Ng HK Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study Cerebrovasc Dis 2008. 25 74–80 [DOI] [PubMed] [Google Scholar]
- 94. Chen XY Lam WW Ng HK Zhao HL Wong KS Diagnostic accuracy of MRI for middle cerebral artery stenosis: a postmortem study J Neuroimaging 2006. 16 318–322 [DOI] [PubMed] [Google Scholar]
- 95.Chen XY, Wong KS, Lam WW, Ng HK. High signal on T1 sequence of magnetic resonance imaging confirmed to be intraplaque haemorrhage by histology in middle cerebral artery. Int J Stroke. 2014;9:E19. doi: 10.1111/ijs.12277. [DOI] [PubMed] [Google Scholar]
- 96. Majidi S Sein J Watanabe M Hassan AE Van de Moortele PF Suri MF Clark HB Qureshi AI Intracranial-derived atherosclerosis assessment: an in vitro comparison between virtual histology by intravascular ultrasonography, 7T MRI, and histopathologic findings AJNR Am J Neuroradiol 2013. 34 2259–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. van der Kolk AG Zwanenburg JJ Denswil NP Vink A Spliet WG Daemen MJ Visser F Klomp DW Luijten PR Hendrikse J Imaging the intracranial atherosclerotic vessel wall using 7T MRI: initial comparison with histopathology AJNR Am J Neuroradiol 2015. 36 694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]