Abstract
Background:
Intravenous thrombolysis (IVT) remains the only approved systemic reperfusion treatment for acute ischemic stroke (AIS), however there are scarce data regarding outcomes and complications of IVT in Greece. We evaluated safety and efficacy outcomes of IVT for AIS in Greece using the Safe Implementation of Thrombolysis in Stroke: International Stroke Thrombolysis Register (SITS-ISTR) dataset.
Methods:
All AIS patients treated with IVT in Greece between December 2002 and July 2017 and recorded in the SITS-ISTR were evaluated. Demographics, risk factors, baseline stroke severity [defined using National Institutes of Health Stroke Scale (NIHSS)], and onset-to-treatment time (OTT) were recorded. Safety outcomes included symptomatic intracranial hemorrhage (sICH) and 3-month mortality rates. The efficacy outcomes evaluated a reduction in baseline NIHSS score at 2 and 24 h following IVT onset, 3-month favorable functional outcome [FFO; modified Rankin scale (mRS) scores of 0–1] and 3-month functional independence (FI; mRS-scores of 0–2). The safety and efficacy outcomes were assessed comparatively with previously published data from SITS national and international registries.
Results:
A total of 523 AIS patients were treated with IVT in 12 Greek centers participating in the SITS-ISTR during the study period (mean age 62.4 ± 12.7; 34.6% women; median baseline NIHSS score: 11 points; median OTT: 150 min). The rates of sICH were 1.4%, 2.3%, and 3.8% according to the SIST-MOST, ECASS II, and NINDS criteria respectively. The median reduction in NIHSS score at 2 and 24 h was 3 [interquartile range (IQR): 1–5] and 5 (IQR: 2–8) points respectively. The 3-month FI, FFO and mortality were 66.5%, 55.6% and 7.9%. All safety and efficacy outcomes were comparable with available data from SITS-ISTR in other European countries.
Conclusions:
Our study underscores the safety and efficacy of IVT for AIS in Greece. Additional action is necessary in order to increase the availability of IVT in the Greek population and to include more centers in the SITS-ISTR.
Keywords: acute ischemic stroke, Greece, intravenous thrombolysis, outcome, SITS registry
Introduction
In the absence of specific contraindications, intravenous thrombolysis (IVT) remains the only approved systemic reperfusion treatment for acute ischemic stroke (AIS).1–3 The Safe Implementation of Thrombolysis in Stroke (SITS), the SITS Monitoring Study (SITS-MOST) and the SITS International Stroke Thrombolysis Register (SITS-ISTR) have used the same international, multicenter, web-based database monitoring the safety and efficacy of intravenous tPA (tissue plasminogen activator) for AIS in different countries all over the world and in the European Union in particular.4,5 There are scarce data regarding outcomes and complications of IVT for AIS in Greece.6,7 In view of the former considerations, we evaluated safety and efficacy outcomes of systemic thrombolysis for AIS in Greece using the SITS-ISTR dataset that has been available in Greece during the past 15 years. These outcomes were also evaluated comparatively with previously published data from SITS registry in other European countries.
Methods
All AIS IVT patients in Greece treated within 4.5 h from symptom onset recorded in the SITS-ISTR between 25 December 2002 and 1 July 2017 were evaluated. Demographics, risk factors, baseline stroke severity [defined using National Institutes of Health Stroke Scale (NIHSS)], and onset-to-treatment time (OTT) were recorded. Details regarding the definitions of baseline characteristics in SITS registries have been previously published.4,5
Safety outcomes included symptomatic intracranial hemorrhage (sICH) and 3-month mortality. sICH was defined using SITS-MOST, ECASS (European Cooperative Acute Stroke Study) II and NINDS (National Institute of Neurological Disorders) rtPA Stroke criteria. The NINDS definition counts an ICH as symptomatic when any new hemorrhage on a brain computed tomography (CT) scan within 7 days of treatment is associated with any decline in neurologic status quantified as 1 or more points increase in the baseline NIHSS score.8 The ECASS II definition is consistent with any intracranial hemorrhage within 7 days of IVT leading to an increase in the NIHSS score of ⩾4 points compared with the baseline or to the lowest score in 7 days, or leading to death.9 The SITS-MOST defines sICH as a local or remote type II parenchymal hematoma within 22–36 h or sooner after treatment, associated with a worsening on the NIHSS score of at least 4 points from baseline assessment or from the lowest score from baseline to 24 h, or an ICH resulting in death.4
The efficacy outcomes assessed a reduction in the baseline NIHSS score at 2 and 24 h following tPA bolus, 3-month favorable functional outcome [FFO; defined as modified Rankin scale (mRS) scores of 0–1] and 3-month functional independence (FI; defined as mRS-scores of 0–2).4,10,11 The safety and efficacy outcomes were evaluated comparatively with previously published data from the SITS registry in Italy,12 Austria,13 Belgium14 and Poland,15 the SITS-EAST10 and the entire SITS-ISTR16 databases. The participating counties in SITS-EAST registry include Croatia, Czech Republic, Estonia, Greece, Hungary, Lithuania, Poland, Russia, Slovakia, Slovenia and Turkey, representing approximately 30% of the population of the SITS registry.10
The present study is a retrospective analysis of registered Greek AIS patients in the SITS-ISTR dataset. SITS-Greece is embedded within the SITS-ISTR, which was originally approved by the Ethics Committee of Karolinska Institutet, Stockholm.5 Further approval from institutional Ethics Committee was obtained in institutions that required this, while other institutions approved the register for conduct as an anonymized audit. Informed consent was obtained from patients or their relatives according to the existing regulations in each participating institution.
Statistical analysis
Continuous variables are presented as mean values with standard deviation (SD) or median values with the corresponding interquartile range (IQR) as appropriate. To compare the rates of safety and efficacy outcomes between the different patient cohorts, we calculated the percentages, with corresponding 95% confidence intervals (95% CI). We also presented graphically the serial assessments of median NIHSS scores, with their corresponding IQRs, reported in both the SITS-ISTR Greek and SITS-EAST cohorts. Finally, we calculated the differences in serial assessments of the NIHSS scores of AIS patients reported in the SITS-ISTR Greek cohort at 2 h (dNIHSS at 2 h = baseline NIHSS − NIHSS at 2 h) and 24 h (dNIHSS at 24 h = baseline NIHSS − NIHSS at 24 h) following tPA bolus. All statistical analysis were performed with the Stata Statistical Software version 13 for Windows (StataCorp LP, College Station, TX, USA).
Results
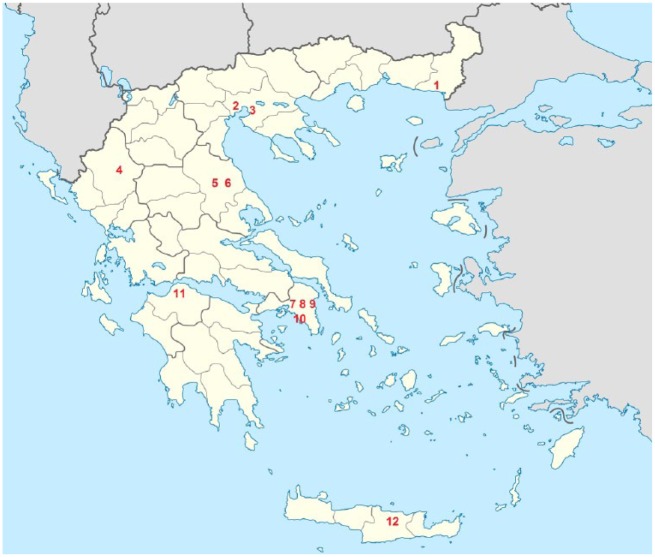
A total of 523 AIS patients were treated with IVT in 12 Greek centers participating in the SITS-ISTR registry during the study period (mean age 62.4 ± 12.7; 34.6% women; median baseline NIHSS score: 11 points, IQR: 7–17). As evident from Figure 1 the aforementioned centers are dispersed across almost all of the Greek territory, while three of them are located in Athens – the capital city of Greece inhabited by nearly the one third of the total Greek population. Baseline characteristics and outcomes of the study population are presented in Table 1. About one quarter of the study population reported pretreatment with antiplatelet and lipid-lowering, while 19.3% were diabetic. Onset-to-door, door-to-needle and OTT times were 70 (IQR: 45–110), 67 (IQR: 50–92) and 150 (IQR: 120–180) min respectively (Table 1).
Figure 1.
Map of Greece indicating the location of centers participating actively in the Safe Implementation of Thrombolysis in Stroke registry and providing treatment with intravenous thrombolysis in acute ischemic stroke patients.
(1): Department of Neurology, University Hospital of Alexandroupolis, Alexandroupolis; (2): Department of Neurology, Papageorgiou Hospital, Thessaloniki; (3): Second Department of Neurology, AHEPA University Hospital, Thessaloniki; (4): Department of Neurology, University Hospital of Ioannina, Ioannina; (5): Department of Neurology, University Hospital of Larissa, Larissa; (6): Intensive Care Unit, General Hospital of Larissa, Larissa; (7): Second Department of Neurology, Attikon University Hospital, Athens, (8): Department of Neurology, Evangelismos Hospital, Athens; (9): Department of Neurology, Athens General Hospital G. Gennimatas, Athens; (10): Stroke Unit, Metropolitan Hospital, Piraeus; (11): Department of Neurology, University Hospital of Patras, Patras; (12): Department of Neurology, University Hospital of Heraklion, Heraklion.
Table 1.
Baseline characteristics and outcomes of acute stroke patients treated with intravenous thrombolysis according to the Safe Implementation of Treatments in Stroke protocol in Greece.
| Variable | n = 523 |
|---|---|
| Baseline characteristics | |
| Age (mean ± SD), years | 62.4 ± 12.7 |
| Females (%) | 34.6% |
| Admission NIHSS (median, IQR) | 11 (7–17) |
| Hypertension (%) | 60.0% |
| Diabetes (%) | 19.3% |
| Hyperlipidemia (%) | 36.6% |
| Current smoking (%) | 33.1% |
| Previous stroke (%) | 11.4% |
| Atrial fibrillation (%) | 15.3% |
| Congestive heart failure (%) | 3.3% |
| Antiplatelet pretreatment (%) | 27.8% |
| Antidiabetic pretreatment (%) | 12.5% |
| Antihypertensive pretreatment (%) | 52.6% |
| Statin pretreatment (%) | 24.9% |
| Admission SBP baseline (mean ± SD), mmHg | 149 ± 24 |
| Admission DBP (mean ± SD), mmHg | 83 ± 13 |
| Admission serum glucose (median, IQR), mg/dl | 115 (100–144) |
| Baseline total cholesterol (mean ± SD), mg/dl | 187 ± 54 |
| Early ischemic changes in baseline CT scan (%) | 8.3% |
| Disability prior to index event (%)* | 2.5% |
| Acute ischemic stroke subtype (%) | Large artery atherosclerosis: 51.4% |
| Cardioembolism: 21.2% | |
| Small vessel disease: 9.3% | |
| Cryptogenic: 9.9% | |
| Other unusual causes: 2.9% | |
| Not available: 5.3% | |
| Times from stroke onset to treatment | |
| Onset-to-door time (min, median, IQR) | 70 (45–110) |
| Door-to-imaging time (min, median, IQR) | 30 (20–45) |
| Door-to-needle time (min, median, IQR) | 67 (50–92) |
| Onset-to-treatment time (min, median, IQR) | 150 (120–180) |
| Outcomes | |
| Any ICH (%, 95% CI) | 58/503 (11.5%, 9.0–14.6%) |
| sICH – SITS-MOST (%, 95% CI) | 3/214 (1.4%, 0.3–4.2%) |
| sICH – ECASS (%, 95% CI) | 5/214 (2.3%, 0.8–5.5%) |
| sICH – NINDS (%, 95% CI) | 7/214 (3.8%, 1.5–6.7%) |
| dNIHSS at 2 h | 3 (1–5) |
| dNIHSS at 24 h | 5 (2–8) |
| mRS at 3-months (median, IQR) | 1 (0–4)** |
| FFO (mRS: 0–1) at 3-months (%, 95% CI) | 262/471 (55.6%, 51.1–60.1%)** |
| FI (mRS: 0–2) at 3-months (%, 95% CI) | 313/471 (66.5%, 62.0–70.7%)** |
| Mortality at 3-months (%, 95% CI) | 37/471 (7.9%, 5.8–10.7%)** |
mRS score prior to stroke onset >1.
last observation carried forward; follow-up was available in 471 patients.
CI, confidence interval; CT, computed tomography; DBP, diastolic blood pressure; dNIHSS, difference in NIHSS score from baseline; ECASS, European Cooperative Acute Stroke Study; FFO, favorable functional outcome; FI, functional independence; ICH, intracerebral hemorrhage; IQR, interquartile range; mRS, modified Rankin scale score; NIHSS, National Institutes of Health Stroke Scale; NINDS, National Institute of Neurological Disorders and Stroke; SBP, systolic blood pressure; SD, standard deviation; sICH, symptomatic ICH; SITS-MOST, Safe Implementation of Treatments in Stroke Monitoring Study.
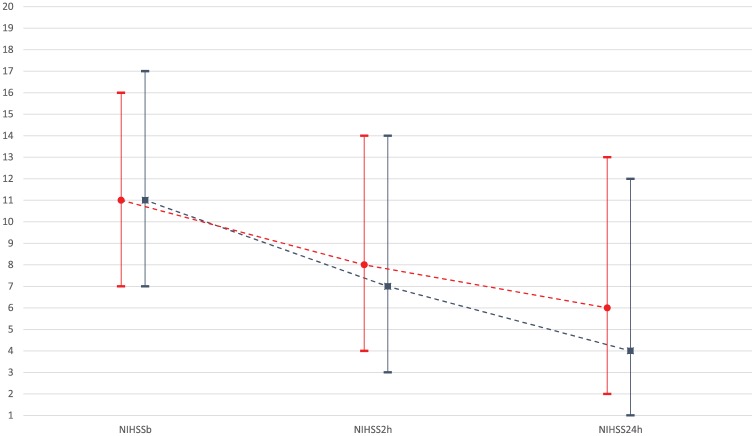
The rates of sICH were 1.4% (95% CI: 0.3–4.2%), 2.3% (95% CI: 0.8–5.5%), and 3.8% (95% CI: 1.5–6.7%) according to the SITS-MOST, ECASS II, and NINDS criteria respectively (Table 1). The median reduction in NIHSS score at 2 and 24 h was 3 (IQR: 1–5) and 5 (IQR: 2–8) points respectively. Figure 2 displays graphically the NIHSS score evolution in the Greek and the SITS-EAST patient cohorts. Early clinical improvement of the neurological deficits attributed to AIS was comparable between the two cohorts. More specifically, in the Greek cohort, the NIHSS score declined from a baseline value of 11 (IQR: 7–17) to 7 (IQR: 3–14) and to 4 points (IQR: 1–12) at 2 and 24 h following tPA bolus. Similarly, in the SITS-EAST cohort, the NIHSS score declined from a baseline value of 11 (IQR: 7–16) to 8 (IQR: 4–14) and to 6 points (IQR: 2–13) at 2 and 24 h following tPA bolus.
Figure 2.
Serial assessments of National Institutes of Health Stroke Scale scores at baseline, 2 and 24 h following tPA bolus in acute ischemic stroke patients reported in the Safe Implementation of Treatments in Stroke Greek (blue line) and EAST registry (red line).
tPA, tissue plasminogen activator.
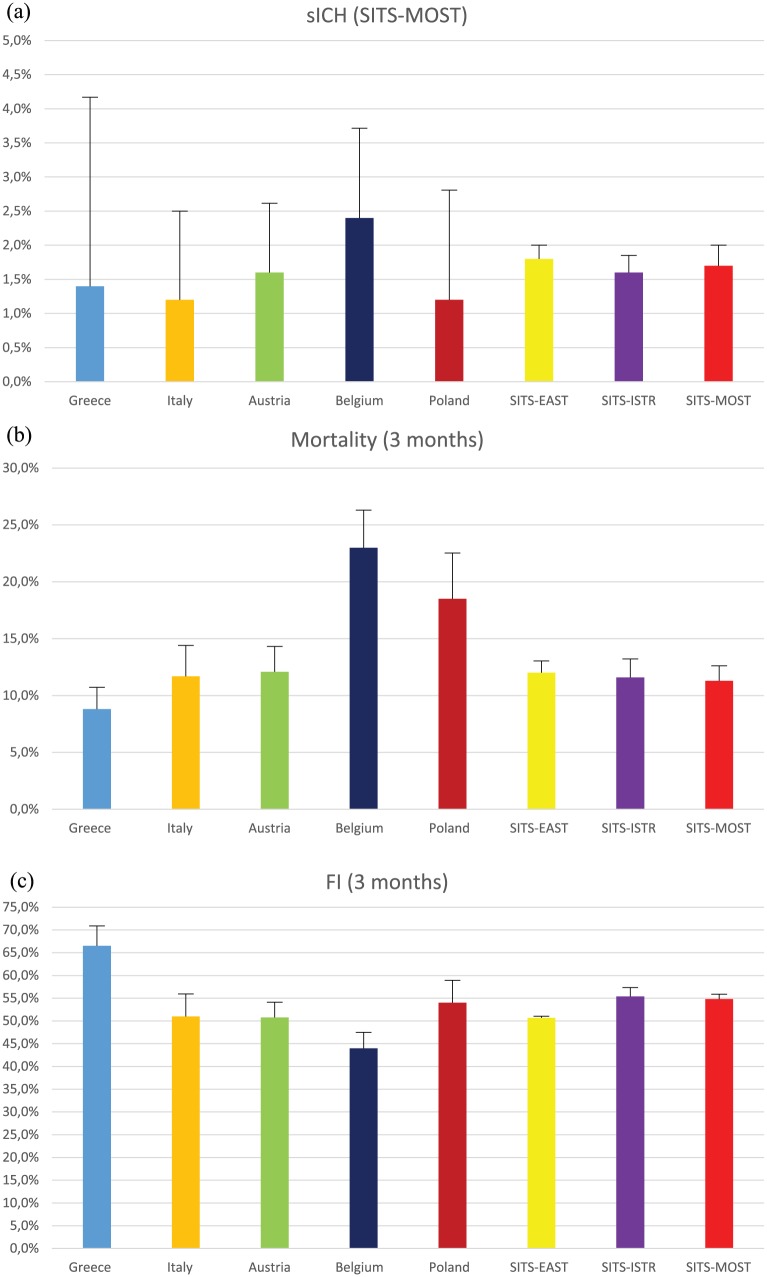
The 3-month FI, FFO and mortality were 66.5% (95% CI: 62.0–70.7%), 55.6% (95% CI: 51.1–60.1%) and 7.9% (95% CI: 5.8–10.7%). All safety and efficacy outcomes of IVT in Greece were comparable with available data from SITS-ISTR registry in other European countries (Table 2 and Figure 3). More specifically, there was substantial overlap in the 95% CI of reported outcomes between the Greek and other available SITS registries (Table 2), while the highest rate of FI at 3 months was documented in the Greek cohort (Figure 3).
Table 2.
Cumulative table of published national and international stroke registries providing data on the outcome of acute stroke patients treated with intravenous thrombolysis.
| Greece | Italy12 | Austria 13 | Belgium14 | Poland15 | SITS-EAST10 | SITS-ISTR16 | SITS-MOST4 | |
|---|---|---|---|---|---|---|---|---|
| Patients (N) | 523 | 586 | 896 | 743 | 481 | 19077 | 12529 | 6483 |
| Age (years, IQR) | 63 (55–72) | 68 (21–80) | 70 (60–77) | 71 | 66 (59–75) | 70 (61–77) | 68 | 68 (59–75) |
| Females (%) | 34.6% | 39.4% | 44.6% | 47% | 41% | 44.1% | 39.2% | 39.8% |
| Therapeutic window (hours) | 0–4.5 | 0–3 | 0–4.5 | 0–4.5 | 0–4.5 | 0–4.5 | 0–3/3–4.5*** | 0–3 |
| Admission NIHSS (IQR) | 11 (7–17) | 13 (2–25) | 13 (8–18) | 14 (9–19) | 11 (7–17)** | 11 (7–16) | 12 | 12 (8–17) |
| Stroke onset-to-treatment time (min, IQR) |
150 (120–180) |
152 (40–180) |
135 (105–160) |
140 (N/A) |
160 (130–175) |
150 (120–180) |
140 (N/A) |
140 (115–165) |
| sICH – NINDS (%, 95%CI) |
3.8% (1.5–6.7%) |
6.7% (4.8–9.1%) |
N/A | 9.5% (7.5–11.9%) |
7.0% (5.0–9.7%)* |
N/A | 7.3% (6.9–7.8%) |
7.3% (6.7–7.9%) |
| sICH – ECASS (%, 95%CI) |
2.3% (0.8–5.5%) |
4.5% (2.9–6.5%) |
7.6% (6–9.6%) |
6.9% (5.1–9%) |
N/A | 4.9% (4.6–5.2%) |
4.8% (4.5–5.2%) |
4.6% (4.1–5.1%) |
| sICH – SITS-MOST (%, 95%CI) |
1.4% (0.3–4.2%) |
1.2% (0.5–2.5%) |
1.6% (0.9–2.6%) |
2.4% (1.4–3.7%) |
1.2% (0.5–2.8%)* |
1.8% (1.6–2.0%) |
1.6% (1.4–1.8%) |
1.7% (1.3–2.0%) |
| 3-month FFO (%, 95%CI) |
55.6% (51.1–60.1%) |
40.6% (36.6–44.7%) |
38.1% (34.8–41.6%) |
29.2% (25.8–32.9%) |
N/A | 42.4% (41.5–43.3%) |
39.9% (39.0–40.9%) |
38.9% (37.7–40.1%) |
| 3-month FI (%, 95%CI) |
66.5% (62.0–70.7%) |
51.6% (47.4–55.7%) |
50.8% (47.2–54.4%) |
44% (39.5–47.5%) |
54% (50–59%)* |
50.7% (49.9–51.6%) |
56.3% (55.4–57.3%) |
54.8% (53.5–56.0%) |
| 3-month mortality (%, 95%CI) |
7.9% (5.8–10.7%) |
11.7% (9.2– 14.6%) |
12.1% (9.9–14.5%) |
23% (19.6–26.3%) |
18.6% (15.3–22.5%)* |
12%, (11.5–12.6%) |
12.2% (11.6–12.8%) |
11.3% (10.5–12.1%) |
Due to the report of percentages instead of absolute values the 95% CI for Poland have been estimated approximately.
mean value (95% CI).
For the SITS-ISTR cohort we present pooled data from both the 0–3 h and 3–4.5 h cohorts.
CI, confidence interval; EAST, EAST registry; ECASS, European Cooperative Acute Stroke Study; FI, functional independence (defined as a modified Rankin scale score ⩽2); IQR, interquartile range; ISTR, International Stroke Thrombolysis Register; MOST, Monitoring Study; NIHSS, National Institutes of Health Stroke Scale; NINDS, National Institute of Neurological Disorders and Stroke; SITS, Safe Implementation of Treatments in Stroke.
Figure 3.
Bar charts with corresponding upper 95% confidence intervals on the rates of (a) symptomatic intracerebral hemorrhage, (b) 3-month mortality and (c) 3-month functional independence reported in available national and international stroke registries of acute stroke patients treated with intravenous thrombolysis.
Discussion
Our study underlines the safety and efficacy of IVT for AIS in Greece. To the best of our knowledge, this is the largest series of AIS patients treated with IVT in Greece over a 15-year period. It should be noted that registration of IVT patient data into SITS-ISTR is not mandatory in Greece, yet is highly recommended. Nevertheless, there are some institutions that do not include AIS patients treated with IVT in the SITS registry. Since there is no Greek national thrombolysis registry, SITS is the only registry where safety and outcomes after IVT can be assessed in the vast majority of tertiary care stroke centers from all over Greece. Therefore, we consider SITS-ISTR data as fully representative of acute stroke care in terms of systemic reperfusion in our country.
Another important aspect deriving from the present analysis is that the median OTT time (150 min) is similar to that of other national and international dataset of SITS registries. OTT is an established measure reflecting the quality of an acute stroke system of care.3,17 Despite the significant impact of the financial crisis on the national health system, depriving Greek hospitals of both human as well as material resources and given the lack of organization of stroke unit care system at a national level,18 the achieved OTT in the present study indicates the tremendous efforts of the healthcare personnel to maintain the quality of stroke care in Greece. On the other hand, the compelling need for improvement of our median door-to-needle time of 67 min needs to be acknowledged. The future efforts of SITS investigators in Greece will focus on implementation of the Helsinki Stroke Thrombolysis model19 in order to reduce delays in door-to-needle time by at least 20 min. Notably, the transferability of this model in stroke systems of care outside Finland has been shown to be feasible and effective.20
The safety and efficacy outcomes in our cohort were comparable with those of larger datasets of SITS registries.4,10,12–16 Compared with other cohorts, in the Greek cohort there was a trend towards a higher rates of 90-day FI (mRS 0–2) or FFO (mRS 0–1) and lower rates of mortality and sICH, particularly according to NINDS and ECASS definitions. This finding could probably be attributed to the younger age and lower median NIHSS scores at baseline of patients included in the Greek cohort (Table 2). The possibility of selection bias should also be taken into consideration due to the presentation of data only from centers actively participating in the SITS registry. However, it should also be noted that the rates of sICH, 3-month mortality and 3-month FFO that we documented in the present study were very close to those reported by two individual patient data meta-analyses pooling data from all available randomized-controlled clinical trials of IVT for AIS.21,22
To our knowledge this is the first and only study so far providing data on the outcomes of IVT-treated AIS patients in Greece. Despite these encouraging results, there are still several actions to be undertaken in order to further increase tPA availability in Greece. There are numerous organizational obstacles that have to be overcome, as well as innovations that have to be adopted. For instance, the availability of IVT has to be expanded beyond the main large cities to smaller cities, islands and rural areas by developing stroke-oriented and efficient transfer protocols, by emergency medical services and telestroke facilities focused on efficient recognition and fast triage of eligible candidates for IVT.23,24 Finally, a media campaign, aimed to enhance public awareness on stroke symptoms and the beneficial effects of timely reperfusion,25,26 would definitely increase acute reperfusion treatment rates in Greece.
Certain limitations of the present study need to be addressed. First, we should acknowledge that the number of patients included in our cohort, although numerically comparable to the previously published nationwide studies (Table 2), is relatively small given the extended time period of 15 years. Apart from the low number of included patients, it should be noted that potential imbalances in thrombolysis rates and heterogeneity in stroke care delivery between participating centers might also be present. Second, we did not perform formal statistical comparisons of baseline characteristics and outcomes between the Greek and the other SITS registry cohorts, since the individual patient data of the other national and international registries were not available. Third, as we have already mentioned the SITS-ISTR registry does not capture all institutions in Greece treating AIS patients with IVT. Moreover, it should be noted that there is a potential risk of selection bias given that data for all potentially eligible IVT patients are not provided from participating centers. Fourth, the outcomes in SITS registry are self-reported and this may introduce numerous sources of bias. Finally, given the limited availability of endovascular reperfusion therapies in Greece,27 we did not report separate outcomes in patients treated with bridging therapy (IVT followed by mechanical thrombectomy) in the present cohort.
In conclusion, our study underscores the tolerability and efficacy of IVT for AIS in Greece. The present findings may serve as a roadmap for the improvement of organization of acute stroke care at a national level. Additional action is necessary in order to increase the availability of systemic thrombolysis in the Greek population, to shorten door-to-needle times and to include more centers in the SITS-ISTR registry.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Apostolos Safouris  https://orcid.org/0000-0002-9630-6949
https://orcid.org/0000-0002-9630-6949
Contributor Information
Georgios Tsivgoulis, Second Department of Neurology, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece; Department of Neurology, University of Tennessee Health Science Center, Memphis, Tennessee, USA.
Odysseas Kargiotis, Stroke Unit, Metropolitan Hospital, Piraeus, Greece.
Jobst Rudolf, Department of Neurology, Papageorgiou Hospital, Thessaloniki, Greece.
Apostolos Komnos, Intensive Care Unit, General Hospital of Larissa, Greece.
Antonios Tavernarakis, Department of Neurology, Evangelismos Hospital, Athens, Greece.
Theodoros Karapanayiotides, Second Department of Neurology, AHEPA University Hospital, Aristotle University of Thessaloniki, School of Medicine, Thessaloniki, Greece.
John Ellul, Department of Neurology, University Hospital of Patras, School of Medicine, University of Patras, Patras, Greece.
Aristeidis H. Katsanos, Department of Neurology, University of Tennessee Health Science Center, Memphis, Tennessee, USA Department of Neurology, University of Ioannina School of Medicine, Ioannina, Greece.
Sotirios Giannopoulos, Department of Neurology, University of Ioannina School of Medicine, Ioannina, Greece.
Maria Gryllia, Department of Neurology, Athens General Hospital G. Gennimatas, Athens, Greece.
Apostolos Safouris, Second Department of Neurology, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece Stroke Unit, Metropolitan Hospital, Piraeus, Greece.
Panagiotis Papamichalis, Intensive Care Unit, General Hospital of Larissa, Greece.
Konstantinos Vadikolias, Department of Neurology, Democritus University of Thrace, School of Medicine, Alexandroupolis, Greece.
Panayiotis Mitsias, Department of Neurology Medical School, University of Crete, Heraklion, Crete, Greece; Department of Neurology, Henry Ford Hospital Detroit, Michigan, USA.
Georgios Hadjigeorgiou, Department of Neurology, School of Medicine, University of Thessaly, Larissa, Greece.
References
- 1. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 2. European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25: 457–507. [DOI] [PubMed] [Google Scholar]
- 3. Tsivgoulis G, Kargiotis O, Alexandrov AV. Intravenous thrombolysis for acute ischemic stroke: a bridge between two centuries. Expert Rev Neurother 2017; 17: 819–837. [DOI] [PubMed] [Google Scholar]
- 4. Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
- 5. Wahlgren N Ahmed N Dávalos A et al.;. SITS investigators. Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 2008; 72: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 6. Rudolf J, Tsivgoulis G, Deretzi G, et al. Feasibility and safety of intravenous thrombolysis for acute ischaemic stroke in Northern Greece. Int J Stroke 2011; 6: 91–92. [DOI] [PubMed] [Google Scholar]
- 7. Tsivgoulis G, Tavernarakis A, Vadikolias K, et al. Effectiveness and safety of intravenous thrombolysis for acute ischemic stroke: the experience in Greece. Neyrologia 2015; 24: 6–13. [Google Scholar]
- 8. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 9. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 10. Tsivgoulis G, Katsanos AH, Kadlecová P, et al. Intravenous thrombolysis for ischemic stroke in the golden hour: propensity-matched analysis from the SITS-EAST registry. J Neurol 2017; 264: 912–920. [DOI] [PubMed] [Google Scholar]
- 11. Tsivgoulis G, Zand R, Katsanos AH, et al. Safety of intravenous thrombolysis in stroke mimics: prospective 5-year study and comprehensive meta-analysis. Stroke 2015; 46: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 12. Lorenzano S, Ahmed N, Rosselli A, et al. Safe implementation of thrombolysis in stroke-monitoring study in Italy. Eur J Neurol 2010; 17: 163–167. [DOI] [PubMed] [Google Scholar]
- 13. Topakian R, Brainin M, Eckhardt R, et al. Thrombolytic therapy for acute stroke in Austria: data from the Safe Implementation of Thrombolysis in Stroke (SITS) register. Eur J Neurol 2011; 18: 306–311. [DOI] [PubMed] [Google Scholar]
- 14. Vanacker P, Thijs V, Peeters A, et al. The Belgian experience with intravenous thrombolysis for acute ischemic stroke. Acta Neurol Belg 2010; 110: 157–162. [PubMed] [Google Scholar]
- 15. Kobayashi A, Czlonkowska A, Ahmed N, et al. Intravenous recombinant tissue plasminogen activator for acute stroke in Poland: an analysis based on the Safe Implementation of Thrombolysis in Stroke (SITS) registry. Acta Neurol Scand 2010; 122: 229–236. [DOI] [PubMed] [Google Scholar]
- 16. Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 2008; 372: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 17. Tsivgoulis G, Safouris A, Alexandrov AV. Safety of intravenous thrombolysis for acute ischemic stroke in specific conditions. Expert Opin Drug Saf 2015; 14: 845–864. [DOI] [PubMed] [Google Scholar]
- 18. Kerasidou A, Kingori P, Legido-Quigley H. “You have to keep fighting”: maintaining healthcare services and professionalism on the frontline of austerity in Greece. Int J Equity Health 2016; 15: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meretoja A, Strbian D, Mustanoja S, et al. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology 2012; 79: 306–313. [DOI] [PubMed] [Google Scholar]
- 20. Meretoja A, Weir L, Ugalde M, et al. Helsinki model cut stroke thrombolysis delays to 25 minutes in Melbourne in only 4 months. Neurology 2013; 81: 1071–1076. [DOI] [PubMed] [Google Scholar]
- 21. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 22. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kodankandath TV, Wright P, Power PM, et al. Improving transfer times for acute ischemic stroke patients to a comprehensive stroke center. J Stroke Cerebrovasc Dis 2017; 26: 192–195. [DOI] [PubMed] [Google Scholar]
- 24. Kepplinger J, Barlinn K, Deckert S, et al. Safety and efficacy of thrombolysis in telestroke: a systematic review and meta-analysis. Neurology 2016; 87: 1344–1351. [DOI] [PubMed] [Google Scholar]
- 25. Zinkstok SM, Beenen LF, Luitse JS, et al. Thrombolysis in stroke within 30 minutes: results of the acute brain care intervention study. PLoS One 2016; 11: e0166668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flynn D, Ford GA, Rodgers H, et al. A time series evaluation of the FAST National Stroke Awareness Campaign in England. PLoS One 2014; 9: e104289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsivgoulis G, Safouris A, Mantatzis M, et al. Mechanical thrombectomy for acute ischemic stroke due to large vessel occlusion: a two year experience of Greek centers in SITS registry. Neyrologia 2017; 24: 6–17. [Google Scholar]