Abstract
The aim of this study was to evaluate CyberKnife-based radioablation as a salvage treatment for prostate cancer postirradiation relapses based on a group of patients disqualified from available conventional methods of salvage treatment. Thirty-eight patients were treated with a fraction dose varying from 5.5 to 10 Gy (median 7.35) to a total dose of 18 to 36.25 Gy (median 36.25). In all, 55.3% of patients had androgen deprivation therapy during this time. Nine patients had oligometastases in the salvage time. The follow-up varied from 1.6 to 46.4 months (mean 19.7, median 14.4). In all, 92.6% to 97.4% of patients had no gastrointestinal acute adverse effects; no effects higher than G1 were noted. There were particular (up to 4.8%) G2 late gastrointestinal effects. The percentage without genitourinary acute effects varied from 59.1% to 78.9%; 3.7% had G3 toxicity. G3 late genitourinary toxicity appeared 3 times, the maximal percentage being 12.5% (24 months after salvage treatment). The nadir of prostate-specific antigen median was 0.24 ng/mL (9 months after treatment). Twelve (31.6%) patients failed in the timeline of 6 to 42 months after salvage treatment (mean 18.7, median 16.5)—5 due to dissemination. In 2 cases, progression in existing metastases was identified. Five (13.2%) patients had biochemical failure without additional metastases (local relapses); hence, local control was 86.8%. The failure risk is strongly influenced by initial disease stage and presalvage prostate-specific antigen concentration. The obtained results permit us to conclude that such a treatment could be an effective and safe option for prostate cancer postirradiation relapse salvage treatment.
Keywords: SABR, SBRT, salvage radiotherapy, prostate cancer relapse, prostate cancer radioablation, prostate cancer salvage treatment
Introduction
There is a wide variety of retreatment options for patients with prostate cancer (PCP) with local relapses, after a definitive radiation treatment. The most common is probably androgen deprivation therapy (ADT), which cannot be considered as a radical treatment. When we take into account radical salvage modalities, surgery seems to be the treatment of choice and for a considerable length of time was the only option.1–6 Later on, some new possibilities such as cryotherapy,7,8 high-intensity focused ultrasound (HIFU),9,10 and brachytherapy (BT)11–18 appeared. All the aforementioned modalities have yielded promising results but not consistently—primarily due to limited availability, patient performance status and/or comorbidities, local anatomical conditions, and a lack of patient agreement.
With the development of various radioablative techniques, it has become clear that they can be applied in the case of postirradiation relapse of PCP. This idea has been introduced into the practice, but to date, the number of publications in this field is considerably low, and results are inconclusive mainly due to the limited number of patients treated and lack of comparisons in the frame of randomized clinical trials.19–25
Purpose
The aim of this study was to evaluate salvage CyberKnife (CK)-based stereotactic ablative radiotherapy (SABR) local toxicity and the effectiveness of previously irradiated PCP relapses.
Materials and Methods
The material consisted of 38 previously irradiated PCP with local relapse—with or without synchronic oligometastases. Patient age on the day of salvage CK varied from 59 to 89 years (mean 73.2, median 71.6). All these patients were deemed unsuitable for surgery based on the stage of disease, medical reasons, or their unwillingness to engage in such a treatment. As a second option, salvage BT was considered; yet no patient from this group was qualified because of the aforementioned reasons. The patients were informed about alternative treatment methods, respectable prognoses, and adverse effects and signed informed consent forms.
The ADT was used in 21 cases before the first irradiation, in 27 cases between the first irradiation and relapse and in 21 cases during or after the salvage therapy. We do not consider SABR to be a replacement but rather a part of salvage treatment, complimentary with ADT in applicable cases, just as ADT should not be considered to be a radical treatment alone.
The First Irradiation
Four patients were previously operated on and irradiated for prostatic bed. Before the first definitive RT, 21 patients used ADT. Prostate-specific antigen (PSA) concentration varied in this time from 1.1 to 331 ng/mL (mean 24.4, median 12). Before the first treatment, 6 patients were from the low-risk group, 15 from the intermediate-risk group, and 17 from the high-risk group. All patients were treated with radical intention; however, 1 patient during this time had node, and another one bone, oligometastases. The Gleason and T stage of patients before the first treatment are presented in Table 1.
Table 1.
Gleason Score and the T Stage of Patients Before First Radical Radiotherapy.
| GS | 2 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Lack of GS |
| N | 1 | 3 | 4 | 11 | 8 | 5 | 3 | 2 | 1 |
| T stage | 1b | 1c | 2a | 2c | 3a | 3b | 2 unspecified | ||
| N | 1 | 15 | 3 | 7 | 1 | 4 | 7 | ||
Abbreviation: GS, Gleason score; N, number of cases; T stage, primary tumor stage according to TNM staging system.
The low Gleason score (3 patients with 4 points) and a lack of score in 1 case—assessed on the basis of biopsy—now in discordance with pathological rules stemmed from the time of evaluation (patients were irradiated between 2000 and 2014). In these 4 cases, biopsies were performed in small remote centers, and reevaluation was not feasible.
In all, 34 patients were treated with external beam radiotherapy (EBRT), 1 with BT, and 3 with EBRT combined with a BT boost. In the EBRT subgroup, the total dose (TD) varied from 45 Gy (postoperative patient) to 78 Gy (median 76 Gy). Five patients were irradiated using a fraction dose (fd) of 1.8 Gy and 29 using an fd of 2 Gy. The BT patient was irradiated (high-dose rate [HDR]) with an fd of 12 Gy up to a TD of 36 Gy. In the combined irradiation subgroup, 2 patients were treated using an fd of 2 Gy up to 54 Gy and the next boosted with 1 (HDR) BT fraction of 10 Gy, and the last one was treated with up to 68 Gy (2 Gy per fraction) postoperatively, and the next—after 14 years—3× 10 Gy were delivered for local relapse with HDR BT. Detailed data concerning prior RT are presented in Table 2.
Table 2.
Previous Radiotherapy Schemes.
| Number of Patients Treated | Prior Operation | RT Total Dose | RT Fraction Dose | BT Total Dose | BT Fraction Dose |
|---|---|---|---|---|---|
| 1 | Yes | 45 | 1.8 | NA | NA |
| 2 | No | 54 | 2 | 10 | 10 |
| 1 | Yes | 68 | 2 | 30 | 10 |
| 1 | No | 70 | 2 | NA | NA |
| 5 | No | 72 | 2 | NA | NA |
| 1 | Yes | 72 | 2 | NA | NA |
| 2 | No | 73.8 | 1.8 | NA | NA |
| 5 | No | 74 | 2 | NA | NA |
| 15 | No | 76 | 2 | NA | NA |
| 1 | Yes | 76 | 2 | NA | NA |
| 2 | No | 77.4 | 1.8 | NA | NA |
| 1 | No | 78 | 2 | NA | NA |
| 1 | No | NA | NA | 36 | 12 |
Abbreviations: BT, brachytherapy; NA, not applicable; RT, radiotherapy.
The PSA nadir after the first treatment appeared in the 1 to 134 months period (mean 27.2, median 18.1) and varied from 0.00 to 3.03 ng/mL (mean 0.45, median 0.17).
The Salvage CK-Based SABR
The period between the first irradiation and CK-based salvage varied from 22 to 179 months (mean 100, median 101).
The maximal PSA concentration between the first and the salvage irradiation varied from 0.44 to 66.0 ng/mL (mean 7.39, median 4.3). In some cases of PSA increase, antihormonal treatment was started; thus, before the salvage treatment, 27 patients had ADT. This was the reason for a lower PSA value just before CK-based SABR, which was in the range of 0.12 to 48.83 ng/mL (mean 5.98, median 3.26).
In 12 cases, local relapse was diagnosed based on multiparametric magnetic resonance imaging and positron-emission tomography (PET; Fluorocholine or prostatic-specific membrane antigen]). In 26 cases, a biopsy was performed. In 9 cases, the Gleason score was not evaluated, in 5 it was assessed as 6, in 6 as 7, in 4 as 8, and in 2 cases as 9 points.
The CK-based salvage SABR was rendered between August 2012 and July 2017. Twenty-four patients were reirradiated using the same regimen as utilized for definitive radical radioablation; 5× 7.25 Gy (in 1 case as a focal treatment), which is actually performed routinely as a salvage radioablation in our center. Detailed data concerning the RT schemes administered are shown in Table 3.
Table 3.
Radiotherapy Regimens Used for Salvage SABR of Recurrent PCP.
| N | SABR Scheme (Gy) | TD (Gy) |
|---|---|---|
| 1 | 3 × 6 | 18.0 |
| 1 | 2 × 10 | 20.0 |
| 1 | 3 × 7.5 | 22.5 |
| 3 | 5 × 5.5 | 27.5 |
| 3 | 5 × 6 | 30.0 |
| 1 | 2 × 10 on prostate + 1 × 10 boost on relapse | 30.0 |
| 1 | 3 × 10 | 30.0 |
| 2 | 2 × 10 on prostate (3 × 15 on relapse—SIB) | 35.0 |
| 1 | 6 × 6 | 36.0 |
| 24 | 5 × 7.25 (1 focal treatment) | 36.25 |
Abbreviations: PCP, prostate cancer patients; SABR, stereotactic ablative radiotherapy; SIB, simultaneous boost; TD, total dose.
The diversity of irradiation regimens among the patients is due to fact that we had no prior experience in the field of salvage post-RT CK-based SABR, and the available literature was not clear on the subject of optimal dose and fractionation. Additional data from newly published works and attained experience enabled us to reach the conclusion that 5× 7.25 Gy modality is optimal for the patients.
The prescribed dose was always delivered to planning target volume (PTV). For 23 patients irradiated with an fd of 7.25 Gy to 36.25 Gy, we used clinical target volume (CTV) and margins for PTV as for a primary radical CK-based treatment. The CTV comprised prostate (or relapse—2 cases) and proximal 1 cm of seminal vesicles. Then, 3 mm of posterior margin and 5 mm margin in the remaining directions were added. In 1 case of focal treatment (5× 7.25 Gy), we added a 5 mm margin to the lesion (gross tumor volume [GTV]). In 3 cases of patients with boost, margins as above were added to prostate and next 5 mm margin to the GTV (boost).
Among patients treated with others fractionation schedules, 6 were irradiated for whole prostate (CTV), 3 for 1 lobe (CTV), and 2 (postoperative) for local relapse (GTV = CTV). For PTV, typical (aforedescribed) margins (5 and 3 mm) were used.
Nine patients had prostate cancer oligometastases during and before CK-based salvage SABR, 5 of them in the lymph nodes (4 in retroperitoneal and 1 in inguinal; one simultaneously with metastases in the pelvis and spine) and 4 in the bones only (2 in the spine, 1 in the spine and pelvis, and 1 in the clavicle and rib). In the first subgroup, in 3 cases, CK-based SABR was used for metastases (also for patients with nodal and bone lesions) and in the next 2 cases, a combination of fractionated RT and ADT was performed. In the subgroup with bone metastases in 3 cases, CK-based SABR was also applied and in 1 case chemotherapy (in this case, metastases developed 68.1 months prior to local relapse salvage treatment).
The patients were subject to control 1 and 3 months posttreatment and subsequently every 3 months up to 24 months and then every 6 months up to 42 months. The last control was performed 45 months after the salvage reirradiation. The ADT usage, PSA concentration, adverse effects (acute up to 3 months, next late) in accordance with the European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group (EORTC/RTOG) system, and pain intensity in the pelvic region (according to the Brief Pain Inventory ranging from 0 “pain free” to 10 “maximal imaginable pain”) were evaluated. Neither mailing nor phone controls were conducted.
While analyzing the adverse effects, the subjectivity of EORTC/RTOG gastrointestinal (GI) toxicity evaluation should be considered. We did not use rigid protocols of antidiarrheal agents administration; the decision was dependent on the patient’s feeling/needs; thus, it can have an impact on the given grades.
Statistical Analysis
To model survival of patients, we used parametric (Weibull's) regression implemented in the “flexsurv” package (R package version 1.1) which is intended as a general platform for survival modeling in R platform.26 We decided to use “flexsurv” because it allows avoiding the risk of model misspecification due to analysis of a relatively small group of patients.
Results
The follow-up (FU) varied from 1.6 to 46.4 months (mean 19.7, median 14.4). The PSA nadir, after CK-based SABR, varied from 0.003 to 29.87 ng/mL (mean 2.11, median 0.38) and appeared in the period of 1 to 12 months (mean 6.6, median 6.0) post-treatment.
Detailed data concerning the evaluated endpoints are presented in Table 4. Due to the clinical situation (relapse/metastases), the percentage of patients using ADT was high and exceeded 50% usually. The percentage and intensity of acute as well as late adverse effects was unexpectedly low (Figures 1 –4). No G2-GI acute effects were noted, and G2-GI late reactions were observed in 4.8% of cases only in months 9 and 12. The genitourinary (GU) effects were more severe. The percentage of G2 acute reactions varied from 5.3% to 9.1%, and 3.7% of G3 effects were noted in third month. Also, in the case of late GU reactions, up to 16.7% were observed. During 3 controls (12th, 18th, and 24th), G3 late GU reactions were noted (12.5% 1 year after treatment). The above-described adverse effects reflect local (prostate/prostate bed) toxicity. Irradiation of oligometastases (mainly due to their location) did not produce any substantial additional GI and GU toxicity as evaluated in the EORTC/RTOG grading system.
Table 4.
Percentage of Evaluated Patients Without ADT, GI and GU Adverse Effects, PSA Concentration, and Pain Intensity of Evaluated Patients During FU.
| Time After SABR | SABR End | 1 Month | 3 Months | 6 Months | 9 Months | 12 Months | 15 Months | 18 Months | 21 Months | 24 Months | 30 Months | 36 Months | 42 Months | 48 Months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | 38 (100) | 23 (61) | 29 (76) | 27 (71) | 24 (63) | 24 (63) | 13 (34) | 9 (24) | 9 (24) | 9 (24) | 7 (18) | 9 (24) | 9 (24) | 5 (13) |
| Without ADT (%) | 44.7 | 43.5 | 48.3 | 47.8 | 50 (5% CT) | 47.6 (9.5% CT) | 30.8 | 55.6 | 55.6 | 33.3 | 28.6 | 55.6 | 33.3 | 20 |
| GI 0 (%) | 97.4 | 95.5 | 92.6 | 95.7 | 90.4 | 85.7 | 92.3 | 100 | 100 | 100 | 100 | 88.9 | 100 | 100 |
| GI 1 (%) | 2.6 | 4.5 | 7.4 | 4.3 | 4.8 | 9.5 | 7.7 | 11.1 | ||||||
| GI 2 (%) | 4.8 | 4.8 | ||||||||||||
| GI 3 (%) | ||||||||||||||
| GU 0 (%) | 78.9 | 59.1 | 70.4 | 69.6 | 81.0 | 71.4 | 75.0 | 88.9 | 77.8 | 87.5 | 85.7 | 88.9 | 77.8 | 80 |
| GU 1 (%) | 15.8 | 31.8 | 18.5 | 17.4 | 4.8 | 20.8 | 8.3 | 11.1 | 14.3 | 11.1 | 22.2 | 20 | ||
| GU 2 (%) | 5.3 | 9.1 | 7.4 | 13.0 | 9.4 | 4.8 | 16.7 | 11.1 | ||||||
| GU 3 (%) | 3.7 | 4.8 | 11.1 | 12.5 | ||||||||||
| PSA mean | 5.98 | 2.14 | 2.76 | 3.70 | 2.32 | 12.20 | 21.90 | 176.83 | 14.20 | 4.42 | 21.06 | 20.22 | 35.97 | 130.45 |
| PSA median | 3.26 | 0.87 | 0.65 | 0.27 | 0.24 | 0.42 | 1.11 | 1.20 | 1.50 | 0.84 | 0.84 | 1.09 | 2.19 | 0.80 |
| BPI mean | 0.026 | 0.182 | 0.259 | 0.044 | 0.667 | 0.250 | 0.000 | 0.111 | 0.000 | 0.500 | 0.000 | 0.000 | 0.556 | 1.25 |
Abbreviations: ADT, androgen deprivation therapy; BPI, Brief Pain Inventory; CT, chemotherapy; FU, follow-up; GI, gastrointestinal; GU, genitourinary; PSA, prostate-specific antigen; SABR, stereotactic ablative radiotherapy.
Figure 1.
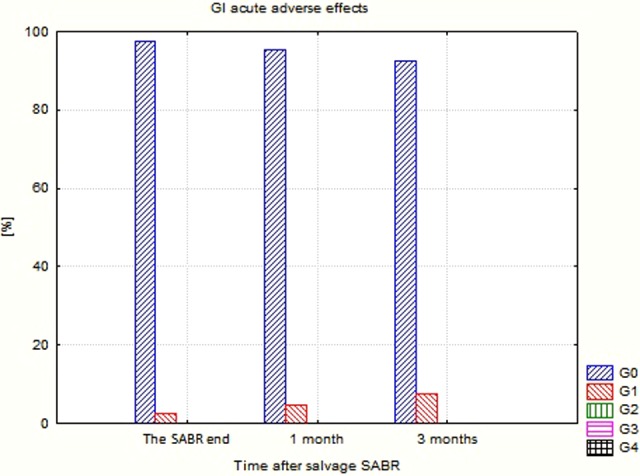
Gastrointestinal (GI) acute adverse effects after CyberKnife-based salvage SABR. SABR indicates stereotactic ablative radiotherapy.
Figure 2.
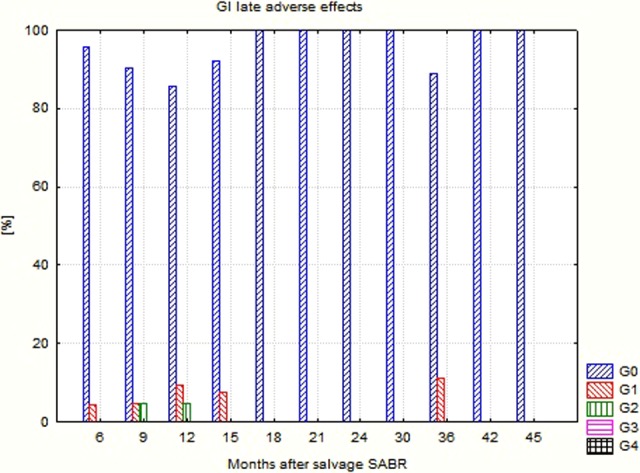
Gastrointestinal (GI) late adverse effects after CyberKnife-based salvage SABR. SABR indicates stereotactic ablative radiotherapy.
Figure 3.
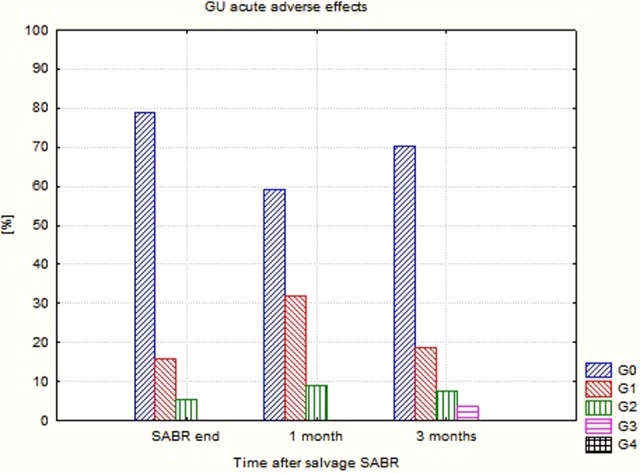
Genitourinary (GU) acute adverse effects after CyberKnife-based salvage SABR. SABR indicates stereotactic ablative radiotherapy.
Figure 4.
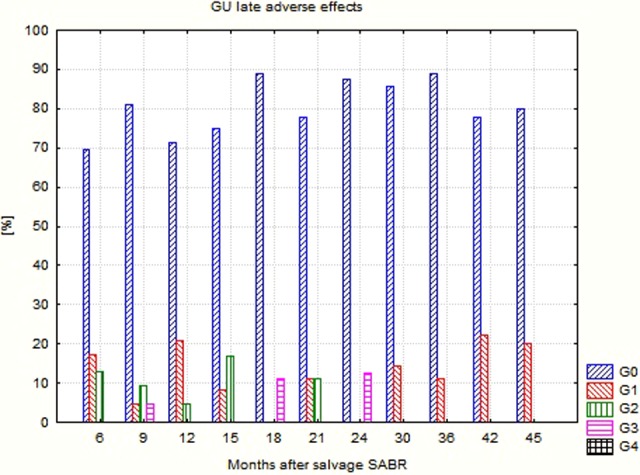
Genitourinary (GU) late adverse effects after CyberKnife-based salvage SABR. SABR indicates stereotactic ablative radiotherapy.
The decline in the PSA concentration mean value was very rapid in the first month after irradiation and continued until ninth month. Later on, the course of this value fluctuated partially as a result of failures and partially because of the decreasing number of monitored patients.
Pain intensity during FU was relatively low and only once, in the 45th month (5 patients controlled) did exceed 1 point in the 11-grade scale (Figure 5). This meant that pain in the pelvis (probably provoked by both the disease and treatment adverse effects) was not a significant concern in the observed group.
Figure 5.
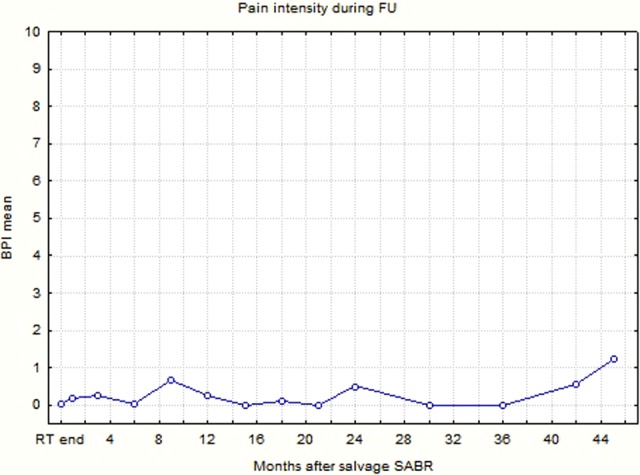
Changes of pain intensity mean value during the follow-up (Brief Pain Inventory grading system).
Twelve (31.6%) patients failed in the 6- to 42-month time frame after salvage treatment (mean 18.7, median 16.5).
Five of them failed due to dissemination (in 2 cases—bones and in 3—bones and lymph nodes). Two of these patients (1 with bone and lymph node metastases and 1 with solitary lymph node involved) were subsequently treated with CK radioablation; the 1 with bone and lymph node metastases was administered docetaxel-based chemotherapy and the 2 with bone dissemination received ADT. In 3 cases, metastases were diagnosed using Fluorocholine PET and in 2 using bone scintigraphy.
In 2 cases, a progression in the existing metastases (one case in bone and another in lymph nodes metastases) was discovered. Five (13.2%) patients had biochemical failure (BF) without additional metastases (local relapses) in the period ranging from 10.3 to 22.2 months (mean 15.9, median 13.9); hence, local control was 86.8%. The estimated hazard ratios for the studied clinical events are reported in Table 5.
Table 5.
Hazards Ratios: Factors Influencing BT and Failure of the Treatment.
| Clinical Event | Risk Factor | HR (95% CI) | P Value |
|---|---|---|---|
| Failure | Neoadjuvant HT | 4.82 (1.25-18.5) | .0218 |
| T | 1.55 (1.04-2.33) | .0326 | |
| PSA before CK | 1.04 (1.01-1.07) | .0474 | |
| Biochemical failure | Prior TD | 0.90 (0.81-0.99) | .0275 |
| Months to PSA nadir after the first RT | 1.03 (1.02-1.05) | .0005 |
Abbreviations: BT, brachytherapy; CI, confidence interval; CK, CyberKnife; HT, hormone therapy; PSA, prostate-specific antigen; RT, radiotherapy.
It can be seen in Table 5 that patients using neoadjuvant ADT before initial RT had increased risk of clinical failure nearly 5 times (Figure 6).
Figure 6.
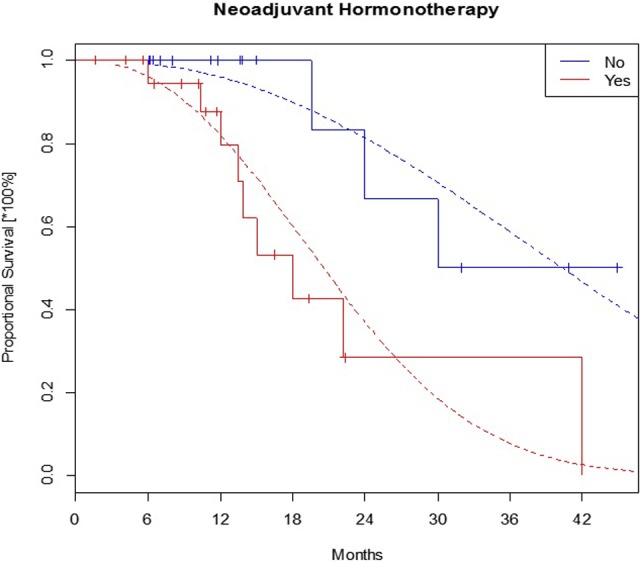
The failure-free survival in subgroups with and without neoadjuvant ADT before first radiotherapy. ADT indicates androgen deprivation therapy.
Moreover, an increase in T degree also generated the risk for an earlier failure in patients as well as PSA before CK salvage SABR. As regards the latter, the difference of 10 ng/mL elevated the risk up to (1.0410 − 1) × 100% = 48%, that is, almost by a half. Prior to delivery, a TD (during the first RT) had apparent inverse effect on the BF; 1 Gy reduced the risk for this clinical event by 10%. Analogously, the difference of 2 Gy generated (1 − 0.92) × 100% = 19% risk reduction. Whereas prolongation of time to PSA nadir after first radiation treatment increased the risk of BF in patients.
Discussion
Initially, we should try to define the clinical significance of post-RT salvage EBRT. As much as 30% to 50% of the patients treated with radical RT for local disease prostate cancer will develop BF, according to the Phoenix criterion,27 within 10 years of the treatment.6 Many of these could profit from local therapy yet will not meet the requirements for well-established methods28 such as salvage surgery, cryotherapy, or BT. In these cases, because of its low risk, feasibility, and availability, EBRT could prove to be an alternative treatment modality.
Due to the technical implications of local salvage treatment, most of the methods result in considerable toxicity. Radical prostatectomy (RP) can cause anastomotic stricture in 17% to 32% and incontinence in 48% to 68% of patients.29 Similar adverse effects accompany HIFU, such as urinary incontinence (in approximately 49.5%) and bowel obstruction (in 20%).30 Cryotherapy, which is typically connected with less adverse effect, can still lead to mild to moderate (6%-13%) or severe (2%-4%) incontinence and urinary retention (2%-21%).29 The BT tends to induce grade 3 to 4 GU toxicity in a mean of 17% (range 0-47) and grade 3 to 4 GI toxicity in 5.6% (0-24) as a late complication.29 In comparison, EBRT seems to be well tolerated by patients. Apart from the work published by Zilli et al 19 (patients treated with a higher TD and a concomitant BT boost), acute and late radiation toxicities were mostly contained within grade 0 and grade 2, with few to no cases of grade 3+–in terms of both GU and GI adverse effects (Tables 6 and 7). It should be noted that the percentage of patients with FU varied from 61% to 76% during the first year of observation and consecutively decreased, which could have affected the evaluation of adverse effects.
Table 6.
Acute GI and GU Toxicity at the Time of Completion of SBRT According to CTCAE Criteria.
| Acute GI Toxicity | Acute GU Toxicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | 0 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Zilli et al 19 | 43% | 43% | 14% | 0% | 0% | 14% | 14% | 72% | 0% | 0% |
| Vavassori et al 20 | “After a median follow-up of 11.3 months none of the patients had developed severe urinary or rectal acute toxicity” | |||||||||
| Zerini et al 21 | 88% | 6% | 3% | 0% | 0% | 75% | 19% | 6% | 0% | 0% |
| Fuller et al 22 | 100% | 0% | 0% | 0% | 97% | 0% | 3% | 0% | ||
| Janoray et al 23 | 90% | 10% | 0% | 0% | 0% | 81% | 14% | 5% | 0% | 0% |
| Mbeutcha24 | 72% | 6% | 11% | 0% | 0% | 44% | 28% | 17% | 6% | 0% |
| Miszczyk et al | 97% | 3% | 0% | 0% | 0% | 79% | 16% | 5% | 0% | 0% |
Abbreviations: GI, gastrointestinal; GU, genitourinary; SABR, stereotactic ablative radiotherapy.
The toxicity in our work was assessed according EORTC/RTOG grading system.
Table 7.
Highest Reported Late GI and GU Toxicity After Completion of SBRT According to CTCAE Criteria.
| Late GI Toxicity | Late GU Toxicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| Zilli et al 19 | 7% | 7% | 21% | 29% | 36% | 0% | 21% | 21% | 29% | 29% |
| Vavassori et al 20 | “No patients experienced adverse late effects higher than EORTC/RTOG grade 2” | |||||||||
| Zerini et al 21 | 78% | 16% | 0% | 0% | 0% | 72% | 19% | 3% | 0% | 0% |
| Fuller et al 22 | 100% | 0% | 0% | 0% | 83% | 10% | 3% | 3% | ||
| Janoray et al 23 | 100% | 0% | 0% | 0% | 0% | 95% | 5% | 0% | 0% | 0% |
| Mbeutcha et al 24 | 50% | 0% | 6% | 0% | 0% | 33% | 22% | 6% | 0% | 6% |
| Miszczyk et al | 76% | 8% | 3% | 0% | 0% | 50% | 16% | 16% | 5% | 0% |
Abbreviations: CTCAE, common terminology criteria for adverse events; EORTC/RTOG, European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group; GI, gastrointestinal; GU, genitourinary; SABR, stereotactic ablative radiotherapy.
The toxicity in our work was assessed according EORTC/RTOG grading system.
The treatment results, however, are difficult to discuss in terms of comparison to other methods due to short FU (median of 11.2-24 and 94 months in 1 study), limited samples (6-38 patients), and different end points. To our knowledge, a 5-year biological disease-free survival (bDFS) ranges from 47% to 82% for RP29 and 50% to 70% for cryotherapy.31 In carefully selected patients, HDR or low-dose rate BT can also be implemented, resulting in 5-year bDFS varying from 20% to 70%.16,29 Compared to these methods, it seems that CK salvage EBRT with a TD of 34 to 36.25 Gy delivered in 5 fractions could prove to be a suitable alternative. Furthermore, it is associated with manageable toxicity and promising treatment outcomes (2-year bDFS of 82%, 1-year bDFS of 85.7%, 1-year biochemical non-evidence of disease of 55.6%, 1-year local control of 86.8%).22–24 The treatment plans used in other studies, however, were connected with either high-radiation toxicity19 or mediocre treatment results.20,21 The comparison of PCP salvage reirradiation results is presented in Table 8.
Table 8.
Results of PCP Salvage Reirradiation.
| Author | Number of Patients | Years of Treatment | TD (Gy) | Equipment Used | Percent Receiving ADT | Median FU (Months) | Endpoint | Results (%) |
|---|---|---|---|---|---|---|---|---|
| Zilli et al 19 | 14a | 2003-2008 | 44-72 + 18-25h | Unspecified | 79 | 94 | 5 years bDFS | 35.7 |
| Vavassori et al 20 | 6b | 2007-2008 | 30 | CyberKnife | 60 | 11.2 | bDFS | 40 |
| Zerini et al 21 | 32c | 2008-2013 | 25-30 | Saturno 43, Clinac 600, Clinac 2100, RapidArc, Vero, CyberKnife | 34 | 21.3 | NED | 40.6 |
| Fuller et al 22 | 29d | 2009-2014 | 34 | CyberKnife | 0 | 24 | 2 years bDFS | 82 |
| Janoray23 | 11e | 2011-2014 | 36.25 | CyberKnife | 9 | 11.7 | 1 year bDFS | 85.7 |
| Mbeutcha24 | 18f | 2011-2015 | 35 | CyberKnife | 55.6 | 14.5 | bNED | 55.6 |
| Miszczyk et al | 38g | 2012-2017 | 18-36.25 | CyberKnife | 55.3 | 14.4 | NED LC | 68.4 86.8 |
Abbreviations: ADT, androgen deprivation therapy; bDFS, biological disease-free survival; LC, local control; FU, follow-up; NED, non-evidence of disease; PCP, prostate cancer patients; TD, total dose.
aModalities of treatment used during prior treatment: 12 EBRT, 2 EBRT + BT boost.
bModalities of treatment used during prior treatment: 6 EBRT.
cModalities of treatment used during prior treatment: 10 RT, 22 RP + RT, including 29 cases of EBRT and 3 cases of BT.
dModalities of treatment used during prior treatment: 28 EBRT, 1 125I BT.
eModalities of treatment used during prior treatment: 11 EBRT.
fModalities of treatment used during prior treatment: 18 EBRT.
gModalities of treatment used during prior treatment: 34 EBRT, 1 BT, 3 EBRT + BT boost.
fBT/CK boost.
Interpretation of statistical analysis results in terms of the salvage treatment failure seems to be clear. Negative impact of neoadjuvant ADT probably is caused by patient selection for this treatment; neoadjuvant ADT was administered mainly in the group of high-risk patients with worse prognosis. Also, the impact of T stage is understandable; the higher disease stage is associated with larger risk of failure. The PSA concentration is one of the strongest factors influencing final result of PCP treatment; hence, PSA before CK salvage SABR impacts significantly the failure risk.
It is more difficult to interpret an impact of TD delivered in first RT and time to PSA nadir after this treatment on the risk of BF. We could suppose that higher TD delivered during first RT influences a risk of local relapse (in prostate region), which is often a reason of BF, but we cannot find any explanation for dependency between time to PSA nadir and BF risk.
Conclusion
The obtained results permit us to conclude that EBRT, utilizing a TD of 34 to 36.25 Gy, delivered in 5 fractions, could be an effective and safe option for local salvage treatment of prostate cancer postirradiation relapses. This treatment failure is strongly influenced by initial disease stage and presalvage PSA concentration.
Abbreviations
- ADT
androgen deprivation therapy
- bDFS
biological disease-free survival
- BF
biochemical failure
- BPI
Brief Pain Inventory
- BT
brachytherapy
- CTV
clinical target volume
- CK
CyberKnife
- EBRT
external beam radiotherapy
- fd
fraction dose
- FU
follow-up
- GTV
gross tumor volume
- HDR
high-dose rate
- HIFU
high-intensity focused ultrasound
- PSA
prostate-specific antigen
- PCP
prostate cancer patients
- PET
positron-emission tomography
- PSMA
prostatic-specific membrane antigen
- PTV
planning target volume
- RP
radical prostatectomy
- SABR
stereotactic ablative radiotherapy
- TD
total dose
Footnotes
Ethics Approval: Ethics approval was not required for this study—this treatment modality was described previously in the literature and was used as a routine treatment. All patients signed a consent for such a treatment.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: I gave paid lectures for Accuray in the past.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Leszek Miszczyk  http://orcid.org/0000-0002-9668-6056
http://orcid.org/0000-0002-9668-6056
References
- 1. Bates AS, Samavedi S, Kumar A, et al. Salvage robot assisted radical prostatectomy: a propensity matched study of perioperative, oncological, and functional outcomes. Eur J Surg Oncol. 2015;41(11):1540–1546. doi:10.1016/j.ejso.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 2. Linares Espinós E, Sánchez-Salas R, Sivaraman A, et al. Minimally invasive salvage prostatectomy after primary radiation or ablation treatment. Urology. 2016;94:111–116. doi:10.1016/j.urology.2016.04.040 [DOI] [PubMed] [Google Scholar]
- 3. Kenney PA, Nawaf CB, Mustafa M, et al. Robotic-assisted laparoscopic versus open salvage radical prostatectomy following radiotherapy. Can J Urol. 2016;23(3):8271–8277. [PMC free article] [PubMed] [Google Scholar]
- 4. Nunes-Silva I, Barret E, Srougi V, et al. Effect of prior focal therapy on perioperative, oncologic, and functional outcomes of salvage robotic assisted radical prostatectomy. J Urol. 2017;198(5):1069–1076. doi:10.1016/j.juro.2017.05.071 [DOI] [PubMed] [Google Scholar]
- 5. Metcalfe MJ, Troncoso P, Guo CC, et al. Salvage prostatectomy for postradiation adenocarcinoma with treatment effect: pathological and oncological outcomes. Can UrolAssoc J. 2017;11(7): E277–E284. doi:10.5489/cuaj.4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Artibani W, Porcaro AB, De Marco V, Cerruto MA, Siracusano S. Management of biochemical recurrence after primary curative treatment for prostate cancer: a review. Urol Int. 2018;100(3):251–262. doi:10.1159/000481438 [DOI] [PubMed] [Google Scholar]
- 7. Lian H, Yang R, Lin T, Wang W, Zhang G, Guo H. Salvage cryotherapy with third-generation technology for locally recurrent prostate cancer after radiation therapy. Int Urol Nephrol. 2016;48(9):1461–1466. doi:10.1007/s11255-016-1339-3 [DOI] [PubMed] [Google Scholar]
- 8. KvorningTernov K, KragJakobsen A, Bratt O, Ahlgren G. Salvage cryotherapy for local recurrence after radiotherapy for prostate cancer. Scand J Urol. 2015;49(2):115–119. doi:10.3109/21681805.2014.968869 [DOI] [PubMed] [Google Scholar]
- 9. Shah TT, Peters M, Kanthabalan A, et al. PSA nadir as a predictive factor for biochemical disease-free survival and overall survival following whole-gland salvage HIFU following radiotherapy failure. Prostate Cancer Prostatic Dis. 2016;19(3):311–316. doi:10.1038/pcan.2016.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanthabalan A, Peters M, Van Vulpen M, et al. Focal salvage high-intensity focused ultrasound in radiorecurrent prostate cancer. BJU Int. 2017;120(2):246–256. doi:10.1111/bju.13831 [DOI] [PubMed] [Google Scholar]
- 11. Rose JN, Crook JM, Pickles T, Keyes M, Morris WJ. Salvage low-dose-rate permanent seed brachytherapy for locally recurrent prostate cancer: association between dose and late toxicity. Brachytherapy. 2015;14(3):342–349. doi:10.1016/j.brachy.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 12. Kukiełka AM, Strnad V, Stauffer P, et al. Salvage brachytherapy in combination with interstitial hyperthermia for locally recurrent prostate carcinoma following external beam radiation therapy: a prospective phase II study. J Contemp Brachytherapy. 2015;7(3):254–258. doi:10.5114/jcb.2015.51871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guimas V, Quivrin M, Bertaut A, et al. Focal or whole-gland salvage prostate brachytherapy with iodine seeds with or without a rectal spacer for postradiotherapy local failure: how best to spare the rectum? Brachytherapy. 2016;15(4):406–411. doi:10.1016/j.brachy.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 14. Baumann BC, Baumann JC, Christodouleas JP, Soffen E. Salvage of locally recurrent prostate cancer after external beam radiation using reduced-dose brachytherapy with neoadjuvant plus adjuvant androgen deprivation. Brachytherapy. 2017;16(2):291–298. doi:10.1016/j.brachy.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 15. Kollmeier MA, McBride S, Taggar A, et al. Salvage brachytherapy for recurrent prostate cancer after definitive radiation therapy: a comparison of low-dose-rate and high-dose-rate brachytherapy and the importance of prostate-specific antigen doubling time. Brachytherapy. 2017;16(6):1091–1098. doi:10.1016/j.brachy.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 16. Wojcieszek P, Szlag M, Głowacki G, et al. Salvage high-dose-rate brachytherapy for locally recurrent prostate cancer after primary radiotherapy prostate cancer salvage brachytherapy failure. Radiother Oncol. 2016;119(3):405–410. doi:10.1016/j.radonc.2016.04.032 [DOI] [PubMed] [Google Scholar]
- 17. Barbera F, Triggiani L, Buglione M, et al. Salvage low dose rate brachytherapy for recurrent prostate cancer after external beam radiotherapy: results from a single institution with focus on toxicity and functional outcomes. Clin Med Insights Oncol. 2017;11 doi:10.1177/1179554917738765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamada Y, Okihara K, Iwata T, et al. Salvage brachytherapy for locally recurrent prostate cancer after external beam radiotherapy. Asian J Androl. 2015;17(6):899 doi:10.4103/1008-682X.151391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zilli T, Benz E, Dipasquale G, Rouzaud M, Miralbell R. Reirradiation of prostate cancer local failures after previous curative radiation therapy: long-term outcome and tolerance. Int J Radiat Oncol Biol Phys. 2016;96(2):318–322. doi:10.1016/j.ijrobp.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 20. Vavassori A, Jereczek-Fossa BA, Beltramo G, et al. Image-guided robotic radiosurgery as salvage therapy for locally recurrent prostate cancer after external beam irradiation: retrospective feasibility study on six cases. Tumori. 2010;96(1):71–75. doi:10.1700/479.5654 [DOI] [PubMed] [Google Scholar]
- 21. Zerini D, Jereczek-Fossa BA, Fodor C, et al. Salvage image-guided intensity modulated or stereotactic body reirradiation of local recurrence of prostate cancer. Br J Radiol. 2015;88(1052). doi:10.1259/bjr.20150197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fuller DB, Wurzer J, Shirazi R, Bridge SS, Law J, Mardirossian G. High-dose-rate stereotactic body radiation therapy for postradiation therapy locally recurrent prostatic carcinoma: preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract Radiat Oncol. 2015;5(6):e615–e623. doi:10.1016/j.prro.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 23. Janoray G, Reynaud-Bougnoux A, Ruffier-Loubière A, Bernadou G, Pointreau Y, Calais G. Stereotactic body re-irradiation therapy for locally recurrent prostate cancer after external-beam radiation therapy: initial report. Cancer Radiother. 2016;20(4):275–281. doi:10.1016/j.canrad.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 24. Mbeutcha A, Chauveinc L, Bondiau PY, et al. Salvage prostate re-irradiation using high-dose-rate brachytherapy or focal stereotactic body radiotherapy for local recurrence after definitive radiation therapy. Radiat Oncol. 2017;12(1):49 doi:10.1186/s13014-017-0789-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arcangeli S, Gambardella P, Agolli L, et al. Stereotactic body radiation therapy salvage reirradiation of radiorecurrent prostatic carcinoma relapsed in the prostatic bed. Tumori. 2015;101(2):e57–e59. doi:10.5301/tj.5000251 [DOI] [PubMed] [Google Scholar]
- 26. Jackson CH. flexsurv: a platform for parametric survival modeling in R. J Stat Softw. 2016;70(8):1–33. doi:10.18637/jss.v070.i08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol. 2006;65(4):965–974. doi:10.1016/j.ijrobp.2006.04.029 [DOI] [PubMed] [Google Scholar]
- 28. Tran H, Kwok J, Pickles T, Tyldesley S, Black PC. Underutilization of local salvage therapy after radiation therapy for prostate cancer. Urol Oncol. 2014;32(5):701–706. doi:10.1016/j.urolonc.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 29. Kimura M, Mouraviev V, Tsivian M, Mayes JM, Satoh T, Polascik TJ. Current salvage methods for recurrent prostate cancer after failure of primary radiotherapy. BJU Int. 2010;105(2):191–201. doi:10.1111/j.1464-410X.2009.08715.x [DOI] [PubMed] [Google Scholar]
- 30. Murat FJ, Poissonnier L, Rabilloud M, et al. Mid-term results demonstrate salvage high-intensity focused ultrasound (HIFU) as an effective and acceptably morbid salvage treatment option for locally radiorecurrent prostate cancer. Eur Urol. 2009;55(3):640–649. doi:10.1016/j.eururo.2008.04.091 [DOI] [PubMed] [Google Scholar]
- 31. Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630–642. doi:10.1016/j.eururo.2016.08.002 [DOI] [PubMed] [Google Scholar]


