Abstract
Background:
Pharmacotherapy, including prokinetics and proton pump inhibitors for functional dyspepsia (FD) have limited effectiveness, and their safety has been recently questioned. Chinese herbal medicine (CHM) could be considered as an alternative. A systematic review (SR) of SRs was performed to evaluate the potential effectiveness and safety of CHM.
Method:
We conducted a comprehensive literature search for SRs with meta-analyses in eight international and Chinese databases. Pooled effect estimation from each meta-analysis was extracted. The AMSTAR instrument was used to assess the methodological quality of the included SRs.
Results:
A total of 14 SRs of mediocre quality assessing various CHMs, alone or in combination with conventional pharmacotherapy, were included. Meta-analyses showed that CHM was more effective than prokinetic agents for the alleviation of global dyspeptic symptoms. Three specific CHM formulae appeared to show superior results in the alleviation of global dyspeptic symptoms, including Si Ni San, modified Xiao Yao San and Xiang Sha Liu Jun Zi decoction. No significant difference in the occurrence of adverse events in using CHM or pharmacotherapy was reported.
Conclusion:
CHM can be considered as an alternative for the treatment of FD symptoms when prokinetic agents and proton pump inhibitors are contraindicated. Future trial design should focus on measuring changes in individual dyspeptic symptoms and differentiate the effectiveness of different CHM for postprandial distress syndrome and epigastric pain syndrome. A network meta-analysis approach should be used to explore the most promising CHM formula for FD treatment in the future.
Keywords: functional dyspepsia, Chinese herbal medicine, Chinese herbs, prokinetics, proton pump inhibitors, systematic reviews
Introduction
Functional dyspepsia (FD) is a common cause of dyspeptic symptoms in the world.1 The annual incidence of FD approximates 9–10%, while 15% of patients have chronic (>3 months per year) and frequent (>3 episodes per week) symptoms.1 In the general population, the prevalence of FD is 11.5–14.7%.2 The prevalence of FD varies between 8% and 23% among Asia population.3 It can also be as high as 23.9% in Spain, 32% in the United States (US) and 38–41% in the United Kingdom.1 FD is more prevalent in women (24.4%) than men (16.6%) and its occurrence was found to increase significantly with age.5,6
Currently defined by the Rome IV criteria, FD is categorized into two types: postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS).7 PDS is characterized by postprandial fullness and early satiety, while EPS is characterized by epigastric pain or burning.8 Both do not carry any structural disorders explaining the symptoms.8 FD does not seem to pose severe or fatal threats to patients. Nevertheless, it greatly reduces patient quality of life;9 the impact of FD on quality of life is comparable in patients with other chronic diseases such as asthma and inflammatory bowel disease.10,11 Moreover, FD incurs heavy economic burdens, attributable to medical care and diagnostic procedure costs. Although the cost data on FD is limited, statistics show that FD created an $18.4 billion medical cost on US patients in 2009.12
There are several guideline-recommended pharmacotherapies for functional dyspepsia.8,9,13–15 In a population with high Helicobacter pylori infection rate (>20%), FD patients should be tested and treated by antibiotics if such infection exists. In contrast, in a population with low H. pylori prevalence (<20%), proton pump inhibitor (PPI) should be used for reducing gastric acid secretion in patients with epigastric pain, while prokinetic agents can be used if the symptoms include postprandial fullness or early saiety.8,9,13–15
However, due to the heterogeneous nature of the symptoms of FD,16 probably no single pharmacotherapy is able to manage the symptoms fully.9 Besides, individual symptoms may be caused by different mechanisms. As a result, the same drug may not be able to deal with the same symptom if it is originated from different mechanisms in different patients.9 In addition, despite being widely in use, prokinetic agents only demonstrate limited effectiveness. For example, acotiamide has a number needed to treat (NNT) of 1617 for symptom elimination, while the NNT figures of omeprazole and lansoprazole are 9.18 The side effects of pharmacotherapy also trigger concerns among medical practitioners. Current evidence has shown its association with extra-pyramidal reactions, cardiac arrhythmia and neurological disorders, such as Parkinsonism.19–22 There is a need for addressing the effectiveness gap between patients who are experiencing limited benefits from prokinetic agents and PPI, or patients who are contraindicated to them.
Chinese herbal medicine (CHM) is part of the routine in the treatment of FD in China and is gaining popularity in other countries. In traditional Chinese medicine (TCM), FD is equivalently termed as ‘distension and fullness’ (in Chinese pinyin: Pi Man), ‘stomach pain’ (in Chinese pinyin: Wei Wan Tong) and ‘retention’ (in Chinese pinyin: Ji Zhi).23 FD can also be divided into various TCM syndromes, which may include, but not limited to: (a) liver–stomach disharmony syndrome; (b) dampness–heat of spleen and stomach syndrome; (3) cold and heat in complexity syndrome; and (4) spleen–stomach weakness syndrome.24 TCM aims at harmonizing or improving the relationship between different organs and systems in the human body in order to relieve the symptoms of FD. Traditionally, accurate diagnosis of TCM syndrome type is considered a key for guiding the appropriate choice of individualized CHM formula, although the use of CHM without TCM diagnosis is also common.
Existing meta-analyses indicated that, in general, CHM was superior to placebo for improvement of quality of life, alleviation of global dyspeptic symptoms, and reduction of severity from a CHM perspective.25,26 When CHM is compared with pharmacotherapy, evidence from existing meta-analyses is inconsistent. Meta-analyses comparing CHM25,27 or selected specific CHM formula28 versus pharmacotherapy in general showed that CHM was more effective than pharmacotherapy for the alleviation of global dyspeptic symptoms. Meta-analyses showed CHM in general,29,30 or selected specific CHM formulae31–37 were more effective than prokinetic agents. However, another meta-analysis and one separate trial showed similar effectiveness between CHM and prokinetic agents for the alleviation of global dyspeptic symptoms and global PDS, respectively.38 For alleviation of FD individual symptoms, meta-analyses showed individual CHM formula was more effective than prokinetic agents, including alleviation of early satiety and epigastric pain, but not for postprandial fullness and epigastric burning.37
Objective
The heterogeneous results from existing systematic reviews (SRs) make it difficult to draw conclusions on the effectiveness of CHM over pharmacotherapy. There is a need to perform a SR of SRs to resolve such inconsistency. We conducted a systematic review of SRs to critically appraise and synthesize current clinical evidence on the effectiveness and safety of CHM on FD.
Method
Eligibility criteria
To be included, the SRs should meet the criteria as follows: (a) patients were diagnosed with FD according to Rome criteria I, II or III, or other criteria stated by the authors; (b) CHM must be included as one of the treatments, and any herbal formula, dosage, and route of administration were eligible; (c) the control group should employ conventional pharmacotherapy, including but not limited to: prokinetic agents, PPIs, H. Pylori eradication, or placebo of CHM; (d) a meta-analysis should be conducted in the SR; (e) SRs should be published in English or Chinese.
We excluded data from meta-analyses which included randomized controlled trials (RCTs) evaluating the following pharmacological therapies, of which substantial side effects had been reported:39–43 cisapride, combined therapy of PPIs and prokinetic agents, as well as combined therapy of H2 histamine receptor antagonist (H2RA) and prokinetic agents.
A comprehensive literature search was conducted by searching both international and Chinese databases from their inception till Jan 2017. International databases included the Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, MEDLINE and EMBASE. Chinese databases included Chinese Biomedical Databases, Wanfang Digital Journals, China National Knowledge Infrastructure and Taiwan Periodical Literature Databases. A specialized search filter for reviews was used in MEDLINE44 and EMBASE.45 Detailed search strategies and related results for each database are reported in Table A1 in the appendix. No language restriction was set during the literature search.
Literature selection, data extraction, and methodological quality assessment
All the retrieved citations were screened and assessed for eligibility. Data that were extracted from the included SRs were as follows: characteristics of the SR, including first author’s name, year of publication, eligibility criteria for selecting primary studies, and number of included studies and patients; details on patient characteristics, details of CHM and control interventions; effect sizes on each outcome of interest, and adverse effects; and risk of bias among RCTs included in the SRs.
The methodological quality of included SRs was appraised by the AMSTAR tool, a reliable and valid tool for methodological quality assessment of SRs.46,47 Eleven aspects were assessed using AMSTAR, with each aspect being judged as ‘yes’, ‘no’, ‘can’t answer’ or ‘not applicable’, based on the information provided. The detailed operational guide for AMSTAR is provided in Table A2 in the appendix.
Literature selection, data extraction, and methodological quality assessment were performed by two researchers (MC and XY) independently. Disagreements were resolved by thorough discussions and consensus adjudication. A third reviewer (VC) would be consulted if disagreement could not be resolved, and his judgment was considered to be the final decision.
Outcomes of interest
Trials results extracted from each meta-analysis should include at least one of the following primary outcomes:
(1) Alleviation of global dyspeptic symptoms, measured by global symptom improvement scale;
(2) Alleviation of individual dyspeptic symptoms (epigastric burning, epigastric pain, gastric emptying or fullness), measured by individual symptom improvement scale.
Quality-of-life improvement and safety were considered as the secondary outcomes in this SR.
Data synthesis
The effectiveness of CHM treatments was assessed at SR level according to the Cochrane Handbook.48 No reanalyzing of the data using network meta-analysis approach was performed due to the insufficient number of trials sharing a common comparator, as well as head-to-head comparison between interventions. We extracted the pooled effect estimation from each meta-analysis. Pooled relative risk (RR) or odds ratio (OR) for dichotomous outcomes, and weighted mean difference (WMD) or standard mean difference (SMD) for continuous outcomes, accompanied by their respective 95% confidence intervals (CIs) were extracted as reported by the meta-analyses.
I-square (I2) values were also extracted for appraising heterogeneity among RCTs. The I2 value of <25%, 25–50%, >50% were regarded as an indicator for the presence of low, moderate, and high heterogeneity, respectively.49
Results
Study selection
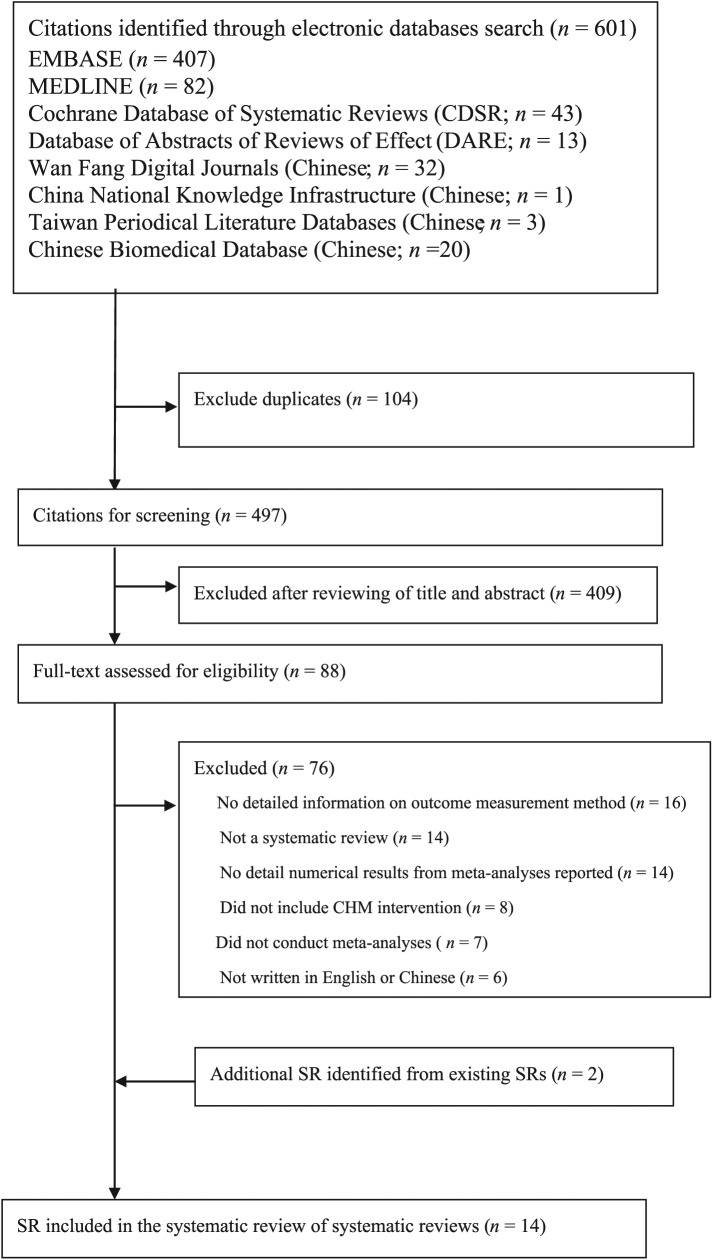
There were a total of 601 citations retrieved from the database searches. Among them, 12 SRs were found to be eligible and were included in the SR of SRs. Two additional SRs were identified from existing SRs.26,28 Therefore, 14 SRs were included.25–38 Details on the study selection process can be found in Figure 1.
Figure1.
Flowchart of literature search for systematic reviews on Chinese herbal medicine for functional dyspepsia.
Characteristics of included systematic reviews
The fourteen SRs were published between 2009 and 2016 and all were conducted in China. Characteristics of these SRs can be found in Table 1. Eight SRs (57.1%) were published in English and the remaining six (42.9%) were published in Chinese. Thirteen SRs (92.9%) searched both international and Chinese databases. Thirteen SRs (92.9%) included only RCTs, while the remaining one (7.1%) included both RCTs and quasi-RCTs. Seven SRs (50%) conducted a literature search in or after 2013, with the most recent search conducted in February 2016.
Table 1.
Main characteristics of randomized controlled trials reported in included systematic reviews.
| Author(s) (country) | Studies, n
(patients, n) |
Study design | Age range (years): | Treatment duration (weeks) |
Diagnostic criteria | Intervention | Control | Reported adverse events | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Liu et al.38
(China) |
4 (309) | RCT | Median age: 37–52 |
4 | Rome criteria and TCM diagnosis standard | CHM | Pharmacotherapy | NR | AGDS; alleviation of fullness |
| Qin et al.31
(China) |
11 (1072) | RCT | 16–72 | 2–8 | Reported as FD patients | CHM; CHM + pharmacotherapy |
Pharmacotherapy | Yes | AGDS |
| Cui and Shen29 (China) | 39 (3546) | RCT; quasi-RCT |
NR | NR | Rome II/III criteria | CHM | Pharmacotherapy | NR | AGDS |
| Wang et al.30
(China) |
13 (1153) | RCT | Mean age: 34.5–45.6 |
2–4 | Rome II/III criteria and TCM diagnosis standard | CHM | Pharmacotherapy | Yes | AGDS |
| Xiao et al.32
(China) |
11 (884) | RCT | 18–70 | 2–4 | Reported as FD patients | CHM | Pharmacotherapy | Yes | AGDS; plasma acylated ghrelin |
| Xiao et al.33
(China) |
9 (918) | RCT | 16–85 | 4–6 | Rome III criteria | CHM + pharmacotherapy | Pharmacotherapy | Yes | AGDS |
| Yang et al.34
(China) |
14 (1198) | RCT | Mean age: 33.9–56.0 | 4 | Rome II/III criteria and TCM diagnosis standard | CHM; CHM + pharmacotherapy |
Pharmacotherapy | Yes | AGDS |
| Gan et al.35
(China) |
9 (872) | RCT | NR | 2–4 | Rome II/III criteria | CHM | Pharmacotherapy | Yes | AGDS |
| Fan and He36
(China) |
9 (907) | RCT | 14–87 | 2–6 | Rome II/III Criteria and TCM diagnosis standard | CHM | Pharmacotherapy | Yes | AGDS |
| Liu25
(China) |
53 (5142) | RCT | 16–87 | 2–8 | Rome II/III criteria | CHM; CHM + pharmacotherapy | Placebo; pharmacotherapy | NR | AGDS; quality of life; gastric emptying rate; |
| Zhang27
(China) |
20 (1998) | RCT | NR | 2–4 | Reported as FD patients | CHM | Pharmacotherapy | NR | AGDS |
| Akarayosapong37
(China) |
17 (1715) | RCT | 18–79 | 2–4 | Rome I/II/III criteria and TCM diagnosis standard |
CHM | Pharmacotherapy | Yes | AGDS; alleviation of postprandial fullness; epigastric pain; epigastric burning; early satiety; gastric emptying rate; |
| Ling et al.28
(China) |
27 (2793) | RCT | NR | 2–4 | Rome III criteria | CHM | Pharmacotherapy | Yes | AGDS |
| Guo et al.26
(China) |
10 (1424) | RCT | NR | 4–8 | Rome III criteria | CHM | Placebo | NR | AGDS; alleviation of Chinese medicine
syndrome; quality of life; |
AGDS, alleviation of global dyspeptic symptoms; FD, functional dyspepsia; NR, not reported; CHM, Chinese herbal medicine; RCT, randomized controlled trial; TCM, traditional Chinese medicine.
Characteristics of patients
The patients included ranged from age 14 to 87 years. Three SRs (21.4%) included FD patients based on Rome III criteria, six (42.9%) based on Rome II or III criteria, two (14.3%) employed Rome I, II or III criteria. The remaining three SRs (21.4%) followed the criteria set by the authors. The treatment duration was 2–4 weeks in eight SRs (57.1%), but it was as long as 8 weeks in three SRs (21.4%).
Characteristics of intervention and control
In the intervention arm, CHM was administered either alone or in combination with conventional pharmacotherapy. Eight SRs reviewed nine specific CHM formulae, namely (a) modified Xiao Yao San, (b) Liu Jun Zi decoction, (c) Xiang Sha Liu Jun Zi decoction, (d) Shu Gan Jie Yu capsule, (e) modified Chai Hu Shu Gan powder, (f) Ban Xia Xie Xin decoction, (g) Liu Wei An Xiao capsule, (h) Si Ni San and (i) Da Li Tong granule. On the other hand, the remaining six SRs summarized outcomes from different CHM formulae. Details of intervention dosage and frequency, and details of CHM evaluated are shown in Table A3 in the appendix.
In the control arm, the included trials applied either conventional pharmacotherapy or placebo of CHM. Six SRs included only trials evaluating domperidone or mosapride in the control group, while seven SRs set no restrictions on conventional pharmacotherapy used in the control group. Among these seven SRs, one of them also included CHM placebo as a control. Therefore, together with another SR accepting CHM placebo as a control, there are two SRs synthesizing evidence on CHM versus CHM placebo comparison.
Methodological quality of included systematic reviews
Overall, the methodological quality of the included SRs was moderate, with 13 SRs (92.9%) having reported the basic characteristics of the included studies. Twelve SRs (85.7%) combined the studies’ findings using appropriate methods, assessed the likelihood of publication bias, assessed and documented the scientific quality of the included studies, and their scientific quality was considered appropriately in conclusion formulation. However, only three (21.4%) searched for grey literature and none of them provided a list of included and excluded studies. Details on the methodological quality of included SRs is shown in Table 2.
Table 2.
Methodological quality of included systematic reviews.
| Author(s) | AMSTAR items |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Liu et al. 38 | N | Y | Y | N | N | Y | Y | Y | NR | Y | N |
| Qin et al. 31 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y |
| Cui and Shen 29 | N | NR | N | N | N | N | N | N | N | Y | N |
| Wang et al. 30 | N | Y | N | N | N | Y | Y | Y | Y | Y | N |
| Xiao et al. 32 | N | Y | Y | N | N | Y | Y | Y | Y | Y | Y |
| Xiao et al. 33 | N | Y | Y | N | N | Y | Y | Y | Y | Y | Y |
| Yang et al. 34 | N | Y | Y | N | N | Y | Y | Y | Y | Y | Y |
| Gan et al. 35 | N | Y | Y | N | N | Y | Y | Y | Y | Y | N |
| Fan and He 36 | N | NR | N | N | N | Y | N | N | Y | N | N |
| Liu 25 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N |
| Zhang 27 | N | NR | N | NR | N | Y | Y | Y | Y | Y | N |
| Akarayosapong 37 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N |
| Ling et al. 28 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y |
| Guo et al. 26 | N | Y | Y | Y | N | Y | Y | Y | Y | N | Y |
| Number of Yes (%) | 4 (28.6) | 11 (78.6) | 10 (71.4) | 3 (21.4) | 0 (0.0) | 13 (92.9) | 12 (85.7) | 12 (85.7) | 12 (85.7) | 12 (85.7) | 6 (42.9) |
Y, yes; N, no; NR, not reported.
AMSTAR items:
(1) Was an a priori design provided?
(2) Was there duplicate study selection and data extraction?
(3) Was a comprehensive literature search performed?
(4) Was the status of publication (i.e. grey literature) used as an inclusion criterion?
(5) Was a list of studies (included and excluded) provided?
(6) Were the characteristics of the included studies provided?
(7) Was the scientific quality of the included studies assessed and documented?
(8) Was the scientific quality of the included studies used appropriately in formulating conclusions? (9) Were the methods used to combine the findings of studies appropriate?
(10) Was the likelihood of publication bias assessed?
(11) Was the conflict of interest included?
Effectiveness of Chinese herbal medicine for functional dyspepsia
Primary outcomes
Alleviation of global dyspeptic symptoms
All the included SRs, with a total of 24 comparisons, summarized evidence of CHM on FD (Table 3). For the intervention arm, there are 13 comparisons focusing on a specific CHM formula. CHM was administered either alone (9 comparisons), or in combination with pharmacotherapy (4 comparisons). There are also 11 comparisons that included multiple types of CHM formulae as intervention, either alone (10 comparisons) or as an add-on to pharmacotherapy (1 comparison). Table 4 presents a summary of the main findings in this SR.
Table 3.
Chinese herbal medicine for functional dyspepsia: systematic review of meta-analysis results.
| Author(s) | Studies, n (patients, n) | Comparison* | Pooled results (95% CI) | Heterogeneity I2 (%) |
|---|---|---|---|---|
| Alleviation of global dyspeptic symptoms | ||||
| Liu et al.38** | 4 (309) | CHM versus prokinetic agents | RR: 1.02 (0.90, 1.15) | 62$ |
| Qin et al.31 | 5 (649) | Modified Xiao Yao San versus pharmacotherapy | OR: 3.53 (2.32, 5.36) | 0 |
| Cui29 | 23 (2013) | CHM versus domperidone | RR: 1.20 (1.15, 1.25) | 14 |
| Cui and Shen29 | 16 (1533) | CHM versus mosapride | RR: 1.14 (1.06, 1.24) | 62$ |
| Wang et al.30 | 12 (1069) | CHM versus pharmacotherapy | OR: 3.20 (2.27, 4.51) | 0 |
| Wang et al.30 | 2 (120) | CHM versus mosapride | OR: 3.98 (1.50, 10.54) | 0 |
| Wang et al.30 | 8 (663) | CHM versus domperidone | OR: 2.99 (1.93, 4.63) | 0 |
| Xiao et al.32 | 3 (350) | Liu Jun Zi decoction versus prokinetic agents | OR: 1.99 (0.71, 5.60) | 63$ |
| Xiao et al.33 | 7 (507) | Xiang Sha Liu Jun Zi decoction versus prokinetic agents | OR: 3.29 (1.99, 5.43) | 0 |
| Yang et al.34 | 8 (728) | Modified Chai Hu Shu Gan powder versus pharmacotherapy | RR: 1.22 (1.13, 1.32) | 0 |
| Gan et al.35 | 9 (872) | Ban Xia Xie Xin decoction versus prokinetic agents | OR: 2.58 (1.72, 3.85) | 0 |
| Fan and He36‡ | 9 (907) | Da Li Tong granule versus prokinetic agents | OR: 1.17 (0.88, 1.55) | 0 |
| Liu25 | 37 (3339) | CHM versus pharmacotherapy | RR: 1.15 (1.10, 1.20) | 52$ |
| Zhang27 | 20 (1998) | CHM versus pharmacotherapy | OR: 3.73 (2.85, 4.88) | 0 |
| Akarayosapong37 | 9 (786) | Liu Wei An Xiao capsule versus pharmacotherapy | RR: 1.29 (1.12, 1.49) | 76$ |
| Ling et al.28 | 27 (2793) | Si Ni San versus pharmacotherapy | OR: 3.81 (3.05, 4.77) | 0 |
| Qin et al.31 | 6 (423) | Modified Xiao Yao San +
pharmacotherapy versus pharmacotherapy |
OR: 4.71 (2.69, 8.25) | 0 |
| Xiao et al.33 | 9 (918) | Shu Gan Jie Yu + mosapride versus mosapride | OR: 3.57 (2.53, 5.05) | 0 |
| Yang et al.34 | 6 (470) | Modified Chai Hu Shu Gan powder + pharmacotherapy versus pharmacotherapy | RR: 1.15 (1.07, 1.23) | 0 |
| Liu25 | 8 (827) | CHM + pharmacotherapy versus pharmacotherapy | RR: 1.23 (1.14, 1.34) | 28 |
| Akarayosapong37 | 5 (681) | Liu Wei An Xiao capsule + pharmacotherapy versus pharmacotherapy | RR: 1.22 (1.14, 1.32) | 0 |
| Liu25 | 4 (459) | CHM versus placebo | RR: 1.47 (1.29, 1.68) | 0 |
| Guo et al.26 | 7 (1074) | CHM versus placebo | RR: 1.45 (1.31, 1.60) | 8 |
| Alleviation of individual dyspeptic symptoms** | ||||
| (a) Alleviation of fullness sensation | ||||
| Liu et al.38 | 1 (55) | CHM versus domperidone | RR: 1.07 (0.93, 1.24) | NA |
| (b) Gastric emptying# | ||||
| Liu25 | 1 (16) | CHM versus pharmacotherapy | RR: 0.80 (0.33, 1.92) | NA |
| Akarayosapong37 | 4 (357) | Liu Wei An Xiao capsule versus prokinetic agents | MD: 2.16 (0.26, 4.17) | 4 |
| (c) Alleviation of epigastric pain** | ||||
| Akarayosapong37 | 6 (NR) | Liu Wei An Xiao capsule versus pharmacotherapy | RR: 1.18 (1.06, 1.31) | 28 |
| (d) Alleviation of epigastric burning** | ||||
| Akarayosapong37 | 3 (NR) | Liu Wei An Xiao capsule versus pharmacotherapy | RR: 0.98 (0.76, 1.26) | 0 |
| (e) Alleviation of postprandial fullness** | ||||
| Akarayosapong37 | 3 (NR) | Liu Wei An Xiao capsule versus pharmacotherapy | RR: 1.06 (0.97, 1.16) | 46 |
| (f) Alleviation of early satiety** | ||||
| Akarayosapong37 | 5 (NR) | Liu Wei An Xiao capsule versus pharmacotherapy | RR: 1.17 (1.04, 1.32) | 18 |
| Quality of life | ||||
| Liu25‖ | 3 (367) | CHM versus placebo | MD: 37.87 (20.01, 55.73) | 44 |
| Guo et al.26‖ | 6 (698) | CHM versus placebo | SMD: 0.30 (0.15, 0.45) | 0 |
| Liu25‖ | 5 (387) | CHM versus pharmacotherapy | MD: 4.82 (2.13, 7.50) | 98$ |
| Alleviation of Chinese medicine syndrome‡‡ | ||||
| Guo et al.26 | 5 (741) | CHM versus placebo | RR: 1.36 (1.23, 1.50) | 31 |
| Plasma acylated ghrelin level | ||||
| Xiao et al.32 | 1 (27) | Liu Jun Zi Tang versus domperidone | MD: 9.00 (−0.27, 18.27) | NA |
| ‘Obviously effective’ rate‡ | ||||
| Fan and He36 | 9 (907) | Da Li Tong granule versus prokinetic agents | OR: 1.38 (0.90, 2.11) | 0 |
| Safety¶ | ||||
| Liu25 | 9 (761) | CHM versus pharmacotherapy | OR: 0.98 (0.45, 2.12) | 0 |
| Zhang27 | 4 (388) | CHM versus pharmacotherapy | OR: 0.14 (0.03, 0.63) | 0 |
| Akarayosapong37 | 6 (425) | Liu Wei An Xiao capsule versus pharmacotherapy | OR: 1.11 (0.38, 3.23) | 0 |
| Liu25 | 2 (222) | CHM + pharmacotherapy versus pharmacotherapy | OR: 1.22 (0.30, 4.89) | 0 |
| Guo et al.26 | 4 (771) | CHM versus placebo | RR: 1.06 (0.66, 1.70) | 0 |
CHM denotes the inclusion of multiple types of Chinese herbal medicine. The generic term ‘prokinetic agents’ is used to denote that multiple types of prokinetic agents were included in the comparison. The term ‘pharmacotherapy’ was used when both proton pump inhibitor and prokinetic agents were included in the comparison. If the authors evaluated multiple CHM formulations and provided detailed information, we reported the compositions of herbal formulations in Table A3 in the appendix.
Alleviation of global or individual dyspeptic symptoms is measured by Gastrointestinal Symptom Rating Scale (GSRS) or other standardized scales. GSRS is a score in which patients report dyspeptic symptom severity on a four-point Likert scale (symptom free, marked improvement, slight improvement, and no improvement).
p < 0.10 for the heterogeneity test.
‘Obviously effective’ rate denotes a numerical result ⩾ 60% in the following calculation: [(Severity score of symptoms before treatment – Severity score of symptoms after treatment) / Severity score of symptoms before treatment] × 100%, with symptoms measured by the GSRS and other standardized scales.
Quality of life is measured with the 36-Item Short Form Survey (SF-36).
Quality of life is measured with the 36-Item Short Form Survey (SF-36), Quality of Life Questionnaire for Functional Digestive Disorders, Functional Dyspepsia-Related Quality of Life or other validated scales.
Safety denotes the occurrence of any side effects or adversities due to the use of interventions or placebo. This definition applies to all results under the ‘Safety’ section in Table 3.
Gastric emptying rate is defined as the GSRS scores in emptying the stomach25 and as the percentage of substances remaining in stomach 4–5 h after a meal.37
Alleviation of Chinese medicine syndrome measures symptom improvement according to traditional Chinese medical theories and standards.
CHM, Chinese herbal medicine; CI, confidence interval; RR, risk ratio; OR, odds ratio; MD, mean difference; SMD, standard mean difference; NA, not applicable.
Table 4.
Chinese herbal medicine for functional dyspepsia: summary of findings for the main comparison.
| Chinese herbal medicine compared with pharmacotherapy for functional dyspepsia | ||||
|---|---|---|---|---|
|
Population:
Patients with functional
dyspepsia Intervention: Chinese herbal medicine Comparison: Pharmacotherapy Settings: Any | ||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) |
Participants, n (studies, n) | |
| Assumed risk | Corresponding risk | |||
| Alleviation of global dyspeptic symptoms | Pharmacotherapy | Modified Xiao Yao San | OR: 3.53 (2.32, 5.36) | 649 (5) |
| 694 per 1000 | 890 per 1000 | |||
| Alleviation of global dyspeptic symptoms | Prokinetic agents | Xiang Sha Liu Jun Zi decoction | OR: 3.29 (1.99, 5.43) | 507 (7) |
| 740 per 1000 | 934 per 1000 | |||
| Chinese herbal medicine + pharmacotherapy compared with pharmacotherapy for functional dyspepsia | ||||
|
Population:
Patients with functional
dyspepsia Intervention: Chinese herbal medicine + pharmacotherapy Comparison: Pharmacotherapy Settings: Any | ||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) |
Participants, n (studies, n) | |
| Assumed risk | Corresponding risk | |||
| Pharmacotherapy | CHM + pharmacotherapy | |||
| Alleviation of global dyspeptic symptoms | Pharmacotherapy | Modified Xiao Yao San + pharmacotherapy | OR: 4.71 (2.69, 8.25) | 423 (6) |
| 705 per 1000 | 917 per 1000 | |||
| Alleviation of global dyspeptic symptoms | Mosapride | Shu Gan Jie Yu capsule + mosapride | OR: 3.57 (2.53, 5.05) | 918 (9) |
| 678 per 1000 | 880 per 1000 | |||
Alleviation of global or individual dyspeptic symptoms is measured by the Gastrointestinal Symptom Rating Scale (GSRS) or other standardized scales. GSRS measures patient reported dyspeptic symptom severity on a four-point Likert scale (symptom free, marked improvement, slight improvement, and no improvement).
CHM, Chinese herbal medicine; OR, odds ratio; CI, confidence interval.
For the control arm, eight comparisons summarized evidence from specific prokinetic agents, namely mosapride (five comparisons) and domperidone (two comparisons). The remaining one comparison combined the outcome using mosapride or domperidone against CHM to generate a pooled result. On the other hand, 14 comparisons summarized evidence from multiple types of pharmacotherapy, either comparing CHM + pharmacotherapy with pharmacotherapy (4 comparisons) or comparing CHM with pharmacotherapy (10 comparisons). There are also two comparisons between multiple types of CHM and placebo of CHM on global symptom improvement. Details on the results are listed in Table 3.
Chinese herbal medicine versus pharmacotherapy
In total, there are 17 pooled results on this comparison, either including multiple types of CHM formulae (8 comparisons) or focusing on a single CHM (9 comparisons). Results showed that CHM is generally superior to pharmacotherapy in improving FD global symptoms, as 13 out of 17 comparisons revealed that CHM had higher treatment effectiveness than pharmacotherapy on FD. Among the eight pooled results that included multiple types of CHM formulae, the effect size ranged from a pooled OR of 3.98 (95% CI = 1.50–10.54, I2 = 0%, two RCTs) to a pooled RR of 1.02 (95% CI = 0.90–1.15, I2 = 62%, four RCTs).
There were three specific CHM formulae which appeared to show larger effect sizes. They are Si Ni San (pooled OR = 3.81, 95% CI = 3.05–4.77, I2 = 0%, 27 RCTs), modified Xiao Yao San (pooled OR = 3.53, 95% CI = 2.32–5.36, I2 = 0%, 5 RCTs) and Xiang Sha Liu Jun Zi decoction (pooled OR = 3.29, 95% CI = 1.99–5.43, I2 = 0%, 7 RCTs). Heterogeneity was low across all three meta-analyses.
Chinese herbal medicine plus pharmacotherapy versus pharmacotherapy
A total of five SRs reported pooled results of this comparison and four of them restricted the type of CHM formula used. With the combined use of CHM and pharmacotherapy, the add-on effectiveness was obvious, as all of the summarized evidence favored the combined treatment group. Modified Xiao Yao San has a relatively better performance, with pooled OR = 4.71 (95% CI = 2.69–8.25, I2 = 0%, six RCTs).
Chinese herbal medicine versus placebo
There were two pooled results summarized from 11 RCTs comparing CHM with placebo on the effectiveness on FD. Both showed that CHM was more effective than placebo, with pooled RR being 1.45 (95% CI = 1.31–1.60, I2 = 8%, seven RCTs) and 1.47 (95% CI = 1.29–1.68, I2 = 0%, four RCTs), respectively. Both of them did not restrict the types of CHM formula in the analyses.
Alleviation of individual dyspeptic symptoms
Alleviation of fullness sensation
There is one RCT comparing CHM and domperidone on this outcome, showing that CHM performed slightly better in reducing fullness sensation, but the difference did not reach significance (RR: 1.07, 95% CI = 0.93–1.24, 55 patients).
Gastric emptying
There were two meta-analyses on this outcome. One demonstrated that Liu Wei An Xiao capsule had a better gastric emptying effect than prokinetic agents (pooled MD = 2.16, 95% CI = 0.26–4.17, I2 = 4%, four RCTs). The other one comparing CHM and pharmacotherapy found no significant difference between the two interventions.
Alleviation of epigastric pain
One meta-analysis based on six RCTs revealed that Liu Wei An Xiao capsule was more favorable than pharmacotherapy in reducing epigastric pain, with a pooled RR = 1.18 (95% CI = 1.06–1.31, I2 = 28%).
Alleviation of epigastric burning
One meta-analysis based on three RCTs showed that there was no significant difference between Liu Wei An Xiao capsule and prokinetic agents, with a pooled RR = 0.98 (95% CI = 0.76–1.26, I2 = 0%).
Alleviation of postprandial fullness
One meta-analysis summarized from three RCTs revealed that there was no significant difference between Liu Wei An Xiao capsule and pharmacotherapy in alleviation of postprandial fullness, with a pooled RR = 1.06 (95% CI = 0.97–1.16, I2 = 46%).
Alleviation of early satiety
One meta-analysis from five RCTs demonstrated that Liu Wei An Xiao capsule could relieve early satiety better than prokinetic agents (pooled RR = 1.17, 95% CI = 1.04–1.32, I2 = 18%).
Secondary outcomes
Quality of life
Three pooled results were reported from two SRs, which did not restrict the use of particular CHM formula. They all showed that CHM improved the quality-of-life scoring of patients to a greater extent as compared with pharmacotherapy or placebo. The most significant pooled MD is 37.87 (95% CI = 20.01–55.73, I2 = 44%, three RCTs), but moderate heterogeneity is present in this meta-analysis.
Alleviating Chinese medicine syndrome severity
One meta-analysis derived from five RCTs demonstrated that CHM was more effective in reducing severity of Chinese medicine syndrome than placebo, with pooled RR = 1.36 (95% CI = 1.23–1.50, I2 = 31%).
Safety
Five SRs reported pooled results for safety, with three comparing CHM with pharmacotherapy, one comparing CHM plus pharmacotherapy with pharmacotherapy alone, and the remaining one comparing CHM with placebo. Four pooled results showed that there were no significant differences between the treatment and control groups in causing adverse events. The remaining one reported that CHM was safer than pharmacotherapy, with pooled OR of 0.14 (95% CI = 0.03–0.63, I2 = 0%, four RCTs) (Table 3). Adverse events that could be attributed to CHM treatment include epigastric discomfort, increased passing of flatus, abdominal fullness and more frequent egestion. Details on reported adverse events are shown in Table A3 in the appendix.
Discussion
Evidence from included SRs showed that CHM outperformed pharmacotherapy at improving global and individual dyspeptic symptoms. Three specific CHM formulae which appeared to show superior results in the alleviation of global dyspeptic symptoms are Si Ni San, modified Xiao Yao San and Xiang Sha Liu Jun Zi decoction. Taking into consideration the safety of using CHM, available evidence suggested that CHM might be safer than pharmacotherapy. Moreover, even if negative effects occurred, they were mild and tolerated by the patients. Therefore, CHM could be an alternative, or used as an add-on treatment, to pharmacotherapy. Yet, future trials should address methodological shortcomings of existing trials. Many trials contributing to the SR result had unclear risk of bias in terms of sequence generation, allocation concealment and blinding. To improve the reporting quality, future randomized controlled trials should also comply with the CONSORT reporting guideline.50
Among the total of 14 SRs, we identified 8 SRs that used specific CHM formulae as intervention, while the remaining six did not restrict their CHM formulae. When there is no restriction on the CHM formulae, the results could only indicate the general effectiveness of CHM. The applicability of such results is limited, as the generalized results from different formulae did not indicate their comparative effectiveness. Therefore, it is difficult to conclude which formula has the highest probability in improving different FD symptoms. Future network meta-analysis and follow-up trials on the most promising CHM formula should be conducted.51
In routine practice, the prescription of Chinese herbal medicine is often tailored in accordance to the patient’s Chinese medicine syndrome type, which is diagnosed via the ‘four diagnostic procedure’ in Chinese medicine: inspection, auscultation and olfaction, inquiry and palpation. However, only five30,34,36–38 of the 14 SRs considered Chinese medicine syndrome type as a trial eligibility criterion. In order words, more than 60% of the results reported in this SR only considered the use of CHM for managing FD, without additional consideration of aligning Chinese medicine syndrome type and choice of CHM. This provides a potential explanation on variations in effect sizes reported in different meta-analyses and trials. In improved model validity of future trials, investigators should incorporate Chinese medicine diagnosis into the inclusion criteria. Nevertheless, standardizing Chinese medicine diagnosis for FD has proved difficult,52 but such obstacles could be resolved by group consensus technique or emerging machine learning methods.53,54
During the process of literature selection, we observed a noteworthy problem among the SRs, which is a lack of detailed description of the outcome measurement method. We have therefore excluded 16 SRs from our SR of SRs for this reason. Without a validated outcome measurement approach, the data derived would become hard to interpret. This common flaw should be rectified in future research by providing information on the validity, reliability and responsiveness of the outcome measurement scale or approach. With regards to outcome selection, both objectives, as well as validated patient-centered outcomes should be reported in future trials, including: (a) individual symptom assessment; (b) disease-specific quality of life questionnaire; (c) nutrient drink test; and (d) gastric emptying test.55 Also, follow-up duration of our included RCTs was only 2–8 weeks. Longer-term benefits of CHM should be evaluated by following the recommended follow-up duration of at least 12 weeks.55
Also, the external validity of our results is limited by the use of heterogeneous diagnostic criteria for inclusion in different trials. Rome III has been adopted in more than half of the included RCTs, and its application is limited by the fact that it may lead to exclusion of a substantial number of patients with endoscopically verified FD.56 The application of such a strict inclusion criteria implies that trial patients are likely to differ from the average patient seen in clinical practice.56 In the newly released Rome IV criteria, only minor modifications were made with regards to symptom description57 and this problem could persist if future trials continue to adopt Rome IV criteria. In future, more flexible diagnostic criteria might be used in recruiting patients in FD clinical trials.58 Additionally, since the evidence summarized was obtained from reports published in China, we should take note of the potential publication bias.59,60 All participants from our included trials were Chinese, the applicability of our results to other ethnicities is therefore limited.
Among the included SRs, CHM has demonstrated a good safety profile, with adverse drug reactions (ADRs) induced by herbal medicine being generally mild. However, existing evidence has suggested that certain CHM may induce more series adverse events,61–64 which highlights the importance of supervision when CHM is prescribed for the treatment of FD.
Finally, it should be highlighted that the methodological quality of SRs regarding CHM is mediocre. Future SR teams should be providing lists of included and excluded studies, and including grey literature in their synthesis. Also, reporting quality of the SRs is less than satisfactory. Only 2 out of the 14 SRs reported the duration of FD diagnosis, which is an importing piece of information for guiding the choice of appropriate treatment for patients with different duration of diagnosis. Six of them did not report details of treatment regimens, including the frequency and dosage. This hinders the translation of research findings into daily practice. It is also noteworthy that some meta-analysis pooled trial data used OR instead of RR. This is not a preferred practice, as RR should be use for quantifying RCT results. The use of OR would lead to exaggeration of effect sizes and readers should be cautious when interpreting these results.65
In conclusion, current clinical evidence has revealed that CHM can be considered an option for FD treatment. Future trials can focus on the improvement of individual dyspeptic symptoms, including PDS and EPS, together with global symptom improvement, in order to provide a more comprehensive and detailed analysis. In addition, the methodological and reporting qualities of future SRs and clinical trials have to be improved urgently, with adherence to international standards.
Appendices
Table A1.
Search strategies and results for systematic reviews on Chinese herbal medicine for functional dyspepsia.
(1) Cochrane Database of Systematic Reviews from inception to 23 January 2017.
| 1 | dyspepsia*.mp. | 227 |
| 2 | functional dyspepsia*.mp. | 15 |
| 3 | non ulcer dyspepsia*.mp. | 11 |
| 4 | 1 or 2 or 3 | 227 |
| 5 | Chinese herb*.mp. | 209 |
| 6 | herb*.mp. | 709 |
| 7 | Traditional Chinese medic*.mp. | 243 |
| 8 | phytother*.mp. | 137 |
| 9 | (chinese adj5 (traditional or medic*)).mp. | 500 |
| 10 | (plant or plants).mp. | 407 |
| 11 | (traditional adj5 medic*).mp. | 380 |
| 12 | Chinese medic*.mp. | 374 |
| 13 | oriental medic*.mp. | 30 |
| 14 | herbaceous agent.mp. | 13 |
| 15 | medicinal plant*.mp. | 34 |
| 16 | 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 1232 |
| 17 | 4 and 16 | 43 |
(2) Database of Abstracts of Reviews of Effects from inception to 23 January 2017.
| 1 | dyspepsia*.mp. | 113 |
| 2 | functional dyspepsia*.mp. | 34 |
| 3 | non ulcer dyspepsia*.mp. | 22 |
| 4 | 1 or 2 or 3 | 113 |
| 5 | Chinese herb*.mp. | 356 |
| 6 | herb*.mp. | 508 |
| 7 | Traditional Chinese medic*.mp. | 82 |
| 8 | phytother*.mp. | 260 |
| 9 | (chinese adj5 (traditional or medic*)).mp. | 350 |
| 10 | (plant or plants).mp. | 336 |
| 11 | (traditional adj5 medic*).mp. | 216 |
| 12 | Chinese medic*.mp. | 150 |
| 13 | oriental medic*.mp. | 19 |
| 14 | herbaceous agent.mp. | 0 |
| 15 | medicinal plant*.mp. | 5 |
| 16 | 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 914 |
| 17 | 4 and 16 | 13 |
(3) MEDLINE from inception to 23 January 2017:
| 1 | search:.tw. | 305990 |
| 2 | meta analysis.mp,pt. | 116082 |
| 3 | review.pt. | 2320501 |
| 4 | 1 or 2 or 3 | 2542882 |
| 5 | exp Dyspepsia/ | 8491 |
| 6 | dyspepsia*.mp. | 12156 |
| 7 | functional dyspepsia*.mp. | 2311 |
| 8 | non ulcer dyspepsia*.mp. | 1020 |
| 9 | 5 or 6 or 7 or 8 | 12156 |
| 10 | exp Drugs, Chinese Herbal/ | 41477 |
| 11 | Chinese herb*.mp. | 44028 |
| 12 | exp Medicine, Chinese Traditional/ | 18731 |
| 13 | Traditional Chinese medic*.mp. | 13289 |
| 14 | exp Phytotherapy/ | 38429 |
| 15 | phytother*.mp. | 38495 |
| 16 | (chinese adj5 (traditional or medic*)).mp. | 36373 |
| 17 | (herbs or herbal).mp. | 63413 |
| 18 | (plant or plants).mp. | 629861 |
| 19 | (traditional adj5 medic*).mp. | 52240 |
| 20 | 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 | 705515 |
| 21 | 4 and 9 and 20 | 82 |
(4) EMABSE from inception to 23 January 2017:
| 1 | meta-analys:.mp. | 197526 |
| 2 | search:.tw. | 408294 |
| 3 | review.pt. | 2232214 |
| 4 | 1 or 2 or 3 | 2619917 |
| 5 | exp dyspepsia/ | 30355 |
| 6 | dyspepsia*.mp. | 32323 |
| 7 | functional dyspepsia*.mp. | 4326 |
| 8 | non ulcer dyspepsia*.mp. | 1340 |
| 9 | 5 or 6 or 7 or 8 | 32323 |
| 10 | exp Chinese medicine/ | 36292 |
| 11 | exp oriental medicine/ | 3094 |
| 12 | exp herbaceous agent/ | 44067 |
| 13 | exp medicinal plant/ | 208652 |
| 14 | exp Chinese herb/ | 5354 |
| 15 | Chinese medic*.mp. | 51163 |
| 16 | oriental medic*.mp. | 3892 |
| 17 | herbaceous agent.mp. | 44069 |
| 18 | medicinal plant*.mp. | 88505 |
| 19 | Chinese herb*.mp. | 12932 |
| 20 | herb*.mp. | 148695 |
| 21 | 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 | 339476 |
| 22 | 4 and 9 and 21 | 431 |
| 23 | Limit 22 to human | 407 |
(5) Wan Fang Digital Journals (Chinese) from inception to 23 January 2017:
(“系统综述” OR “荟萃分析” OR “META”) AND (“中药” OR “中草药” OR “中成药” OR“中西医” OR “中医”) AND (“功能性消化不良” OR “消化不良”) yielded 32 citations.
(6) China National Knowledge Infrastructure (Chinese) from inception to 23 January 2017:
(KY = ‘系统综述’ OR KY = ‘荟萃分析’ OR KY = ‘META’) AND (KY = ‘中药’ OR KY = ‘中草药’ OR KY = ‘中成药’ OR KY = ‘中西医’ OR KY = ‘中医’) AND (KY = ‘功能性消化不良’ OR KY = ‘消化不良’) yielded one citation.
(7) Taiwan Periodical Literature Databases (Chinese) from inception to 23 January 2017:
(TX = 系統綜述 OR 薈萃分析 OR META) [AND] (TX = 中藥 OR 中草藥 OR 中成藥 OR 中西醫OR 中醫) [AND] (TX = 功能性消化不良 OR 消化不良) yielded three citations.
(8) Chinese Biomedical Database (Chinese) from inception to 2 November 2015:
(“系统综述”[全字段] OR “荟萃分析”[全字段] OR “META”[全字段]) AND (“中药”[全字段] OR “中草药”[全字段] OR “中成药”[全字段] OR “中西医”[全字段] OR “中医”[全字段]) AND (“功能性消化不良”[全字段] OR “消化不良”[全字段]) yielded 20 citations.
Table A2.
Detailed operational guide for applying the AMSTAR tool*.
| AMSTAR 1: Was an a priori design
provided? The research question and inclusion criteria should be established before the conduct of the review. Operational definition (OD): a ‘Yes’ will be given if the review has published a protocol, ethics approval or predetermined/a priori published research objectives. |
❑ Yes ❑ No |
| AMSTAR 2: Was there duplicate study selection and data
extraction? There should be at least two independent data extractors and a consensus procedure for disagreements should be in place. OD: a ‘Yes’ will be given if:(a) two reviewers performed study selection, and (b) two reviewers performed data extraction and(c) the consensus process was implemented for resolving disagreement. |
❑ Yes (a + b + c) ❑ No (these three criteria were not fulfilled) ❑ Could not answer (i.e. not reported) |
| AMSTAR 3: Was a comprehensive literature search
performed? At least two electronic sources should be searched. The report must include years and databases used (e.g. CENTRAL, EMBASE, and MEDLINE). Key words and MESH terms must be stated and where feasible, the search strategy should be provided. All searches should be supplemented by consulting current contents, reviews, textbooks, specialized registers, or experts in the particular field of study, and by reviewing the references in the studies found. OD: a ‘Yes’ will be given if at least two electronic sources plus one supplementary strategy were used (e.g. Cochrane register/CENTRAL counts as two sources; a grey literature search counts as supplementary; the SIGLE database, dissertations, conference proceedings, and trial registries are all considered grey literature). |
❑ Yes ❑ No ❑ Could not answer (i.e. not reported) |
| AMSTAR 4: Was the status of publication (i.e. grey
literature) used as an inclusion criterion?The authors
should state that they searched for reports regardless of
their publication type. The authors should state whether or
not they excluded any reports from the systematic review,
based on their publication status, language, etc. If review indicates that there was a search for ‘grey literature’ or ‘unpublished literature,’ indicate ‘Yes.’ The SIGLE database, dissertations, conference proceedings and trial registries are all considered grey for this purpose. If searching a source that contains both grey and nongrey, authors must specify that they were searching for grey/unpublished literature. OD: if grey literature or unpublished literature was searched, a ‘Yes’ will be given. If eligibility criterion is restricted to ‘nongrey’ literature, a ‘No’ will be given, which indicates a methodological shortcoming in this domain. |
❑ Yes ❑ No ❑ Could not answer (i.e. not reported) |
| AMSTAR 5: Was a list of studies (included and excluded)
provided? A list of included and excluded studies should be provided. OD: a ‘Yes’ will be given if the included and excluded studies are referenced, otherwise a ‘No’ will be given. |
❑ Yes ❑ No |
| AMSTAR 6: Were the characteristics of the included studies
provided? In an aggregated form such as a table, data from the original studies should be provided on the participants, interventions and outcomes. The ranges of characteristics in all the studies analyzed e.g. age, race, sex, relevant socioeconomic data, disease status, duration, severity, or other diseases should be reported. OD: a ‘Yes’ will be given if the information abovementioned were provided in detail, otherwise a ‘No’ will be given. |
❑ Yes ❑ No |
| AMSTAR 7: Was the scientific quality of the included studies
assessed and documented? A priori methods of assessment should be provided (e.g. the use of Cochrane Risk of Bias tool or Jadad scale as a mean to assessment); for other types of studies, alternative tools will also be acceptable. OD: to score a ‘Yes,’ the authors should report risk of bias level in each of the methodological domain included in the risk of bias assessment tool that the authors have chosen to use. |
❑ Yes ❑ No |
| AMSTAR 8: Was the scientific quality of the included studies
used appropriately in formulating conclusions? The results of the methodological rigor and scientific quality should be considered in the analysis and the conclusions of the review, and considerations on how risk of bias among included study may impact conclusion should be explicitly stated. OD: to score a ‘Yes,’ the reviewers must consider risk of bias explicitly when writing the conclusion. For example, a reviewer may state: the results should be interpreted with caution due to high risk of bias among included studies. |
❑ Yes ❑ No |
| AMSTAR 9: Were the methods used to combine the findings of
studies appropriate? For meta-analysis, statistical tests should be done to ensure that the studies were combinable by assessing their homogeneity. This can be done by using the Cochran Q test or reporting the I2 value. OD: ‘Yes’ will be given when one of the following two situation applies: (1) homogeneity was found, and authors used fixed effect model or random effect model, or (2) heterogeneity was found, and authors performed appropriate subgroup analysis or meta-regression. ‘No’ will be given when one of the following two situations apply: (1) heterogeneity was found, and authors used fixed or random effect model and reported the results directly without highlighting the role of heterogeneity; heterogeneity was not assessed or reported. |
❑ Yes ❑ No |
| AMSTAR 10: Was the likelihood of publication bias
assessed? An assessment of publication bias should include a combination of graphical aids (e.g. funnel plot) or statistical tests (e.g. Egger regression test). OD: a ‘No’ will be given if no relevant test values or funnel plot was reported. However, a ‘Yes’ will still be given if authors mentioned that publication bias could not be assessed because there were fewer than 10 included studies. |
❑ Yes ❑ No |
| AMSTAR 11: Was the conflict of interest
included? Potential sources of support should be clearly acknowledged in both the systematic review and the included studies. OD: To score a “Yes”, the authors must indicate source of funding or support for the systematic review AND for each of the included studies. A “Yes” will still be scored if the authors acknowledged that funding sources for included randomized controlled trials were unknown. |
❑ Yes ❑ No |
Adapted from the official AMSTAR website (http://www.amstar.ca/).
Table A3.
Details of intervention and adverse events among included systematic reviews.
| Author(s) | Intervention (dosage, frequency) | Control (dosage, frequency) | Adverse Events |
|---|---|---|---|
| Liu et al.38 | (1) Jian Pi Yi Qi formula (NR); (2) Li Qi Fu Wei solution (NR); (3) Jian Pi Xiao Zhang granule (NR); (4) He Wei Xiao Pi capsule (NR) |
(1) Mosapride (NR); (2) Domperidone (NR) |
NR |
| Qin et al.31 | (1) Modified Xiao Yao San (dosage: NR, q.d./
b.i.d.); (2) Modified Xiao Yao San (dosage: NR, b.i.d.) + domperidone (10 mg, t.i.d.); (3) Modified Xiao Yao San (dosage: NR, b.i.d.) + deanxit (1–2 tablets, t.i.d.); (4) Modified Xiao Yao San (dosage: NR, b.i.d.) + mosabilium (5 mg, t.i.d.) |
(1) Domperidone (10 mg, t.i.d.); (2) Domperidone (10 mg, t.i.d.) + oryzanol (20 mg, t.i.d.); (3) Deanxit (1–2 tablets, t.i.d.); (4) Mosabilium (5 mg, t.i.d.) |
Sleepiness, insomnia, passage of small amount loose stools |
| Cui and Shen29 | Details of CHM are not reported | (1) Domperidone (NR); (2) Mosapride (NR) |
NR |
| Wang et al.30 | (1) Gan Wei Shu capsule (three capsules, t.i.d.,
p.o.) (2) Chai Hu Shu Gan powder/decoction (NR) (3) He Wei Xiao Yao decoction (NR) (4) Jia Wei Si Ni powder (NR) (5) Xing Qi Tong Jian pill (4 Pills, t.i.d., p.o.) + Ju Pi porridge (NR) (6) Li Qi He Wei oral liquid (10 ml, t.i.d., p.o.) (7) Jiang Qi He Zhong decoction (NR) (8) Shu Wei decoction (NR) (9) Jian Pi Shu Gan decoction (NR) (10) Shu He decoction (NR) (11) Modified Si Ni powder (NR) (12) Shu Gan Jian Pi formula and acupuncture (NR) |
(1) Domperidone (10 mg t.i.d.); (2) Mosapride (5 mg, t.i.d.); (3) Polyzyme tablets (three tablets, t.i.d.) |
Intervention group: epigastric discomfort and increased passing of flatus |
| Xiao et al.32 | (1) Xiang Sha Liu Jun Zi decoction (dosage: NR,
b.i.d.); (2) Liu Jun Zi decoction (dosage: NR, b.i.d.) |
(1) Domperidone (10 mg, t.i.d.); (2) Mosapride (5 mg, t.i.d.) |
Control group: Tolerable: abdominal pain, diarrhea, rugitus, loose stools |
| Xiao et al.33 | Shu Gan Jie Yu capsule (dosage: NR, b.i.d.)
+ mosapride (5 mg, t.i.d.) |
Mosapride (5 mg, t.i.d.) | Intervention group: gastrointestinal discomfort, dry mouth,
dizziness Control group: dry mouth, diarrhea, dizziness Both groups: Mild and tolerable: increasing frequency of defecation |
| Yang et al.34 | (1) Modified Chai Hu Shu Gan powder (dosage: NR,
b.i.d.); (2) Modified Chai Hu Shu Gan powder (dosage: NR, b.i.d.) + mosapride (5 mg, t.i.d.); (3) Modified Chai Hu Shu Gan powder (dosage: NR, b.i.d.) + flupentixol melitracen tablets (one pill, q.d.); (4) Modified Chai Hu Shu Gan powder (dosage: NR, b.i.d.) + domperidone (10 mg, t.i.d.); (5) Modified Chai Hu Shu Gan powder (dosage: NR, b.i.d.) + trimebutine maleate tablets (100 mg, q.d.) |
(1) Domperidone (10 mg, t.i.d.); (2) Trimebutine maleate tablets (100 mg, q.d.); (3) Flupentixol melitracen tablets (two pills, q.d.); (4) Domperidone (10 mg, t.i.d.) + compound azintamide Tablets (1–2 Pills, t.i.d.); (5) Mosapride (5 mg, t.i.d.) |
Reported as no adverse events |
| Gan et al.35 | Ban Xia Xie Xin decoction (NR) | (1) Domperidone (NR) (2) Mosapride (NR) |
Control group: gastrointestinal symptoms, headache |
| Fan and He36 | Da Li Tong granule (6 g, t.i.d.) | (1) Mosapride (5 mg, t.i.d.); (2) Domperidone (10 mg, t.i.d.) |
Intervention group: abdominal fullness Control group: abdominal pain, dizziness, diarrhea, borborygmi |
| Liu25 | (1) Xiao Pi formula no. 1 or 2 (NR) (2) Li Zhong decoction (NR) (3) Jian Pi Xiao Zhang powder decoction (NR) (4) Xiang Sha Liu Jun Zi decoction (NR) (5) He Wei Xiao Pi capsules (NR) (6) He Wei powder (NR) (7) San Jiu Wei Tai granule (NR) (8) Chai Hu Shu Gan San (NR) (9) Qing Song decoction (NR) (10) Shu Gan Yang Xin decoction (NR) (11) Huo Xiang Zheng Qi capsule (NR) (12) Ban Xia Xie Xin decoction (NR) (13) Can Ling Bai Shu San (NR) (14) Zuo Jin decoction (NR) (15) San Xiang decoction (NR) (16) Jian Wei Xiao Zhang granule (NR) (17) Si Ni San (NR) (18) Da Li Tong granule (NR) (19) Ping Wei capsule (NR) (20) Xiao Yao San (NR) (21) He Wei An Xiao decoction (NR) (22) Shu Gan Jie Yu capsule (NR) |
(1) Details of placebo are not reported; (2) Prokinetic agents/H2 receptor/antagonists/antidepressants (NR) |
NR |
| Zhang27 | (1) Ban Xia Xie Xin decoction (NR) (2) Si Ni Xiexin decoction (NR) (3) Xiao Pi Tong Jiang decoction (NR) (4) Chai Hu Wen Dan decoction (NR) (5) Zhi Shi Xiao Pi decoction (NR) (6) Fu He Xie Xin decoction (NR) (7) Chai Zhi Ban Xia Xie Xin decoction (NR) |
(1) Domperidone (NR); (2) Trimebutine (NR); (3) Mosapride (NR); (4) Omeprazole (NR) |
NR |
| Akarayosapong37 | Liu Wei An Xiao capsule (500 mg × 2–6 pills, t.i.d.) |
(1) Mosapride (5 mg, t.i.d.); (2) Domperidone (10 mg, t.i.d.); (3) Metoclopramide (10 mg, t.i.d.); (4) Digestive enzymes (500 mg, two pills t.i.d.); (5) Domperidone (10 mg, t.i.d.) + oryzanol (20 mg, t.i.d.); (6) Domperidone (10 mg, t.i.d.) + doxepin (12.5 mg, t.i.d.); (7) Flupentixol + melitracen (one pill, b.i.d.); (8) Domperidone (10 mg, t.i.d.) + amoxicillin (0.5 g, t.i.d.) |
Intervention group: more frequent egestion Control group: constipation, dizziness, insomnia Both groups: abdominal pain, borborygmi, more frequent urination, loose stool, diarrhea, headache |
| Ling et al.28 | Si Ni San (NR) | (1) Domperidone (NR); (2) Domperidone + deanxit (NR); (3) Domperidone + oryzanol (NR); (4) Mosapride (NR) |
Mild and tolerable nausea, vomiting, abdomen discomfort |
| Guo et al.26 | (1) Ri Kun Shi To powder (2.5 g, t.i.d.) (2) Gastrosis no. 3 compound granules (150 ml, b.i.d.) (3) Qing Hua He Wei Tablets (four tablets, 1.2 g, t.i.d.) (4) Gastrosis no. 1 compound granules (150 ml, b.i.d.) (5) Ban Xia Xie Xin Tang granules (150 ml, b.i.d.) (6) Modified Liu Jun Zi Tang granules (150 ml, b.i.d.) (7) Nos.1–4 compound granules (b.i.d./t.i.d.) (8) Ban Ha Sa Si decoction (3 g, t.i.d.) (9) Yang Sa Yang Wei San powder (9.68 g, t.i.d.) |
Placebo in the forms of powder (2.5 g/9.68 g,
t.i.d.), granule (150 mL/3 g, b.i.d./t.i.d.) or tablets (four pills, 1.2 g, t.i.d.) |
NR |
NR, not reported; CHM, Chinese herbal medicine; b.i.d., twice a day; t.i.d., three times a day; q.d., once a day.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Michael H. K. Chu, Global Physician-Leadership Stream, MBChB program, The Chinese University of Hong Kong, Hong Kong
Irene X. Y. Wu, Hong Kong Institute of Integrative Medicine, The Chinese University of Hong Kong, 5/F, School of Public Health Building, Prince of Wales Hospital, Shatin, New Territories, Hong Kong.
Robin S. T. Ho, Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
Charlene H. L. Wong, Hong Kong Institute of Integrative Medicine, The Chinese University of Hong Kong, Hong Kong Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong
Anthony L. Zhang, China–Australia International Research Centre for Chinese Medicine School of Health and Biomedical Sciences, RMIT University, Melbourne, Australia Australian Research Centre in Complementary and Integrative Medicine (ARCCIM), University of Technology Sydney, Sydney, Australia
Yan Zhang, Australian Research Centre in Complementary and Integrative Medicine (ARCCIM), University of Technology Sydney, Sydney, Australia Department of Family and Community Medicine, Texas Tech University Health Sciences Center, Lubbock, TX, USA.
Justin C. Y. Wu, Hong Kong Institute of Integrative Medicine, The Chinese University of Hong Kong, Hong Kong
Vincent C. H. Chung, Hong Kong Institute of Integrative Medicine, The Chinese University of Hong Kong, Hong Kong Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
References
- 1. Kumar A, Pate J, Sawant P. Epidemiology of functional dyspepsia. J Assoc Physicians India 2012; 60(Suppl.): 9–12. [PubMed] [Google Scholar]
- 2. El-Serag HB, Talley NJ. Systemic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther 2004; 19: 643–654. [DOI] [PubMed] [Google Scholar]
- 3. Ghoshal U, Singh R, Chang FY, et al. Epidemiology of uninvestigated and functional dyspepsia in Asia: facts and fiction. J Neurogastroenterol Motil 2011; 17: 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar D, Bhat ZA, Singh P, et al. Ailanthus excelsa Roxb is really a plant of heaven. Int J Pharmacol 2010; 6: 535–550. [Google Scholar]
- 5. Piessevaux H, De Winter B, Louis E, et al. Dyspeptic symptoms in the general population: a factor and cluster analysis of symptom groupings. Neurogastroenterol Motil 2009; 21: 378–388. [DOI] [PubMed] [Google Scholar]
- 6. Welen K, Faresjo A, Faresjo T. Functional dyspepsia affects women more than men in daily life: a case-control study in primary care. Gened Med 2008; 5: 62–73. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki H. The application of the Rome IV criteria to functional esophagogastroduodenal disorders in Asia. J Neurogastroenterol Motil 2017; 23: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 130: 1466–1479. [DOI] [PubMed] [Google Scholar]
- 9. Camilleri M, Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013; 10: 187–194. [DOI] [PubMed] [Google Scholar]
- 10. Sundberg R, Palmqvist M, Tunsäter A, et al. Health-related quality of life in young adults with asthma. Respir Med 2009; 103: 1580–1585. [DOI] [PubMed] [Google Scholar]
- 11. Lix L, Graff LA, Walker JR, et al. Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis 2008; 14: 1575–1584. [DOI] [PubMed] [Google Scholar]
- 12. Lacy B, Weiser KT, Kennedy AT, et al. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther 2013; 38: 170–177. [DOI] [PubMed] [Google Scholar]
- 13. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection–the Maastricht IV/ Florence consensus report. Gut 2012; 61: 646–664. [DOI] [PubMed] [Google Scholar]
- 14. Moayyedi P, Soo S, Deeks J, et al. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev 2006; (2): CD001960. [DOI] [PubMed] [Google Scholar]
- 15. Xiao YL, Peng S, Tao J, et al. Prevalence and symptom pattern of pathologic esophageal acid reflux in patients with functional dyspepsia based on the Rome III criteria. Am J Gastroenterol 2010; 105: 2626–2631. [DOI] [PubMed] [Google Scholar]
- 16. Rosch W, Liebregts T, Gundermann KJ, et al. Phytotherapy for functional dyspepsia: a review of the clinical evidence for the herbal preparation STW 5. Phytomedicine. 2006; 13(Suppl. 5): 114–121. [DOI] [PubMed] [Google Scholar]
- 17. Hojo M, Nagahara A, Asaoka D, et al. Emerging pharmacological therapy for functional dyspepsia. Clin J Gastroenterol 2013; 6: 352–356. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki H, Okada S, Hibi T. Proton-pump inhibitors for the treatment of functional dyspepsia. Therap Adv Gastroenterol 2011; 4: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quigley EMM. Prokinetics in the Management of Functional Gastrointestinal Disorders. J Neurogastroenterol Motil 2015; 21: 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leelakanok N, Holcombe A, Schweizer ML. Domperidone and risk of ventricular arrhythmia and cardiac death: a systematic review and meta-analysis. Clin Drug Investig 2016; 36: 97–107. [DOI] [PubMed] [Google Scholar]
- 21. Shin HW, Chung SJ. Drug-induced parkinsonism. J Clin Neurol 2012; 8: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bosco D, Plastino M, Marcello MG, et al. Acute hemifacial dystonia possibly induced by clebopride. Clin Neuropharmacol 2009; 32: 107–108. [DOI] [PubMed] [Google Scholar]
- 23. Spleen and Stomach Diseases Committee CAoCM. Consensus of diagnosis and treatment of Chinese medicine on dyspepsia. Chin J Integ Med 2009; 30: 533–537. [Google Scholar]
- 24. Zheng XY. Guiding principle of clinical research on new drugs of traditional Chinese Medicine. Beijing: China Medic-Pharmaceutical Sciences and Technology Publishing House, 2002, p.143. [Google Scholar]
- 25. Liu PF. Systematic review of Chinese medicine in treating functional dyspepsia. China: Guangxi Medical University, 2015. [Google Scholar]
- 26. Guo Y, Zhu JJ, Su XL, et al. Efficacy of Chinese herbal medicine in functional dyspepsia: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Tradit Chin Med Sci 2016; 3: 147–156. [Google Scholar]
- 27. Zhang SZ. Systematic review on acrid opening and bitter downbearing method in treating functional dyspepsia. China: Shaanxi University of Chinese Medicine, 2015. [Google Scholar]
- 28. Ling W, Li Y, Jiang W, et al. Common mechanism of pathogenesis in gastrointestinal diseases implied by consistent efficacy of single Chinese medicine formula. Medicine (United States) 2015; 94: e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cui YP, Shen H. Meta-analysis of Chinese Medicine in Treating Functional Dyspepsia. World federation of Chinese medicine societies digestive disease professional committee 3rd international academic conference cum Guangxi society of integrated traditional Chinese and western medicine digestive disease annual conference proceedings Nanning, China, 2012, p.1–8. [Google Scholar]
- 30. Wang C, Zhu M, Xia W, et al. Meta-analysis of traditional Chinese medicine in treating functional dyspepsia of liver-stomach disharmony syndrome. J Tradit Chin Med 2012; 32: 515–522. [DOI] [PubMed] [Google Scholar]
- 31. Qin F, Huang X, Ren P. Chinese herbal medicine modified Xiao Yao San for functional dyspepsia: meta-analysis of randomized controlled trials. J Gastroenterol Hepatol (Australia). 2009; 24: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 32. Xiao Y, Liu YY, Yu KQ, et al. Chinese herbal medicine Liu Jun Zi Tang and Xiang Sha Liu Jun Zi Tang for functional dyspepsia: meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. Epub ahead of print 12 December 2012. DOI: 10.1155/2012/936459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao ZJ, Guo JW, Xu F. Efficacy of Chinese medicine Shu Gan Jie Yu capsule for functional dyspepsia. Acta Pharmacologica Sinica 2013; 34: 23. [Google Scholar]
- 34. Yang N, Jiang X, Qiu X, et al. Modified Chai Hu Shu Gan powder for functional dyspepsia: meta-analysis for randomized controlled trial. Evid Based Complement Alternat Med 2013; 2013: no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gan Y, Liu H, Yang L, et al. Effect of Ban Xia Xie Xin Tang on treatment of functional dyspepsia: a meta-analysis of randomized controlled trials. J Tradit Chin Med 2014; 34: 140–144. [DOI] [PubMed] [Google Scholar]
- 36. Fan CS, He MM. Cost-effectiveness analysis and evaluation of Da Li Tong granule in treating functional dyspepsia. Eval Med 2015; 6: 24–34. [Google Scholar]
- 37. Akarayosapong P. Systematic review on the effectiveness and safety of Liu Wei An Xiao Capsule in treating functional dyspepsia. China: Guangxi Medical University, 2015. [Google Scholar]
- 38. Liu JL, Zhang YJ, Song YX, et al. Systematic review on randomized control trials of Chinese and Western medicine in treating functional dyspepsia. Chin J Evid Based Med 2009; 9: 1315–1322. [Google Scholar]
- 39. Wysowski D, Corken A, Gallo-Torres H, et al. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol 2001; 96: 1698–1703. [DOI] [PubMed] [Google Scholar]
- 40. Ren L, Chen WX, Qian LJ, et al. Addition of prokinetics to PPI therapy in gastroesophageal reflux disease: a meta-analysis. World J Gastroenterol 2014; 20: 2412–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eom CS, Jeon CY, Lim JW, et al. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ 2011; 183: 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lam J, Schneider JL, Zhao W, et al. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310: 2435–2442. [DOI] [PubMed] [Google Scholar]
- 43. Hanlon J, Landerman LR, Artz MB, et al. Histamine2 receptor antagonist use and decline in cognitive function among community dwelling elderly. Pharmacoepidemiol Drug Saf 2004; 13: 781–787. [DOI] [PubMed] [Google Scholar]
- 44. Haynes R, McKibbon KA, Wilczynski NL, et al. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ 2005; 330: 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wong SSL, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc 2006; 94: 41–47. [PMC free article] [PubMed] [Google Scholar]
- 46. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009; 62: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 47. Pieper D, Buechter RB, Li L, et al. Systematic review found AMSTAR, but not R(evised)-AMSTAR, to have good measurement properties. J Clin Epidemiol 2015; 68: 574–583. [DOI] [PubMed] [Google Scholar]
- 48. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Hoboken, New Jersey: John Wiley & Sons, 2011. [Google Scholar]
- 49. Higgins J, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schulz K, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ 2013; 346: f2914. [DOI] [PubMed] [Google Scholar]
- 52. Shuldiner SR, Chung VC, Wu X, et al. Methodological challenges in mapping chinese medicine syndrome with conventional diagnosis: implications for multi-centre trials in integrative medicine. Eur J Integr Med 2015; 7: 358–364. [Google Scholar]
- 53. Chung VC, Ho RS, Wu X, et al. Incorporating traditional Chinese medicine syndrome differentiation in randomized trials: methodological issues. Eur J Integr Med 2016; 8: 898–904. [Google Scholar]
- 54. Zhang NL, Fu C, Liu TF, et al. A data-driven method for syndrome type identification and classification in traditional Chinese medicine. J Integr Med 2017; 15: 110–123. [DOI] [PubMed] [Google Scholar]
- 55. Lacy BE, Talley NJ, Camilleri M. Functional dyspepsia: time to change clinical trial design[quest]. Am J Gastroenterol 2010; 105: 2525–2529. [DOI] [PubMed] [Google Scholar]
- 56. Van Kerkhoven L, Laheij RJ, Meineche-Schmidt V, et al. Functional dyspepsia: not all roads seem to lead to Rome. J Clin Gastroenterol 2009; 43: 118–122. [DOI] [PubMed] [Google Scholar]
- 57. Sood R, Ford AC. Diagnosis: Rome IV criteria for FGIDs - an improvement or more of the same? Nat Rev Gastroenterol Hepatol 2016; 13: 501–502. [DOI] [PubMed] [Google Scholar]
- 58. Scott RS, Chung VCH, Wu XY, et al. Methodological challenges in mapping chinese medicine syndrome with conventional diagnosis: implications for multi-centre trials in integrative medicine. Eur J Integr Med 2015; 7: 358–364. [Google Scholar]
- 59. Tang JL, Zhan SY, Ernst E. Review of randomised controlled trials of traditional Chinese medicine. BMJ 1999; 319: 160–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vickers A, Goyal N, Harland R, et al. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials 1998; 19: 159–166. [DOI] [PubMed] [Google Scholar]
- 61. Zeng Z-P, Jiang J-G. Analysis of the adverse reactions induced by natural product-derived drugs. Br J Pharmacol 2010; 159: 1374–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Meyer MM, Chen T-P, Bennett WM. Chinese herb nephropathy. Proc (Bayl Univ Med Cent). 2000; 13: 334–337. [PMC free article] [PubMed] [Google Scholar]
- 63. Douros A, Bronder E, Andersohn F, et al. Herb-induced liver injury in the Berlin Case-Control Surveillance Study. Int J Mol Sci 2016; 17: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cho J-H, Oh D-S, Hong S-H, et al. A nationwide study of the incidence rate of herb-induced liver injury in Korea. Arch Toxicol 2017; 91: 4009–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Viera AJ. Odds ratios and risk ratios: what’s the difference and why does it matter? South Med J 2008; 101: 730–734. [DOI] [PubMed] [Google Scholar]