Abstract
Primary angiitis of the central nervous system (PACNS) represents a rare inflammatory disease affecting the brain and spinal cord. Stroke, encephalopathy, headache and seizures are major clinical manifestations. The diagnosis of PACNS is based on the combination of clinical presentation, imaging findings (magnetic resonance imaging and angiography), brain biopsy, and laboratory and cerebral spinal fluid (CSF) values. PACNS can either be confirmed by magnetic resonance angiography (MRA)/conventional angiography or tissue biopsy showing the presence of typical histopathological patterns. Identification of PACNS mimics is often challenging in clinical practice, but crucial to avoid far-reaching treatment decisions. In view of the severity of the disease, with considerable morbidity and mortality, early recognition and treatment initiation is necessary. Due to the rareness and heterogeneity of the disease, there is a lack of randomized data on treatment strategies. Retrospective studies suggest the combined administration of cyclophosphamide and glucocorticoids as induction therapy. Immunosuppressants such as azathioprine, methotrexate or mycophenolate mofetil are often applied for maintenance therapy. In addition, the beneficial effects of two biological agents (anti-CD20 monoclonal antibody rituximab and tumour necrosis factor-α blocker) have been reported. Nevertheless, diagnosis and treatment is still a clinical challenge, and further insights into the immunopathogenesis of PACNS are required to improve the diagnosis and management of patients. The present review provides a comprehensive overview of diagnostics, differential diagnoses, and therapeutic approaches of adult PACNS.
Keywords: diagnosis, inflammation, primary angiitis of the central nervous system, stroke, treatment
Introduction
Primary angiitis of the central nervous system (PACNS) is a rare and severe disease. It was first described by Harbitz in 1922 as an unknown form of angiitis in the CNS.1 Isolated vasculitis of the central nervous system, primary CNS vasculitis, and isolated angiitis of the CNS are common synonyms for the disease. Histologically, inflammation affects the blood vessels supplying the brain parenchyma, spinal cord and leptomeninges, and less frequently veins and venules.2 In PACNS, pathological findings can affect both small and large vessels of the CNS.3 The pathophysiological mechanism is based on infiltration of immune cells within CNS blood vessel walls leading to destruction of the vessel walls.4 As a result, thickening of vessel walls with alternating segments of stenosis can occur, resulting in poor blood circulation.4 On the other hand, vessel wall weakening, due to inflammatory processes, may cause blood vessel ruptures and intracranial haemorrhage.4
PACNS mainly affects younger stroke patients lacking cerebrovascular risk factors.5 Approximately 3–5% of cerebrovascular events in patients aged <50 years are caused by primary CNS vasculitis.6 The mean age of the initial manifestation of adult PACNS is at the end of the fourth decade.6 In retrospective analyses a similar frequency in male and female patients has been shown.7 An incidence of 2.4 person-years was determined in an epidemiologic study of a White population in North America (Olmsted County, Minnesota).7
Clinical features at diagnosis are highly variable and nonspecific; classic or pathognomonic clinical symptoms are lacking. Major symptoms of cerebral vasculitis are headache (60%), altered cognition (50%) and focal neurologic deficits (e.g. hemiparesis, ataxia, aphasia, dysarthria and visual disturbances).5 Furthermore seizures and encephalopathy have been reported. In general, the onset of disease is insidious and slowly progressive, but an acute beginning of symptoms can occur.5 Marked constitutional symptoms such as fever, weight loss and night sweats are less frequent and can be indicative of systemic vasculitides.8 In addition to cerebral manifestation, in about 5% of patients primary CNS vasculitis can affect the spinal cord.9 Isolated vasculitic myelopathies have also been reported.10
Diagnostic criteria have been implemented in 1988 to distinguish between PACNS and mimics, and to make a definite diagnosis.2 The diagnostic criteria of cerebral vasculitis are as follows: (1) a history of clinical findings of an acquired otherwise unexplained neurologic deficit, (2) presence of classic angiographic or histopathologic features of angiitis within the CNS, and (3) no evidence of systemic vasculitis or of any other disorder that could cause or mimic the angiographic or pathologic features. In 2009 Birnbaum and colleagues suggested a modification of these criteria to avoid misdiagnosis, especially because of its most important and challenging mimic, that is, reversible cerebral vasoconstriction syndrome (RCVS).8 The level of certainty of diagnosis was subdivided into ‘definite’, when the analysis of a tissue biopsy specimen confirms the presence of vasculitis, and ‘probable’, if there are high probability findings on an angiogram with abnormal findings on magnetic resonance imaging (MRI) and a CSF profile consistent with PACNS (in the absence of tissue confirmation).8
Pathophysiologically, infectious agents, such as varicella-zoster virus (VZV) and mycoplasma-like structures, have been thought to predispose to manifestation of cerebral vasculitis.11–13 Memory T-cells, interpreted as indicators of an antigen-specific immune response occurring in the wall of cerebral arteries, have been suggested to be involved in immunological mechanisms. In a single case report, immunohistochemical staining of a biopsy sample showed predominant infiltration by CD45R0+-T-cells in and around small cerebral vessels.14 However, the etiology, pathogenesis and immunological mechanisms of primary CNS vasculitis have not yet been conclusively elucidated.
Retrospective studies suspected two subtypes of primary angiitis of the CNS, distinguished by the size of vessels being involved: a small vessel disease (SV-PACNS) and a large/medium-vessel disease (LV-PACNS).15 Accordingly, isolated SV-PACNS is associated with a more severe encephalopathic presentation with cognitive or vigilance impairment and seizures, more abnormal CSF analysis and more diffuse gadolinium-enhanced lesions on MRI.15,17 In patients with SV-PACNS, relapses were more frequently observed.15,18 In contrast, LV-PACNS is more likely to present with focal deficits and acute ischaemia on MRI.15,19
Besides the size of affected vessels and typical histopathological patterns, patients suffering from cerebral vasculitis can be classified by clinical, imaging, and pathological characteristics, and age of onset in the following six disease subtypes (Table 1):4,16 (1) In angiogram-negative biopsy-positive cases cognitive impairment is often noticed in clinical examination.17 Further characteristics are an increased CSF protein, meningeal and parenchymal enhancing lesions on MRI and a favourable response to treatment. (2) In amyloid-β-related cerebral angiitis (ABRA) histopathological analysis shows granulomatous vasculitis with β-amyloid deposition in vessel walls often present with a more acute onset.20,21 Patients are generally older, predominantly male and show cognitive impairment. (3) Another subset of patients is defined by prominent meningeal enhancement in MRI with a rapid response to therapy.22 (4) Spinal cord involvement is the less frequently observed disease subtype. Spinal cord symptoms may develop at disease onset or during the disease course, cerebral manifestations are usually present in these disease subtypes.9 (5) PACNS presenting with intracranial or subarachnoid haemorrhage often shows a favourable response to immunosuppressive treatments and necrotizing vasculitis is a predominant histopathological pattern.23 Women are more frequently affected: in a retrospective cohort of patients all 16 patients with intracranial haemorrhage were women.23 (6) Rapidly progressive PACNS, characterized by bilateral, multiple, large cerebral vessel lesions and multiple CNS infarctions, appears to be a more aggressive and serious disease course, less responsive to therapy, and often with a fatal outcome.24 Angiogram findings are frequently positive in ABRA, intracranial haemorrhage and rapidly progressive subtypes.4 Given the fact that any combination of clinical and diagnostic features may be found at presentation, overlapping symptoms among the aforementioned six disease types exist.
Table 1.
Disease subtypes of PACNS.
| Subtype | Clinical features | MRI | Angiogram | Biopsy |
|---|---|---|---|---|
|
Angiogram
-negative biopsy-positive |
cognitive impairment, greater CSF abnormalities, favourable response to treatment | meningeal and parenchymal enhancing lesions on MRI |
negative | granulomatous pattern |
|
Amyloid-β-
related cerebral angiitis (ABRA) |
older age, predominantly males, cognitive impairment | contrast-enhanced meningeal lesions |
positive | granulomatous pattern with β- amyloid deposition in vessel walls |
| Meningeal enhancement in MRI | predominantly males, cognitive impairment, rapid response to treatment | prominent leptomeningeal enhancement |
negative | granulomatous pattern |
|
Spinal cord
involvement |
spinal cord symptoms, cerebral manifestations usually present | enhanced spinal (especially thoracic) lesions |
negative | necrotizing pattern |
|
Intracranial
or subarachnoid haemorrhage |
predominantly women, favourable response to treatment | intracranial or subarachnoid haemorrhage |
positive | necrotizing pattern |
| Rapidly progressive | aggressive disease course, less responsive to treatment, often fatal outcome | bilateral, multiple, vessel lesions and infarctions |
positive | granulomatous or necrotizing pattern |
Classification according to Giannini and colleagues, 2012.4 Clinical and diagnostic features can overlap among disease subtypes.
CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; PACNS, primary angiitis of the central nervous system
Diagnostics
Diagnostic work-up is based on detailed anamnesis, clinical examination, and laboratory and imaging findings (Table 2). CSF examinations show inflammatory findings, typically mild lymphocytic pleocytosis combined with an elevated protein level. CSF examination is abnormal in 80–90% of patients.3 MRI of the brain is abnormal in more than 90% of patients, but the pattern of abnormal findings is not specific.3,25 The diagnosis of PACNS can be established by angiography showing characteristic features of vasculitis. In patients with noncontributive angiography the definite diagnosis can be confirmed by biopsy.15
Table 2.
Findings in diagnostic work-up in suspected PACNS.
| Clinical features | Laboratory tests | CSF | MRI | DSA | Biopsy |
|---|---|---|---|---|---|
| Headache, seizures, stroke, encephalopathy, altered cognition | usually normal serum findings usually absence of systemic vasculitis associated antibodies |
mild lympho- monocytic pleocytosis or protein elevation; occasionally presence of oligoclonal bands | ischaemic infarctions, signs of microangiopathy,
haemorrhage, contrast enhancement, tumour-like lesions |
vessel beading (segmental arterial wall narrowing, followed by post-stenotic dilation) |
granulomatous, lymphocytic or acute necrotizing patterns |
CSF, cerebrospinal fluid; DSA, digital subtraction angiography; MRI, magnetic resonance tomography PACNS, primary angiitis of the central nervous system
Imaging
Conventional cerebral angiography is a cornerstone for establishing the diagnosis of PACNS. Multiple areas of narrowing and dilatation or multilocular occlusions of intracranial vessels are typical imaging features highly indicative of primary CNS vasculitis.26 Additional findings are fusiform arterial dilatations, the development of a collateral circulation, and a delayed contrast enhancement.27 In contrast, long segment stenoses, microaneurysms and complete occlusions are rather unlikely in PACNS.28
Sufficient visualization of intracranial vessels for detection of stenosis and dilatation (‘vessel beading’) is recommended in suspected PACNS patients.5,29 For this approach, MRA and conventional angiography are implemented in diagnostic work-up.5,30 In contrast to conventional angiography, MRA is a less invasive imaging modality and therefore can be used to monitor the disease course.31 Nonetheless, in comparison with digital subtraction angiography (DSA), MRA is less sensitive in the detection of both lesions localized in the posterior circulation and in distal arteries.30 Overall, vessel beading is not specific and is also seen in noninflammatory vasculopathies and nonvasculitis conditions such as atherosclerosis, after radiation, neurofibromatosis, atrial myxomas, neurofibromatosis, infections, and vasospasm.32–34
Although the spatial and temporal resolution of standard angiography is currently the best of all imaging techniques in use, it often appears normal when the inflammatory changes are limited to arteries of <500 µm in diameter.35 Therefore, in case of negative angiographic findings due to small vessel involvement, brain biopsy should be considered as an important step towards diagnosing PACNS. In contrast, in patients diagnosed by angiogram primarily large/proximal vessels are affected.7 However, it should be born in mind that even in the presence of classic angiographic findings, an alternative diagnosis is often made when biopsy is done.36
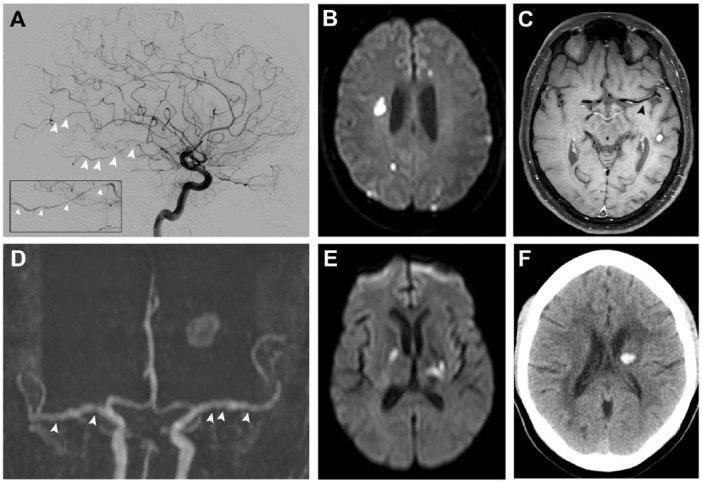
Besides angiograms, conventional MRI is an important and highly sensitive imaging modality in the diagnostic approach of PACNS. The majority of patients (more than 90%) show MRI abnormalities.8 However, MRI findings are not specific and clinicians need to be aware of the main differential diagnoses. MRI should include T1-weighted, T2-weighted and fluid-attenuated inversion-recovery (FLAIR) sequences as well as apparent diffusion coefficient maps, and diffusion and gradient echo sequences, with and without gadolinium administration.27 Typical findings of PACNS are multifocal bilateral T2 or FLAIR sequence abnormalities in the cortical and subcortical structure, and deep and grey white matter.27 Both, new and older ischaemic lesions, usually coexistent with bilateral stenoses and dilatations in multiple large and small vessels, are a cardinal feature and highly suspicious of PACNS (Figure 1).3 Ischaemic lesions can be accompanied by subarachnoid and intraparenchymal haemorrhagic lesions (Figure 1).37 MRI may also demonstrate mass lesions that can mimic a tumour or abscess.8 Gadolinium enhancement of parenchymal lesions and the leptomeninges have been reported.3,22
Figure 1.
Imaging of patients with PACNS. (A) A 44-year-old patient presenting with multifocal segmental narrowing of intracranial arteries on cerebral angiogram, (B) multiple DWI-lesions in different vascular territories, and (C) concentric enhancement of the M1-segment of the left middle cerebral artery on black blood MRI. (D) A 48-year-old patient with vessel beading on MRI-TOF-angiography, (E) bilateral infarctions of variable size (affecting different vascular territories and in various stages of healing), and (F) intracerebral haemorrhage.
DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging; PACNS, primary angiitis of the central nervous system; TOF, time of flight.
In comparison with MRI, computed tomography (CT) is less sensitive in the assessment of signs of vasculitis-related infarcts.28 CT angiography (CTA) can be used to evaluate large vessel vasculitis. Nevertheless, the resolution of DSA remains to be superior to that of CTA.38
High resolution contrast-enhanced MRI (HR-MRI) such as ‘black blood MRI’ may be helpful in characterization of pathologic findings in and around the wall of intracranial vessels. Technically, pathological changes like wall thickening and wall enhancement can be investigated (Figure 1). In particular, differentiating between inflammation, intracranial atherosclerotic plaques, and other wall abnormalities based on the typical enhancement patterns was reported.39–41 Improvement of this emerging noninvasive technique should be a possible future perspective.
An additional aim of future studies could be the implementation of nuclear medicine molecular diagnostic tools. Positron emission tomography with [11C]-PK11195 has been demonstrated to detect vascular inflammatory activity in patients with large vessel vasculitis.42 In PACNS patients with inconclusive imaging results, this could be a useful future diagnostic approach.
Biopsy
In patients with suspicion of primary angiitis of the CNS but inconclusive imaging findings, brain biopsy for definite diagnosis is mandatory. Due to the focal and segmental distribution of the disease, the sensitivity of brain biopsy is as a result of sampling errors between 53–74%,43,44 but the diagnostic yield can be increased to over 80% by targeting areas of imaging abnormality.44 If affected lesions are not accessible for surgery, a biopsy from the right (nondominant) frontal lobe with its overlying leptomeninges tissue is recommended.29,35,45
As described above, a negative biopsy cannot rule out the diagnosis of cerebral vasculitis, but histopathological examination often results in alternative diagnoses.46 Thus, special attention is paid to PACNS mimics like infections, neoplasms (especially lymphoma) and degenerative disease.47 Brain biopsies are associated with rather low morbidity and mortality rates.5,48 Therefore, a biopsy should be obtained, if the diagnosis is uncertain. The characteristic biopsy finding, which confirms the diagnosis of PACNS, is a transmural inflammation with subsequent injury of the vessel wall.49 The main histopathological patterns include granulomatous inflammation, lymphocytic cellular infiltrates, and acute necrotizing vasculitis.34,49 In a review of surgical biopsies from patients with PACNS the granulomatous pattern was seen in 56% (associated with deposition of β-A4 amyloid in 27%), purely lymphocytic in 20% and acute necrotizing pattern in 22% of cases.4 The characteristic features of granulomatous vasculitis are vasculocentric destructive mononuclear infiltrate with well-formed granulomas and multinucleated giant cells.4 Biopsy samples, particularly of the granulomatous vasculitis group, can show extensive β-A4 amyloid deposition in vessel walls with associated inflammation, recognized as amyloid-β-related angiitis.50 As reported in the literature, an association between granulomatous vasculitis with prominent meningeal enhancement, and with cerebral amyloid angiopathy (CAA) has been suggested.22,50 The second most prevalent pattern in PACNS is lymphocytic vasculitis showing lymphocytes with a variable number of plasma cells, histiocytes, neutrophils, and eosinophils.4,27 Necrotizing vasculitis is characterized by acute necrotizing vessel wall changes, transmural fibrinoid necrosis, and acute inflammation; it commonly presents with haemorrhage.4 Fibrinoid necrosis and inflammatory reaction are pathologic mechanisms supposed to cause a thickening of vessel walls which increases the risk of vascular rupture and aneurysmal dilatation.51 The presence of intimal fibrosis is characteristic for healed lesions.27 Further histopathological abnormalities detected in patients with biopsy-proven PACNS are neointimal proliferation and thromboses in vessels.8
In conclusion, brain biopsy can be considered a relatively safe and effective procedure for determining the pathologic nature of intracranial lesions, and thus can help to prevent unnecessary immunosuppressive treatments in alternative diagnoses.
Laboratory tests and CSF study
All patients suspected of PACNS need a laboratory work-up focusing on inflammation and antibody-mediated diseases. Serum screening can show mild systemic symptoms or increased acute phase reactants (erythrocyte sedimentation rate/serum C-reactive protein),52 but in the majority of patients these parameters are completely unaltered.7,53 Detection of antinuclear antibodies, antineutrophil cytoplasmic antibodies, antiphospholipid antibodies, rheumatoid factor, cryoglobulins or bacterial or viral antibodies is highly suggestive of an underlying systemic vasculitis or autoimmune disease.5 Serological examinations are needed to rule out infectious disorders, including varicella, mycobacteria, syphilis, human immunodeficiency virus (HIV), and fungi.
Lumbar puncture should be performed in all patients. CSF analysis reveals abnormal (inflammatory findings) in 80–90% of patients with cerebral vasculitis.3 Typically, PACNS patients exhibit mild lymphocytic pleocytosis or an elevated protein level. Normal CSF findings (cell count <5 cells/μl or total protein concentration <45 mg/dl) should direct diagnostic work-up towards possible differential diagnoses.54 In patients with PACNS oligoclonal bands or immunoglobulin (Ig)G synthesis are only occasionally detectable.27,43 An increase of the proportion of interleukin (IL)17-producing CD41 cells in CSF might be useful in discriminating cerebral vasculitis from ischaemic stroke.55 Values of more than 250 cells/μl are indicative for infection and further analyses should include appropriate staining and cultures, polymerase chain reaction assays and antibody indices for detection of infectious agents.7,8 Malignant vasculitis can be detected with CSF cytology and flow cytometry.
Serial CSF analyses might be useful for treatment monitoring. For instance, improvements of CSF changes and neurological deficits have been reported in an individual case report.56
Differential diagnosis
The differential diagnosis of cerebral vasculitis is broad, as diverse neurological diseases of inflammatory, infectious, malignant, and vascular aetiologies can resemble primary angiitis of the CNS (Table 3). Therefore, serious consideration of possible differential diagnoses in the diagnostic approach is crucial to avoid unnecessary immunosuppressive treatments.
Table 3.
Overview of (selected) differential diagnosis of PACNS.
| Type of disease | Selected example |
Similarities with PACNS | Differences to PACNS | Diagnostic approach |
| RCVS | cerebral angiographic abnormalities (multifocal segmental
cerebral artery vasoconstriction), cerebral infarctions, intracerebral haemorrhage |
acute onset, monophasic course, thunderclap headache, usually normal CSF analysis, normal MRI in 20%, no vasculitis changes in cerebral biopsy, precipitating factors, reversible angiographic abnormalities | angiographic follow-up after 12 weeks shows resolution of abnormalities, nimodipine (reverse vessel narrowing in DSA?) | |
| Non-inflammatory vasculopathies |
Atherosclerosis | multiple cerebral infarctions, vessel beading, vessel wall enhancement | older age, vascular risk factors (hypertension, diabetes
mellitus), hetergeneous lesions, calcifications and irregular
focal stenoses of proximal arteries, normal CSF analysis,
infarcts usually restricted to a single vascular territory |
eccentric enhancement patterns of intracranial atherosclerotic plaques in high-resolution 3-Tesla contrast-enhanced MRI, calcified proximal cerebral arteries |
| CADASIL | disease course, headache, psychiatric disturbances, sensory, motor and cognitive deficits, seizures, cerebral infarctions, diffuse white matter abnormalities on brain MRI | strokes or dementia in the history of the first-degree
relatives, bilateral external capsule and
anterior temporal lobe hyperintensities |
genetic testing (mutation of the notch 3 gene), pathologic findings characteristic of CADASIL on brain or skin punch biopsies | |
| MELAS | enzephalopathy, stroke-like episodes before age 40, seizures, dementia, multiple hyperintensities on T2 and FLAIR-sequences | bilateral basal ganglia calcifications | genetic testing (point mutation A3243G), muscle biopsy (ragged-red fibers) | |
| Moyamoya angiopathy |
cerebral infarctions, headache |
younger age, normal CSF analysis, no inflammatory signs in the vessel wall, triggering of ischaemic events with hyperventilation, orthostatic stress, etc.,watershed infarctions on brain MRI, no gadolinium-enhancement, effect on extracranial or proximal intracranial cerebral arteries | angiography shows typical collateral network of small leptomeningeal and transdural vessels | |
| Radiation vasculopathy |
vessel wall enhancement, leukoaraiosis | normal CSF analysis | history of cranial irradiation | |
| CNS manifestations as part of a primary systemic vasculitis | Systemic vasculitis |
headache, encephalopathy, seizures, cranial nerve palsies, visual symptoms, myelopathy, cerebral infarctions, intracranial haemorrhage, signs of mural inflammation on brain MRI | systemic signs and symptoms (fever, malaise, weight loss), renal insufficiency, pulmonary haemorrhage, unexplained sinusitis, abdominal pain, PNS can be affected | Elevated erythrocyte sedimentation rate or serum C reactive protein; Testing for antineutrophil cytoplasmic antibodies, antinuclear antibodies, cryoglobulins, hepatitis B/C serologies, p ANCA, c ANCA, anti-PR3, anti-MPO, extraneuronal biopsy |
ANCA, Anti-neutrophil cytoplasmic antibodies; CSF, cerebrospinal fluid; DSA, digital subtraction angiography; MPO, Myeloperoxidase; MRI, magnetic resonance imaging; PACNS, primary angiitis of the central nervous system; PNS, peripheral nervous system; RCVS, reversible cerebral vasoconstriction syndrome
The RCVS is the most important noninflammatory differential diagnosis to PACNS. Formerly RCVS was described as a subtype of PACNS, called benign angiopathy of the CNS.57 RCVS occurs significantly more frequently in female patients and the mean age of onset is 42 years.58,59 Typical clinical features are sudden and recurrent attacks of severe (usually thunderclap) headache with or without focal neurological deficits or seizures.58,59 RCVS is more often detected in patients with exposure to vasoactive drugs, migraine, hypertension, eclampsia or in the postpartum period.58,60 Neuroimaging may show cerebral infarctions, usually located in the superficial border zone or watershed regions, cortical subarachnoid haemorrhages and lobar intracerebral haemorrhage.61 Angiogram shows multifocal segmental cerebral artery vasoconstriction; dissection and unruptured aneurysms occur significantly more frequently than in cerebral vasculitis.61 Angiographic follow up 12 weeks after clinical onset usually shows complete or substantial resolution of abnormalities in RCVS and therefore remains crucial in differentiation to primary angiitis of the CNS.60 Additionally, two emerging diagnostic tools might be useful in distinguishing PACNS from RCVS. Black blood MRI in PACNS can visualize inflammatory changes in multiple vessels characterized by long and smooth circumferential concentric wall thickening with diffuse gadolinium enhancement of the inflamed wall. In contrast, angiogram in RCVS patients usually shows a short stenosis without, or with only moderate, wall thickening.62,63 In individual cases the use of intra-arterial nimodipine during conventional angiography showed reverse vessel narrowing in RCVS patients in contrast with PACNS.64 Distinguishing PACNS from RCVS was recently demonstrated to be crucial since corticosteroids worsen the outcome of RCVS.58
The spectrum of diseases causing a secondary vasculitis of the CNS is broad. In general, systemic inflammatory symptoms or increased acute phase reactants are suspicious for systemic vasculitis, malignoma or infectious disease (e.g. viral, bacterial or fungal).8
Important vasculites secondary to connective tissue diseases that can involve the CNS are systemic lupus erythematosus, Sjögren’s syndrome, rheumatoid arthritis, mixed connective tissue disease, and dermatomyositis.65 CNS involvement in systemic vasculitis can be due to giant cell arteritis,66 Takayasu’s arteritis,67 polyarteritis nodosa,68 Behçet’s disease,69 granulomatosis with polyangiitis (Wegener’s granulomatosis),70 eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome),71 Henoch–Schönlein purpura,72 and Kawasaki disease.73 CNS involvement most often arises in patients with Behçet’s disease, Wegener’s granulomatosis and Churg–Strauss syndrome.52
Other noninflammatory vascular diseases like Moyamoya angiopathy,74 Divry-van Bogaert syndrome,75 fibromuscular dysplasia,76 Fabry disease77 or Sneddon’s syndrome77 should also be taken into account as differential diagnoses.
Whereas PACNS can affect people of all ages, prevalence of atherosclerosis increases with higher age and is more likely in patients with presence of vascular risk factors (e.g. diabetes and hypertension).78 Likewise, in older patients, especially in combination with intracerebral haemorrhage and dementia, CAA should be considered as a possible differential diagnosis.50 In patients with subarachnoid haemorrhage, cerebral infarctions due to transient cerebral vasospasm can mimic PACNS.
In single cases, the occurrence of PACNS in association with Hodgkin’s lymphoma, non-Hodgkin’s lymphoma and angioimmunolymphoproliferative lesions has been reported.37 Furthermore, intravascular lymphoma (IVL), a subtype of extranodal diffuse large B-cell lymphoma, is an important differential diagnosis of primary CNS vasculitis characterized by intravascular proliferation of lymphoma cells with a predilection for the CNS and skin.79 The differentiation of PACNS from neurosarcoidosis can be difficult as the angiographic pictures share similarities. In these cases, brain biopsy is an essential component of the diagnostic approach.80
VZV vasculopathy is an infectious arteritis that causes ischaemic infarction of the brain and spinal cord, and cerebral haemorrhage, as well as aneurysm and carotid dissection.79,81–83 Diagnosis of VZV infection can be made by detection of anti-VZV IgG antibody in the CSF; amplifiable VZV DNA often cannot be determined.84
In addition, PACNS should be distinguished from other infectious agents that can affect the CNS including hepatitis C virus,85 Streptococcus pneumoniae,86 Neisseria meningitidis,87 Bartonella spp.,88 Mycobacterium tuberculosis,89 Borrelia burgdorferi,90 and Treponema pallidum.91
Special attention is drawn to patients with immunosuppression, for example, due to infection with HIV, diabetes or alcohol abuse. In patients with acquired immune deficiency syndrome-associated CNS disease encephalitis, leptomeningitis, and vasculitis (mostly in cause of opportunistic infections) have been described.92 In patients with immunosuppression, bacterial endocarditis needs to be excluded.8 In bacterial endocarditis, valvular emboli may cause cerebrovascular occlusions and a vasculitic pattern on cerebral angiography.54
Other important mimics of primary angiitis of the CNS are cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), Susac syndrome and thrombophilasis such as antiphospholipid syndrome. CADASIL is an autosomal-dominant arteriopathy with manifestation in early adulthood. Clinical symptoms include migraine, recurrent transient ischaemic attacks or strokes, psychiatric disorders, cognitive impairment, and epilepsy.93 Brain MRI typically shows large or small white matter abnormalities. The most typical MRI findings in CADASIL patients are white matter hyperintensities in the anterior temporal lobe (temporal pole) seen on T2-weighted sequences.94 As an autoimmune-mediated endotheliopathy characterized by the clinical triad of branch retinal artery occlusions, encephalopathy and neurosensorial hearing loss, Susac syndrome causes microangiopathic occlusion of precapillary arterioles in the brain, retina, and inner ear.95 In patients with antiphospholipid antibodies diverse neurologic manifestations including stroke, transient ischaemic attack, seizures, movement disorders, neuropsychiatric manifestations, and cognitive decline with arteriographic intracranial abnormalities suggestive of vasculitis occur.96,97 Beyond that, it can be challenging to discriminate PACNS with small disseminated lesions or T2 hypersignals in the periventricular white matter against the demyelinating lesions of autoimmune diseases such as multiple sclerosis.98,99 Further demyelinating differential diagnoses are acute disseminated encephalomyelitis (ADEM) or progressive multifocal leukoencephalopathy.100,101 In case of early-onset vasculitis, STING-associated vasculopathy with onset in infancy (SAVI) defined as an autoinflammatory disease with vasculopathy, interstitial lung disease, and ulcerative skin lesions caused by sporadic/dominant mutation in the TMEM173 gene, should be considered.102 In addition, deficiency of adenosine deaminase type 2 (DADA2), an autosomal recessive disease, representing with lacunar stroke, vasculitic peripheral neuropathy, livedo racemosa and systemic inflammation is a possible differential diagnosis to PACNS with manifestation in early childhood.103
Therapy
Induction therapy
Treatment recommendations for PACNS are mainly based on retrospective studies and expert opinions. Prospective and randomized treatment trials to define evidence-based strategies for the management of PACNS are lacking so far. Therefore, current therapeutic regimens are adapted from those validated in systemic vasculitis on ground of pathologic concordance. In principle, three treatment approaches are available for PACNS, that is, corticosteroids, immunosuppressants and biologicals (Table 4).
Table 4.
Therapeutic agents used in PACNS.
| Treatment | Regimen |
|---|---|
| Acute therapy | |
| Corticosteroids | oral prednisone at 1 mg/ kg/day or methylprednisolone pulse IV (1000 mg daily for 3–5 days) |
| Cyclophosphamide | daily oral dose (2 mg/kg/day) or by monthly intravenous pulse dose (e.g. starting at 750 mg/m2) |
| Maintenance therapy | |
| Azathioprine | 1–2 mg/kg daily |
| Methotrexate | 20–25 mg/week |
| Mycophenolate mofetil | 1–2 g daily |
| Biological agents | |
| Rituximab | 375 mg/m2/week for 4 weeks or 2 IV doses of 1 g each, administered 2 weeks apart |
| Tumour necrosis factor-α blockers | |
| Infliximab | single IV infusion (5 mg/kg) |
| Etanercept | 25 mg twice weekly for 20 months, then 25 mg/kg, once weekly for 8 months |
IV, intravenous; PACNS, primary angiitis of the central nervous system.
In a retrospective cohort study with 163 patients, induction therapy with combined glucocorticoids and cyclophosphamide has been shown to be equally effective compared with glucocorticoid monotherapy.19 However, combination therapy with cyclophosphamide and glucocorticoids was associated with fewer disease relapses.19 Furthermore, a multicentre cohort study showed that the most patients treated with a combination therapy have a significant reduction in disability, suggesting that an early diagnosis and aggressive treatment might improve outcomes.104 Therefore, in patients with a severe and rapidly progressive disease course, treatment-refractory disease or relapses, a combination with an immunosuppressant should strictly be considered.27
High-dose administration of intravenous corticosteroids (1000 mg daily for 3–5 days) or oral prednisone (1 mg/kg per day) are currently the most frequently used therapies.7,104 To date, no evidence exists that methylprednisolone pulses are more effective than oral prednisone. Overall, intravenous corticosteroids can be preferred in severe and rapidly progressive disease course to induce remission immediately after diagnosis.7 Concomitant gastritis/ulcer and thrombosis prophylaxis are essential. Cyclophosphamide, given either as an oral dose (2 mg/kg/day) for 3–6 months or as intravenous pulse (750 mg/m2/month) for 6 months, is usually recommended in combination with corticosteroids.7 During therapy, cyclophosphamide dosage must be adjusted according to leukocyte nadir and disease course. Furthermore, clinicians should be aware of the most relevant side effects of cyclophosphamide, that is, infection (especially bladder problems), cancer and infertility. In addition, it is contraindicated during pregnancy.105 Prior to beginning a therapy with corticosteroids or immunosuppressants infectious disorders need to be excluded.
Likewise, there are positive data in support of the use of biological agents (rituximab and tumour necrosis factor-α blockers) with an equal potential to induce remission in PACNS patients compared with glucocorticoids and immunosuppressants.106–108 Tumour necrosis factor-α blockers (infliximab and etanercept) and rituximab might be a therapeutic option in those patients who are intolerant to conventional immunosuppressive therapeutic regimens or in patients failing to respond to cyclophosphamide therapy.19 In case of severe and rapidly progressive disease course a combination therapy with cyclophosphamide and rituximab might be considered. Further biological agents, like tocilizumab, are currently being tested in systemic vasculitis.109 However, only a few case series on the implementation of biological agents in PACNS treatment exist and further clinical studies are needed.
Maintenance therapy
While treatment in the induction period aims at achieving remission and avoiding worse outcomes, the goals of maintenance therapies are limiting the risk of relapses and preventing long-term disabilities. PACNS maintenance therapy is typically started 4–6 months after initiation of the induction therapy.7, 53 Disease-modifying therapies include corticosteroid-sparing low-risk immunosuppressive agents such as azathioprine (1–2 mg/kg daily), mycophenolate mofetil (1–2 g daily) and methotrexate (20–25 mg/week).7,54
In a cohort study of fourteen children with SV-PACNS, it was shown that treatment with mycophenolate mofetil for maintenance therapy led to less adverse events compared with azathioprine.110 Comorbidities, side effects and the clinician’s preference are factors influencing therapeutic decisions. In order to compensate for the delayed onset of drug effects of azathioprine, an overlapping therapy with oral prednisone following an initial steroid pulse might be useful. Commonly reported side effects associated with azathioprine are elevated liver enzymes.111
During immunosuppressive therapies, the administration of calcium and vitamin D for osteoporosis prophylaxis, gastric ulcer prophylaxis and Pneumocystis infection prophylaxis are recommended.112 Additionally, supportive therapies such as anticonvulsants in case of seizures, and antipsychotic agents for patients suffering from hallucinations or severe behavioural disorders, should complement the therapeutic regimen.112
The optimal duration of induction and maintenance therapy is a source of uncertainty and validated data are lacking. In principle, induction therapy should be administered for 6–12 months based on individual response to treatment.19 Decisions on de-escalation of induction therapy should depend on the achievement of clinical stability. Repeated clinical and radiological monitoring is needed to determine the ideal duration of maintenance therapy.
The two recent retrospective cohort studies showed associations between the clinical course of PACNS and different disease subtypes (e.g. size of affected vessels) as well as patient characteristics.19,104 Older patients and those with infarctions on the MRI scan had an increased mortality rate.19 Gadolinium-enhanced lesions were associated with an increased survival,19 whereas meningeal gadolinium enhancements on MRI and seizures were associated with an increased risk of relapse.104 Involvement of larger or rather proximal cerebral vessels seems to be associated with a higher mortality rate and poorer prognosis, requiring a more aggressive treatment.7
In view of high morbidity and mortality among PACNS patients,7 adequate treatment monitoring is required. Combination of repeated neurological examinations and periodic neuroradiological imaging (e.g. MRI and MRA) during therapy and afterwards is recommended for assessing disease activity.54 MRI should be performed 4–6 weeks after the initiation of therapy and afterwards every 3–4 months in the first year of treatment.54 CSF examinations can additionally be helpful in the follow up to document improvement in the inflammatory response. For instance, a drop in CSF abnormalities is reported to correspond with clinical improvement.56 In patients with severe clinical course and worsening neurological symptoms, serial conventional angiography might be necessary.54 Colour duplex sonography might be useful for follow-up examinations in patients with cerebral artery stenoses.113
Conclusion
PACNS is a rare inflammatory disease and only little is known about its immunopathogenesis and appropriate treatment. Knowledge regarding clinical, laboratory and imaging findings mainly derives from observational studies. Given the fact, that clinical and neuroimaging features are nonspecific, diagnosing PACNS remains challenging. In LV-PACNS the diagnosis can be confirmed by detection of typical angiographic abnormalities. However, the definite diagnosis in SV-PACNS can only be proven by brain biopsy. Due to a lack of randomized control trials validated treatment regimens do not exist. Therefore, prospectively designed controlled trials are imperative to establish uniform diagnostic criteria and common treatment guidelines. In addition, the implementation of an animal model for further research on PACNS immunopathogenesis and for the development of more targeted therapies could be a promising future perspective.
Acknowledgments
Images are published with the kind permission of the Department of Clinical Radiology of the University Hospital Münster (Director: Univ. Prof. Dr Walter Heindel).
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: CB, AS, DS, and PBS declare no conflict of interest.
HW receives honoraria for acting as a member of Scientific Advisory Boards and as consultant for Biogen, Evgen, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG, Sanofi-Genzyme, as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Sanofi-Genzyme, TEVA, and WebMD Global. Prof. Wiendl is acting as a paid consultant for Abbvie, Actelion, Biogen, IGES, Novartis, Roche, Sanofi-Genzyme, and the Swiss Multiple Sclerosis Society. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster and RE Children’s Foundation, Biogen GmbH, GlaxoSmithKline GmbH, Roche Pharma AG, Sanofi-Genzyme.
SGM has received honoraria for lecturing, travel expenses for attending meetings and financial research support from Almirall, Bayer Health Care, Biogen, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS and Teva.
JM has received grants from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung (BMBF), Else Kröner-Fresenius-Stiftung, EVER Pharma Jena GmbH, Ferrer International, travel grants from Boehringer Ingelheim and speaking fees from Bayer Vital.
Contributor Information
Carolin Beuker, Department of Neurology, University Hospital Münster, Albert-Schweitzer-Campus 1, Münster, D-48149, Germany.
Antje Schmidt, Department of Neurology, University of Münster, Albert-Schweitzer-Campus 1, Münster, Germany.
Daniel Strunk, Department of Neurology, University of Münster, Albert-Schweitzer-Campus 1, Münster, Germany.
Peter B. Sporns, Department of Clinical Radiology, University of Münster, Albert-Schweitzer-Campus 1, Münster, Germany
Heinz Wiendl, Department of Neurology, University of Münster, Albert-Schweitzer-Campus 1, Münster, Germany.
Sven G. Meuth, Department of Neurology, University of Münster, Albert-Schweitzer-Campus 1, Münster, Germany
Jens Minnerup, Department of Neurology, University of Münster, Albert-Schweitzer-Campus 1, Münster, Germany.
References
- 1. Harbitz F. Unknown forms of arteritis, with special reference to their relation to syphilitic arteritis and periarteritis nodosa. Am J Med Sci 1922; 163: 250–271. [Google Scholar]
- 2. Calabrese LH, Mallek JA. Primary angiitis of the central nervous system: report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine 1988; 67: 20–39. [DOI] [PubMed] [Google Scholar]
- 3. Salvarani C, Brown RD, Calamia KT, et al. Primary central nervous system vasculitis: analysis of 101 patients. Ann Neurol 2007; 62: 442–451. [DOI] [PubMed] [Google Scholar]
- 4. Giannini C, Salvarani C, Hunder G, et al. Primary central nervous system vasculitis: pathology and mechanisms. Acta Neuropathol 2012; 123: 759–772. [DOI] [PubMed] [Google Scholar]
- 5. Berlit P, Kraemer M. Cerebral vasculitis in adults: what are the steps in order to establish the diagnosis? Red flags and pitfalls. Clin Exp Immunol 2014; 175: 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferro JM. Vasculitis of the central nervous system. J Neurol 1998; 245: 766–76. [DOI] [PubMed] [Google Scholar]
- 7. Salvarani C, Brown RD, Jr, Christianson T, et al. An update of the Mayo Clinic cohort of patients with adult primary central nervous system vasculitis: description of 163 patients. Medicine 2015; 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol 2009; 66: 704–709. [DOI] [PubMed] [Google Scholar]
- 9. Salvarani C, Brown RD, Calamia KT, et al. Primary CNS vasculitis with spinal cord involvement. Neurology 2008; 70: 2394 [DOI] [PubMed] [Google Scholar]
- 10. Goertz C, Wegner C, Brück W, et al. Primary angiitis of the CNS with pure spinal cord involvement: a case report. J Neurol 2010; 257: 1762–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagel MA, Cohrs RJ, Mahalingam R, et al. The varicella zoster virus vasculopathies. Clinical, CSF, imaging, and virologic features. Neurology 2008; 70: 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas L, Davidson M, McCluskey RT. Studies of PPLO infection. J Exp Med 1966; 123: 897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arthur G, Margolis G. Mycoplasma-like structures in granulomatous angiitis of the central nervous system. Case reports with light and electron microscopic studies. Arch Pathol Lab Med 1977; 101: 382–387. [PubMed] [Google Scholar]
- 14. Iwase T, Ojika K, Mitake S, et al. Involvement of CD45RO+ T lymphocyte infiltration in a patient with primary angiitis of the central nervous system restricted to small vessels. Eur Neurol 2001; 45: 184–185. [DOI] [PubMed] [Google Scholar]
- 15. de Boysson H, Boulouis G, Aouba A, et al. Adult primary angiitis of the central nervous system: isolated small vessel vasculitis represents distinct disease pattern. Rheumatology (Oxford) 2017; 56: 439–444. [DOI] [PubMed] [Google Scholar]
- 16. Giannini C, Salvarani C, Hunder G. Primary central nervous system vasculitis: pathology and mechanisms. Acta Neuropathol 2012; 123: 759–772. [DOI] [PubMed] [Google Scholar]
- 17. Salvarani C, Brown RD, Jr, Calamia KT, et al. Angiography-negative primary central nervous system vasculitis: a syndrome involving small cerebral vessels. Medicine 2008; 87: 264–271. [DOI] [PubMed] [Google Scholar]
- 18. Cellucci T, Tyrrell PN, Sheikh S, et al. Childhood primary angiitis of the central nervous system: identifying disease trajectories and early risk factors for persistently higher disease activity. Arthritis Rheum 2012; 64: 1665–1672. [DOI] [PubMed] [Google Scholar]
- 19. Salvarani C, Brown RD, Christianson TJ, et al. Adult primary central nervous system vasculitis treatment and course: analysis of one hundred sixty-three patients. Arthritis Rheum 2015; 67: 1637–1645. [DOI] [PubMed] [Google Scholar]
- 20. Salvarani C, Brown RD, Jr, Calamia KT, et al. Primary central nervous system vasculitis: comparison of patients with and without cerebral amyloid angiopathy. Rheumatology 2008; 47: 1671–1677. [DOI] [PubMed] [Google Scholar]
- 21. Melzer N, Harder A, Gross CC, et al. CD4+ T cells predominate in cerebrospinal fluid and leptomeningeal and parenchymal infiltrates in cerebral amyloid β–related angiitis. Arch Neurol 2012; 69: 773–777. [DOI] [PubMed] [Google Scholar]
- 22. Salvarani C, Brown RD, Calamia KT, et al. Primary central nervous system vasculitis with prominent leptomeningeal enhancement: a subset with a benign outcome. Arthritis Rheum 2008; 58: 595–603. [DOI] [PubMed] [Google Scholar]
- 23. Salvarani C, Brown RD, Calamia KT, et al. Primary central nervous system vasculitis presenting with intracranial hemorrhage. Arthritis Rheum 2011; 63: 3598–3606. [DOI] [PubMed] [Google Scholar]
- 24. Salvarani C, Brown RD, Jr, Calamia KT, et al. Rapidly progressive primary central nervous system vasculitis. Rheumatology 2010; 50: 349–358. [DOI] [PubMed] [Google Scholar]
- 25. Woolfenden AR, Tong DC, Marks MP, et al. Angiographically defined primary angiitis of the CNS: is it really benign? Neurology 1998; 51: 183–8. [DOI] [PubMed] [Google Scholar]
- 26. Berlit P. Primary angiitis of the CNS–an enigma that needs world-wide efforts to be solved. Eur J Neurol 2009; 16: 10–11. [DOI] [PubMed] [Google Scholar]
- 27. Hajj-Ali RA, Singhal AB, Benseler S, et al. Primary angiitis of the CNS. Lancet Neurol 2011; 10: 561–572. [DOI] [PubMed] [Google Scholar]
- 28. Zuccoli G, Pipitone N, Haldipur A, et al. Imaging findings in primary central nervous system vasculitis. Clin Exp Rheumatol 2011; 29: S104. [PubMed] [Google Scholar]
- 29. Hajj-Ali RA, Calabrese LH. Diagnosis and classification of central nervous system vasculitis. J Autoimmun 2014; 48: 149–152. [DOI] [PubMed] [Google Scholar]
- 30. Eleftheriou D, Cox T, Saunders D, et al. Investigation of childhood central nervous system vasculitis: magnetic resonance angiography versus catheter cerebral angiography. Dev Med Child Neurol 2010; 52: 863–867. [DOI] [PubMed] [Google Scholar]
- 31. Korogi Y, Takahashi M, Nakagawa T, et al. Intracranial vascular stenosis and occlusion: MR angiographic findings. Am J Neuroradiol 1997; 18: 135–143. [PMC free article] [PubMed] [Google Scholar]
- 32. Alhalabi M, Moore PM. Serial angiography in isolated angiitis of the central nervous system. Neurology 1994; 44: 1221–1221. [DOI] [PubMed] [Google Scholar]
- 33. Duna GF, Calabrese LH. Limitations of invasive modalities in the diagnosis of primary angiitis of the central nervous system. J Rheumatol 1995; 22: 662–667. [PubMed] [Google Scholar]
- 34. Alrawi A, Trobe JD, Blaivas M, Musch DC. Brain biopsy in primary angiitis of the central nervous system. Neurology 1999; 53: 858–858. [DOI] [PubMed] [Google Scholar]
- 35. Zuber M. Isolated angiitis of the central nervous system. Uncom Cau Stroke 2001; p.1. [Google Scholar]
- 36. Kadkhodayan Y, Alreshaid A, Moran CJ, et al. Primary angiitis of the central nervous system at conventional angiography. Radiology 2004; 233: 878–882. [DOI] [PubMed] [Google Scholar]
- 37. Calabrese LH, Duna GF, Lie JT. Vasculitis in the central nervous system. Arthritis Rheum 1997; 40: 1189–201. [DOI] [PubMed] [Google Scholar]
- 38. Gomes LJ. The role of imaging in the diagnosis of central nervous system vasculitis. Curr Allergy Asthma Rep 2010; 10: 163–170. [DOI] [PubMed] [Google Scholar]
- 39. Mandell DM, Matouk CC, Farb RI, et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: preliminary results. Stroke 2012; 43: 860–862. [DOI] [PubMed] [Google Scholar]
- 40. Küker W, Gaertner S, Nägele T, et al. Vessel wall contrast enhancement: a diagnostic sign of cerebral vasculitis. Cerebrovasc Dis 2008; 26: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 2009; 72: 627–634. [DOI] [PubMed] [Google Scholar]
- 42. Pugliese F, Gaemperli O, Kinderlerer AR, et al. Imaging of vascular inflammation with [11 C]-PK11195 and positron emission tomography/computed tomography angiography. J Am Coll Cardiol 2010; 56: 653–661. [DOI] [PubMed] [Google Scholar]
- 43. Kraemer M, Berlit P. Primary central nervous system vasculitis: clinical experiences with 21 new European cases. Rheumatol Int 2011; 31: 463–472. [DOI] [PubMed] [Google Scholar]
- 44. Calabrese LH, Furlan AJ, Gragg LA, et al. Primary angiitis of the central nervous system: diagnostic criteria and clinical approach. Cleve Clin J Med 1992; 59: 293–306. [DOI] [PubMed] [Google Scholar]
- 45. Schmidley JW. 10 questions on central nervous system vasculitis. Neurologist 2008; 14: 138. [DOI] [PubMed] [Google Scholar]
- 46. Torres J, Loomis C, Cucchiara B, et al. Diagnostic yield and safety of brain biopsy for suspected primary central nervous system angiitis. Stroke 2016; 47: 2127–2129. [DOI] [PubMed] [Google Scholar]
- 47. Lie JT. Primary (granulomatous) angiitis of the central nervous system: a clinicopathologic analysis of 15 new cases and a review of the literature. Hum Pathol 1992; 23: 164–171. [DOI] [PubMed] [Google Scholar]
- 48. Hall A. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer 1998; 82: 1749–1755. [DOI] [PubMed] [Google Scholar]
- 49. Miller DV, Salvarani C, Hunder GG, et al. Biopsy findings in primary angiitis of the central nervous system. Am J Surg Pathol 2009; 33: 35–43. [DOI] [PubMed] [Google Scholar]
- 50. Scolding NJ, Joseph F, Kirby PA, et al. Aβ-related angiitis: primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain 2005; 128: 500–515. [DOI] [PubMed] [Google Scholar]
- 51. Bajaj BK, Pandey S, Ramanujam B, et al. Primary angiitis of central nervous system: the story of a great masquerader. J Neurosci Rural Pract 2015; 6: 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peter B. Review: diagnosis and treatment of cerebral vasculitis. Ther Adv Neurol Disord 2010; 3: 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Boysson H, Parienti J-J, Arquizan C, et al. Maintenance therapy is associated with better long-term outcomes in adult patients with primary angiitis of the central nervous system. Rheumatology (Oxford) 2017; 56: 1684–1693. [DOI] [PubMed] [Google Scholar]
- 54. Salvarani C, Brown RD, Hunder GG. Adult primary central nervous system vasculitis. Lancet 2012; 380: 767–777. [DOI] [PubMed] [Google Scholar]
- 55. Thom V, Schmid S, Gelderblom M, et al. IL-17 production by CSF lymphocytes as a biomarker for cerebral vasculitis. Neurol Neuroimmunol Neuroinflamm 2016; 3: e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oliveira V, Póvoa P, Costa A, et al. Cerebrospinal fluid and therapy of isolated angiitis of the central nervous system. Stroke 1994; 25: 1693–1695. [DOI] [PubMed] [Google Scholar]
- 57. Calabrese LH, Gragg LA, Furlan AJ. Benign angiopathy: a distinct subset of angiographically defined primary angiitis of the central nervous system. J Rheumatol 1993; 20: 2046–2050. [PubMed] [Google Scholar]
- 58. Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol 2011; 68: 1005–1112. [DOI] [PubMed] [Google Scholar]
- 59. Ducros A, Boukobza M, Porcher R, et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain 2007; 130: 3091–3101. [DOI] [PubMed] [Google Scholar]
- 60. Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol 2012; 11: 906–917. [DOI] [PubMed] [Google Scholar]
- 61. Singhal AB, Topcuoglu MA, Fok JW, et al. Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol 2016; 79: 882–894. [DOI] [PubMed] [Google Scholar]
- 62. Elstner M, Linn J, Müller-Schunk S, et al. Reversible cerebral vasoconstriction syndrome: a complicated clinical course treated with intra-arterial application of nimodipine. Cephalalgia 200; 29: 677–682. [DOI] [PubMed] [Google Scholar]
- 63. Mandell DM, Matouk CC, Farb RI, et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. Stroke 2012; 43: 860–862. [DOI] [PubMed] [Google Scholar]
- 64. Saam T, Habs M, Cyran CC, et al. Neue Aspekte der MRT-Bildgebung zur Diagnostik der Großgefäßvaskulitiden sowie der primären Angiitis des zentralen Nervensystems. Radiologe 2010; 50: 861–871. [DOI] [PubMed] [Google Scholar]
- 65. Hajj-Ali RA, Calabrese LH. Central nervous system vasculitis. Curr Opin Rheumatol 2009; 21: 10–18. [DOI] [PubMed] [Google Scholar]
- 66. Salvarani C, Giannini C, Miller DV, et al. Giant cell arteritis: involvement of intracranial arteries. Arthritis Rheum 2006; 55: 985–989. [DOI] [PubMed] [Google Scholar]
- 67. Ringleb PA, Strittmatter EI, Loewer M, et al. Cerebrovascular manifestations of Takayasu arteritis in Europe. Rheumatology 2005; 44: 1012–1015. [DOI] [PubMed] [Google Scholar]
- 68. Schmidley JW. Central nervous system angiitis. Boston: Butterworth-Heinemann, 2000. [Google Scholar]
- 69. Mirsattari SM, McGinn GJ, Halliday WC. Neuro-Behçet disease with predominant involvement of the brainstem. Neurology 2004; 63: 382–384. [DOI] [PubMed] [Google Scholar]
- 70. Nishino H, Rubino FA, DeRemee RA, et al. Neurological involvement in Wegener’s granulomatosis: an analysis of 324 consecutive patients at the Mayo Clinic. Ann Neurol 1993; 33: 4–9. [DOI] [PubMed] [Google Scholar]
- 71. Sehgal M, Swanson JW, DeRemee RA, et al. Neurologic manifestations of Churg–Strauss syndrome. Mayo Clinic Proc 1995; 70: 337–341. [DOI] [PubMed] [Google Scholar]
- 72. Gonçalves C, Ferreira G, Mota C, et al. Cerebral vasculitis in Henoch–Schönlein purpura. An Pediatr (Barc) 2004; 60: 188–189. [DOI] [PubMed] [Google Scholar]
- 73. Tabarki B, Mahdhaoui A, Selmi H, et al. Kawasaki disease with predominant central nervous system involvement. Pediatr Neurol 2001; 25: 239–241. [DOI] [PubMed] [Google Scholar]
- 74. Kraemer M, Berlit P. Primary central nervous system vasculitis and moyamoya disease: similarities and differences. J Neurol 2010; 257: 816–819. [DOI] [PubMed] [Google Scholar]
- 75. Bersano A, Morbin M, Ciceri E, et al. The diagnostic challenge of Divry van Bogaert and Sneddon Syndrome: report of three cases and literature review. J Neurol Sci 2016; 364: 77–83. [DOI] [PubMed] [Google Scholar]
- 76. Bhattacharyya S, Berkowitz AL. Primary angiitis of the central nervous system: avoiding misdiagnosis and missed diagnosis of a rare disease. Pract Neurol 2016; 16: 195–200. [DOI] [PubMed] [Google Scholar]
- 77. Viana-Baptista M. Stroke and Fabry disease. J Neurol 2012; 259: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 78. Kis B, Liebig T, Berlit P. Severe supraaortal atherosclerotic disease resembling Takayasu’s arteritis. Vasc Health Risk Manag 2007; 3: 351. [PMC free article] [PubMed] [Google Scholar]
- 79. Dutra LA, de Souza AWS, Grinberg-Dias G, et al. Central nervous system vasculitis in adults: an update. Autoimmun Rev 2017; 16: 123–131. [DOI] [PubMed] [Google Scholar]
- 80. Nowak DA, Widenka DC. Neurosarcoidosis: a review of its intracranial manifestation. J Neurol 2001; 248: 363–372. [DOI] [PubMed] [Google Scholar]
- 81. Sigal LH. The neurologic presentation of vasculitic and rheumatologic syndromes: a review. Medicine 1987; 66: 157–180. [DOI] [PubMed] [Google Scholar]
- 82. Kleinschmidt-DeMasters BK, Gilden DH. Varicella-Zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med 2001; 125: 770–780. [DOI] [PubMed] [Google Scholar]
- 83. Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 2009; 8: 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gilden DH, Bennett JL, Kleinschmidt-DeMasters BK, et al. The value of cerebrospinal fluid antiviral antibody in the diagnosis of neurologic disease produced by varicella zoster virus. J Neurol Sci 1998; 159: 140–144. [DOI] [PubMed] [Google Scholar]
- 85. Dawson TM, Starkebaum G. Isolated central nervous system vasculitis associated with hepatitis C infection. J Rheumatol 1999; 26: 2273–2276. [PubMed] [Google Scholar]
- 86. Kastenbauer S, Pfister H-W. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 2003; 126: 1015–1025. [DOI] [PubMed] [Google Scholar]
- 87. Küker W. Cerebral vasculitis: imaging signs revisited. Neuroradiology 2007; 49: 471–479. [DOI] [PubMed] [Google Scholar]
- 88. Marra M. Neurologic complications of Bartonella henselae infection. Curr Opin Neurol 1995; 8: 164–169. [DOI] [PubMed] [Google Scholar]
- 89. Starke JR. Tuberculosis of the central nervous system in children. In: Seminars in pediatric neurology Elsevier, 1999, pp.318–331. [DOI] [PubMed] [Google Scholar]
- 90. Heinrich A, Khaw AV, Ahrens N, et al. Cerebral vasculitis as the only manifestation of Borrelia burgdorferi infection in a 17-year-old patient with basal ganglia infarction. Eur Neurol 2003; 50: 109–112. [DOI] [PubMed] [Google Scholar]
- 91. Kakumani PL, Hajj-Ali RA. A forgotten cause of central nervous system vasculitis. J Rheumatol 2009; 36: 655–655. [DOI] [PubMed] [Google Scholar]
- 92. Mossakowski MJ, Zelman IB. Neuropathological syndromes in the course of full blown acquired immune deficiency syndrome (AIDS) in adults in Poland (1987–1995). Folia Neuropathol 1997; 35: 133–143. [PubMed] [Google Scholar]
- 93. Dichgans M, Mayer M, Uttner I, et al. The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann Neurol 1998; 44: 731–739. [DOI] [PubMed] [Google Scholar]
- 94. Williamson EE, Chukwudelunzu FE, Meschia JF, et al. Distinguishing primary angiitis of the central nervous system from cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy: the importance of family history. Arthritis Rheum 1999; 42: 2243–2248. [DOI] [PubMed] [Google Scholar]
- 95. García-Carrasco M, Mendoza-Pinto C, Cervera R. Diagnosis and classification of Susac syndrome. Autoimmun Rev 2014; 13: 347–350. [DOI] [PubMed] [Google Scholar]
- 96. Rodrigues CEM, Carvalho JF, Shoenfeld Y. Neurological manifestations of antiphospholipid syndrome. Eur J Clin Invest 2010; 40: 350–359. [DOI] [PubMed] [Google Scholar]
- 97. Provenzale JM, Barboriak DP, Allen NB, et al. Antiphospholipid antibodies: findings at arteriography. Am J Neuroradiol 1998; 19: 611–616. [PMC free article] [PubMed] [Google Scholar]
- 98. Scolding N. Can diffusion-weighted imaging improve the diagnosis of CNS vasculitis? Nat Rev Neurol 2007; 3: 608–609. [DOI] [PubMed] [Google Scholar]
- 99. Kis B, Rumberg B, Berlit P. Clinical characteristics of patients with late-onset multiple sclerosis. J Neurol 2008; 255: 697–702. [DOI] [PubMed] [Google Scholar]
- 100. Verhey LH, Branson HM, Laughlin S, et al. Development of a standardized MRI scoring tool for CNS demyelination in children. Am J Neuroradiol 2013; 34: 1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Terrier B, Martinez V, Seilhean D, et al. Progressive multifocal leukoencephalopathy mimicking cerebral vasculitis in systemic granulomatosis. J Infect 2007; 54: 133–135. [DOI] [PubMed] [Google Scholar]
- 102. Liu Y, Jesus AA, Marrero B, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med 2014; 371: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Brogan P, Eleftheriou D. Vasculitis update: pathogenesis and biomarkers. Pediatr Nephrol 2018; 33: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Boysson H, Zuber M, Naggara O, et al. Primary angiitis of the central nervous system: description of the first fifty-two adults enrolled in the french cohort of patients with primary vasculitis of the central nervous system. Arthritis Rheum 2014; 66: 1315–1326. [DOI] [PubMed] [Google Scholar]
- 105. Molloy ES, Langford CA. Advances in the treatment of small vessel vasculitis. Rheum Dis Clin North Am 2006; 32: 157–172. [DOI] [PubMed] [Google Scholar]
- 106. Salvarani C, Brown RD, Calamia KT, et al. Efficacy of tumor necrosis factor α blockade in primary central nervous system vasculitis resistant to immunosuppressive treatment. Arthritis Care Res (Hoboken) 2008; 59: 291–296. [DOI] [PubMed] [Google Scholar]
- 107. Salvarani C, Brown RD, Huston J, et al. Treatment of primary CNS vasculitis with rituximab: case report. Neurology 2014; 82: 1287–1288. [DOI] [PubMed] [Google Scholar]
- 108. De Boysson H, Arquizan C, Guillevin L, et al. Rituximab for primary angiitis of the central nervous system: report of 2 patients from the French COVAC cohort and review of the literature. J Rheumatol 2013; 40: 2102–2103. [DOI] [PubMed] [Google Scholar]
- 109. Addimanda O, Pipitone N, Pazzola G, et al. Tocilizumab for severe refractory neuro-Behçet: three cases IL-6 blockade in neuro-Behçet. In: Seminars in arthritis and rheumatism Elsevier, 2015. pp.472–475. [DOI] [PubMed] [Google Scholar]
- 110. Hutchinson C, Elbers J, Halliday W, et al. Treatment of small vessel primary CNS vasculitis in children: an open-label cohort study. Lancet Neurol 2010; 9: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 111. de Abajo FJ, Montero D, Madurga M, et al. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol 2004; 58: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Alba MA, Espígol-Frigolé G, Prieto-González S, et al. Central nervous system vasculitis: still more questions than answers. Curr Neuropharmacol 2011; 9: 437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hou WH, Liu X, Duan YY, et al. Evaluation of transcranial color-coded duplex sonography for cerebral artery stenosis or occlusion. Cerebrovasc Dis 2009; 27: 479–484. [DOI] [PubMed] [Google Scholar]