Abstract
Purpose:
To evaluate the clinical effectiveness and feasibility of simultaneous integrated boost-proton beam therapy in patients with localized pancreatic cancer.
Methods:
Thirty-seven patients with localized pancreatic cancer underwent simultaneous integrated boost-proton beam therapy, and 8 (21.6%) patients received induction chemotherapy. The internal target volume was obtained by summing the gross tumor volumes in exhalation phase computed tomography images. Planning target volume 1 included internal target volume plus 3 to 5 mm margins, excluding the 5 mm expanded volume of gastrointestinal structures, and planning target volume 2 included the internal target volume plus 7 to 12 mm margins. The prescribed doses to planning target volume 1 and planning target volume 2 were 45 GyE (equivalent dose in 2 Gy, 54.4 GyE10) and 30 GyE (equivalent dose in 2 Gy, 32.5 GyE10) in 10 fractions, respectively.
Results:
Overall, treatment was well tolerated, with no grade of toxicity ≥3. Median overall survival was 19.3 months, and 1-year local progression-free survival, relapse-free survival, and overall survival rates were 64.8%, 33.2%, and 75.7%, respectively. Patients treated with simultaneous integrated boost-proton beam therapy after induction chemotherapy had a significantly higher median overall survival time compared to those with simultaneous integrated boost-proton beam therapy alone (21.6 months vs 16.7 months, P = .031). Multivariate analysis showed that induction chemotherapy was a significant factor for overall survival (P < .05).
Conclusions:
Simultaneous integrated boost-proton beam therapy could be feasible and promising for patients with localized pancreatic cancer.
Keywords: pancreas cancer, proton beam therapy, overall survival
Introduction
At diagnosis, approximately 30% of patients with pancreatic cancer present with locally advanced disease.1 Although chemotherapy and/or radiotherapy (RT) have usually been performed, the role of RT is controversial because of conflicting results from clinical trials over the past decades comparing concurrent chemoradiotherapy (CRT) with chemotherapy alone in these patients.2,3 In the LAP07 trial,3 although CRT after induction chemotherapy did not show a survival benefit compared to chemotherapy alone, CRT resulted in a significantly longer period without treatment (6.1 months vs 3.7 months) and reduction in the local tumor progression (32% vs 46%; P < .05 for each). Additionally, several autopsy studies demonstrated that approximately 30% of patients with pancreatic cancer had no evidence of distant metastases at the time of death.4,5 One population-based study showed that 41% of patients with locally advanced cancer treated with chemotherapy died without evidence of distant metastases.6 These findings suggested that RT could be a valuable treatment option for selected patients with locally advanced disease.
When administering RT for patients with locally advanced disease, conventional fractionated courses of RT with concurrent chemotherapy have been typically used which has been associated with a significant grade 3 or 4 toxicity rate and a median overall survival (OS) of 9 to 15 months.2,3,7,8 With recent advances in RT techniques, intensity-modulated RT (IMRT), stereotactic body RT (SBRT), and proton beam therapy (PBT) can deliver high doses to the tumor as well as minimizing the radiation dose to surrounding normal tissues.9–17 Because of the apparent physical properties of proton beams that can deposit high doses of radiation to the target without an exit dose outside the target, PBT has been attracting attention. Conceptually, PBT has potential advantages of an accelerated RT, known as simultaneous integrated boost (SIB), which different doses can be delivered simultaneously to different targets. That is, higher doses can be delivered to the tumor, while lower doses can be delivered simultaneously to surrounding normal tissues, such as gastrointestinal structures close to the tumor. This accelerated hypofractionated RT can potentially improve the therapeutic ratio compared to conventional fractionated RT because it can reduce radiation damage to surrounding normal tissues, shorten overall treatment time, and avoid the need for prolonged chemotherapy breaks. Since June 2013, SIB-PBT has been applied for patients with localized pancreatic cancer in our institution, and the aim of this study was to evaluate the clinical effectiveness and safety of SIB-PBT in these patients.
Materials and Methods
Patients
Between June 2013 and July 2016, forty-one consecutive patients with pancreatic cancer were treated with SIB-PBT at our institute. Among them, there were 4 patients who had locoregional recurrent disease after surgical resection (n = 2), neuroendocrine carcinoma (n = 1), or distant metastasis (n = 1). The remaining 37 patients were retrospectively analyzed according to the guidelines of National Cancer Center (NCC) institutional review board (NCC20180158), and informed consent was not required because of the retrospective nature of this study.
All patients were given physical examinations, and complete blood count, liver function test, measurement of serum CA 19-9, chest radiography, computed tomography (CT) of the abdomen and pelvis, and/or positron-emission tomography (PET) were performed. All tumors were staged using the American Joint Committee on Cancer, Sixth edition, and were classified as stage cT4 (unresectable disease) based on the CT scans, with tumor extension to the celiac axis or superior mesenteric artery or occlusion of the superior mesenteric–portal venous confluence. Positive lymph node involvement was defined by the presence of a lymph node of at least 1 cm in the short axis, with a spiculated or indistinct border, or with a mottled heterogenic pattern on CT and/or PET scans (n = 35).18
Treatment
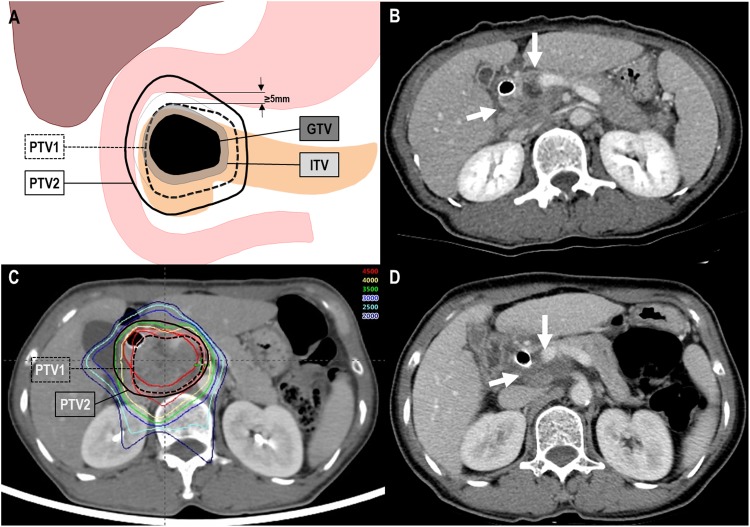
For SIB-PBT planning, contrast-enhanced 4-dimensional CT images, with a 2.5-mm slice thickness, were obtained under shallow respiration while monitoring with a real-time position management (RPM) system (Varian Medical Systems, Palo Alto, California), and the CT images in 10 equally spaced respiratory phases were reconstructed. The average intensity projection (AIP) CT images were reconstructed using the CT images in gated (exhalation) phases (30% of the total respiratory cycle). The sum of contours of organs at risk (OARs) in each CT images during gated phase was delineated in AIP-CT image to account for residual organ motion. All tumors detected in AIP-CT images were defined as the gross tumor volume (GTV), and the sum of the GTVs in each CT images of the gated phases was defined as the internal target volume (ITV). Similar to other studies,10–12 clinical target volume expansion from GTV was not utilized. Planning target volume 1 (PTV1) included the ITV with a margin of 3 to 5 mm in all directions, excluding the 5 mm expanded volume of gastrointestinal structures to avoid gastrointestinal toxicity. The PTV2 included the ITV with a margin of 7 to 12 mm in all directions. The definition of target volumes is illustrated in Figure 1. Planning for SIB-PBT (Eclipse treatment planning system, version 8.1; Varian Medical System) was undertaken using 2 nonplanar or coplanar beams of 230 MeV protons (Proteus 235; Ion Beam Applications, S.A., Louvain-la-Neuve, Belgium) covering the PTV2 and 1 beam covering the PTV1, which was manually selected based on geometrical relationships of PTVs and OARs. The proximal, distal, border smoothing, smearing, and aperture margins of proton beams using the double scattering mode to the PTV were set to 5 to 10 mm each by considering the uncertainties by inter- and intrafractional organs motion. The doses for the target volumes and OARs were expressed in Gray equivalents (GyE = proton physical dose [in Gray] × relative biologic effectiveness [1.1]), and the equivalent dose in 2 Gy fractions (EQD2, GyE10 or GyE3) was calculated using a linear quadratic model with α–β ratios of 10 and 3 for acute and late effects on tumor and OARs, respectively, EQD2 = total dose [GyE, physical dose × 1.1] × (fraction dose [GyE] +10) / (2 + α/β).19 The beam weights of the plan were optimized to maximize the coverage of the target volumes and minimize the maximal doses of OARs, and the SIB-PBT plan was designed with the intent to cover at least 90% of the PTV1 and PTV2 with 100% of each prescribed dose and with minimum and maximum doses of >80% and <110%, respectively. The prescribed doses for PTV1 and PTV2 were 45 GyE (EQD2, 54.4 GyE10) and 30 GyE (EQD2, 32.5 GyE10) in 10 fractions, 5 times a week, respectively (Figure 1). The dose–volume constraints for the normal tissues have been described in our previous reports9,20–23: The maximum dose to the spinal cord were <27 GyE; the absolute volumes of the stomach and esophagus receiving ≥37 GyE were <2 cm3; the absolute volumes of the duodenum and bowel receiving ≥35 GyE were <2 cm3; the relative volumes of the liver receiving ≥27 GyE were below 60%; and the relative volumes of the kidney receiving ≥18 GyE were below 35%. The dose–volumetric parameters for target volumes and OARs are summarized in Supplementary Table 1. To reduce stomach movement and interfractional position problems for all patients, fasting was required at least 4 hours prior to treatment. At each treatment, digital orthogonal fluoroscopy was used to verify each patient’s position and the isocenter, and irradiation was delivered during the exhalation phase using the RPM system.
Figure 1.
Partial response (PR) of a primary tumor to simultaneous integrated boost-proton beam therapy (SIB-PBT). (A) Definition of target volumes depending on the proximity of gastrointestinal structures, (B) pretreatment CT scans showing the primary tumor (arrow), (C) the patient underwent SIB-PBT, and (D) CT scans 3 months after SIB-PBT demonstrating PR of the primary tumor (arrow). CT indicates computed tomography; GTV, gross tumor volume; ITV, internal target volume; PTV, planning target volume.
Of the 37 patients, 8 (21.6%) patients received a median of 4 cycles (range, 2-10) of induction chemotherapy, with 5-fluorouracil (5-FU), irinotecan and oxaliplatin (n = 4), gemcitabine and erlotinib (n = 3), and gemcitabine and cisplatin (n = 1) prior to SIB-PBT. Median interval from the date of the start of induction chemotherapy to SIB-PBT was 4.2 months (range, 1.8-7.1). During SIB-PBT, 31 (83.8%) patients received concurrent chemotherapy, with capecitabine (n = 29) and 5-FU (n = 2); the remaining 6 (16.2%) patients did not receive concurrent chemotherapy due to poor performance status because of advanced age (n = 5) and refusal (n = 1). After completion of SIB-PBT, patients who had resectable disease were considered for surgical resection, whereas patients who still had unresectable disease were considered for maintenance chemotherapy until disease progression or treatment-limiting toxicity. Chemotherapy regimens were chosen according to physician preference, and patients who refused further chemotherapy or had poor performance status received supportive care.
Follow-Up and Statistical Considerations
The assessment of patients was performed weekly during SIB-PBT, 1 month after completion of SIB-PBT, every 2 to 3 months in the first 3 years, and every 6 months thereafter. Follow-up evaluations included a physical examination, complete blood count, liver function tests, measurement of serum CA 19-9, chest X-ray, and abdominopelvic CT scan. The responses of the primary tumor were defined as the maximal tumor response observed during the follow-up period in the absence of a progressive disease, which was assessed according to the Response Evaluation Criteria in Solid Tumours criteria24 by comparing CT scans before and after SIB-PBT. The objective response rates were the sum of the partial response (PR) and complete response (CR) rates. Patients who had PR or CR were defined as “responders,” and those who had stable disease (SD) or progressive disease (PD) were defined as “nonresponders.” Acute hematological and nonhematological toxicities occurring within 3 months of PBT in the absence of disease progression were assessed using the Common Terminology Criteria for Adverse Events (v4.0).
Recurrence was demonstrated by radiological findings, such as increased size with time and/or pathologic findings. Local failure was defined as progression of the primary tumor or recurrence at the primary tumor bed, regional failure was defined as progression or recurrence of disease in regional lymph nodes and soft tissues located near the primary tumor, while distant failure was defined as the development of distant metastasis. The OS, relapse-free survival (RFS), and locoregional progression-free survival (LPFS) were defined as the intervals from the date of start of induction chemotherapy or SIB-PBT (whichever came first) to the date of death or last follow-up, any detection of recurrence, and detection of locoregional progression, respectively. The probabilities of OS were calculated using the Kaplan-Meier method. Univariate analysis of factors related to OS were evaluated using log-rank tests, and multivariate analysis was performed using Cox proportional hazard model with a stepwise forward selection procedure including all variables of P < .1 in univariate analysis. All statistical tests were 2 sided and were performed using STATA software (version 14.0; StataCorp, College Station, Texas). A P value <.05 was considered statistically significant.
Results
Patient characteristics are summarized in Table 1. Clinical T classification was cT3, medically inoperable due to a combination of advanced age (range, 72-87 years) and comorbidities (eg, heart disease, diabetes, and/or chronic renal insufficiency) in 5 (13.5%) patients and cT4 in 32 (86.5%) patients. After completion of SIB-PBT, overall and primary tumor response was as follows: PR in 8 (21.6%) and 14 (37.8%) patients, SD in 17 (45.9%) and 23 (62.2%) patients, and PD in 12 (32.4%) and 0 (0%) patients, respectively (Figure 1). Of the 5 patients with cT3 disease, 3 patients received concurrent chemotherapy with capecitabine, and none of the patients received induction and maintenance chemotherapy. Of the 32 patients with cT4 disease, 2 patients underwent surgical resection with resection margin negative after SIB-PBT (Table 1). After completion of SIB-PBT, 24 patients received maintenance chemotherapy, with gemcitabine (n = 9); gemcitabine and erlotinib (n = 9); tegafur/gemeracil/oteracil (n = 2); 5-FU, irinotecan, and oxaliplatin (n = 2); gemcitabine and nab-paclitaxel (n = 1); and capecitabine (n = 1). The remaining 13 patients did not receive maintenance chemotherapy because of patient refusal (n = 6), advanced age (n = 6), and poor performance status (n = 1; Table 1).
Table 1.
Patient Characteristics.
| Characteristic | Distribution, n (%) |
|---|---|
| Gender | |
| Male | 20 (54.1) |
| Female | 17 (45.9) |
| Age, years | |
| Median (range) | 72 (52-92) |
| <70 | 17 (45.9) |
| ≥70 | 20 (54.1) |
| ECOG PS | |
| 0 | 31 (83.8) |
| 1 | 6 (16.2) |
| Histology | |
| Adenocarcinoma | 37 (100) |
| Tumor location | |
| Head | 23 (62.2) |
| Body/tail | 14 (37.8) |
| Tumor size,a cm | |
| Median (range) | 3.6 (2.0-7.3) |
| <4 | 23 (54.1) |
| ≥4 | 14 (37.8) |
| T classification | |
| T3 | 5 (13.5) |
| T4 | 32 (86.5) |
| N classification | |
| N0 | 34 (91.9) |
| N1 | 3 (8.1) |
| Pretreatment CA 19-9 level, U/mL | |
| Median (range) | 35.2 (2.0-1707) |
| ≤37 | 20 (54.1) |
| >37 | 17 (45.9) |
| Induction chemotherapyb | |
| No | 29 (78.4) |
| Yes | 8 (21.6) |
| Pre-SIB-PBT CA 19-9 level, U/mL | |
| Median (range) | 34.0 (2.0-1707) |
| ≤37 | 21 (56.8) |
| >37 | 16 (43.2) |
| Concurrent chemotherapyc | |
| No | 6 (16.2) |
| Yes | 31 (83.8) |
| Post-SIB-PBT CA 19-9 level, U/mL | |
| Median (range) | 24.3 (2.0-705) |
| ≤37 | 21 (56.8) |
| >37 | 16 (43.2) |
| Post-SIB-PBT surgeryd | |
| No | 35 (94.6) |
| Yes | 2 (5.4) |
| Maintenance chemotherapye | |
| No | 13 (35.1) |
| Yes | 24 (64.9) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; CA 19-9, carbohydrate antigen 19-9; SIB-PBT, simultaneous integrated boost-proton beam therapy.
a Maximum diameter of the primary tumor.
b 5-Flurouracil (5-FU), irinotecan, plus oxaloplatin (FOLFINOX; n = 4), gemcitabine plus erlotinib (GT; n = 3), and gemcitabine plus cisplatin (GP; n = 1).
c Capectabine (n = 29) and 5-FU (n = 2).
d Pylorus-preserving pancreaticoduodenectomy (n = 1) and distal pancreatectomy (n = 1).
e Gemcitabine (n = 9), GT (n = 9), tegafur/gemeracil/oteracil (TS-1; n = 2), FOLFINOX (n = 2), gemcitabine plus nab-paclitaxel (n = 1), and capecitabine (n = 1).
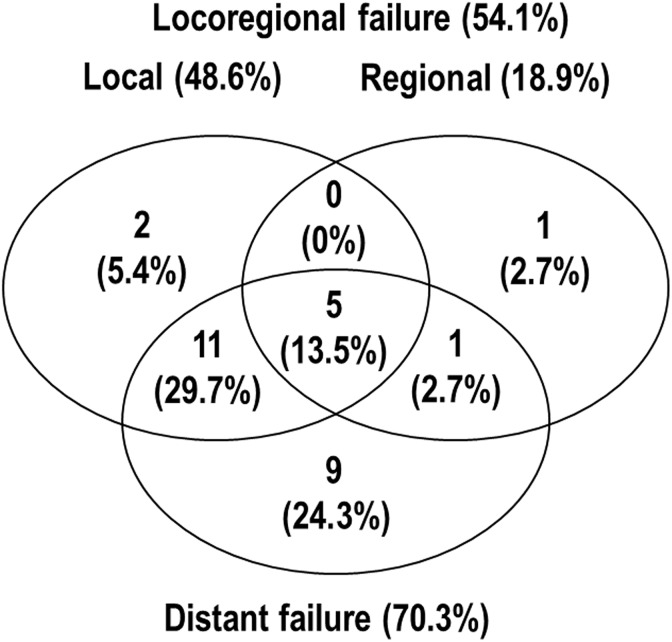
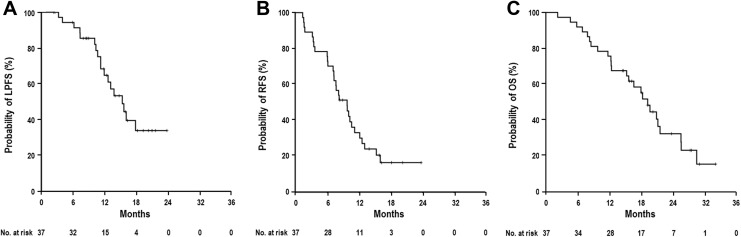
At the time of analysis, 25 patients had died from the disease and 12 remained alive. The median follow-up period was 16.7 months (range, 2.3-32.1 months) for all patients and 19.8 months (range, 14.5-32.1 months) for living patients. Of the 37 patients, 29 (75.6%) developed disease progression, including 18 (48.6%) with local progression, 7 (18.9%) with regional progression, and 26 (70.3%) with distant metastases (Figure 2). Two patients who received surgical resection remained alive at 18.2 months and 27.5 months after SIB-PBT, with regional recurrence at 10 months after surgical resection (n = 1) and controlled disease (n = 1), respectively. The median times of LPFS, RFS, and OS in all patients were 15.3 months (95% confidence interval [CI], 11.6-19.0 months), 9.8 months (95% CI, 7.1-12.4 months), and 19.3 months (95% CI, 16.5-22 months), respectively, and the actuarial 1-year LPFS, RFS, and OS rates were 64.8% (95% CI, 47.7%-81.9%), 33.2% (95% CI, 17.5%-48.9%), and 75.7% (95% CI, 61.8%-89.6%), respectively (Figure 3).
Figure 2.
Patterns of treatment failure in all patients.
Figure 3.
Locoregional progression-free survival (LPFS; A), relapse-free survival (RFS; B), and overall survival (OS; C) curves in all patients.
Univariate and multivariate analyses were performed to identify parameters predicting OS (Table 2). Univariate analysis demonstrated that induction chemotherapy, concurrent chemotherapy, and maintenance chemotherapy were significantly associated with OS (P < .05; Table 2). The patients who received surgical resection had a trend toward higher OS (remained alive at 18.2 and 27.5 months after SIB-PBT) than those who did not receive surgical resection (median, 18.3 months), but the difference was not significant due to the small number of patients who received surgical resection (n = 2; P = .139). Patient age (<70 years vs ≥ 70 years) showed marginal associations with OS (25.6 months vs 16.7 months, P = .066), whereas none of the other factors was significantly associated with OS (P > .05; Table 2). In multivariate analysis, induction chemotherapy was a significant factor independently associated with OS (P < .05; Table 2). Median OS time from SIB-PBT showed higher trend in the patients who received induction chemotherapy than those who did not (19 months vs 16.7 months), but the difference was not significant (P = 0.299).
Table 2.
Univariate Analysis of Clinical Characteristics Associated With Overall Survival (OS).a
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Characteristic | OS, Median (95% CI), months | P Valueb | Hazard Ratio (95% CI) | P Valuec |
| Gender | ||||
| Male | 20.9 (9.0-32.8) | .286 | – | – |
| Female | 18.3 (14.6-22.0) | – | ||
| Age, years | ||||
| <70 | 25.6 (13.2-32.9) | .066 | – | – |
| ≥70 | 16.7 (12.0-21.3) | – | ||
| ECOG PS | ||||
| 0 | 19.3 (15.5-23.0) | .746 | – | – |
| 1 | 20.9 (-) | – | ||
| Tumor location | ||||
| Head | 18.0 (14.0-22.0) | .122 | – | – |
| Body/tail | 20.9 (18.1-23.7) | – | ||
| Tumor size,b cm | ||||
| <4 | 18.0 (14.2-21.8) | .259 | – | – |
| ≥4 | 20.9 (17.7-24.1) | – | ||
| T classification | ||||
| T3 | 15.7 (4.2-27.2) | .156 | – | – |
| T4 | 19.7 (16.4-23.0) | – | ||
| N classification | ||||
| N0 | 19.3 (15.8-22.8) | .390 | – | – |
| N1 | 25.6 (4.4-46.8) | – | ||
| Pretreatment CA 19-9 level, U/mL | ||||
| ≤37 | 16.7 (8.3-25.0) | .749 | – | – |
| >37 | 19.7 (14.8-24.4) | – | ||
| Induction chemotherapy | ||||
| No | 16.7 (13.5-19.8) | .031 | 1.000 | .040 |
| Yes | 21.6 (-) | 0.317 (0.106-0.949) | ||
| Pre-SIB-PBT CA 19-9 level, U/mL | ||||
| ≤37 | 19.3 (12.6-25.9) | .719 | – | – |
| >37 | 19.7 (15.6-23.8) | – | ||
| Concurrent chemotherapy | ||||
| No | 8.3 (2.9-13.7) | .036 | – | – |
| Yes | 20.9 (16.8-25.1) | – | ||
| Post-SIB-PBT CA 19-9 level, U/mL | ||||
| ≤37 | 19.3 (14.3-24.3) | .541 | – | – |
| >37 | 21.6 (15.8-27.4) | – | ||
| Primary tumor response | ||||
| Responder | 21.2 (19.3-23.1) | .432 | – | – |
| Nonresponder | 16.7 (12.6-20.7) | – | ||
| Post-SIB-PBT surgery | ||||
| No | 18.3 (12.2-21.6) | .139 | – | – |
| Yes | NR (-) | – | ||
| Maintenance chemotherapy | ||||
| No | 15.7 (8.2-23.2) | .044 | – | – |
| Yes | 21.2 (17.4-25.0) | – | ||
Abbreviations:CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; CA 19-9, carbohydrate antigen 19-9; NR, not reached; SIB-PBT, simultaneous integrated boost-proton beam therapy.
a Responder denotes complete or partial response and nonresponder denotes stable disease or progressive disease.
b Log-rank test.
c Cox proportional hazards model.
During SIB-PBT, acute toxicities were transient, easily manageable, and caused no interruption in the treatment course, and the details of the distribution of acute toxicities are summarized in Table 3. The most common toxicities were grade 1 anemia (32.4%), grade 1 leukopenia (21.6%), and grade 1 abdominal pain (16.2%), and no cases of grade ≥3 acute toxicity were detected. No late radiation toxicities of grade ≥3, such as gastrointestinal bleeding or duodenal ulcer, were observed.
Table 3.
Acute toxicities During Simultaneous Integrated Boost-Proton Beam Therapy.a
| Type of Toxic Effectb | Grade 0, n (%) | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Grade 5, n (%) |
|---|---|---|---|---|---|---|
| Hematologic toxicity | ||||||
| Leukopenia | 28 (75.7) | 8 (21.6) | 1 (2.7) | 0 (0) | 0 (0) | 0 (0) |
| Anemia | 22 (59.4) | 12 (32.4) | 3 (8.1) | 0 (0) | 0 (0) | 0 (0) |
| Thrombocytopenia | 36 (97,3) | 1 (2.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nonhematologic toxicity | ||||||
| Hand-foot syndrome | 37 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Anorexia | 30 (81.1) | 4 (10.8) | 3 (8.1) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 32 (86.5) | 3 (8.1) | 2 (5.4) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhoea | 37 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Abdominal pain | 31 (83.8) | 6 (16.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Stomatitis | 35 (94.6) | 1 (2.7) | 1 (2.7) | 0 (0) | 0 (0) | 0 (0) |
a Some patients experienced more than 1 toxicity.
b National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Discussion
In patients with locally advanced pancreatic cancer, the role of RT has been disputed because of conflicting results with regard to OS benefit, whether CRT was added or chemotherapy alone. In addition, there was the concern of significant high toxicity following CRT.2,3,7,8 In particular, the Eastern Cooperative Oncology Group 4201 trial demonstrated a 77% incidence of grade ≥3 toxicity in patients with locally advanced disease receiving RT with concurrent gemcitabine. These previous trials used conventionally fractionated RT with large RT volume, including elective nodal irradiation.2,3,7 Recently, conventional or hypofractionated IMRT with 5 to 39 fractions or SBRT with 1 to 8 fractions, using a limited RT volume without elective nodal irradiation, has been used to improve the sparing of surrounding normal structures while increasing the dose to the target volume. This treatment has resulted in median OS of 6 to 20 months and grade ≥3 toxicity of 0% to 26%.9–15,25 Several studies have showed a dosimetric superiority of PBT compared to RT with X-ray, including IMRT, for the delivery of radiation dose to the target while significantly reducing exposure to surrounding normal tissues and the dosimetric feasibility of hypofractionated PBT for pancreatic cancer.26,27 Terashima et al 14 reported on the outcomes for 50 patients with pancreatic cancer with locally advanced disease treated with PBT, with 50 to 70.2 GyE in 25 to 26 fractions, and concurrent gemcitabine. They found promising outcomes in terms of 1-year LPFS, RFS, and OS rates of 81.7%, 64.3%, and 76.8%, respectively, and grade ≥3 toxicity of 10%. In addition, Nichols et al 15 analyzed 22 patients with pancreatic and ampullary cancer who were treated with PBT, 50 to 59.4 GyE in 28 to 33 fractions, and concurrent capecitabine. They reported a favorable toxicity profile including no grade ≥3 gastrointestinal toxicity. In the present study, we applied SIB-PBT, prescribed 45 or 30 GyE in 10 fractions to target volumes depending on the closeness of the gastrointestinal structures, and observed a median OS of 19.7 months for patients with locally advanced disease (ie, cT4) and no grade ≥3 toxicity. Direct comparison of data among previous studies and those of our present study is difficult due to heterogeneous baseline characteristics, particularly in performance status and tumor burden (ie, tumor size, lymph node involvement) and the various agents and sequence of chemotherapy administered. However, the median OS and incidence of grade ≥3 toxicity in the present study were at the higher and lower end of the wide range reported previously, respectively.9–15,25
Due to the high propensity of patients with locally advanced disease to develop distant metastases, induction chemotherapy before CRT has been proposed to select the subset of patients who will benefit from RT.28–30 Although several retrospective studies28,29 showed an OS benefit of CRT after induction chemotherapy compared to chemotherapy alone, the recent randomized LAP07 trial3 did not show an additional benefit by adding CRT after induction chemotherapy. However, the LAP07 trial demonstrated a significant decrease in the rate of local progression using CRT after induction chemotherapy compared to chemotherapy alone, and recent cohort studies showed an OS benefit of CRT after induction chemotherapy compared to chemotherapy alone.31,32 In the present study, the patients treated with SIB-PBT after induction chemotherapy had a significantly higher median OS time from commencement of treatment and higher trend in median OS time from SIB-PBT compared to those treated with SIB-PBT alone (21.6 months vs 16.7 months, P = .031, and 19 months vs 16.7 months, P = .299, respectively; Table 2). Although the study population was small and did not include those patients who were treated with chemotherapy alone, these findings implied that induction chemotherapy may help to select the patients who benefit from RT.
Chemotherapy and/or RT are often the treatment of choice for patients with medically inoperable disease, and its outcomes have been reported. Low-dose gemcitabine may improve survival compared to best supportive care in unresectable disease (median OS, 7.6 months vs 2.3 months, P < .05),33 and chemotherapy and/or RT has shown a median OS time of 8.6 to 12.2 months in medically inoperable disease.34,35 Recently, SBRT has been tried in medically inoperable disease based on its promising outcomes, in terms of high local control with minimal toxicity; it was used in unresectable disease, and it showed a median OS time of 6.4 to 7.6 months.36,37 To date, the question remains of how best to manage these patients who cannot tolerate aggressive treatments, such as surgery, chemotherapy, and/or a conventional course of RT. In the present study, SIB-PBT showed a median OS time of 15.7 months in medially inoperable disease. Although the number of these patients was small (n = 5), outcomes of SIB-PBT for patients with medically inoperable disease were promising.
This study was retrospective and thus had certain inherent limitations. First, our data were from a single institutional study with a relatively small and heterogeneous population, which included patients with medically inoperable and locally advanced disease and had the heterogeneity of various chemotherapeutic agents and sequence; thus, the effects of systemic chemotherapy and probable selection bias were not thoroughly evaluated. Second, the assessment of toxicity in a retrospective analysis inherently underestimates risks owing to incomplete reporting of side effects in clinic notes, recall bias, and lack of continuity of follow-up at 1 institution to capture all possible adverse events. Despite these study limitations, SIB-PBT with 10 factions offers the advantage of delivering RT over 2 weeks and thereby minimizes the delay in administration of chemotherapy. In addition, due to superior dose localization of the proton beams to the target than is achieved by X-ray, SIB-PBT can potentially result in minimizing RT dose to surrounding gastrointestinal structures. In the present study, the median OS in the patients with localized inoperable disease treated with SIB-PBT was 19.3 months, and grade ≥3 toxicity was not observed. However, because of the aforementioned limitations and lack of comparison of RT with X-ray, such as IMRT and SBRT, further large-scale prospective studies are warranted, including combinations of SIB-PBT and modern systemic chemotherapy regimens, such as 5-FU, irinotecan plus oxaliplatin, and gemcitabine plus nab-paclitaxel,38,39 and/or dose escalation using SIB-PBT techniques.
In conclusion, we found that SIB-PBT for patients with localized pancreatic cancer showed promising results, including a median OS time of 19.3 months and no grade ≥3 toxicity. Because we could not analyze subgroups in detail due to the relatively small number of the study population and heterogeneity of pre- and post-RT treatments, further larger scaled prospective studies are warranted. However, our data suggest that SIB-PBT could be a feasible and promising component of combination therapy for these patients.
Supplementary Material
Abbreviations
- AIP
average intensity projection
- CI
confidence interval
- CR
complete response
- CRT
concurrent chemoradiotherapy
- CT
computed tomography
- CTCAE
common terminology criteria for adverse events
- GTV
gross tumor volume
- GyE
Gray equivalent
- IMRT
intensity-modulated radiotherapy
- ITV
internal target volume
- LPFS
locoregional progression-free survival
- OARs
organs at risk
- OS
overall survival
- PBT
proton beam therapy
- PD
progressive disease
- PET
positron-emission tomography
- PR
partial response
- PTV
planning target volume
- RFS
relapse-free survival
- RPM
real-time position management
- RT
radiotherapy
- SBRT
stereotactic body RT
- SD
stable disease
- SIB
simultaneous integrated boost.
Footnotes
Authors’ Note: THK, WJL, DYK, and SMW are responsible for the study design. THK, WJL, SMW, HJK, ESO, JHL, SSH, SJP, YKS, SHM, SSK, and DYK collected the clinical data and drafted the manuscript. THK, WJL, SMW, SSH, SJP, ESO, HJK, and DYK were responsible for the treatment and evaluation of the patients. SSH, SJP, SHM, SSK, DYK, and THK provided oversight of the analysis of data and reviewing of the manuscript. All authors read and approved the final manuscript. THK, WJL, and SMW contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Cancer Center Grant (NCC 1710060 and 1710030). The funding source had no role in study design, data collection, analysis or interpretation of data.
ORCID iD: Tae Hyun Kim, MD  http://orcid.org/0000-0001-8413-3385
http://orcid.org/0000-0001-8413-3385
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189(1):1–7. [DOI] [PubMed] [Google Scholar]
- 2. Loehrer PJ, Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29(31):4105–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–1853. [DOI] [PubMed] [Google Scholar]
- 4. Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamisawa T, Isawa T, Koike M, Tsuruta K, Okamoto A. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas. 1995;11(4):345–349. [DOI] [PubMed] [Google Scholar]
- 6. Peixoto RD, Speers C, McGahan CE, Renouf DJ, Schaeffer DF, Kennecke HF. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med. 2015;4(8):1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14(4):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80(10):751–755. [PubMed] [Google Scholar]
- 9. Woo SM, Kim MK, Joo J, et al. Induction chemotherapy with gemcitabine and cisplatin followed by simultaneous integrated boost-intensity modulated radiotherapy with concurrent gemcitabine for locally advanced unresectable pancreatic cancer: results from a feasibility study. Cancer Res Treat. 2017;49(4):1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badiyan SN, Olsen JR, Lee AY, et al. Induction chemotherapy followed by concurrent full-dose gemcitabine and intensity-modulated radiation therapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2016;39(1):1–7. [DOI] [PubMed] [Google Scholar]
- 11. Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84(5):1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86(3):516–522. [DOI] [PubMed] [Google Scholar]
- 13. Maemura K, Mataki Y, Kurahara H, et al. Comparison of proton beam radiotherapy and hyper-fractionated accelerated chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology. 2017;17(5):833–838. [DOI] [PubMed] [Google Scholar]
- 14. Terashima K, Demizu Y, Hashimoto N, et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother Oncol. 2012;103(1):25–31. [DOI] [PubMed] [Google Scholar]
- 15. Nichols RC, Jr, George TJ, Zaiden RA, Jr, et al. Proton therapy with concomitant capecitabine for pancreatic and ampullary cancers is associated with a low incidence of gastrointestinal toxicity. Acta Oncol. 2013;52(3):498–505. [DOI] [PubMed] [Google Scholar]
- 16. Bae JS, Kim DH, Kim WT, Kim YH, Park D, Ki YK. The role of surgical clips in the evaluation of interfractional uncertainty for treatment of hepatobiliary and pancreatic cancer with postoperative radiotherapy. Radiat Oncol J. 2017;35(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avanzo M, Chiovati P, Boz G, Sartor G, Dozza F, Capra E. Image-guided volumetric arc radiotherapy of pancreatic cancer with simultaneous integrated boost: optimization strategies and dosimetric results. Phys Med. 2016;32(1):169–175. [DOI] [PubMed] [Google Scholar]
- 18. Roche CJ, Hughes ML, Garvey CJ, et al. CT and pathologic assessment of prospective nodal staging in patients with ductal adenocarcinoma of the head of the pancreas. AJR Am J Roentgenol. 2003;180(2):475–480. [DOI] [PubMed] [Google Scholar]
- 19. Joiner MC, Bentzen SM. Time–dose relationships: the linear-quadrantic approach In: Steel GG, ed. Basic Clinical Radiobiology. 3rd ed New York: Oxford University Press; 2003:121–133. [Google Scholar]
- 20. Kim DY, Park JW, Kim TH, et al. Risk-adapted simultaneous integrated boost-proton beam therapy (SIB-PBT) for advanced hepatocellular carcinoma with tumour vascular thrombosis. Radiother Oncol. 2017;122(1):122–129. [DOI] [PubMed] [Google Scholar]
- 21. Kim TH, Park JW, Kim YJ, et al. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res Treat. 2015;47(1):34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim TH, Park JW, Kim YJ, et al. Simultaneous integrated boost-intensity modulated radiation therapy for inoperable hepatocellular carcinoma. Strahlenther Onkol. 2014;190(10):882–890. [DOI] [PubMed] [Google Scholar]
- 23. Lee SU, Park JW, Kim TH, et al. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol. 2014;190(9):806–814. [DOI] [PubMed] [Google Scholar]
- 24. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 25. Park JJ, Hajj C, Reyngold M, et al. Stereotactic body radiation vs. intensity-modulated radiation for unresectable pancreatic cancer. Acta Oncol. 2017;56(12):1746–1753. [DOI] [PubMed] [Google Scholar]
- 26. Bouchard M, Amos RA, Briere TM, Beddar S, Crane CH. Dose escalation with proton or photon radiation treatment for pancreatic cancer. Radiother Oncol. 2009;92(2):238–243. [DOI] [PubMed] [Google Scholar]
- 27. Kozak KR, Kachnic LA, Adams J, et al. Dosimetric feasibility of hypofractionated proton radiotherapy for neoadjuvant pancreatic cancer treatment. Int J Radiat Oncol Biol Phys. 2007;68(5):1557–1566. [DOI] [PubMed] [Google Scholar]
- 28. Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25(3):326–331. [DOI] [PubMed] [Google Scholar]
- 29. Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110(1):47–55. [DOI] [PubMed] [Google Scholar]
- 30. Yoo T, Lee WJ, Woo SM, et al. Pretreatment carbohydrate antigen 19-9 level indicates tumor response, early distant metastasis, overall survival, and therapeutic selection in localized and unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e623–e630. [DOI] [PubMed] [Google Scholar]
- 31. Faisal F, Tsai HL, Blackford A, et al. Longer course of induction chemotherapy followed by chemoradiation favors better survival outcomes for patients with locally advanced pancreatic cancer. Am J Clin Oncol. 2016;39(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Torgeson A, Lloyd S, Boothe D, et al. Multiagent induction chemotherapy followed by chemoradiation is associated with improved survival in locally advanced pancreatic cancer. Cancer. 2017;123(19):3816–3824. [DOI] [PubMed] [Google Scholar]
- 33. Matsumoto K, Miyake Y, Kato H, et al. Effect of low-dose gemcitabine on unresectable pancreatic cancer in elderly patients. Digestion. 2011;84(3):230–235. [DOI] [PubMed] [Google Scholar]
- 34. Miyamoto DT, Mamon HJ, Ryan DP, et al. Outcomes and tolerability of chemoradiation therapy for pancreatic cancer patients aged 75 years or older. Int J Radiat Oncol Biol Phys. 2010;77(4):1171–1177. [DOI] [PubMed] [Google Scholar]
- 35. Tas F, Sen F, Odabas H, Kilic L, Keskin S, Yildiz I. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol. 2013;18(5):839–846. [DOI] [PubMed] [Google Scholar]
- 36. Kim CH, Ling DC, Wegner RE, et al. Stereotactic body radiotherapy in the treatment of pancreatic adenocarcinoma in elderly patients. Radiat Oncol. 2013;8:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yechieli RL, Robbins JR, Mahan M, Siddiqui F, Ajlouni M. Stereotactic body radiotherapy for elderly patients with medically inoperable pancreatic cancer. Am J Clin Oncol. 2017;40(1):22–26. [DOI] [PubMed] [Google Scholar]
- 38. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. [DOI] [PubMed] [Google Scholar]
- 39. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.