Abstract
Background:
Although step counters are popularly employed for physical rehabilitation in chronic obstructive pulmonary disease (COPD) patients, their effectiveness is inconsistent and even questioned. This meta-analysis aimed to investigate whether step counter use increases physical activity or improves exercise capacity in COPD patients.
Methods:
Electronic databases were searched for randomized controlled trials that assessed the efficacy of step counter use in increasing physical activity or in improving exercise capacity. Data were aggregated using a random-effects model to get the overall effect sizes [standard mean difference (SMD) with 95% confidence interval (CI)], and subgroup analyses were performed.
Results:
A total of 15 trials enrolling 1316 patients with moderate to severe COPD were included. Step counter use increased physical activity compared with controls (SMD = 0.57, 95% CI 0.31–0.84), which is equal to a magnitude of 1026 steps/day in daily steps. It also enhanced exercise capacity with an effect size of 0.30 (95% CI 0.16–0.45), approximating to a magnitude of 11.6 m in the 6-min walking distance. Step counter use could augment physical activity (SMD = 0.64, 95% CI 0.19–1.08) and exercise capacity (SMD = 0.32, 95% CI 0.01–0.62) for patients receiving pulmonary rehabilitation. Yet it cannot enhance physical activity or exercise capacity in patients with severe COPD or among studies with intervention durations ⩾6 months (both p > 0.50).
Conclusions:
Step counter use increases physical activity and improves exercise capacity in COPD patients, at least in the short term, which supports the notion of recommending step counter use in COPD management.
Keywords: chronic obstructive pulmonary disease, exercise capacity, physical activity, step counter
Introduction
Physical activity remains a cornerstone in the management of chronic obstructive pulmonary disease (COPD).1,2 Emerging evidence has suggested that increased physical activity is correlated with low levels of airway obstruction and systemic inflammation,3 but also associated with reduced risks of hospital admission and all-cause mortality in patients with COPD.4–6 However, some studies have pointed out that most patients with COPD show significantly lower levels of physical activity on a daily basis compared with healthy controls even at relatively early stages of disease.3,7,8 Moreover, physical inactivity may deteriorate the exercise capacity of patients with COPD and worsen their disease severity progressively, leading to a subsequently further enlarged reduction in physical activity.9,10 As a result, efficacious approaches aimed at enhancing physical activity for patients with COPD are needed.
Step counters, like pedometers and accelerometers, are inexpensive but smart and simple wearable devices that can be used to monitor physical activity by counting the number of steps walked daily.11 Since they are also able to provide real-time feedback to individuals about their physical activity for possible behavioral reinforcement (e.g. walking more), step counters have experienced a surge in popularity recently 12 and have been emerged as essential components in walking programs for health promotion, in particular for patients with chronic diseases such as type 2 diabetes or COPD.13–30 Previous meta-analyses have highlighted the efficacy of step counters in increasing physical activity among patients with type 2 diabetes.31,32 However, it remains unclear and even doubtful whether there is a similar effect for patients with COPD, especially for those attending pulmonary rehabilitation (PR) programs,28 since individual studies provided inconsistent findings,13–21,25–28 with some showing significantly increased physical activity 14,25,26 but others showing nonsignificant outcomes.15,20,28 Moreover, there exists some heterogeneity regarding the use of step counters in patients with COPD (e.g. differences in intervention durations or diary use),18,19,28 while it remains unknown whether they were crucial for the observed changes in physical activity.
Therefore, the primary aim of this meta-analysis was to evaluate the effectiveness of step counter use on physical activity in patients with COPD and to establish the potential moderators for predicting changes in physical activity. In addition, since reduced exercise capacity may reflect the inability of performing physical tasks and is closely related to physical activity levels,3,33 the secondary aim was to determine whether step counter use would also improve the exercise capacity of patients.
Materials and methods
Data sources and search strategy
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline,34 and was registered in PROSPERO as CRD42017077223. A comprehensive literature search up to May 2018 was performed in databases including PubMed, Cochrane Library, and Web of Science using terms associated with ‘step counter’ and ‘chronic obstructive pulmonary disease’ (Supplementary Table S1). All bibliographic details of retrieved articles were stored in a reference-managing software named EndNote (version X7, Thomson Reuters, New York, NY, USA). In addition, a hand search of the bibliographic references of the included or relevant articles was conducted to seek for other potentially eligible studies.
Inclusion criteria
Studies published in English were included if they fulfilled the following criteria: (1) enrolled only adults (mean age >18 years) with COPD diagnosed according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria; (2) received interventions using step counters as a key component for physical activity promotion with minimum durations of 4 weeks; (3) compared with controls receiving no step counter-related intervention or utilizing step counters simply for recording daily steps; (4) provided data on physical activity objectively measured using pedometers or accelerometers (including multisensors), or on exercise capacity assessed by the 6-min walking distance (6MWD); and (5) were randomized controlled trials (RCTs). Studies were excluded if they enrolled a mixed population with chronic respiratory disease, failed to allow for the investigation of the impact of step counter use on outcomes of interest, or used step counters only for recording steps in intervention groups. Studies were also excluded if they were review articles, editorials, or congress abstracts.
Data extraction
After removing the duplicates and based on the inclusion criteria, two authors (SQ and XC) initially screened the title and abstract of each article, and then evaluated the full texts for the final selection in an unblinded manner. Any possible disagreement between the two authors during the study selection process was discussed with a third author (US) for resolution. If studies reported outcomes with different intervention durations, data from the longest study were included in the primary analysis.35 When a study provided data based on intention-to-treat and per-protocol analyses, priorities of selection were given to the data from the intention-to-treat analyses.36
For each eligible study, a predesigned standardized Excel (Microsoft, USA) form was used to collect data by one author (SQ) on the following items: author information (including the name of first author and the year of publication), participant characteristics [including origin of country, baseline mean age and body mass index (BMI), sex distribution, baseline mean steps/day and 6MWD, the presence of comorbidity, and lung function assessed by forced expiratory volume in 1s (FEV1)%pred], intervention details (including intervention duration, step goal setting, use of diary recording, application of internet or mobile phone), outcome variables (physical activity, exercise capacity, and the related assessment approaches/tools). These data were then rechecked by another author (XC). The details for data abstraction are shown in Supplementary Table S2.
Statistical analysis and quality assessment
All statistical analyses were performed using Stata Software (version 11.0, College Station, TX, USA). Change scores with the corresponding standard deviation (SD) for the outcome of interest were used in the meta-analysis to obtain the overall effect size represented by standard mean difference (SMD) with 95% confidence interval (CI) given the different measurement approaches/tools, with a threshold p < 0.05 considered as significant.36 The reason for choosing a random-effects model rather than a fixed-effects model was due to the differences in the intervention details and also because the former model provides more conservative outcomes and better accounts for heterogeneity than the latter.36
The Cochrane Collaboration’s tool was employed to assess the methodological quality of each study by two authors (SQ and XC) on the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.36,37 For each of these domains, it was rated as a ‘low’, ‘high’, or ‘unclear’ risk of bias (Supplementary Table S3).
Heterogeneity in this meta-analysis was assessed by I2 value, with a value ⩾50% indicative of significant heterogeneity.36 In order to address the potential sources of heterogeneity, subgroup and meta-regression analyses were performed based on the participant and intervention characteristics aforementioned, which mainly included baseline mean values of age, BMI, disease severity, physical activity, and exercise capacity, gender distribution, intervention duration, step goal setting, diary use, internet or mobile phone use, and outcome assessment approaches/tools. Moderator effects were considered statistically significant at p < 0.05. Sensitivity analysis by restricting studies using intention-to-treat analysis was conducted to assess the robustness of the summary effect size. Publication bias was assessed by Egger’s test, with p value <0.10 deemed apparent publication bias.
Results
Characteristics of patients and step counter interventions
The search strategy resulted in a total of 2406 potentially relevant articles. After removing duplicates and screening on titles/abstracts, 66 articles remained for the full text assessment. After further excluding 51 of them based on the reasons specified in Figure 1, which included no regular use of step counters,30,38 14 were considered eligible for inclusion.13–21,25–29 Yet because two articles enrolled the same groups of patients but with different follow-up periods,18,19 which were then treated as 1 RCT,19 and one article provided three different comparisons,13 resulting in 3 separate RCTs, a total of 15 RCTs were finally included in this meta-analysis.
Figure 1.
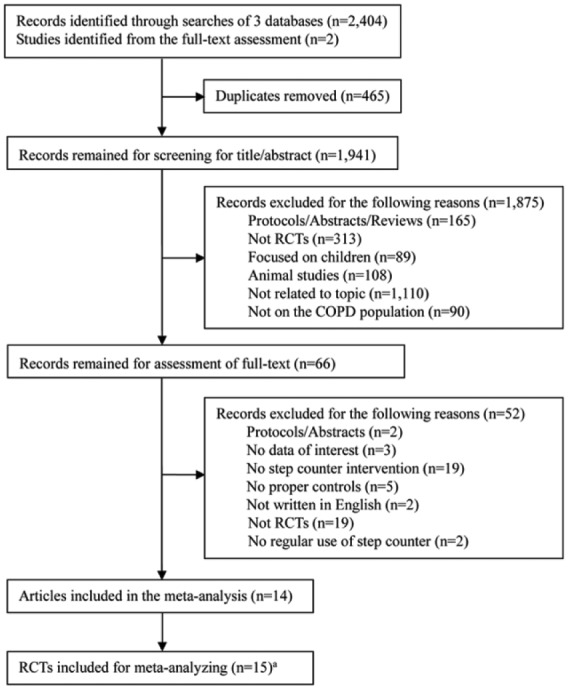
Flow diagram of literature search and selection processes.
COPD, chronic obstructive pulmonary disease; RCT, randomized controlled trial.
aTwo articles enrolled the same groups of patients but with different follow-up periods,18,19 which were treated as 1 RCT,19 while one article provided three different comparisons,13 resulting in 3 separate RCTs, 15 RCTs in total were therefore included.
Characteristics of included RCTs are summarized in Table 1. These RCTs were published from 2006 to 2017, and 12 of them were conducted in European countries (with 7 in The Netherlands13,16,20,21,26). A total of 1316 patients (about 66% men) were enrolled, with a median sample size of 40 (range 16–299). These patients had a mean age of 66 years (range 54–74 years) and a mean BMI of 27 kg/m2 (range 21.9–29.5 kg/m2), and their FEV1%pred ranged from 42 to 78, indicative of moderate to severe COPD. Almost all patients were physically inactive at baseline with a mean value of 4322 steps/day (range 2237–7313 steps/day) and showed impaired exercise capacity with a mean 6MWD of 393.3 m (range 96.3–519 m). The percentages of smokers at baseline in the intervention and control groups were comparable in general.
Table 1.
Characteristics of included studies in the meta-analysis.
| Source | Descriptions of intervention and control groups | Duration (weeks) | Telehealtha | Disease severityb | PA assessment |
|---|---|---|---|---|---|
| Compared with usual care group | |||||
| Altenburg and colleagues (a)c13 | Intervention: received usual care and a lifestyle PA counselling program, which included 5 individual 30 min counselling sessions, a pedometer, and a diary. | 12 | No | 78 | steps/day; pedometer |
| Control: received usual care | |||||
| Altenburg and colleagues (b)d13 | Intervention: received usual care and a lifestyle PA counselling program, which included 5 individual 30 min counselling sessions, a pedometer, and a diary. | 12 | No | 58 | steps/day; pedometer |
| Control: received usual care | |||||
| Demeyer and colleagues14 | Intervention: received a semiautomated telecoaching program, which included a one-to-one interview, a pedometer, a smartphone with applications, a booklet, weekly group text message, and telephone contacts, plus usual care using a standard leaflet explaining the importance and recommendations of PA. | 12 | Yes | 56 | steps/day; accelerometer |
| Control: received the same usual care as the intervention group. | |||||
| Hornikx and colleagues15 | Intervention: received a PA counselling program, which included a pedometer and personal, telephone-based PA counselling, and advice to increase PA. | 4 | Yes | 42 | steps/day; accelerometer |
| Control: received advice about increasing PA. | |||||
| Hospes and colleagues16 | Intervention: received a PA counselling program, which included 5 individual 30-min counselling sessions, and a pedometer. | 12 | No | 64.7 | steps/day; pedometer |
| Control: received usual care | |||||
| Mendoza and colleagues17 | Intervention: received a pedometer-based program, which included PA counselling, a pedometer and a diary | 12 | No | 66.1 | steps/day; accelerometer |
| Control: received PA counselling | |||||
| Moy and colleagues19 | Intervention: received an internet-mediated pedometer-based program, which included a pedometer, motivational contents, an online community forum, and educational content at the first 4 months. | 52 | Yes | NS | steps/day; accelerometer |
| Control: received a pedometer but no information about PA and goal setting. | |||||
| Tabak and colleagues20 | Intervention: received a tele-rehabilitation intervention, which included an accelerometer, a smartphone, and feedback text messages, plus usual care that could consist of medication and physiotherapy. | 4 | Yes | 52.6 | steps/day; pedometer |
| Control: received usual care as the intervention group. | |||||
| Vorrink and colleagues21 | Intervention: received usual care and an mHealth intervention, which included a smartphone with an accelerometer embedded, a phone/internet contract and an individual face-to-face instruction on the use of the smartphone and the application | 24 | No | 56 | steps/day (weekday); accelerometer |
| Control: received usual care. | |||||
| Compared with PR programmes | |||||
| Altenburg and colleagues (c)e13 | Intervention: received a lifestyle PA counselling program, which included 5 individual 30-min counselling sessions, a pedometer, and a diary, plus a PR program that included 9 weeks of exercise training (3 sessions/week, 1–2 h/session). | 12 | No | 43 | steps/day; pedometer |
| Control: received the same PR program as the intervention group. | |||||
| Cruz and colleagues25 | Intervention: received a PA-focused behavioral intervention, which included psychosocial support, education sessions, health contracts, a pedometer, and a diary, plus a PR program that included psychosocial support and education sessions once a week and 12 weeks of exercise training (3 sessions/week, 60 min/session). | 12 | No | 67.1 | steps/day; accelerometer |
| Control: received the same PR program as the intervention group. | |||||
| de Blok and colleagues26 | Intervention: received a lifestyle PA counselling program, which included 4 separate 30-min counselling sessions and a pedometer, plus a PR programme containing exercise training, dietary intervention and psycho-educational modules according to evidence-based guidelines. | 9 | No | 48 | steps/day; pedometer |
| Control: received the same PR program as the Intervention group. | |||||
| Kawagoshi and colleagues27 | Intervention: received a low-intensity and home-based PR program, which included upper and lower limb exercises, and a monthly 45-min education program, plus a pedometer | 52 | No | 56.6 | walking time; accelerometer |
| Control: received the same PR program as the intervention group. | |||||
| Nolan and colleagues28 | Intervention: received a pedometer intervention, plus a twice weekly supervised, 8-week PR program comprised two supervised sessions of exercise and education and at least one additional home-based exercise session per week (2 h/session). | 8 | No | 50.5 | steps/day; accelerometer |
| Control: received the same PR program as the Intervention group. | |||||
| Holland and colleagues 29 | Intervention: received a pedometer with PA counselling, a home visit, and weekly telephone calls, plus an 8-week, twice weekly home-based PR program that included aerobic exercise training (at least 30 min), resistance training, and self-management education. | 8 | Yes | 51 | steps/day; accelerometer |
| Control: received a center-based PR program with the core components the same as the intervention group. | |||||
NS, not specified; PA, physical activity; PR, pulmonary rehabilitation.
It included components like telephone calls, text messages, or internet applications.
It was assessed by baseline mean values of forced expiratory volume in 1s, %pred in both groups.
This study enrolled patients from general practices.
This study enrolled in outpatient hospital clinics.
This study enrolled patients from a PR center (i.e. receiving PR programs).
The duration of step counter interventions varied from 1 to 12 months, with seven of them being 3 months,13,14,16,17,25 and the details of interventions were different across studies (Table 1). However, all interventions employed components of physical activity counselling and step goal setting for physical activity promotion. There were six RCTs assessing the effectiveness of step counter intervention as an adjunct to PR on physical activity and exercise capacity,13,25–29 where such influence from PR was minimized by a matched comparator. For the remaining nine RCTs, they were compared with controls receiving usual care in general.13–17,19–21
Of the 15 RCTs, 11 had proper descriptions of the randomization process,13,14,17,19–21,25,28,29 while only 6 were judged to utilize the intention-to-treat analyses.14,17,19,21,28,29 Since it is intended to use step counters to promote physical outcomes, and the outcomes of interest are measured using objective approaches, the risk of blinding of participants and personnel and blinding of outcome assessment was all judged to be low. The mean drop-out rate was about 16.5% for the intervention group and 12.8% for the controls. The compliance to step counter use was found to be good (>80%). The most commonly reported adverse events among step counter users were mild musculoskeletal injuries and exacerbations, where the rate of musculoskeletal injury was higher than controls but the rate of exacerbations was lower than or comparable to controls.14,17,18,28
Step counter use and physical activity
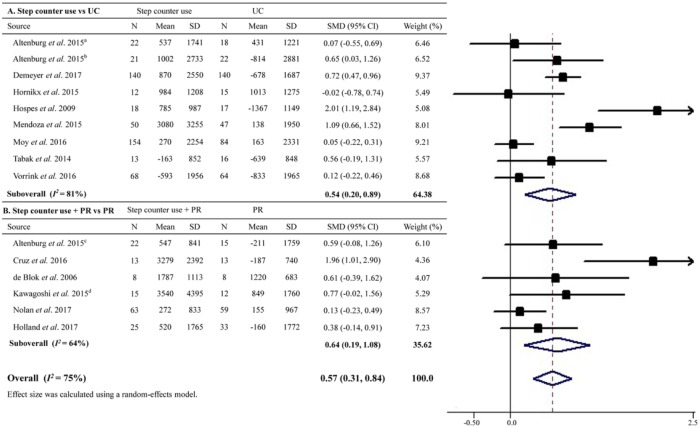
A total of 15 RCTs enrolling 1207 patients assessed the impact of step counter use on physical activity. Pooled results showed that step counter use significantly increased physical activity compared with the controls with an overall effect size of 0.57 (95% CI 0.31–0.84; Figure 2), which is equal to a magnitude of 1026 steps/day in daily steps. However, the result was of considerable heterogeneity (I2 = 75%).
Figure 2.
Effect sizes of step counter use on physical activity in patients with chronic obstructive pulmonary disease.
CI, confidence interval; PR, pulmonary rehabilitation; SD, standard deviation; SMD, standard mean difference; UC, usual care.
aThis study enrolled patients from general practice.
bThis study enrolled in outpatient hospital clinics.
cThis study enrolled patients from a pulmonary rehabilitation center (i.e. receiving pulmonary rehabilitation programs).
dData were collected using the methods described in Supplementary Table S2.
Subgroup analyses showed that step counter use was associated with an increase in physical activity compared with usual care (SMD = 0.54, 95% CI 0.20–0.89), which was also significant versus PR when step counter was added as an adjunct to PR (SMD = 0.64, 95% CI 0.19–1.08; Figure 2). Moreover, the increases in physical activity associated with step counter use were comparable among studies with or without use of diary or telehealth elements, or in those employing pedometers or accelerometers for physical activity quantification in the overall or further subgroup analyses (all pfor interaction >0.5; Table 2). However, in studies with longer intervention durations (⩾6 months) or among patients with severe COPD, step counter use was associated with a nonsignificant and smaller overall increase in physical activity compared with their counterparts (Table 2). Meta-regression analyses suggested that baseline age, sex, BMI, and exercise capacity were not significant predictors of increased physical activity (all p > 0.80), except for baseline physical activity (β coefficient = 0.0002, p = 0.046). Sensitivity analysis by excluding studies using per-protocol analyses also suggested an increased physical activity associated with step counter use (Table 2). Egger’s test suggested no evidence of publication bias (p = 0.19).
Table 2.
Subgroup analyses on physical activity outcomes.
| Subgroups | Overall analysis |
Step counter use versus usual care |
Step counter use + PR versus PR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Effect sizea |
I2,% | No. | Effect sizea |
I2,% | No. | Effect sizea |
I2,% | ||||
| SMD | 95% CI | SMD | 95% CI | SMD | 95% CI | |||||||
| Diary use | ||||||||||||
| With | 9 | 0.62 | 0.33–0.91 | 67 | 5 | 0.68 | 0.39 to 0.97 | 45 | 4 | 0.65 | 0.05 to 1.25 | 77 |
| Without | 6 | 0.51 | 0.02 to 0.99 | 78 | 4 | 0.45 | −0.16 to 1.05 | 85 | 2 | 0.71 | 0.09 to 1.33 | 1 |
| Telehealth elementsb | ||||||||||||
| With | 5 | 0.39 | 0.07 to 0.71 | 59 | 4 | 0.38 | −0.02 to 0.78 | 69 | 1 | 0.38 | −0.14 to 0.91 | NA |
| Without | 10 | 0.72 | 0.32 to 1.12 | 81 | 5 | 0.73 | 0.09 to 1.36 | 88 | 5 | 0.73 | 0.15 to 1.31 | 71 |
| Step counter | ||||||||||||
| Pedometer | 6 | 0.72 | 0.22 to 1.22 | 64 | 4 | 0.79 | 0.04 to 1.54 | 78 | 2 | 0.60 | 0.04 to 1.15 | 1 |
| Accelerometer | 9 | 0.50 | 0.18 to 0.82 | 80 | 5 | 0.41 | 0.00 to 0.82 | 85 | 4 | 0.70 | 0.06 to 1.34 | 78 |
| Intervention duration | ||||||||||||
| <6 months | 12 | 0.67 | 0.38 to 0.97 | 71 | 7 | 0.72 | 0.34 to 1.10 | 71 | 5 | 0.63 | 0.11 to 1.14 | 69 |
| ⩾6 months | 3 | 0.15 | −0.12 to 0.42 | 31 | 2 | 0.08 | −0.13 to 0.29 | 1 | 1 | 0.77 | −0.02 to 1.56 | NA |
| Disease severitycd | ||||||||||||
| Moderate | 11 | 0.68 | 0.37 to 0.99 | 77 | 7 | 0.70 | 0.31 to 1.08 | 78 | 4 | 0.70 | 0.06 to 1.34 | 78 |
| Severe | 3 | 0.38 | −0.07 to 0.83 | 1 | 1 | −0.02 | −0.78 to 0.74 | NA | 2 | 0.60 | 0.04 to 1.15 | 1 |
| Data analysing | ||||||||||||
| ITT | 6 | 0.41 | 0.08 to 0.73 | 82 | 4 | 0.48 | 0.03 to 0.93 | 88 | 2 | 0.21 | −0.08 to 0.50 | 1 |
| Per-protocol | 9 | 0.76 | 0.32 to 1.20 | 67 | 5 | 0.63 | −0.02 to 1.28 | 76 | 4 | 0.94 | 0.35 to 1.54 | 50 |
CI, confidence interval; ITT, intention-to-treat; NA, not applicable; PR, pulmonary rehabilitation; SMD, standard mean difference.
Effect sizes were analyzed using a random-effects model.
It included components like telephone calls, text messages, or internet applications.
Disease severity was assessed based on mean values of forced expiratory volume in 1s, %pred, in both groups.
Disease severity was not clearly reported in the study by Moy and colleagues19
Step counter use and exercise capacity
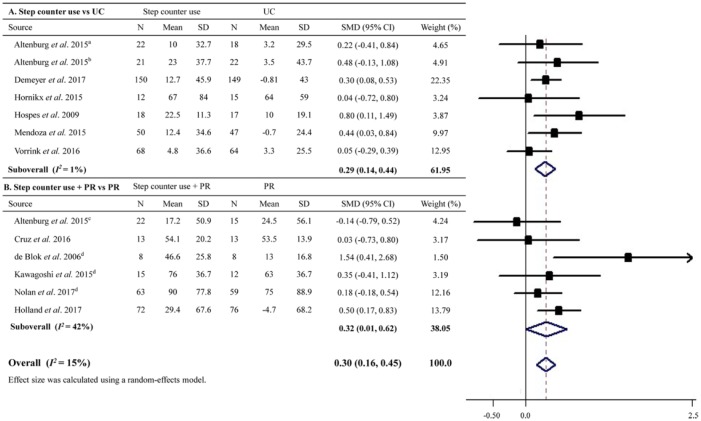
A total of 13 RCTs with a total of 1049 patients evaluated the influence of step counter use on exercise capacity. Meta-analysis showed that step counter use improved exercise capacity better than the controls with an overall effect size of 0.30 (95% CI 0.16–0.45; Figure 3), approximating to a magnitude of 11.6 m in 6MWD. There was no significant evidence of heterogeneity (I2 = 15%).
Figure 3.
Effect sizes of step counter use on exercise capacity in patients with chronic obstructive pulmonary disease.
CI, confidence interval; PR, pulmonary rehabilitation; SD, standard deviation; SMD, standard mean difference; UC, usual care.
aThis study enrolled patients from general practice.
bThis study enrolled in outpatient hospital clinics.
cThis study enrolled patients from a pulmonary rehabilitation center (i.e. receiving pulmonary rehabilitation programs).
dData were collected using the methods described in Supplementary Table S2.
Subgroup analyses suggested that step counter use was related to an improvement in exercise capacity compared with usual care (SMD = 0.29, 95% CI 0.14–0.44), or compared with PR when step counter was employed as an adjunct to PR (SMD = 0.32, 95% CI 0.01–0.62; Figure 3). Step counter use was also not associated with any significant changes on exercise capacity among studies with intervention durations lasting longer than 6 months or among patients having severe COPD in the overall or further subgroup analyses (Table 3). Improvements in exercise capacity associated with step counter use were comparable between studies with or without the use of diary or telehealth elements among the overall and further subgroup analyses (all pfor interaction > 0.2; Table 3). Meta-regression analyses revealed that none of the variables including baseline age, sex, BMI, physical activity, and exercise capacity could significantly predict the overall changes in exercise capacity (all p > 0.17). Sensitivity analysis by removing studies with per-protocol analyses also showed an improved exercise capacity resulted from step counter use (Table 3). No evidence of publication bias was detected by Egger’s test (p = 0.55).
Table 3.
Subgroup analyses on exercise capacity outcomes.
| Subgroups | Overall analysis |
Step counter use versus usual care |
Step counter use + PR versus PR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Effect sizea |
I2,% | No. | Effect sizea |
I2,% | No. | Effect sizea |
I2,% | ||||
| SMD | 95% CI | SMD | 95% CI | SMD | 95% CI | |||||||
| Diary use | ||||||||||||
| With | 8 | 0.31 | 0.17 to 0.45 | 1 | 4 | 0.34 | 0.16 to 0.52 | 1 | 4 | 0.25 | −0.02 to 0.51 | 25 |
| Without | 5 | 0.43 | −0.03 to 0.89 | 56 | 3 | 0.25 | −0.21 to 0.71 | 48 | 2 | 0.87 | −0.28 to 2.03 | 66 |
| Telehealth elementsb | ||||||||||||
| With | 4 | 0.28 | 0.08 to 0.47 | 24 | 3 | 0.21 | 0.03 to 0.40 | 1 | 1 | 0.50 | 0.17 to 0.83 | NA |
| Without | 9 | 0.34 | 0.12 to 0.56 | 20 | 4 | 0.46 | 0.19 to 0.73 | 1 | 5 | 0.25 | −0.14 to 0.64 | 41 |
| Intervention duration | ||||||||||||
| <6 months | 11 | 0.34 | 0.19 to 0.50 | 14 | 6 | 0.35 | 0.18 to 0.52 | 1 | 5 | 0.31 | −0.05 to 0.67 | 54 |
| ⩾6 months | 2 | 0.10 | −0.21 to 0.41 | 1 | 1 | 0.05 | −0.29 to 0.39 | NA | 1 | 0.35 | −0.41 to 1.12 | NA |
| Disease severityc | ||||||||||||
| Moderate | 10 | 0.31 | 0.18 to 0.44 | 1 | 6 | 0.30 | 0.15 to 0.46 | 1 | 4 | 0.33 | 0.11 to 0.55 | 1 |
| Severe | 3 | 0.37 | −0.49 to 1.24 | 70 | 1 | 0.04 | −0.72 to 0.80 | NA | 2 | 0.64 | −1.01 to 2.28 | 84 |
| Data analysing | ||||||||||||
| ITT | 5 | 0.29 | 0.14 to 0.44 | 10 | 3 | 0.40 | 0.06 to 0.73 | 1 | 2 | 0.35 | 0.03 to 0.66 | 41 |
| Per-protocol | 8 | 0.34 | 0.04 to 0.64 | 27 | 4 | 0.26 | 0.07 to 0.45 | 15 | 4 | 0.33 | −0.27 to 0.93 | 55 |
CI, confidence interval; ITT, intention-to-treat; NA, not applicable; PR, pulmonary rehabilitation; SMD, standard mean difference.
Effect sizes were analyzed using a random-effects model.
It included components like telephone calls, text messages, or internet applications.
Disease severity was assessed based on mean values of forced expiratory volume in 1 s, %pred, in both groups.
Discussion
Summary of the main findings
This meta-analysis of 14 RCTs shows that step counter use was effective in increasing physical activity and improving exercise capacity in patients with COPD. It further shows that step counter use can augment physical activity as well as exercise capacity in COPD patients receiving PR programs. Moreover, this meta-analysis suggests that step counter use may not be able to enhance physical activity or exercise capacity in patients with severe COPD, and its long-term effects also remain inconclusive.
Interpretation of the results
In agreement with parts of our findings, previous meta-analyses or systematic reviews conducted in patients with chronic diseases or specifically with type 2 diabetes have also shown that step counter use can significantly increase physical activity.31,32,39 Despite a smaller magnitude of the increase in physical activity observed in our study (1026 steps/day) compared with those in aforementioned studies (2042 steps/day and 1822 steps/day, respectively),31,39 such an increase is of clinically significant importance for patients with COPD, who may walk only about 4500 steps/day on average. This is because for every 1845 steps/day increase in physical activity for patients with COPD, the risk of all-cause mortality would be reduced up to approximately 50%.40 On the other hand, increased physical activity might be also dose-dependently associated with lower risks of COPD admissions and respiratory mortality.4,41 Moreover, the nearly 25% increase over baseline physical activity that resulted from step counter use seems to be much larger than those from traditional interventions like exercise training programs, long-term oxygen therapy, or neuromuscular electrical stimulation, which led to increases in physical activity by 12%, 6%, and 9%, respectively.42 This indicates that step counter use might be more beneficial in increasing physical activity than such alternative interventions.
Our meta-analysis also suggests that step counter use improved exercise capacity among patients with COPD, with a magnitude of increase based on 6MWD larger than that associated with telehealth interventions (11.6 versus 1.3 m) but smaller than that of PR (11.6 versus 25.7 m).43,44 Yet it is worth noting that PR might be not accessible for most patients with COPD because of possible resource constraints,17 yet even a 10 m increase in 6MWD is associated with an approximately 7% risk reduction in all-cause and respiratory mortality in patients with COPD.45 Given such evidence, and in light of the efficacy of step counter use in increasing physical activity, step counters are worth being recommended for patients with COPD for the improvement of physical outcomes.
Remarkably, there is evidence with a relatively large sample size questioning the effectiveness of step counter use on physical activity in patients with COPD attending PR programs recently.28 The authors observed that step counter use did not provide further benefits in enhancing the effects of PR for increasing physical activity or exercise capacity, and concluded that step counters should not be used routinely to augment physical activity during PR programs. In contrast with their results, our meta-analysis suggests that step counter usage was helpful to augment physical activity as well as exercise capacity in patients receiving PR programs. This might be because of the fact that most individual studies have limited statistical power in detecting positive findings and the study by Nolan and colleagues had drop-out rates higher than 15% for the intervention group and 20% for the control group.28
Of note, is that our meta-analysis shows that step counter use might be useless for patients with severe COPD with respect to physical outcome improvements, which is also evidenced by individual results.13,15 It is speculated that the high degree of airflow limitation associated with severe COPD might be the major impediment against the benefits of step counter use. Moreover, our meta-analysis fails to provide adequate evidence supporting the long-term efficacy of step counter use in increasing physical activity or improving exercise capacity in patients with COPD. However, one should be aware that this result might be subject to limited statistical power due to limited number of included studies, and that studies having intervention durations not less than 6 months might be more vulnerable to deteriorated adherence to step counter use over the time course.19 Therefore, future studies with long-term intervention durations together with approaches to improve the adherence to step counter use are required.
Previous meta-analyses have demonstrated that diary use for recording daily steps is an important predictor of increased physical activity among step counter users,12,31 and telehealth cointerventions may help to reinforce self-efficacy to achieve target goals like walking more.46 However, our results did not identify them as determinant factors for neither increased physical activity nor improved exercise capacity in patients with COPD. Yet one should be aware that these comparisons were indirect, and the findings may require further confirmation using ‘head-to-head’ designs. In addition, Nolan and colleagues pointed out that accelerometers are more reliable in measuring physical activity than pedometers, and stated that current discrepancies in physical activity outcomes across studies conducted in COPD populations might partly be attributed to this.28 In disagreement with this statement, our results showed that there was no significant difference between accelerometers and pedometers, which is also supported by another meta-analysis in patients with type 2 diabetes.32
Strengths and limitations
This meta-analysis is the first one that attempts to address the efficacy of step counter use on physical outcomes in patients with COPD, yet several limitations should be noted. Firstly, some heterogeneity existed in the outcome of physical activity, which could be only partially explained by some of our subgroup analyses (e.g. baseline physical activity, intervention duration). Secondly, step goal setting is shown to be associated with increased physical activity among step counter users, and it is suggested that the individualized self-set goal might be the best one for physical activity promotion in patients with type 2 diabetes.31 However, we could not confirm this among patients with COPD, because about half of the included studies did not have clear specifications, albeit they all did set step goals. Thirdly, it is observed that medications like bronchodilators and nutritional supplementations can lead to the increased physical activity and improved exercise capacity in patients with COPD.42 However, very few studies reported changes on medication use or nutrition intake.15 Finally, despite a comprehensive literature search, there still exists the possibility that some eligible studies might be missed. Moreover, the restriction to English studies and the failure to search for unpublished studies may result in selection and publication bias.
Conclusion
In conclusion, step counter use promotes physical activity and exercise capacity in patients with COPD, at least in the short term. Step counter use may also enhance the effects of PR on physical activity as well as on exercise capacity. These data suggest that step counter is worth being recommended as a routine component in the rehabilitation programs for patients with COPD. Future studies with specifications on step goal setting and records of medication changes are required to address the long-term effects of step counter use on physical activity and exercise capacity in such patients.
Supplemental Material
Supplemental material, Supplentary_files for Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis by Shanhu Qiu, Xue Cai, Xuyi Wang, Can He, Martina Zügel, Jürgen Michael Steinacker and Uwe Schumann in Therapeutic Advances in Respiratory Disease
Acknowledgments
SQ conducted the study, collected and analysed the data, and wrote the manuscript. XC collected the data. XW and CH contributed to the introduction and discussion. MZ and JS contributed to the introduction, and reviewed/edited the manuscript. US designed the study, contributed to the discussion, and edited the manuscript. All authors read and approved the final manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Shanhu Qiu  https://orcid.org/0000-0003-2597-3856
https://orcid.org/0000-0003-2597-3856
Contributor Information
Shanhu Qiu, Department of Endocrinology, Zhongda Hospital, Institute of Diabetes, School of Medicine, Southeast University, Dingjiaqiao No. 87, Nanjing, China.
Xue Cai, Department of Endocrinology, Zhongda Hospital, Institute of Diabetes, School of Medicine, Southeast University, Nanjing, China.
Xuyi Wang, Department of Endocrinology, Zhongda Hospital, Institute of Diabetes, School of Medicine, Southeast University, Nanjing, China.
Can He, Department of Pneumology, Zhongda Hospital, Southeast University, Nanjing, China.
Martina Zügel, Division of Sports and Rehabilitation Medicine, Ulm University Medical Center, Ulm, Germany.
Jürgen Michael Steinacker, Division of Sports and Rehabilitation Medicine, Ulm University Medical Center, Ulm, Germany.
Uwe Schumann, Division of Sports and Rehabilitation Medicine, Ulm University Medical Center, Ulm, Germany.
References
- 1. Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J 2014; 44: 1521–1537. [DOI] [PubMed] [Google Scholar]
- 2. Nakazawa A, Cox NS, Holland AE. Current best practice in rehabilitation in interstitial lung disease. Ther Adv Respir Dis 2017; 11: 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bossenbroek L, de Greef MH, Wempe JB, et al. Daily physical activity in patients with chronic obstructive pulmonary disease: a systematic review. COPD 2011; 8: 306–319. [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006; 61: 772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zanoria SJ, ZuWallack R. Directly measured physical activity as a predictor of hospitalizations in patients with chronic obstructive pulmonary disease. Chron Respir Dis 2013; 10: 207–213. [DOI] [PubMed] [Google Scholar]
- 6. Vaes AW, Garcia-Aymerich J, Marott JL, et al. Changes in physical activity and all-cause mortality in COPD. Eur Respir J 2014; 44: 1199-1209. [DOI] [PubMed] [Google Scholar]
- 7. Van Remoortel H, Hornikx M, Demeyer H, et al. Daily physical activity in subjects with newly diagnosed COPD. Thorax 2013; 68: 962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kentson M, Todt K, Skargren E, et al. Factors associated with experience of fatigue, and functional limitations due to fatigue in patients with stable COPD. Ther Adv Respir Dis 2016; 10: 410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al. Physical activity in COPD patients: patterns and bouts. Eur Respir J 2013; 42: 993–1002. [DOI] [PubMed] [Google Scholar]
- 10. Kosteli MC, Heneghan NR, Roskell C, et al. Barriers and enablers of physical activity engagement for patients with COPD in primary care. Int J Chron Obstruct Pulmon Dis 2017; 12: 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bassett DR, Jr., Toth LP, LaMunion SR, et al. Step counting: a review of measurement considerations and health-related applications. Sports Med 2017; 47: 1303–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007; 298: 2296–2304. [DOI] [PubMed] [Google Scholar]
- 13. Altenburg WA, ten Hacken NHT, Bossenbroek L, et al. Short- and long-term effects of a physical activity counselling programme in COPD: a randomized controlled trial. Respir Med 2015; 109: 112–121. [DOI] [PubMed] [Google Scholar]
- 14. Demeyer H, Louvaris Z, Frei A, et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax 2017; 72: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hornikx M, Demeyer H, Camillo CA, et al. The effects of a physical activity counseling program after an exacerbation in patients with Chronic Obstructive Pulmonary Disease: a randomized controlled pilot study. BMC Pulm Med 2015; 15: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hospes G, Bossenbroek L, Ten Hacken NH, et al. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns 2009; 75: 274–278. [DOI] [PubMed] [Google Scholar]
- 17. Mendoza L, Horta P, Espinoza J, et al. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J 2015; 45: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moy ML, Collins RJ, Martinez CH, et al. An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest 2015; 148: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moy ML, Martinez CH. Long-term effects of an internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial. J Med Internet Res 2016; 18: e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tabak M, Vollenbroek-Hutten MM, van der Valk PD, et al. A telerehabilitation intervention for patients with Chronic Obstructive Pulmonary Disease: a randomized controlled pilot trial. Clin Rehabil 2014; 28: 582–591. [DOI] [PubMed] [Google Scholar]
- 21. Vorrink SNW, Kort HSM, Troosters T, et al. Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation. Eur Respir J 2016; 48: 1019–1029. [DOI] [PubMed] [Google Scholar]
- 22. Tubili C, Di Flaviani A, Morviducci L, et al. Pedometer use is beneficial for type 2 diabetes mellitus patients if included in educational programs. Metabolism 2010; 59: E1–; author reply E3–4. [DOI] [PubMed] [Google Scholar]
- 23. Araiza P, Hewes H, Gashetewa C, et al. Efficacy of a pedometer-based physical activity program on parameters of diabetes control in type 2 diabetes mellitus. Metabolism 2006; 55: 1382–1387. [DOI] [PubMed] [Google Scholar]
- 24. De Greef KP, Deforche BI, Ruige JB, et al. The effects of a pedometer-based behavioral modification program with telephone support on physical activity and sedentary behavior in type 2 diabetes patients. Patient Educ Couns 2011; 84: 275–279. [DOI] [PubMed] [Google Scholar]
- 25. Cruz J, Brooks D, Marques A. Walk2Bactive: a randomised controlled trial of a physical activity-focused behavioural intervention beyond pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron Respir Dis 2016; 13: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Blok BM, de Greef MH, ten Hacken NH, et al. The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: a pilot study. Patient Educ Couns 2006; 61: 48–55. [DOI] [PubMed] [Google Scholar]
- 27. Kawagoshi A, Kiyokawa N, Sugawara K, et al. Effects of low-intensity exercise and home-based pulmonary rehabilitation with pedometer feedback on physical activity in elderly patients with chronic obstructive pulmonary disease. Respir Med 2015; 109: 364–371. [DOI] [PubMed] [Google Scholar]
- 28. Nolan CM, Maddocks M, Canavan JL, et al. Pedometer step count targets during pulmonary rehabilitation in Chronic Obstructive Pulmonary Disease. A randomized controlled trial. Am J Respir Crit Care Med 2017; 195: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holland AE, Mahal A, Hill CJ, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax 2017; 72: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burtin C, Langer D, van Remoortel H, et al. Physical activity counselling during pulmonary rehabilitation in patients with COPD: a randomised controlled trial. PLoS One 2015; 10: e0144989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qiu S, Cai X, Chen X, et al. Step counter use in type 2 diabetes: a meta-analysis of randomized controlled trials. BMC Med 2014; 12: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baskerville R, Ricci-Cabello I, Roberts N, et al. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2017; 34: 612–620. [DOI] [PubMed] [Google Scholar]
- 33. Yu T, Frei A, Ter Riet G, et al. Determinants of physical activity in patients with Chronic Obstructive Pulmonary Disease: a 5-year prospective follow-up study. Respiration 2016; 92: 72–79. [DOI] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269, W264. [DOI] [PubMed] [Google Scholar]
- 35. Qiu SH, Xue C, Sun ZL, et al. Attenuated heart rate recovery predicts risk of incident diabetes: insights from a meta-analysis. Diabet Med 2017; 34: 1676–1683. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JPT, Green S. and Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Chichester, UK; Hoboken, NJ: Wiley-Blackwell, 2008, p. xxi, 649 p. [Google Scholar]
- 37. Liu HM, Chiang IJ, Kuo KN, et al. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis 2017; 11: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bernocchi P, Vitacca M, La Rovere MT, et al. Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial. Age Ageing 2018; 47: 82–88. [DOI] [PubMed] [Google Scholar]
- 39. Vaes AW, Cheung A, Atakhorrami M, et al. Effect of ‘activity monitor-based’ counseling on physical activity and health-related outcomes in patients with chronic diseases: a systematic review and meta-analysis. Ann Med 2013; 45: 397–412. [DOI] [PubMed] [Google Scholar]
- 40. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140: 331–342. [DOI] [PubMed] [Google Scholar]
- 41. Garcia-Rio F, Rojo B, Casitas R, et al. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest 2012; 142: 338–346. [DOI] [PubMed] [Google Scholar]
- 42. Mantoani LC, Rubio N, McKinstry B, et al. Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J 2016; 48: 69–81. [DOI] [PubMed] [Google Scholar]
- 43. Lundell S, Holmner A, Rehn B, et al. Telehealthcare in COPD: a systematic review and meta-analysis on physical outcomes and dyspnea. Respir Med 2015; 109: 11–26. [DOI] [PubMed] [Google Scholar]
- 44. Rugbjerg M, Iepsen UW, Jorgensen KJ, et al. Effectiveness of pulmonary rehabilitation in COPD with mild symptoms: a systematic review with meta-analyses. Int J Chron Obstruct Pulmon Dis 2015; 10: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 2008; 134: 746–752. [DOI] [PubMed] [Google Scholar]
- 46. Trief PM, Teresi JA, Eimicke JP, et al. Improvement in diabetes self-efficacy and glycaemic control using telemedicine in a sample of older, ethnically diverse individuals who have diabetes: the IDEATel project. Age Ageing 2009; 38: 219–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplentary_files for Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis by Shanhu Qiu, Xue Cai, Xuyi Wang, Can He, Martina Zügel, Jürgen Michael Steinacker and Uwe Schumann in Therapeutic Advances in Respiratory Disease