Abstract
α-Enolase is a significant subunit of enolase and acts as a glycolytic enzyme responsible for catalyzing the conversion of 2-phosphoglycerate to phosphoenolpyruvate in the anaerobic glycolysis pathway. The research about their role is known little in tumor invasion and metastasis. This research analyzed the effect of α-enolase in proliferation and progression of human gastric cancer. The constructed PLKO.1-ENO1 shRNA vector was transfected into 293 T cells and used to infect gastric cancer cells, MKN45, by using lentivirus method. Negative controls were generated by infection with viruses containing empty vector PLKO.1-scramble-shRNA by the same protocol and using wild-type MKN45 cells as blank control. The silencing effect was confirmed by reverse transcription polymerase chain reaction and Western blotting at messenger RNA and protein levels, respectively. Cell proliferation and chemosensitivity were tested by methyl-thiazolyl-tetrazolium assay. Cell apoptosis was tested by flow cytometry. The cell line α-enolase short hairpin RNA stabling silence α-enolase was successfully constructed. In the α-enolase short hairpin RNA cell lines, messenger RNA and protein expression of α-enolase were significantly lower than those in negative control and blank control groups. The proliferation and clone formation ability were significantly inhibited, cell apoptosis was increased significantly, and the inhibition rate of chemotherapy drugs was increased (P < .05). Our data provide strong evidence that α-enolase short hairpin RNA interference vector can effectively suppress the proliferation and increase chemosensitivity of MKN45 cells, which may provide a novel gene therapy for gastric cancer.
Keywords: gastric cancer, shRNA, ENO1, cell proliferation, chemotherapeutics
Introduction
Among malignant tumors worldwide, gastric cancer has the fourth highest incidence and the second highest mortality rate.1,2 In China, approximately 46.8% of new patients with cancer are diagnosed with gastric cancer each year, and this cancer has the second highest incidence among malignant tumors.3,4 Radical resection is the most effective treatment for gastric cancer, but studies have shown that approximately 80% of patients with gastric cancer have postoperative recurrence. Chemotherapy has become the preferred treatment for gastric cancer, but a standard chemotherapy regimen for this disease does not currently exit. Therefore, its early diagnosis and intervention are particularly important.5,6 As tumors have continued to be studied, other hallmarks of cancer have been identified: reprogrammed energy metabolism, immune destruction evasion, upregulated inflammation, genome instability, and genetic mutation.7 The rapid proliferation of tumor cells not only affects cell numbers but also adjusts energy metabolism to promote cell growth and division. We speculated that gastric cancer cells also have an altered metabolism, and using proteomics and immunohistochemistry, we found that the expression of α-enolase (ENO1), a key enzyme in glycolysis, was increased in the early stages of cancer.8 In this study, we constructed a eukaryotic expression vector encoding a short hairpin RNA (shRNA) that specifically targeted ENO1 and downregulated its expression in the gastric cancer MKN45 cell line. Determining the effects of ENO1 on gastric cancer cell growth and proliferation and on cell sensitivity to chemotherapeutic drugs may provide a new theoretical basis for gastric cancer gene treatment.
Materials and Methods
Materials
The human gastric cancer MKN45 cell line and human embryonic kidney 293 T cell line were provided by the Lanzhou Center for Tuberculosis Research, Lanzhou University. The PLKO.1-TRC cloning vector was used as the empty plasmid (Sigma, USA) from which the ENO1 plasmid was constructed by plasmid recombination, and some of the specific segments were silenced (experimental group). The PLKO.1-scramble-shRNA plasmid was used for the negative control group, and a scrambled fragment was inserted into a disordered segment that did not correspond to human or mouse messenger RNA (mRNA). PsPAX2 and pMD2.G were the packaging and envelope plasmids, respectively, for virus production. These 3 plasmids were purchased from Sigma. The shRNA sequence was synthesized by BGI Diagnosis Co Ltd (Beijing, China). For the ENO1 shRNA sequence, the sense strand was 5′-CCGGCGTACCGCTTCCTTAGAACTTCTCGAGAAGTTCTAAGGAAGCGGTACGTTTTTG-3′ and the antisense strand was 5′-AATTCAAAAACGTACCGCTTCCTTAGAACTTCTCGAGAAGTTCTAAGGAAGCGGTACG-3′. Both Dulbecco Modified Eagle Medium (DMEM) medium and fetal bovine serum (FBS) were purchased from Gibco (USA). The FuGENE 6 transfection reagent was purchased from Roche Applied Biosciences (Basel, Switzerland). The radioimmunoassay precipitation assay (RIPA) buffer was purchased from Sangon Biotech (Shanghai, China), and the bicinchoninic acid (BCA) kit was purchased from Beyotime Biotechnology (Shanghai, China). The rabbit antihuman ENO1 and PKM2 monoclonal antibodies were purchased from Cell Signaling Technology, and the mouse antihuman β-actin antibody was purchased from Sigma-Aldrich. Both horseradish peroxidase–labeled goat antirabbit and goat antimouse immunoglobulin G (IgG) secondary antibody were purchased from ZSGB-BIO (Beijing, China). RNA extraction reagent (TRIzol), Prime Script Reverse Transcription Kit, 2 × Taq polymerase chain reaction (PCR) Green Mix, and SYBR Green qPCR kit were purchased from Dalian Takara Bio Inc (Dalian, China). Polybrene, puromycin, methyl-thiazolyl-tetrazolium (MTT), and dimethyl sulfoxide (DMSO) were purchased from Sigma. Cisplatin (DDP) was manufactured by Qilu Pharmaceutical Co Ltd (Ji'nan, China), and 5-fluorouracil (5-FU) was produced by Shenyang Yaoda Pharmaceutical Co Ltd.
Cells and Cell Culture
The human gastric cancer MKN45 cell line and human embryonic kidney 293 T cell line were provided by the Lanzhou Center for Tuberculosis Research, Lanzhou University. The MKN-45 and 293 T cells were cultured in complete DMEM containing 5% serum (1% penicillin + streptomycin) in an incubator at 37°C, with 5% CO2.
Acquisition of Lentivirus Packaging and Stable Transfection Cell Lines
Two hundred ninety-three T cells in the logarithmic growth phase were inoculated in a 6-well plate at 5 × 103 cells/well and incubated at 37°C, with 5% CO2 until the cell density was approximately 50% to 80% in preparation for transfection. The recombinant lentiviral vector PLKO.1-ENO1 shRNA interference plasmid (experimental group) or PLKO.1-scramble-shRNA plasmid (negative control group) + psPAX2 plasmid + pMD2.G plasmid were transfected into 293 T cells by FuGENE6 for lentiviral packaging and cultured in serum-free DMEM, in accordance with FuGENE6 transfection reagent instructions. The virus supernatant was collected 24 and 48 hours after transfection and centrifuged at 3000 rpm for 10 minutes. Then, the cell pellet was removed, and cells were filtered using a 0.45-μm filter, collected and stored at −80°C for subsequent experiments.
The gastric cancer cell line MKN45 was infected with the packaged lentivirus supernatant, and the effect of the lentiviral infection was increased by adding polybrene. After 48 hours of infection, to select resistant clones, cells were screened with medium containing puromycin (2 μg/mL) for stable transfection. The stably transfected ENO1 shRNA/MKN-45 and Scr shRNA/MKN-45 cell lines were selected and expanded in culture for subsequent experiments.
Real-Time Fluorescent Quantitative PCR Detection of the ENO1 mRNA Expression Level in ENO1 shRNA/MKN-45 Cells
Total RNA was extracted from cells in the blank control group (MKN-45), negative control group (Scr shRNA/MKN-45), and experimental group (ENO1 shRNA/MKN-45) during the logarithmic growth phase; and 1 mL of TRIzol reagent was added. Then, cDNA was synthesized from 2 μg of total RNA, according to the Prime Script RT Reverse Transcription Kit instructions. The mRNA expression of ENO1 was detected by fluorescent quantitative detection using the SYBR Green qPCR kit. The RT-PCR primers were synthesized by Takara (Dalian, China). The sense strand of the ENO1 primer sequence was 5′-CCCAGCTGCCCGATCAGTG-3′ and the antisense strand was 5′-GATGAGCCCAGTTCGGAT CTC-3′; β-actin was used as the internal reference with a sense strand of 5′-GACCACAGTCCATGCCATCAC-3′ and antisense strand of 5′-CATACCAGGAAATGAGCTTGAC-3′. The above genes were amplified by CFX96 fluorescent quantitative PCR (Bio-Rad Laboratories Ltd, USA). The 25-μL PCR reaction system comprised 2.5 μL of 2 × Mix, 1 μL of upstream and downstream primers, 2 μL of cDNA template, and 8.5 μL of sterilized double-distilled water. The reaction conditions were as follows for a total of 40 cycles: 95°C for 30 seconds, 95°C for 5 seconds, and 60°C for 30 seconds. The results were analyzed by Rotor-Gene Real-Time Analysis Software 6.1, and the relative quantitative model was selected by cycle threshold values from each well analyzed. Three replicate wells were tested for each group. The experiment was repeated 3 times. The expression level of the target gene was detected using the 2−ΔΔCt method.
Detection of ENO1 Protein Expression in ENO1 shRNA/MKN-45 Cells by Western Blotting
Cells were taken from the blank control group (MKN-45), negative control group (Scr shRNA/MKN-45), and experimental group (ENO1 shRNA/MKN-45) during the logarithmic growth phase. The medium was discarded, the cells were washed twice with phosphate-buffered saline (PBS), RIPA cell lysate was added, and cells were lysed on ice for 30 minutes. After complete lysis, cells were centrifuged at 12 000g at 4°C for 15 minutes; the supernatant was gently removed. A BCA kit was used to determine the protein concentration. Protein samples of equal concentrations were separated by 6% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane using the wet transfer method. Then, the membrane was blocked in 5% skim milk for 2 hours at room temperature; primary antibody (ENO1 1:2000, PKM2 1:2000, β-actin 1:5000) was added and incubated at 4°C overnight and for 2 hours at room temperature. Then, the membrane was slowly shaken, horseradish peroxidase–labeled goat antirabbit or goat antimouse (IgG) secondary antibody was added, and the membrane was incubated for 2 hours before being washed with Tris-buffered saline Tween-20 (TBST). After washing the membrane 3 times with TBST, the protein band that had been incubated with the secondary antibody was placed into the cartridge, ECL luminescent reaction solution was added, and the photographic film in the cartridge was exposed. The film was exposed to the developer for ≥55 minutes, removed after a clear band appeared, soaked in water for 2 to 3 minutes, and placed in a fixing solution for 5 minutes. Subsequently, the film clip was rinsed and dried. ImageJ software was used to analyze the gray value of the protein band. The experiment was repeated at least 3 times, and β-actin was used as the internal reference.
Detection of the Proliferative Activity of ENO1 shRNA/MKN-45 Cells by MTT
MKN45 cells in the logarithmic growth phase (blank control group), ENO1 shRNA/MKN-45 cells (experimental group), and Scr shRNA/MKN-45 (negative control group) were routinely digested and counted. Then, these 3 cell lines were inoculated in five 96-well plates with 3 replicate wells for each group, 4 × 103 cells/well. Cells were cultured for 1 to 5 days. One plate was evaluated each day; 20 μL of MTT reagent was added (5 mg/mL) and incubated at 37°C with 5% CO2 for 4 hours. The culture medium and MTT were removed, 150 μL of DMSO was added to each well, and the plate was shaken on a level shaking table for 15 minutes. Then, the absorbance (optical density [OD]) of each well was measured using a microplate reader at 490 nm, and the growth curve was plotted with the OD value on the y-axis and the number of days (d) on the x-axis. The experiment was performed in triplicate.
Detection of ENO1 shRNA/MKN-45 Cell Proliferation by Plate Cloning
During the logarithmic growth phase, MKN45, ENO1 shRNA/MKN-45, and Scr shRNA/MKN-45 cells were routinely digested and counted. Then, cells were resuspended at 200 cells/mL with 10% FBS and placed into a 6-well plate at 1 mL/well. Next, 3 mL of 10% FBS medium was added to each well and incubated at 37°C, with 5% CO2 for 10 to 14 days. When the cloned sphere was visible by eye in the medium, the culture was terminated and the supernatant was terminated. Then, cells were carefully washed twice with PBS and fixed in 3 mL of 4% paraformaldehyde for 15 minutes. Afterward, the fixing solution was discarded, 0.1% Giemsa staining was added for 15 to 30 minutes, and the dyed cells were carefully washed and air-dried. The number of colonies was visually counted directly, or the number of cloned spheres with more than 20 cells was counted under a microscope. The experiment was performed in triplicate.
Detection of ENO1 shRNA/MKN-45 Cell Apoptosis by Flow Cytometry
During the logarithmic growth phase, MKN45 and ENO1 shRNA/MKN-45 were digested, blown, suspended in PBS, and cleaned twice. Then removed the supernatant. Next, 1 × binding buffer solution was made into 1 × 106 cell/mL cell suspension of 1 × 106 cell/mL. Then, 100 µL cell suspension, the prepared Annexin V, and the nucleic acid were added and mixed into Falcon test tube gently. It was placed away from light at the room temperature (20°C-25°C) for 15 minutes. FACSCalibur flow cytometry (BD FACSAria II, USA) was used to detect cell apoptosis.
Detection of the Impact of Chemotherapy on ENO1 shRNA/MKN-45 Cell Proliferation by the MTT Assay
In the logarithmic growth phase, MKN45, Scr shRNA/MKN-45, and ENO1 shRNA/MKN-45 cells were routinely digested and counted. Then, in a 96-well plate, 100 μL of cells, at 5 × 104 cells/mL, was added to each well in triplicate for each group and was inoculated. After cell adherence, DDP (the concentration of the solution was 5, 10, and 20 μmol/L) and 5-FU (the concentration of the solution was 10, 20, and 40 μmol/L) were added, and cells in the blank control group were untreated. After 48 hours of culture, the 96-well plates were removed, and 20 μL of MTT (5 mg/mL) was added to each well. After incubating at 37°C, with 5% CO2 for 4 hours, the medium and MTT were removed, 150 μL of DMSO was added to each well, and the plate was shaken on a level shaking table for 15 minutes. The OD of each well was measured using a microplate reader at a wavelength of 490 nm, as well as the number of viable cells affected the OD. Cell inhibition rate = (control group − experimental group)/control group × 100%. The experiment was performed in triplicate, and the average value was taken.
Statistical Analysis
SPSS 19.0 software was used for statistical analysis. The experimental data are expressed as the means (standard deviations). Comparisons among groups were performed using paired t tests. The standard for a statistically significant difference was P < .05.
Results
Decreased mRNA and Protein Expression Level of ENO1 in MKN-45 Cells After ENO1 shRNA Transfection
RT-qPCR results revealed that ENO1 mRNA expression in the ENO1 shRNA/MKN-45 group was significantly decreased compared to that in the blank and negative control groups (P < .05; Figure 1, Figure 2A). Western blot results showed that the protein expression of ENO1 was significantly decreased in ENO1 knockout cells compared to that in the empty vector group and blank group, and the difference in the gray value was statistically significant (P < .05; Figure 2B).
Figure 1.
Amplification curve and dissolution curve of RT-PCR.
Figure 2.
(A) Expression of ENO1 mRNA based on real-timeRT-PCR. (Lane 1) MKN45 ;(Lane 2) ScrshRNA/MKN45; and (Lane 3) ENO1shRNA/MKN45. (B) Level of ENO1 protein expression determined by western blot analysis.
Decreased Proliferation of MKN45 Cells After ENO1 shRNA Transfection
To investigate the effect of ENO1 knockout on the proliferation of MKN45 cells, the absorbance values of cells cultured for 1 to 5 days were measured by MTT assay (Table 1). The growth curves of each group were plotted as the number of days of growth and the absorbance values. The results revealed that for the transfected gastric cancer ENO1 shRNA/MKN-45 cell line, the growth rate was significantly slower than that of the blank control (MKN45) and negative control groups (P < .05; Figure 3).
Table 1.
The OD490 of MKN45, Scr shRNA/MKN45, and ENO1 shRNA/MKN45 in Different Days.a
| Groups | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| MKN45 | 0.22 (0.15) | 0.53 (0.37) | 0.80 (0.73) | 0.96 (0.16) | 1.12 (0.17) |
| Scr shRNA/MKN45 | 0.22 (0.11) | 0.54 (0.19) | 0.83 (0.52) | 0.96 (0.11) | 0.14 (0.71) |
| ENO1 shRNA/MKN45 | 0.20 (0.09)b | 0.30 (0.10)b | 0.33 (0.31)b | 0.41 (0.11)b | 0.49 (0.30)b |
Abbreviations: ENO1, α-enolase; OD, optical density; SD, standard deviation; shRNA, short hairpin RNA.
a Values expressed in (SD).
b P < .05.
Figure 3.
The proliferation of α-enolase (ENO1) short hairpin RNA (shRNA)/MKN45 cells was tested by methyl-thiazolyl-tetrazolium (MTT). The data are represented as the mean (standard deviation [SD]) from triplicate assays and are representative of 3 independent experiments. α-Enolase shRNA/MKN45 group versus Scr shRNA/MKN45 group (P < .05) and ENO1 shRNA/MKN45 group versus MKN45 group (P < .05).
The Cloning Ability and Proliferation of MKN45 Cells Decreased After Transfection With ENO1 shRNA
To investigate the effect of ENO1 knockout on the proliferation of MKN45 cells, the results of the plate cloning assay revealed that the colony formation of MKN45 cells was inhibited by ENO1 shRNA. The number of visually observable cloned spheres formed by ENO1 shRNA/MKN-45 cells was significantly decreased compared to that of the MKN45 blank control group and Scr shRNA/MKN-45 in the negative control group. The experiment was performed in triplicate, and the results were statistically significant (P < .05; Figure 4).
Figure 4.
The proliferation clonal ability of α-enolase (ENO1) short hairpin RNA (shRNA)/MKN45 cells was tested by tablet cloning. The data are represented as the mean (standard deviation [SD]) from triplicate assays and are representative of 3 independent experiments. α-Enolase shRNA/MKN45 group versus Scr shRNA/MKN45 group (P < .05) and ENO1 shRNA/MKN45 group versus MKN45 group (P < .05).
Cell Apoptosis Was Increased Significantly After ENO1 shRNA Transfection
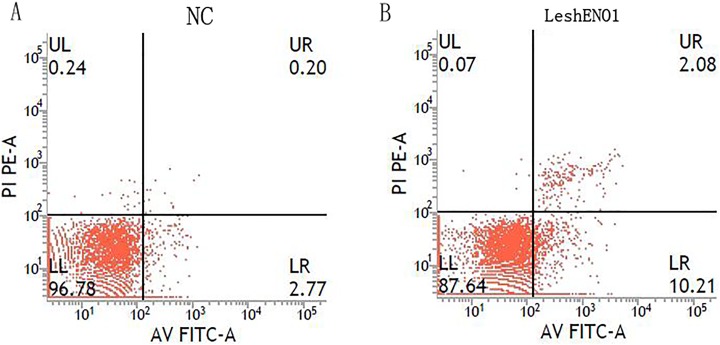
To investigate the effect of ENO1 knockout on the apoptosis of MKN45 cells, flow cytometry was used to detect the blank control group (MKN-45) and experimental group (ENO1 shRNA/MKN-45). The results revealed that the apoptosis rate increased significantly after ENO1 knockout. The average apoptosis rate of ENO1 shRNA/MKN-45 group was 10.21%, while that of blank group was 2.77%, and there was a significant difference between the groups (P < .05; Figure 5).
Figure 5.
The apoptosis rate of α-enolase (ENO1) short hairpin RNA (shRNA)/MKN45 cells was measured by flow cytometry. A, The apoptosis level of MKN45 cells. B, The apoptosis level of ENO1 shRNA/MKN-45 cells. The lower left quadrant showed living cells, the upper right quadrant showed necrotic cells, and the lower right quadrant showed apoptotic cells.
Cell Proliferation Was Significantly Inhibited by Chemotherapy Drugs After ENO1 shRNA Transfection
Forty-eight hours after different concentrations of DDP and 5-FU were administered to the 3 groups of cells, namely, the blank control group (MKN45), negative control group (Scr shRNA/MKN45), and experimental group (ENO1 shRNA/MKN45), it was revealed that the proliferation of cells in all 3 groups was inhibited to varying degrees (Table 2). These results show that proliferation was inhibited in a dose-dependent manner; the inhibition of cells in the experimental group was significantly higher than that in the blank control group or negative control group, and the results were statistically significant. Furthermore, between the blank control group and the negative control group, there was no significant difference in inhibition. Hence, these results show that ENO1 gene silencing increases cell sensitivity to DDP and 5-FU treatment, significantly inhibiting cell proliferation (Figure 6).
Table 2.
The Inhibition Rates of MKN45, Scr shRNA/MKN45, and ENO1 shRNA/MKN45 Groups Using Different Concentration of DDP and 5-Fu at 48 Hours.a
| Groups | DDP | 5-FU | ||||
|---|---|---|---|---|---|---|
| 5 μmol | 10 μmol | 20 μmol | 10 μmol | 20 μmol | 40 μmol | |
| MKN45 | 0.24 (0.03) | 0.44 (0.02) | 0.52 (0.01) | 0.19 (0.03) | 0.23 (0.09) | 0.33 (0.03) |
| Scr shRNA/MKN45 | 0.23 (0.04) | 0.45 (0.02) | 0.54 (0.09) | 0.19 (0.04) | 0.26 (0.04) | 0.35 (0.03) |
| ENO1 shRNA/MKN45 | 0.35 (0.03)b | 0.64 (0.03)b | 0.69 (0.01)b | 0.30 (0.02)b | 0.40 (0.01)b | 0.54 (0.03)b |
Abbreviations: ENO1, α-enolase; DDP, cisplatin; SD, standard deviation; shRNA, short hairpin RNA; 5-FU, 5-fluorouracil.
a Values expressed in (SD).
b P < .05.
Figure 6.
The ability proliferation of α-enolase (ENO1) short hairpin RNA (shRNA)/MKN45 cells was inhibited by different concentrations of chemotherapeutic drugs. A, The drug sensitivity of cisplatin (DDP) after ENO1 silencing. B, The drug sensitivity of 5-fluorouracil (5-Fu) after ENO1 silencing. The data are represented as the mean (standard deviation [SD]) of 3 independent experiments. α-Enolase shRNA/MKN45 group versus Scr shRNA/MKN45 group (P < .05) and ENO1 shRNA/MKN45 group versus MKN45 group (P < .05).
Discussion
Enolase is an important enzyme in glycolysis. As a new tumor marker, it has recently become a popular research topic worldwide. Enolase catalyzes the conversion of 2-phosphoglyceric acid to phosphoenolpyruvate during glycolysis to produce the second glycolytic ATP. Enolase plays an important role in cellular energy metabolism.9,10 Enolase is an intracellular enzyme that is necessary for glycolysis and is widely found in eukaryotic and prokaryotic cells. There are 3 tissue-specific isoenzyme forms (α, β, and γ) of enolase that are each encoded by a different gene; the active form of enolase is composed of a dimer that consists of different subunits. The existing enolase isoform varies according to different development stages. α-Enolase is mainly present in embryonic tissues and in most adult mammalian tissues. Furthermore, αβ- and ββ-enolase are mainly found in skeletal muscles and muscle tissues, while αγ- and γγ-enolase are mainly present in nerve tissues and neuroendocrine tissues.11,12
α-Enolase contains 433 amino acids and is composed of 2 identical subunits that are arranged in a reverse parallel manner, with each subunit having a relative molecular mass of approximately 47 kD. α-Enolase is a metalloenzyme, and its activation requires the participation of metal ions; Mg2+ plays an important role in the activation of enolase.13 Recent studies have shown that the cellular localization of ENO1 and the biological function of diversity play an important role in the occurrence and development of tumor, such as in the cell membrane of ENO1 can promote the invasion and metastasis of tumor cells, while in the cytoplasm of ENO1 can promote the growth and movement of tumor cells, and in the nucleus of ENO1 can inhibit tumor cell.14 No specific inhibitor of ENO1 has been found that regulates the activity of enolase.15 However, few studies have investigated the relationship between ENO1 and tumors, and of those that exist, the research results are inconsistent. Zhang et al 14 detected the protein expression of ENO1 in tissues and serum samples obtained from 42 healthy participants, 34 patients with benign lung lesions, and 84 patients with non-small cell lung cancer (NSCLC); this study found that the protein the expression of ENO1 in NSCLC tissues was significantly higher than in corresponding normal lung tissue (P < .05). Furthermore, the protein expression of ENO1 in the serum obtained from patients with NSCLC was significantly higher than that in the serum of healthy people and patients with benign lung lesions (P < .05). Tsai et al 16 detected the mRNA expression of ENO1 in 44 patients with head and neck cancer and found that the expression of ENO1 in human head and neck cancer tissues was significantly higher than in the adjacent normal tissues. Furthermore, the high expression of ENO1 was significantly correlated with tumor clinical stage, poor prognosis, and distant metastasis. After knocking out ENO1 or using RNA interference (RNAi) to silence ENO1 gene expression, the invasion and metastasis abilities of ENO1enolase-overexpressing cells were significantly reduced. Tu et al 17 demonstrated in vitro that decreasing the expression of the ENO1 gene through small interfering RNA in tamoxifen-resistant breast cancer cells significantly enhanced 4-hydroxytamoxifen-induced cytotoxicity. Zhou et al 8 showed that the ENO1 expression was closely correlated with the tumor differentiation. The expression of ENO1 in 55 cases of gastric carcinoma tissues and 23 cases of gastric ulcer tissue was detected by Ni et al,18 and this study revealed that the expression of ENO1 in gastric cancer tissues was significantly higher than that in gastric ulcer tissues (P < .01) and positively related to differentiation grade, depth of invasion, lymph node metastasis, and TNM staging (P < .05). In poorly differentiated hepatocellular carcinomas, ENO1 expression was significantly increased, and positive ENO1 expression was associated with tumor size and vascular invasion. The interfering RNA-induced decrease in ENO1 expression reduced the rate of proliferation in hepatocellular carcinoma and prolonged the G2/M phase of the cell cycle.19,20 It has been reported recently that in NSCLC, the downregulation of ENO1 decreased proliferation, migration, and invasion through an FAK-mediated PI3K/AKT pathway.21
With the continuous development of RNAi technology, gene therapy for tumors has developed rapidly. RNA interference is an eukaryotic phenomenon that has been highly conserved during biological evolution and uses double-stranded RNA to block specific gene sequences in cells. This effectively inhibits the mRNA and protein expression of the target gene, which plays a very important role in the evolution of many organisms. This technique has been widely used for investigating gene function and tumor gene therapy because RNAi has the advantage of specifically removing or blocking the expression of specific genes. RNA interference technology plays an important role in the study of oncogenes and antioncogenes. In many ways, gene therapy for gastric cancer has made astounding progress. However, most gene therapy approaches are in the early stages of clinical trials or in the initial stage of experimental research, but gene therapy combined with radiotherapy and chemotherapy has become a popular topic for cancer treatment. Studies have shown that compared with the normal group, the reduction of ENO1 expression in an ENO1-knockout breast cancer mouse model decreased the hypoxia response and increased cell sensitivity to radiotherapy.22 RNA interference technology improves the sensitivity of tumor cells to radiotherapy and chemotherapy and offers hope that oncogenes can be treated and patients cured. Gene therapy plays an important role in the treatment of cancer and is a popular topic in tumor therapy research. Our research has also focused on selecting a gene therapy target. To study the effect of ENO1 gene interference on the proliferation of gastric cancer cell lines, the PLKO.1-ENO1 shRNA expression vector was constructed, and the ENO1 shRNA gene was integrated into the gastric cancer MKN45 cell line by lentivirus. After being screened by puromycin, gastric cancer cells that stably expressed the ENO1 shRNA gene were cloned to study ENO1 gene function. RT-qPCR and Western blotting were used to detect the effect of shRNA interference. α-Enolase shRNA effectively inhibited the gene and protein expression of ENO1 in gastric cancer cells. Furthermore, MTT assays were used to detect the growth ability of cells, and the results revealed that cell growth in the ENO1 shRNA group was significantly lower than that in the empty vector and blank control group (P < .05). This indicated that ENO1 shRNA can inhibit the growth of MKN45 cells. Plate cloning assays represent the proliferation ability of cells. The experimental results revealed that the proliferation ability of cells was significantly decreased (P < .05) after ENO1 interference, indicating that the proliferation of ENO1 shRNA was significantly decreased. Furthermore, we used flow cytometry to detect the cell apoptosis and found that it was significantly increased after ENO1 silencing. At the same time, the MTT method was used to detect the sensitivity of cells to chemotherapeutic drugs after ENO1 interference. The results revealed that the ENO1 shRNA group exhibited a significant increase in sensitivity to chemotherapeutic drugs, compared to the empty vector and blank control groups. This indicates that ENO1 gene silencing can enhance the sensitivity of gastric cancer cells to chemotherapeutic drugs, such as DDP and 5-FU. This study focused on the role of ENO1 in cell proliferation and drug sensitivity. However, additional in-depth research and in vivo experiments are needed to further confirm these findings.
Conclusion
In conclusion, ENO1 gene silencing can inhibit the proliferation of the gastric cancer MKN45 cell line and increase its sensitivity to chemotherapeutics. Identifying the key signaling pathway protein involved in ENO1 function may provide a new experimental basis for the further study of gastric cancer and for gene therapies for other tumors. Furthermore, it also provides new ideas for the clinical treatment of cancer.
Acknowledgments
The authors would like to acknowledge Qian-Qian Wang, MM, for helping with completing part of the experiment.
Abbreviations
- BCA
bicinchoninic acid
- DDP
cisplatin
- DMEM
Dulbecco’s Modified Eagle Medium
- DMSO
dimethyl sulfoxide
- ENO1
α-enolase
- FBS
fetal bovine serum
- mRNA
messenger RNA
- MTT
methyl-thiazolyl-tetrazolium
- NSCLC
non-small cell lung cancer
- OD
optical density
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- RIPA
radioimmunoassay precipitation assay
- RNAi
RNA interference
- shRNA
short hairpin RNA
- TBST
Tris-buffered saline Tween-20
- 5-FU
5-fluorouracil.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Gansu Health Industry Planning Project (GSWST2013-16), Gansu Science and Technology Project (1504FKCA084), and The First Hospital of Lanzhou University Hospital Fund (ldyyyn2014-09).
References
- 1. Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11(5):531–546. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. [DOI] [PubMed] [Google Scholar]
- 3. Abbasi SY, Taani HE, Saad A, Badheeb A, Addasi A. Advanced gastric cancer in Jordan from 2004 to 2008: a study of epidemiology and outcomes. Gastrointest Cancer Res. 2011;4(4):122–127. [PMC free article] [PubMed] [Google Scholar]
- 4. Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Radiother Oncol. 2014;110(1):189–194. [DOI] [PubMed] [Google Scholar]
- 5. Ilson DH. Current progress in the adjuvant treatment of gastric cancer. Surg Oncol Clin N Am. 2017;26(2):225–239. [DOI] [PubMed] [Google Scholar]
- 6. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 8. Zhou X, Yao K, Zhang L, et al. Identification of differentiation-related proteins in gastric adenocarcinoma tissues by proteomics. Technol Cancer Res Treat. 2016;15(5):697–706. [DOI] [PubMed] [Google Scholar]
- 9. Brand RA. Biographical sketch: Otto Heinrich Warburg, PhD, MD. Clin Orthop Relat Res. 2010;468(11):2831–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warburg OH. The classic: the chemical constitution of respiration ferment. Clin Orthop Relat Res. 2010;468(11):2833–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsiao KC, Shih NY, Chu PY, et al. Anti-alpha-enolase is a prognostic marker in postoperative lung cancer patients. Oncotarget. 2015;6(33):35073–35086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vizin T, Kos J. Gamma-enolase: a well-known tumour marker, with a less-known role in cancer. Radiol Oncol. 2015;49(3):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Isgro MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125–143. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Li M, Liu Y, et al. ENO1 protein levels in the tumor tissues and circulating plasma samples of non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi. 2010;13(12):1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cappello P, Principe M, Bulfamante S, Novelli F. Alpha-Enolase (ENO1), a potential target in novel immunotherapies. Front Biosci (Landmark Ed). 2017;22:944–959. [DOI] [PubMed] [Google Scholar]
- 16. Tsai ST, Chien IH, Shen WH, et al. ENO1, a potential prognostic head and neck cancer marker, promotes transformation partly via chemokine CCL20 induction. Eur J Cancer. 2010;46(9):1712–1723. [DOI] [PubMed] [Google Scholar]
- 17. Tu SH, Chang CC, Chen CS, et al. Increased expression of enolase alpha in human breast cancer confers tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat. 2010;121(3):539–553. [DOI] [PubMed] [Google Scholar]
- 18. Ni TG, Gao C, Zhou X, Wang N, Zeng F, Guan QL. Expression of enolase-α and tumor M2 pyruvate kinase in gastric cancer and their clinical significances [in Chinese]. Chin J Cancer Biother. 2011;18(5):524–527. [Google Scholar]
- 19. Peng B, Huang X, Nakayasu ES, et al. Using immunoproteomics to identify alpha-enolase as an autoantigen in liver fibrosis. J Proteome Res. 2013;12(4):1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rojpibulstit P, Kittisenachai S, Puthong S, et al. Hep88 mAb-initiated paraptosis-like PCD pathway in hepatocellular carcinoma cell line through the binding of mortalin (HSPA9) and alpha-enolase. Cancer Cell Int. 2014;14:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu QF, Liu Y, Fan Y, et al. Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol. 2015;8:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao J, Zhao R, Xue Y, et al. Role of enolase-1 in response to hypoxia in breast cancer: exploring the mechanisms of action. Oncol Rep. 2013;29(4):1322–1332. [DOI] [PubMed] [Google Scholar]